Abstract
Astrocytes represent an abundant type of glial cell involved in nearly every aspect of central nervous system (CNS) function, including synapse formation and maturation, ion and neurotransmitter homeostasis, blood-brain barrier maintenance, as well as neuronal metabolic support. These various functions are enabled by the morphological complexity that astrocytes adopt. Recent experimental advances in genetic and viral labeling, lineage tracing, and live- and ultrastructural imaging of miniscule astrocytic sub-compartments reveal a complex morphological heterogeneity that is based on the origin, local function, and environmental context in which astrocytes reside. In this minireview, we highlight recent findings that reveal the plastic nature of astrocytes in the healthy brain, particularly at the synapse, and emerging technologies that have advanced our understanding of these morphologically complex cells.
INTRODUCTION
Many of the functions now ascribed to astrocytes were postulated nearly 100 years ago by Santiago Ramón y Cajal (1852–1934), who used metal impregnation staining methods to label astrocytes[1]. His hand-drawn images revealed an intimate relationship shared between astrocytes, neuronal cell bodies and their processes, and the vasculature (Fig 1A). However, these images failed to capture the entirety of the astrocyte volume. Nearly a century later, Bushong et al. utilized iontophoresis to fill single astrocytes, demonstrating that mature protoplasmic astrocytes densely ramify into the brain parenchyma during early postnatal development in the rat hippocampus[2]. Immature astrocytes extend stringy filopodial processes which initially overlap with neighboring astrocyte processes, but ultimately refine to develop non-overlapping domain boundaries comprised of leaflet-like processes[2] (Fig 1B, C). These processes are so small (10–200 nm in width), they fall below the diffraction barrier of traditional light microscopy[3, 4], yet are estimated to comprise nearly 80% of the cell volume[4]. The dense network of astrocyte processes allow single astrocytes to engage in bidirectional communication with neighboring astrocytes, contact 10’s of neurons, 100’s of neuronal dendrites, and up to 100,000 individual synapses[5]. These values vary depending by brain region and are estimated to be 10-fold higher in humans whose astrocyte volumes are as much as 30-fold larger than rodent astrocytes[6]. The complexity of astrocyte branching has recently been classified and organized into sub-compartments defined as primary branches (1st order branches that radiate from the soma), branchlets (2nd and 3rd order branches), leaflets (perisynaptic astrocyte processes), and endfeet (specialized structures that enwrap vasculature)[6]. The study of astrocyte morphology is still very much in its infancy, as cellular heterogeneity remains an ongoing subject of study. Advanced techniques in cell labeling, sequencing, and visualization, however, are now beginning to unravel molecular mechanisms that drive astrocyte morphological complexity and structural plasticity.
FIGURE 1. Astrocytes are morphologically complex cells.
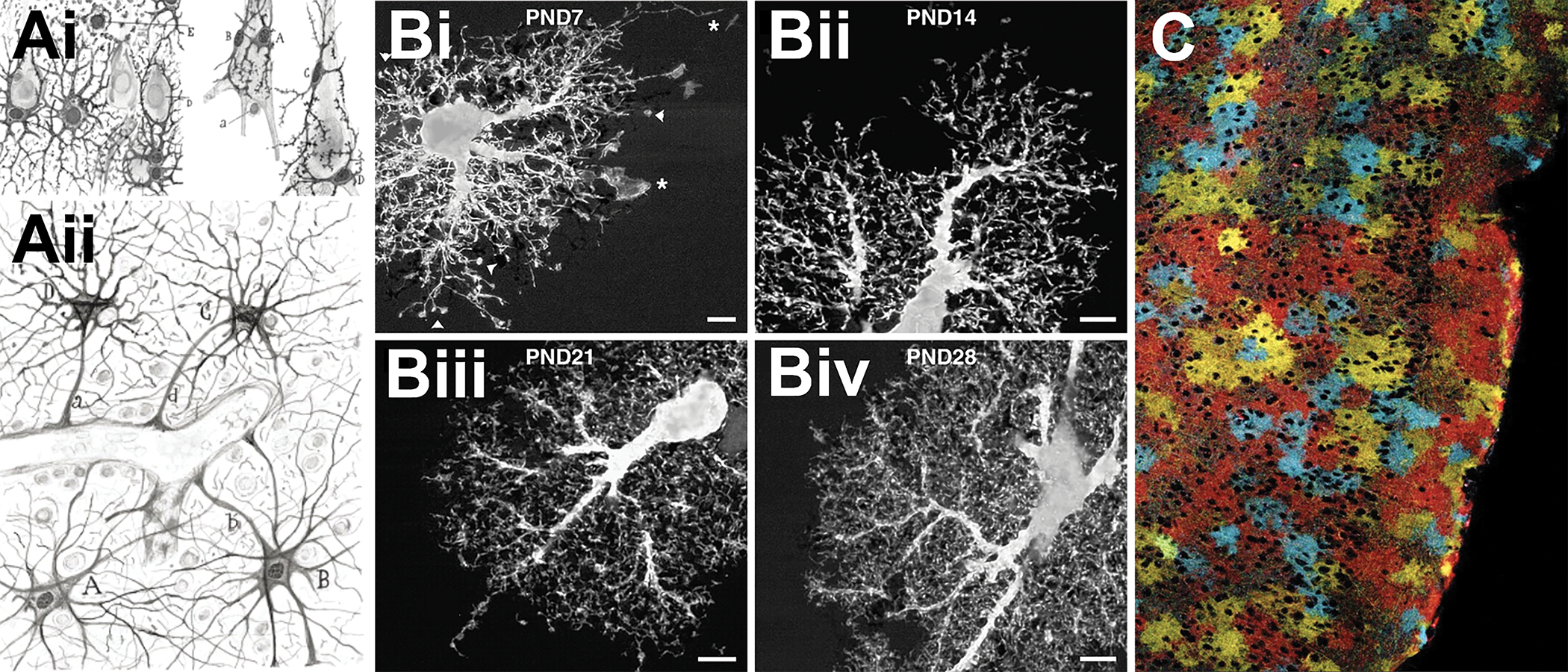
Ai) Hand drawn illustrations by Santiago Ramón y Cajal depicting astrocytes in the brain contacting neurons and Aii) the vasculature. Ramon y Cajal’s illustrations revealed the stellate-nature of astrocyte morphology[1]. Nearly a century after Ramón y Cajal’s illustrations, Bi-Biv) dye-filling of single astrocytes in rat hippocampus revealed that astrocytes are densely ramified cells composed of numerous leaflet-like processes. It was observed that astrocytes undergo a dynamic phase of morphological maturation between the first 4 postnatal weeks [2]. These processes have little overlap with other astrocytes in such a way that astrocytes tile the brain parenchyma. This tiling may not have been fully appreciated until C) the first glial “Brainbow” mice were developed and enabled for mosaic expression of numerous combinations of fluorescent proteins to depict the complexity of astrocytes and their ability to tile and infiltrate the CNS[53].
ASTROCYTE DEVELOPMENT, HETEROGENEITY, AND MATURATION
In the rodent cortex, astrocyte birth and migration take place late in embryonic development through the first few postnatal weeks[7]. Until recently, it was unclear if cortical astrocytes were produced by a singular pool of predetermined progenitors, or from non-specified progenitors. In addition, progenitor cells were considered to migrate radially from the subventricular zone, proliferating on their way out to give rise to clusters of astrocytes in the same cortical column[8]. This idea was recently challenged by applying multiclonal lineage tracing combined with a new large-volume chromatic multiphoton serial microscopy technique (ChroMS)[9]••. Here, a combinatorial MAGIC Marker (MM) approach, which uniquely labeled the nuclei and cytoplasm of individual progenitors marked prior to gliogenesis, enabled lineage tracing of numerous astrocyte clones through early development. This study identified that astrocytes arise from both pre- and early postnatal glial progenitors which migrate stochastically, undergo local proliferation, and disperse across cortical cell layers and columns, generating both pial and protoplasmic cortical astrocytes, which have striking morphological differences (Fig 2a). This is followed by a maturation phase whereby astrocyte morphological complexity increases and is independent of origin cell, but perhaps driven by extrinsic cues[9] (Fig 2a). These findings suggest that astrocyte development and maturation is plastic, rather than stereotyped and supports recent observations regarding astrocyte heterogeneity.
FIGURE 2. Astrocyte Morphological Development.
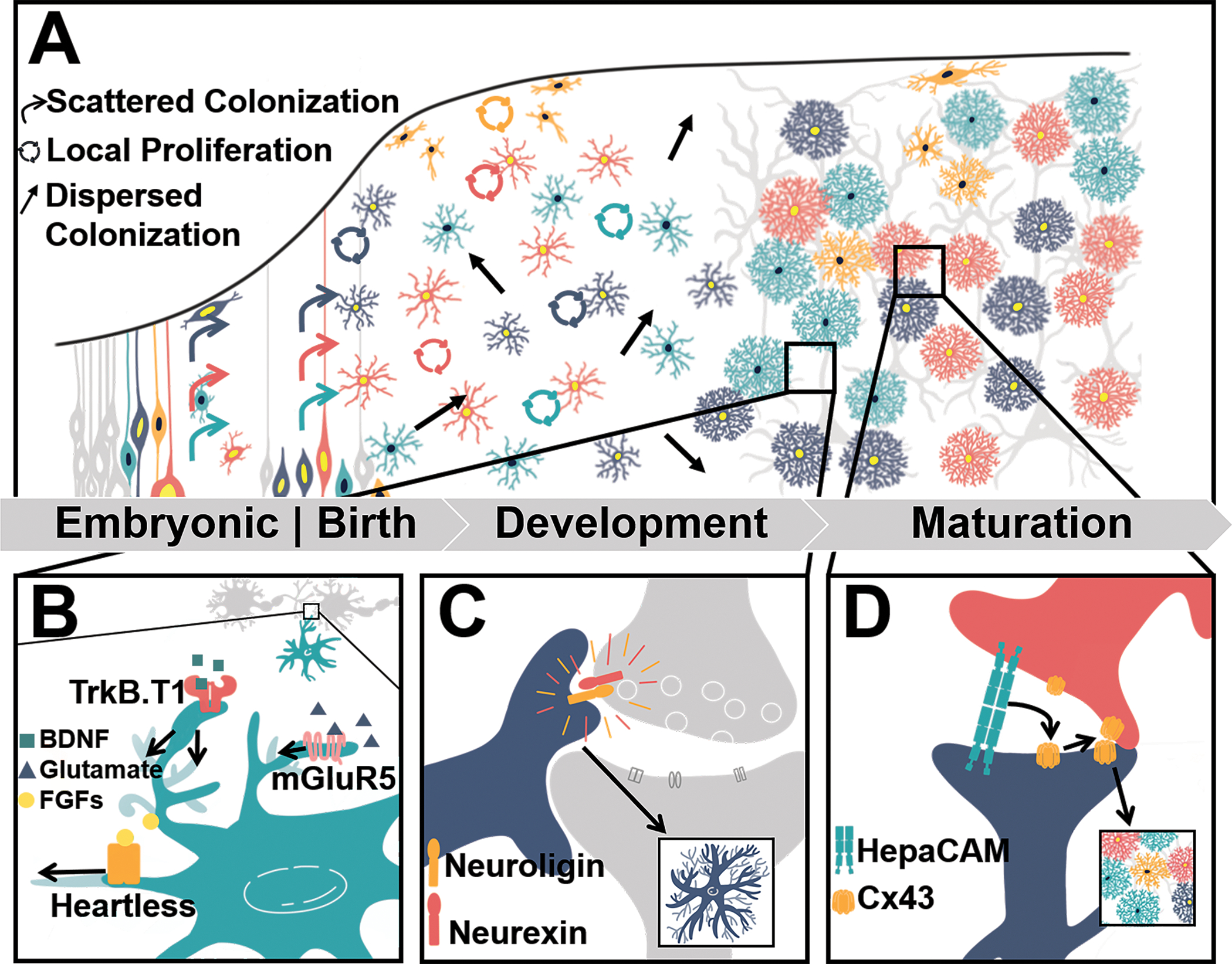
A) Clavreul et al.’s recent work examined astrocyte cortical developmental by tracing numerous astrocyte progenitors marked prior to gliogenesis. This revealed that both pre- and postnatal astrocyte progenitors undergo a period of sporadic proliferation and randomized spreading across the cortex during early development, before finally adopting unique morphological profiles during the period of maturation[13]. The underlying mechanisms of this morphological maturation are beginning to be resolved and they include B) signaling mechanisms such as Fibroblast Growth Factor (FGFs)/astrocytic Heartless signaling[17], glutamate/astrocytic mGluR5 signaling[18], BDNF/astrocytic TrkB.T1 signaling[19]. C) Cell adhesion mechanisms that involve astrocytic neuroligin contact with neuronal neurexins have also been demonstrated to underlie astrocyte morphology[20]. D) Interestingly, the ability for astrocytes to form their non-overlapping territories and gap-junctional networking has been shown to rely on astrocyte-to-astrocyte contacts via astrocyte HepaCAM interactions[21]. Altogether, both neuronal-derived cues and astrocytic collaborations sculpt the morphology of individual astrocytes and give rise to their heterogeneity.
The underlying molecular mechanisms driving astrocyte heterogeneity are not yet understood, yet recent evidence suggests layer and brain region specific heterogeneity is driven in part by environmental and/or extrinsic cues. For example, single cell RNA-sequencing (scRNA-seq) in cortex and hippocampus recently revealed five distinct subpopulations of astrocytes based on molecular profiles, morphology, and Ca2+ dynamics[10]•. Within a given brain region, two- and three-dimensional reconstructions of astrocytes identified distinct morphological profiles across all six layers of the somatosensory cortex[11]••. Electron microscopy (EM) of synapses established that synaptic coverage by astrocyte leaflets also varied across cortical layers such that 78% of upper layer axon-dendrite interfaces contained astrocyte contacts, while this fraction was a mere 30% in deep layer axon-dendrite interfaces. scRNA-sequencing revealed that these morphological differences were also correlated with unique molecular profiles across layers[11]. Similarly, large-area spatial transcriptomic (LaST) mapping, a novel approach which quantifies single-cell gene expression in situ has also revealed distinct astrocytic profiles across cortical layers[12]•. Genetic manipulations to abolish neuronal migration, resulting in an “inside out– inversion” of cortical organization, abolished the morphological[12] and molecular[11, 12] heterogeneity of astrocytes across cortex. Even cell autonomous or intrinsic astrocyte mechanisms are subject to environmental influence. For example, ablation of Sox9 or NFIA, both of which are globally expressed in astrocytes, demonstrates region-specific roles on astrocyte morphology. Sox9 ablation in mature astrocytes impaired astrocyte morphology in olfactory bulb, but spared astrocytes in other brain regions[13]•, while NFIA ablation stunted astrocyte morphology and Ca2+ signaling specifically in hippocampus[14]. Similarly, ablation of the astrocyte cytoskeletal protein, Daam2, increased morphological complexity and Ca2+ dynamics in olfactory bulb but decreased in cortex[15]. This highlights the need to study how transcription factors and even cytoskeletal proteins can interact differentially across brain regions to give rise to morphological and functional heterogeneity. Altogether, these findings suggest that neuronal organization and local environments sculpt astrocyte morphology and heterogeneity. However, the signaling mechanisms through which this occurs remain to be fully elucidated.
Neuronal Cues Sculpt Astrocyte Morphology
Early work identified neuronal-derived Fibroblast Growth Factor (FGF) [16] and glutamate signaling[17] as mediators of astrocyte morphogenesis. In Drosophila, genetic ablation of the astrocytic FGF receptor Heartless (Htl) and manipulations of its ligands, Pyramus and Thisbe, demonstrated the Htl signaling pathway as a key player underlying the infiltration of astrocytic processes into the neuropil and synapses (Fig 2b)[16]. In mice, inhibition of glutamate release via genetic ablation of the vesicular glutamate transporter 1 (VGluT1) resulted in astrocytes with reduced astrocyte volumes and synaptic interactions[17], suggesting glutamate signaling onto astrocytes plays a role in astrocyte morphology. This notion was supported by showing that conditional deletion of the astrocyte metabotropic glutamate receptor, mGluR5, in developing cortical astrocytes stunts morphological complexity[17] (Fig 2b). Similarly, brain derived neurotrophic factor (BDNF), presumably of neuronal origin, drives astrocyte morphogenesis by signaling to the astrocyte TrkB.T1 receptor (Fig 2b). Manipulation of TrkB.T1 using global and astrocyte specific manipulations resulted in astrocytes which fail to attain typical morphological complexities during the critical period of morphological maturation[18]. Contact-dependent cues have also been identified. The neuroligin family cell adhesion molecules (NL1-3) expressed in cortical astrocytes influence astrocyte morphogenesis through interactions with neuronal cell adhesion molecules neurexins (Fig 2c). In vivo, both short hairpin RNA-driven (shRNA) and conditional deletion of NLs led to disrupted astrocyte morphogenesis[19].
While these signaling mechanisms derived from neuronal cues may underlie the morphological maturation of individual astrocytes, this does not explain how astrocytes coordinate with one another to establish their non-overlapping domains. HepaCAM is primarily localized at astrocyte-astrocyte cell junctions by forming interactions that facilitate the targeting of transmembrane proteins. Disruption of astrocytic HepaCAM both in vitro and in vivo using shRNA-driven knockdown, resulted in morphologically immature astrocytes in both astrocyte neuron co-cultures and in cortical astrocytes respectively[20]••. In addition, hepaCAM appears as a key player in the localization of Connexin 43 (Cx43), a gap junction protein highly expressed in astrocytes, thus influencing the ability of astrocytes to form gap-junctional networks and compete for territory as they morphologically mature and infiltrate the brain (Fig 2d).
Together, these studies suggest that extrinsic factors, which may be layer specific, including neuronally derived molecules (Fig 2b–c) and adhesion molecules (Fig 2b–c) have an overlapping role guiding astrocyte morphogenesis. While the signaling mechanisms described above (Figure 2) begin to shed light on molecules that facilitate morphological maturation, little is understood regarding the downstream signaling mechanisms that mediate structural organization in astrocytes
THE ASTROCYTE CYTOSKELETON
Ultimately, the elaboration of astrocytic processes is driven by a combination of microtubules, intermediate filaments, and actin dynamics. The major processes of astrocytes are rich in intermediate filaments such as glia fibrillary acidic protein (GFAP), a protein frequently used as a marker to identify astrocytes[5, 21] and microtubule proteins[22, 23] such as α-tubulin[24]. Both microtubules and intermediate filaments are restricted to the primary and secondary branches that emanate from the soma[23]. The peripheral, leaflet-like processes which comprise much of the astrocyte volume exclude these large filaments and instead rely on actin-dynamics for their structure, much like neuronal spines (Fig. 3a). Early in vitro studies identified the actin-nucleating Arp2/3 complex to be important for stellation, where inhibition of this complex through PICK1 and N-WASP molecules induces the rapid extension of processes in cultured astrocytes[25]. Rho-GTPases, key players in cytoskeletal rearrangements, have also been shown to affect astrocyte morphology in vitro by triggering downstream signaling required for actin dynamics[26, 27]. Further, the activation of PKC-ε was found to regulate actin-interacting elements such as ezrin[28], an actin binding protein recently found to be rich in leaflets[29] and important for leaflet plasticity triggered by glutamate signaling onto astrocytic mGluR receptors[30]. Ezrin has been shown to interact with other cell-adhesion molecules such as CD44 and F-actin to tether membranes[31] (Fig 3a) and ultimately aid in motility.
FIGURE 3. Astrocyte cytoskeletal dynamics give rise to plasticity at the synapse.
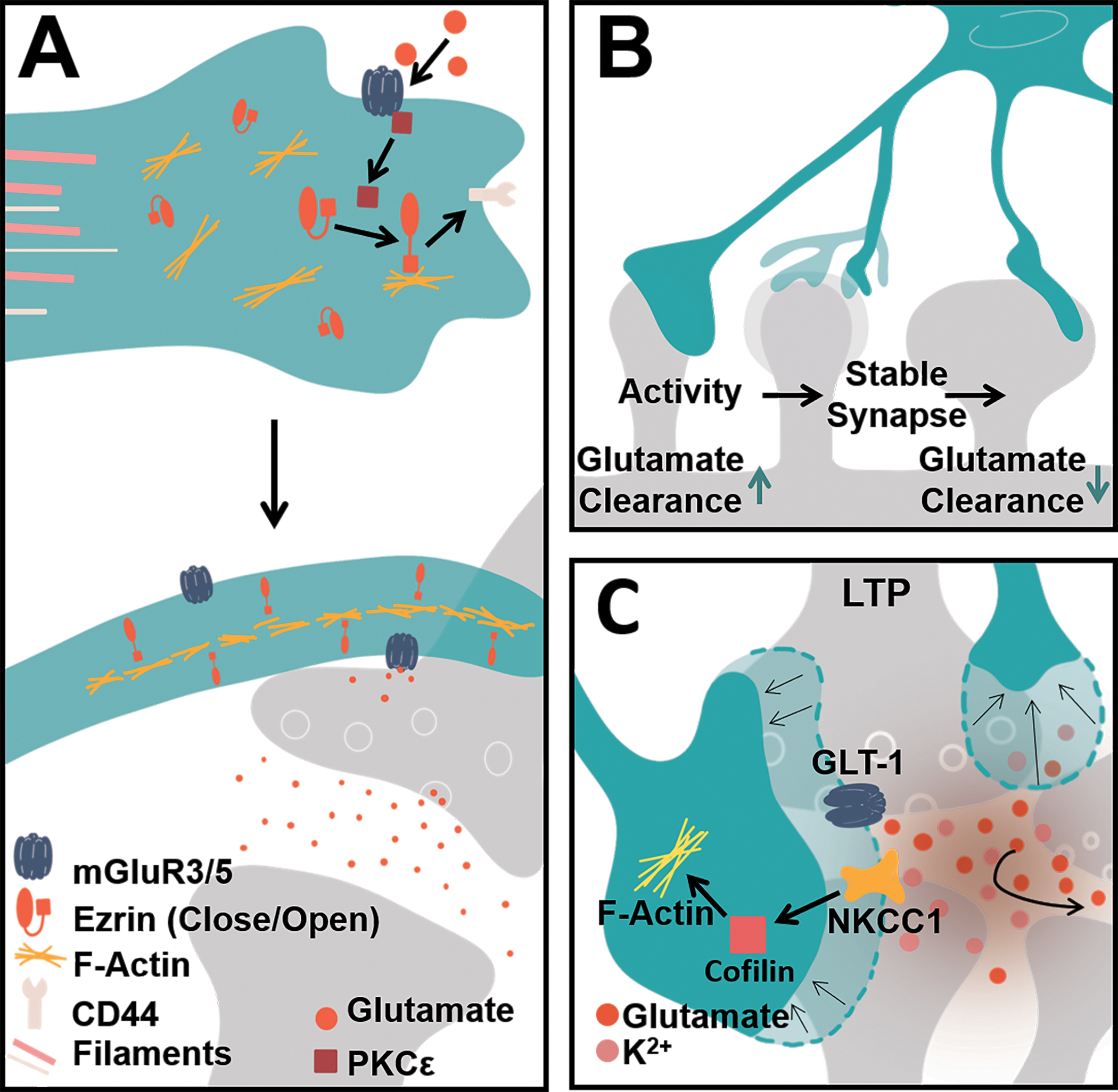
A) The astrocyte cytoskeleton is composed of microtubules and intermediate filaments such as GFAP that reside in the major primary and secondary branches. Actin dynamics such as Ezrin and its kinases (e.g. PKCɛ) have been identified to reside in astrocyte leaflets that associate with synapses. In this way, glutamatergic signaling can trigger astrocyte plasticity. One possible mechanism this occurs through is by direct glutamate signaling onto the astrocytic mGluR5 receptor. Glutamate binding activates and releases receptor-associated PKCɛ which can in turn phosphorylate ezrin. Phosphorylation of ezrin triggers a conformational change to its open state. Activated and open ezrin can then tether actin filaments to the membrane by linking with membrane-bound proteins such as CD44. This enables membrane motility and overall plasticity. B) This plasticity may vary depending on the synapse and the degree of leaflet coverage at a synapse. Smaller spines have been observed to have larger degrees of leaflet coverage and GLT1/GLAST-mediated glutamate clearance, perhaps enabling immature spines to enlarge and mature. Activity such as that from LTP has been observed to trigger leaflet rearrangement at spines. C) In vivo imaging has revealed that leaflet plasticity can occur in the span of minutes. Following LTP, leaflet plasticity at potentiated synapses begins with the initial withdrawal of leaflets. This allows for inter-synaptic glutamate communication. The increase in glutamate at potentiated synapses and consequent release of K+ leads to NKCC1 transporter-mediated actin engagement and leaflet motility at potentiated synapses.
While a handful of recent studies have evaluated the relationship between intermediate filaments and actin organization in the context of injury[32–34] surprisingly few studies have evaluated these proteins in the context of astrocyte morphogenesis or in astrocyte structural plasticity that occurs in response to signaling in the healthy brain. Given the role of astrocyte leaflets in synapse function, elucidating their cytoskeletal dynamics is critical for understanding the morphology, plasticity, and orchestration of protein trafficking in these structures.
UNDERSTANDING ASTROCYTE STRUCTURAL PLASTICITY AT THE TRIPARTITE SYNAPSE
The tripartite synapse[35] is comprised of the pre- and post-synaptic neuronal elements, and terminal leaflet processes whereby astrocytes dynamically cradle synapses[36]. Generally, astrocytic coverage of synapses is thought to allow for sensing of activity, modulation of neuronal signaling, prevention of neurotransmitter spillover, neurotransmitter uptake, ionic homeostasis, stabilization of synapses via cell adhesions, and the release of synaptogenic factors[37]. Studies have shown leaflet processes to be highly plastic in response to various physiological stimuli including changes in metabolism[38], motor learning[39] sleep/wake states[40], and lactation[41, 42]. These findings suggest that astrocytes interact with their environment through leaflet shapeshifting.
Glutamate: a primary driver of astrocyte structural plasticity at excitatory synapses
The mechanisms that engage leaflets to rearrange their association with synapses appear to be highly dependent on glutamate. Astrocytes sense neuronal activity at glutamatergic synapses in part, by high levels of expression of the Na+ dependent glutamate transporters (GLAST and GLT1, in rodents) and metabotropic glutamate receptors (mGluR3/5). In fact, increasing glutamate availability and signaling through direct activation of astrocyte mGluRs or through glutamate uncaging increases astrocyte leaflet motility and cradling at potentiated synapses[43]. This glutamate/mGluR signaling has previously been shown to engage ezrin and actin dynamics[30] (Fig 3a). Expansion microscopy (ExM), an approach where tissues and molecules of interest are isotropically enlarged to allow visualization of sub-diffraction limited samples with conventional light microscopy, identified higher GLT-1 expression on astrocyte leaflets located around immature spines relative to leaflets at mature spines[44]•. This observed phenomenon may serve to limit glutamate spillover as spines develop (Fig 3b). In the context of LTP, high frequency stimulation at the CA3-CA1 synapse, astrocytes demonstrate rapid changes in volume and leaflet plasticity[45]••. Combining stimulated emission depletion (STED) microscopy, which enables the live monitoring of astrocytes and structures beyond the optical diffraction limit, patch-clamping, and optical glutamate sensing, it was demonstrated that astrocyte leaflets at potentiated synapses retract to boost glutamate spillover and facilitate NMDA-receptor-mediated synaptic crosstalk. This LTP-induced leaflet withdrawal was found to depend on activation of NKCC1 transporters, key players in astrocyte volume regulation[46], and cofilin, an actin-associated protein that triggers cytoskeletal remodeling (Fig 3c). While previous EM studies revealed that LTP[43, 47] and chronic stimulation of whiskers[48] increased leaflet synapse coverage, EM fails to track early leaflet plasticity that occurs within minutes. By combining advanced live imaging, EM, and super-resolution microscopy such as STED and ExM, the study of astrocytic morphology is beginning to reveal not only the plastic nature of distal astrocytic processes, but the precise complexity that these processes contain both structurally and functionally.
A CLOSER LOOK AT ASTROCYTE LEAFLET PROCESSES AND MORPHOLOGY
Classically, terminal leaflets emanating from 2nd and 3rd order branchlets were considered the astrocyte compartment of the tripartite synapse. However, recent 3D reconstructions from Serial Block Face Scanning Electron Microscopy (SBF-SEM) images demonstrated that astrocyte/synapse interactions occur in several astrocyte sub compartments including the soma, primary and secondary branches, and even endfeet, challenging the idea that synaptic elements are exclusive to terminal leaflets [49]••. Detailed analysis of nearly 1000 synapses revealed most of these astrocyte/synapse contacts occurred at asymmetric (excitatory) synapses, with the greatest density of synapses abutting terminal leaflets. Intriguingly, it was observed that leaflets often formed loop-like structures, or reflexive contacts[49]. These loop structures do not appear to be an artifact of tissue fixation as recent advances in live imaging also support the notion of astrocyte processes form loop structures. For example, live STED microscopy identified that most synaptic spines in hippocampus and barrel cortex are associated with loop-like structures called Ca2+ “nodes”, so called, because astrocyte Ca2+ signaling is highly circumscribed within these structures in response to spontaneous neuronal activity and whisker barrel stimulation[50]••. Similarly, SU-per-resolution Shadow Imaging (SUSHI), a technique that labels the extracellular space with a membrane-impermeant fluorescent dye so that cellular structures appear as sharp, contrasted shadows under STED microcopy, also identified ring-like microstructures throughout the spongiform domains of astrocytes[51]•. 3D-STED microscopy of astrocyte-labeled organotypic hippocampal slices treated with SUSHI allowed for high resolution visualization of astrocyte arborizations. These ring-like structures were often observed to enwrap pools of interstitial fluid and cellular structures that were assumed to be dendritic spines based on size and morphology[51]. Together, these high-resolution imaging approaches reveal astrocyte loop-like structures represent a specialized compartment, akin to the neuronal spine; a structure that can restrict Ca2+ and potentially other biochemical signaling needed for actin dynamics and overall astrocyte structural plasticity in response to neuronal activity[52]. Altogether, these approaches also begin to uncover novel details regarding astrocyte morphology and illustrate the need to study the functional dynamics of these details in CNS function.
CONCLUDING REMARKS
While accumulating evidence has illustrated the complexity, heterogeneity, and plasticity of astrocyte morphology, many questions remain regarding morphogenesis and structural plasticity. These include intrinsic, cell autonomous mechanisms (i.e. transcription factors and associated gene expression) as well as extrinsic, non-cell autonomous factors, including neurons, glia cells, and the extracellular space. Furthermore, little is known regarding the mechanisms that engage actin reorganization to drive astrocyte plasticity during development and in response to neuronal signaling. Glutamate sensing appears a key player in plasticity at the synapse. Studies at other synapse types (i.e. GABAergic) are needed to determine how different synapses may differentially sculpt astrocyte morphology and function. By combining advanced imaging approaches that zoom into the diffraction-limited structures of astrocytes, and perhaps exploiting tools and technical approaches that helped us understand neuronal structure and plasticity at the synapse, we can begin to understand the intricate mechanisms that underlie astrocyte structural plasticity. The intricately complex morphology of astrocytes gives rise to their ability to interact and respond to thousands of synapses at time, therefore, understanding the mechanisms of astrocyte morphogenesis is critical to understanding CNS health and identifying targets relating to disease.
FIGURE 4. Astrocyte sub-compartments revealed with high resolution microscopy.
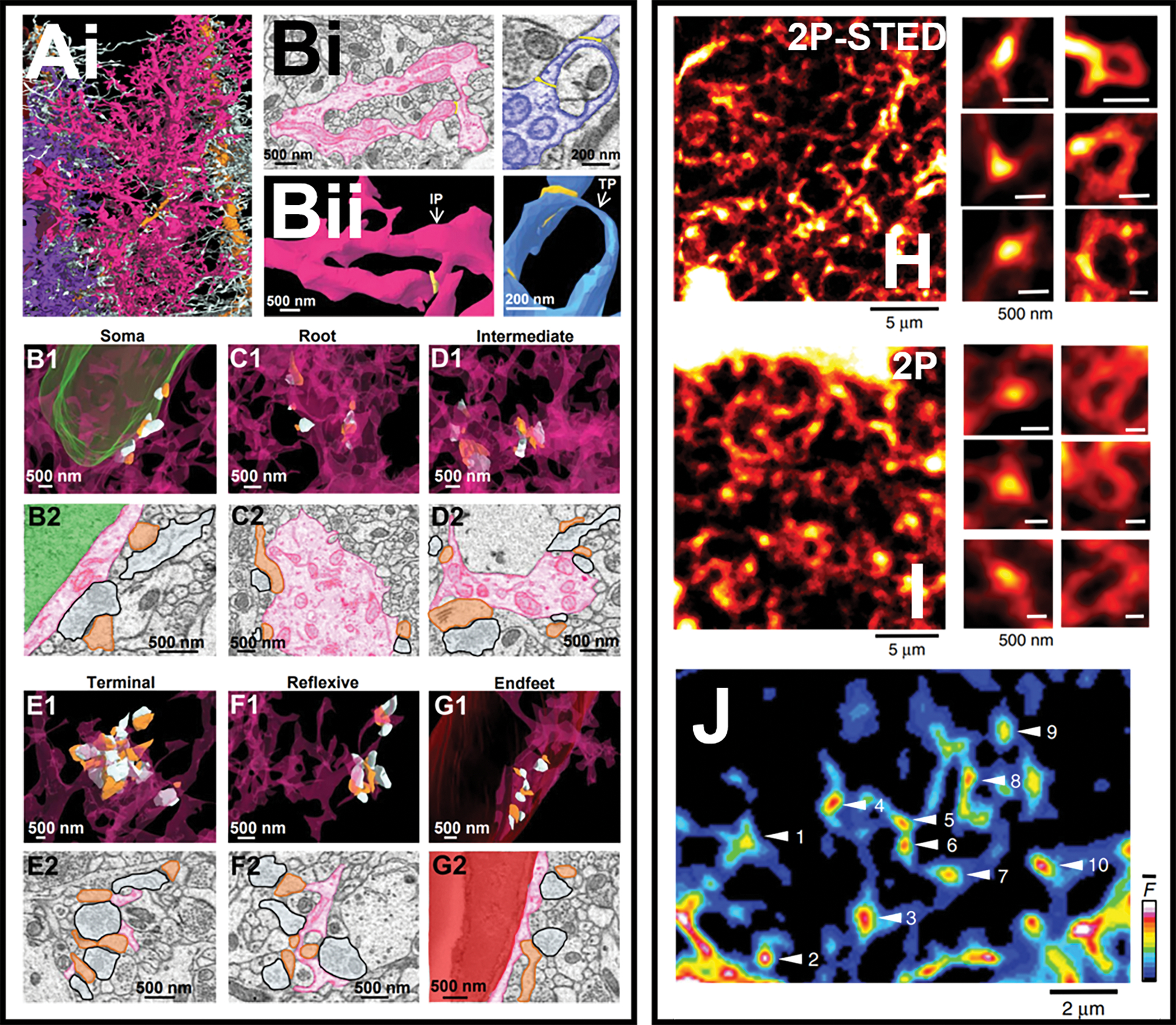
Ai) 3D reconstruction of neighboring astrocytes (purple, pink, white) associated with dendrites (orange). A closer look at astrocytic processes reveals that many leaflets create reflexive processes. Bi) 2D EM traces of reflexive process pseudo-colored as pink and purple with Bii) their corresponding 3D reconstructions reveal these reflexive processes are loops-like structures. In the same study, evaluation of each astrocyte sub-compartment revealed that synapses associate with astrocytic B1-B2) somas, C1-C2) root processes and D1-D2) intermediate processes, E1-E2) terminal leaflets, F1-F2) reflexive loops, and G1-G2) endfeet. Interestingly, similar loop-like structures are also found in situ and in vivo. H) Left: 2Photon-STED image of the spongiform domain of an astrocyte from an acute slice of dentate gyrus. Right: Increased magnification reveals that astrocyte processes form loop-like structures termed Nodes. The same structures are observed using 2Photon microscopy in vivo in the whisker barrel cortex. I) Left: spongiform domain of an astrocyte in barrel cortex imaged with 2 Photon microscopy. Right: Increased magnifications reveal hot spot nodes and loop like structures. J) Ca2+ imaging reveals that astrocyte nodes/loop-like structures are compartments of high Ca2+ dynamics.
HIGHLIGHTS.
Astrocytes rely on their environment to sculpt their morphological complexity.
Astrocyte morphological heterogeneity exists within and across brain regions.
Dynamic astrocyte plasticity has been observed at glutamatergic synapses.
Advanced microscopy reveals novel astrocyte sub-compartments and synaptic contacts.
Acknowledgements
This work was supported by funds from the National Institutes of Health
Grants R01 NS120746 and R01 HL104101 to MLO.
Footnotes
Conflict of interest statement
Nothing declared
References
- 1.Garcia-Marin V, Garcia-Lopez P, and Freire M, Cajal’s contributions to glia research. Trends Neurosci, 2007. 30(9): p. 479–87. [DOI] [PubMed] [Google Scholar]
- 2.Bushong EA, Martone ME, and Ellisman MH, Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci, 2004. 22(2): p. 73–86. [DOI] [PubMed] [Google Scholar]
- 3.Rusakov DA, Disentangling calcium-driven astrocyte physiology. Nat Rev Neurosci, 2015. 16(4): p. 226–33. [DOI] [PubMed] [Google Scholar]
- 4.Medvedev N, et al. , Glia selectively approach synapses on thin dendritic spines. Philos Trans R Soc Lond B Biol Sci, 2014. 369(1654): p. 20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushong EA, et al. , Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci, 2002. 22(1): p. 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oberheim NA, et al. , Uniquely hominid features of adult human astrocytes. J Neurosci, 2009. 29(10): p. 3276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farhy-Tselnicker I and Allen NJ, Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev, 2018. 13(1): p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tabata H, Diverse subtypes of astrocytes and their development during corticogenesis. Front Neurosci, 2015. 9: p. 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••9. Clavreul S, et al. , Cortical astrocytes develop in a plastic manner at both clonal and cellular levels. Nat Commun, 2019. 10(1): p. 4884. By employing a novel large-scale lineage tracing technique in combination with multiphoton serial microscopy, this study observed pre- and postnatal astrocyte progenitors across development, redefining the origin of astrocytes and their ability to morpholligically mature in an indepdent manner.
- •10. Batiuk MY, et al. , Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun, 2020. 11(1): p. 1220. This study employed single cell RNA sequencing to reveal that astrocyte heterogeneity exists down to the molecular level across the central nervous system.
- ••11. Lanjakornsiripan D, et al. , Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat Commun, 2018. 9(1): p. 1623. In this study, advanced two- and three-dimensional imaging approaches and reconstructions of astrocytes revealed a dynamic spectrum of astrocytes across all 6 layers of the somatosensory cortex, revealing inter-regional heterogeneity in morphological and moleculuar profiles.
- •12. Bayraktar OA, et al. , Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci, 2020. 23(4): p. 500–509. This study is of special interest in that in provides a novel approach to investigate single-cell RNA profiles in situ and provides evidence for molecular heterogeneity in cortical astrocytes.
- •13. Ung K, et al. , Olfactory bulb astrocytes mediate sensory circuit processing through Sox9 in the mouse brain. Nat Commun, 2021. 12(1): p. 5230. This study is of special interest in that it identifies the ability for transcription factors such as Sox9, which are globally expressed in astrocytes, to engage in interactions that dictate astrocyte morphology and function in a region-specific manner.
- 14.Huang AY, et al. , Region-Specific Transcriptional Control of Astrocyte Function Oversees Local Circuit Activities. Neuron, 2020. 106(6): p. 992–1008 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo J, et al. , Regional heterogeneity of astrocyte morphogenesis dictated by the formin protein, Daam2, modifies circuit function. EMBO Rep, 2021. 22(12): p. e53200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stork T, et al. , Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron, 2014. 83(2): p. 388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morel L, et al. , VGluT1+ neuronal glutamatergic signaling regulates postnatal developmental maturation of cortical protoplasmic astroglia. J Neurosci, 2014. 34(33): p. 10950–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt LM, et al. , Astrocyte morphogenesis is dependent on BDNF signaling via astrocytic TrkB.T1. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stogsdill JA, et al. , Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature, 2017. 551(7679): p. 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••20. Baldwin KT, et al. , HepaCAM controls astrocyte self-organization and coupling. Neuron, 2021. 109(15): p. 2427–2442 e10. This paper of special interest describes a mechanism through which astrocytes are able to communicate with one other to compete for territorry, establish their non-overlapping domains, and engage in bidirectional communication.
- 21.Kimelberg HK, The problem of astrocyte identity. Neurochem Int, 2004. 45(2–3): p. 191–202. [DOI] [PubMed] [Google Scholar]
- 22.Peters A and Vaughn JE, Microtubules and filaments in the axons and astrocytes of early postnatal rat optic nerves. J Cell Biol, 1967. 32(1): p. 113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eom TY, et al. , Direct visualization of microtubules using a genetic tool to analyse radial progenitor-astrocyte continuum in brain. Nat Commun, 2011. 2: p. 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haseleu J, et al. , Studying subcellular detail in fixed astrocytes: dissociation of morphologically intact glial cells (DIMIGs). Front Cell Neurosci, 2013. 7: p. 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murk K, et al. , The antagonistic modulation of Arp2/3 activity by N-WASP, WAVE2 and PICK1 defines dynamic changes in astrocyte morphology. J Cell Sci, 2013. 126(Pt 17): p. 3873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sit ST and Manser E, Rho GTPases and their role in organizing the actin cytoskeleton. J Cell Sci, 2011. 124(Pt 5): p. 679–83. [DOI] [PubMed] [Google Scholar]
- 27.Hall A, Rho family GTPases. Biochem Soc Trans, 2012. 40(6): p. 1378–82. [DOI] [PubMed] [Google Scholar]
- 28.Burgos M, et al. , PKCepsilon induces astrocyte stellation by modulating multiple cytoskeletal proteins and interacting with Rho A signalling pathways: implications for neuroinflammation. Eur J Neurosci, 2007. 25(4): p. 1069–78. [DOI] [PubMed] [Google Scholar]
- 29.Derouiche A and Frotscher M, Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia, 2001. 36(3): p. 330–41. [DOI] [PubMed] [Google Scholar]
- 30.Lavialle M, et al. , Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc Natl Acad Sci U S A, 2011. 108(31): p. 12915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai FC, et al. , Ezrin enrichment on curved membranes requires a specific conformation or interaction with a curvature-sensitive partner. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stern S, et al. , RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron, 2021. 109(21): p. 3436–3455 e9. [DOI] [PubMed] [Google Scholar]
- 33.Schiweck J, et al. , Drebrin controls scar formation and astrocyte reactivity upon traumatic brain injury by regulating membrane trafficking. Nat Commun, 2021. 12(1): p. 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renault-Mihara F, et al. , Regulation of RhoA by STAT3 coordinates glial scar formation. J Cell Biol, 2017. 216(8): p. 2533–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araque A, et al. , Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci, 1999. 22(5): p. 208–15. [DOI] [PubMed] [Google Scholar]
- 36.Verkhratsky A and Nedergaard M, Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci, 2014. 369(1654): p. 20130595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung WS, Allen NJ, and Eroglu C, Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol, 2015. 7(9): p. a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. , Astrocytic Process Plasticity and IKKbeta/NF-kappaB in Central Control of Blood Glucose, Blood Pressure, and Body Weight. Cell Metab, 2017. 25(5): p. 1091–1102.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kleim JA, et al. , Motor learning induces astrocytic hypertrophy in the cerebellar cortex. Behav Brain Res, 2007. 178(2): p. 244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellesi M, et al. , Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC Biol, 2015. 13: p. 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theodosis DT and Poulain DA, Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience, 1984. 11(1): p. 183–93. [DOI] [PubMed] [Google Scholar]
- 42.Oliet SH, Piet R, and Poulain DA, Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science, 2001. 292(5518): p. 923–6. [DOI] [PubMed] [Google Scholar]
- 43.Bernardinelli Y, et al. , Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol, 2014. 24(15): p. 1679–88. [DOI] [PubMed] [Google Scholar]
- •44. Herde MK, et al. , Local Efficacy of Glutamate Uptake Decreases with Synapse Size. Cell Rep, 2020. 32(12): p. 108182. Here, the use of Expansion Microscopy and glutamate sensing enabled the group to examine the engagement of astrocyte leaflets in immature versus mature spines. The tools employed and the findings make this paper of special interest.
- ••45. Henneberger C, et al. , LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron, 2020. 108(5): p. 919–936 e11. This study is of particular interest due to its use of in vivo imaging to examine astrocyte leaflet plsaticity before and after LTP in potentiated synapses. This is one of the first studies to find that astrocyte leaflets initially retract from potentiated synapses to allow for inter-synaptic glutamate crosstalk.
- 46.Larsen BR, et al. , Contributions of the Na(+)/K(+)-ATPase, NKCC1, and Kir4.1 to hippocampal K(+) clearance and volume responses. Glia, 2014. 62(4): p. 608–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lushnikova I, et al. , Synaptic potentiation induces increased glial coverage of excitatory synapses in CA1 hippocampus. Hippocampus, 2009. 19(8): p. 753–62. [DOI] [PubMed] [Google Scholar]
- 48.Genoud C, et al. , Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol, 2006. 4(11): p. e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••49. Kiyoshi CMA, Sidney; Arzola Emily P.; Patterson Jeremy A.; Taylor Anne T.; Du Yixing; Guiher Allly M.; Philip Merna; Gerviacio Camacho Elizabeth; Mediratta Devin; Collins Kelsey; Benson Emily; Kidd Grahame, Terman David; Zhou Min, Ultrastructural view of astrocyte-astrocyte and astrocyte-synapse contacts within the hippocampus. BioRxIV, 2020. This study is of particular interest. Serial block face scanning electron microscopy and the reconstruction of three individual astrocytes revealed novel sub-compartments in astrocytes, and novel ways through which astrocytes interact with synapses.
- ••50. Arizono M, et al. , Structural basis of astrocytic Ca(2+) signals at tripartite synapses. Nat Commun, 2020. 11(1): p. 1906. In this article of particular interest, Arizono et al visualize loop-like structure sub-compartments in the spongiform domains of astrocytes. The technological approaches employed here allowed for high resolution visualization of these loops and their Ca2+ driven function.
- •51. Arizono M, et al. , Super-resolution shadow imaging reveals local remodeling of astrocytic microstructures and brain extracellular space after osmotic challenge. Glia, 2021. 69(6): p. 1605–1613. Here, super-resolution imaging reveals the functional dynamics of astrocyte sub-compartments that interact at the synapse.
- 52.Molotkov D, et al. , Calcium-induced outgrowth of astrocytic peripheral processes requires actin binding by Profilin-1. Cell Calcium, 2013. 53(5–6): p. 338–48. [DOI] [PubMed] [Google Scholar]
- 53.Livet J, et al. , Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature, 2007. 450(7166): p. 56–62. [DOI] [PubMed] [Google Scholar]


