Abstract
Background.
Endobronchial ultrasound (EBUS) transbronchial needle aspiration (TBNA) has a high diagnostic yield when evaluating mediastinal and hilar lymphadenopathy (LAD). Having previously demonstrated the safety of EBUS-guided cautery-assisted transbronchial nodal forceps biopsy (ca-TBFB), we report disease-specific improvements in diagnostic yield and tissue acquisition when supplementing the EBUS-TBNA–based standard ofcare (SOC) with ca-TBFB.
Methods.
We retrospectively reviewed 213 patients who sequentially underwent SOC and ca-TBFB during the same procedure. We determined 3 clinical scenarios of interest based on preprocedural imaging: isolated mediastinal/hilar LAD, LAD associated with a nodule or mass suspicious for malignancy, and LAD associated with parenchymal findings suggestive of sarcoidosis. Using validated methods, we assessed diagnostic yield on a per-patient basis and specimen quality on a per-node basis on the 136 patients meeting diagnostic criteria.
Results.
Administration of disease-specific SOC with ca-TBFB yielded gains that varied by diagnosis. Diagnostic yields of SOC and its supplementation with ca-TBFB were 91.8% and 93.4% (P = .50) of the 61 patients diagnosed with solid-organ malignancy, 62.7% and 94.9% (P < .001) of the 59 patients diagnosed with sarcoidosis, and 62.5% and 93.8% (P = .042) of the 16 patients diagnosed with lymphoma, the. For each disease process, specimens obtained with ca-TBFB exhibited statistically higher quality.
Conclusions.
We suggest that relative to SOC, ca-TBFB improves diagnostic yield for sarcoidosis and lymphoma while providing uniformly better tissue quality and cellularity. We propose a protocol for use of this innovative technique.
Mediastinal and hilar lymphadenopathy (LAD) are frequently encountered, and according to American College of Chest Physicians (ACCP) guidelines, endobronchial ultrasound (EBUS) transbronchial needle aspiration (TBNA) is considered standard of care (SOC) for tissue acquisition in such patients.1 Its yield varies depending on the clinical scenario, with diagnostic sensitivity for isolated mediastinal LAD (25% to 89%)2,3 and lymphoma (38% to 91%)4–7 being quite variable. The sensitivity for lung cancer is 88% to 93%8–10 and for sarcoidosis has been reported as 79%.11 After nondiagnostic EBUS-TBNA, mediastinoscopy is often recommended, although it incurs more complications and higher costs.12
Substantive limitations of EBUS-TBNA are well acknowledged. Labarca and colleagues13 demonstrated that EBUS-TBNA–derived tissue has a high yield for molecular analysis of epidermal growth factor receptor and anaplastic lymphoma kinase mutations and acknowledged that its suitability for next-generation sequencing is uncertain.
To diagnose sarcoidosis, specimens from additional bronchoscopic techniques, such as endobronchial and transbronchial biopsies, are often required. We consider these to be disease-specific diagnostic procedures that are part of standard of care (SOC) when combined with EBUS-TBNA.14 Although rarely occurring, pneumothorax may be associated with respiratory distress or a need for hospitalization. Finally, given the smaller specimens obtained relative to those from traditional surgical excision and “core biopsies,” questions remain about the efficacy of EBUS-TBNA for the diagnosis of lymphoma, especially Hodgkins, subtype and grade.7 Whether the newer, larger 19-gauge needles confer an advantage over smaller needles is the subject of ongoing investigation.15–17
The use of EBUS for extracting lymph node biopsy specimens with forceps rather than with needle aspiration has been described, and results suggest that the combination of both techniques improves yield over SOC alone.18–20 In a significant number of patients, however, the forceps encounter resistance and consequently does not pass reliably through the bronchial wall and into the targeted lymph node.21 Novel instruments to obtain core biopsy specimens, such as the ProCore needle (Cook Medical, Bloomington, IN), are being studied.22 The direct processing of formalin-fixed specimens as core biopsies has also been proposed.23
To circumvent concerns with insufficient tissue obtained with TBNA, our goal in this study was to supplement tissue collection with a previously described EBUS-guided cautery-assisted transbronchial forceps biopsy (ca-TBFB) technique. We hypothesized that combining the current SOC of EBUS-TBNA with EBUS-guided ca-TBFB would increase the diagnostic yield and specimen quality in patients with solid-organ malignancy, sarcoidosis, and lymphoma.
We assessed the diagnostic yield of each disease-specific SOC procedure based on 3 preexisting clinical scenarios: patients with isolated mediastinal or hilar LAD, LAD associated with a nodule or mass suggestive of malignancy, or LAD associated with parenchymal disease suggestive of sarcoidosis. In this study, disease-specific SOC based on EBUS-TBNA was always supplemented with ca-TBFB as a comparative diagnostic technique. The cellularity and tissue quality of nodal specimens obtained from SOC and ca-TBFB were graded using a standardized protocol.24 Based on these results, we propose a clinical protocol based on preprocedural diagnostic suspicion that promotes individualized patient care in the context of EBUS bronchoscopy.
Patients and Methods
Overall Study Design
We retrospectively reviewed patients who underwent SOC of EBUS-TBNA and concomitant ca-TBFB on the same lymph nodes in a tertiary care academic center between 2011 and 2016. Procedures were performed by 2 interventional pulmonary attendings trained in this technique. Patients consented for the procedure, and institutional approval was obtained for analysis under guidelines for ethical research from the Yale University Human Investigation Committee (#1008007224). Most procedures were performed on an outpatient basis. Patients were selected when sarcoidosis or lymphoma were in the differential diagnosis and when tissue beyond TBNA was requested in patients with probable malignancy. Select subgroup analysis was performed on those patients diagnosed with the index conditions.
Procedure Protocol
Sedation was accomplished using moderate sedation and typically involved a combination of fentanyl and midazolam, along with topical lidocaine, as previously described.25 In addition to SOC of EBUS-TBNA and ca-TBFB, some patients underwent bronchoalveolar lavage (BAL), endobronchial biopsies, transbronchial biopsies, or other bronchoscopic techniques based on the radiographic findings and at the discretion of the bronchoscopist. For suspected sarcoidosis, endobronchial biopsies were performed, and BAL specimens were sent for cultures. When parenchymal disease was present, transbronchial biopsies using 2.0- to 2.8-mm fenestrated alligator forceps were performed using fluoroscopic guidance. In cases of suspected lymphoma, flow cytometry was requested on TBNA nodal samples. These techniques are supplemental to EBUS and are considered disease-specific SOC for positive diagnosis of each disease. These supplemental techniques were therefore grouped with EBUS-TBNA as a component of SOC for analysis.
White light bronchoscopy preceded EBUS evaluation. The EBUS scope (Olympus BF-U180F with EU-ME1 processor; Olympus America Inc, Center Valley, PA) was advanced, and a comprehensive mediastinal and hilar evaluation was performed. Lymph nodes sized greater than 5 mm were typically biopsied by TBNA because in cases of malignancy, full staging by EBUS-TBNA is our SOC. Patients underwent SOC of EBUS-TBNA with 20 to 30 agitations per pass using the 21- or 22-gauge needle (Olympus NA-201Sx-4022), although the 19-gauge needle (Olympus NA-U402SX-4019) was used occasionally once commercially available. Three passes were obtained per station, and specimens were preserved for pathologic evaluation.
After TBNA, EBUS was again used to identify the target lymph nodes sized greater than 10 mm. In these instances, the electrocautery knife (Olympus KD-31C-1) was advanced through the working channel of the EBUS scope and placed against the airway wall with the lymph node in view. Incisions were made at 40 W using the Erbe system (Erbe VIO 300D-40W; Erbe USA, Inc, Marietta, GA), and the knife was seen in real time to extend through the airway wall and into the lymph node. After penetration of the node, the knife was withdrawn, and the 1.9-mm spiked fenestrated alligator forceps (Olympus FB-241K) was advanced through the working channel of the EBUS scope.
We relied upon ultrasound to reenter the lymph node, although occasionally the tract through the bronchus could be seen. Upon entering the lymph node, the forceps was opened at the proximal end of the node, advanced, and then closed at the distal end of the node for each specimen. This process was repeated using approximately 5 biopsies during each of 3 passes, thereby maintaining a technique comparable to that used during TBNA. These specimens were placed in formalin and sent to surgical pathology for analysis. This technique is demonstrated in the Video.
Pathologic Analysis
We analyzed the tissue samples on both per-patient and per-nodal bases. Patient-level diagnosis of sarcoidosis was based on the finding of noncaseating granulomas in the absence of infection. Patient-level diagnosis of lymphoma was based on TBNA and forceps histopathology to determine pathologic subsets or flow cytometry, or both, consistent with a monoclonal lymphocyte proliferation. In this analysis we evaluated all nodal specimens from all patients positively diagnosed with solid-organ malignancy, sarcoidosis, or lymphoma.
In all cases the pathologists making the diagnoses were different. Those who analyzed the TBNA specimens were different from those who examined the forceps specimens. When there were differences in a pathologic diagnosis, a single, blinded pathologist was asked to review all specimens for the patient.
Comparative Analysis of Nodal Specimen Quality
Using a validated scoring system,25 a single pathologist re-reviewed all nodal specimens obtained with SOC (based on EBUS-TBNA) and ca-TBFB from patients who were positively diagnosed with solid-organ malignancy, sarcoidosis, or lymphoma. Specimen quality was assessed by the amount of cellular material, background blood, degree of cellular degeneration and trauma, and retention of normal architecture. Quality in each subdomain was scored on an ordinal 0 to 2 scale where higher scores reflect higher quality, resulting in an overall score ranging from 0 to 8.
Statistical Analysis
The demographic and clinically relevant characteristics of the participants are described using means and 95% confidence intervals (CIs) or medians and interquartile ranges for continuous variables and percentages for dichotomous variables. The rates of diagnostic yield were calculated taking the number of patients determined to have the disease with the index approach (SOC or ca-TBFB) as the numerator and the total number of patients determined to have that disease from all approaches as the denominator (endobronchial biopsies, transbronchial biopsies, and flow cytometry added to those from SOC and ca-TBFB). The point estimates and 95% CIs for each rate were estimated using an intercept-only Poisson model, and rates were compared using the exact Wilcoxon test.
The rates for each of the 3 disease processes were first determined for SOC, defined as TBNA supplemented with appropriate disease-specific bronchoscopic procedures. For malignancy, SOC consisted of TBNA alone. For sarcoidosis, SOC consisted of TBNA, endobronchial biopsy, BAL and transbronchial biopsy, as deemed appropriate. For lymphoma, SOC consisted of TBNA and flow cytometry.
To evaluate a potential explanation for the gains in diagnostic yield for sarcoidosis and lymphoma from supplementation with ca-TBFB, we compared the overall pathology score (0–8) from all nodes obtained with both approaches. After we verified that the overall pathology score followed a symmetric unimodal distribution, this comparison was made using simple linear regression with generalized estimating equations with compound symmetry to adjust for the multiple nodes contributed by individual patients. In all cases statistical significance was defined as a P value of less than .05. All analyses were performed using SAS 9.4 software (SAS Institute Inc, Cary, SC).
Results
Figure 1 depicts the derivation of our study sample. Our initial review consisted of 213 patients who underwent disease-specific SOC supplemented with ca-TBFB. Of these, 37 patients were excluded based on their radiographic findings more consistent with processes such as interstitial lung disease or infections for which EBUS was used. Of the 176 remaining patients, 40 were ultimately excluded based on final diagnoses that did not include our index diseases, yielding our final analytic sample of 136 patients. Demographic and clinical characteristics of patients positively determined to have solid-organ malignancy, sarcoidosis, or lymphoma are presented in Table 1.
Figure 1.
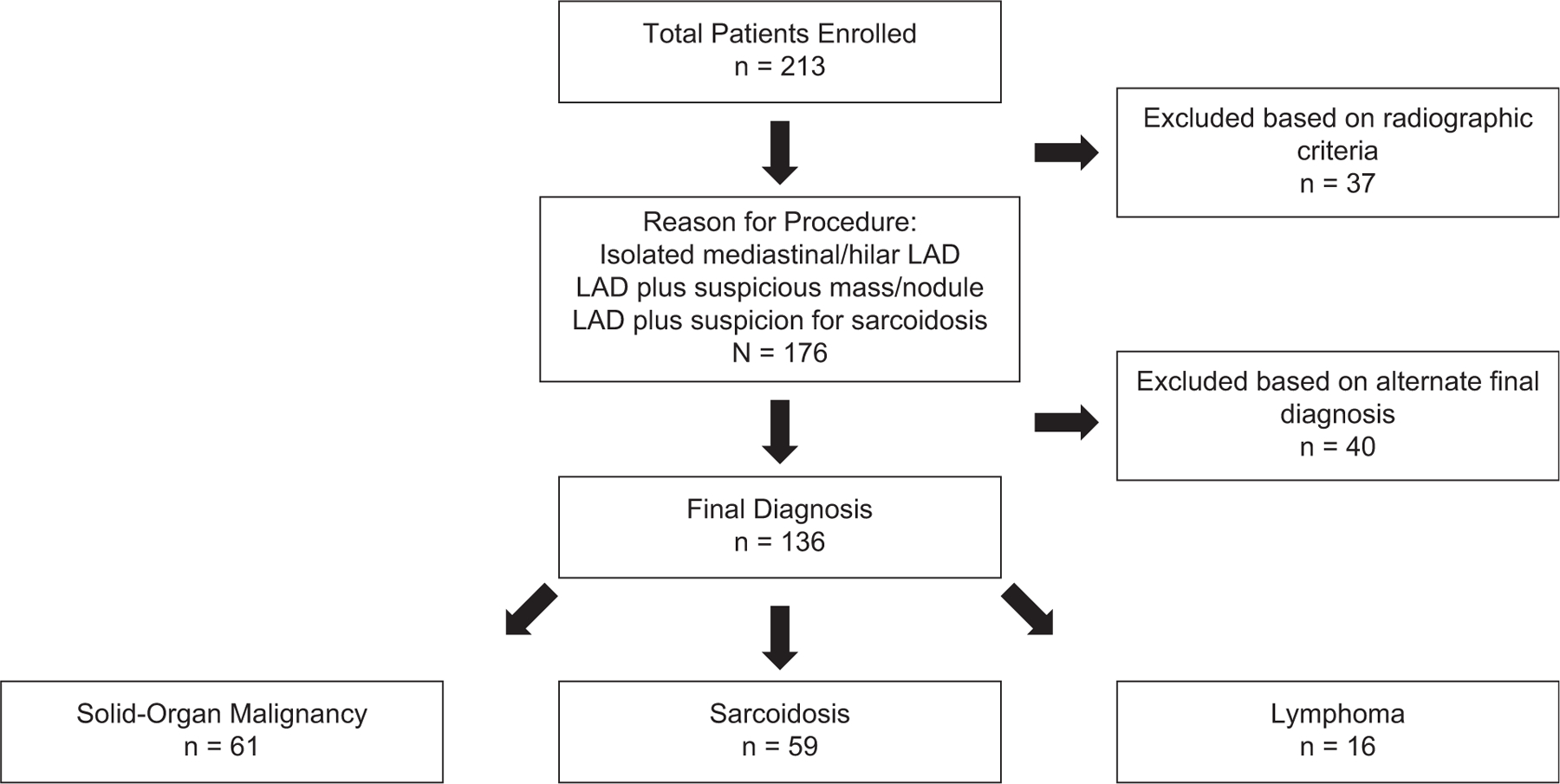
This study retrospectively evaluated 213 patients. We excluded patients in the final analysis who did not meet criteria for any of the 3 clinical radiographic findings: patients with isolated mediastinal lymphadenopathy (LAD), adenopathy associated with a suspicious nodule or mass, and adenopathy associated with other radiographic findings consistent with sarcoidosis. The final analysis includes patients diagnosed by bronchoscopy with solid-organ malignancy, sarcoidosis, or lymphoma.
Table 1.
Characteristics of Patients Diagnosed With Malignancy, Sarcoidosis, or Lymphoma
| Final Diagnoses (N = 1136) |
|||
|---|---|---|---|
| Participant Characteristics | Malignancy (n = 61) | Sarcoidosis (n = 59) | Lymphoma (n = 16) |
| Age, mean (SD), y | 67.5 (10.5) | 53.1 (14.6) | 49.2 (19.5) |
| Male sex | 31 (50.8) | 30 (50.9) | 10 (62.5) |
| Nonwhite race | 4 (6.6) | 17 (2.9) | 4 (25.0) |
| Smoking history with at least 10 pack-years | 49 (83.1) | 9 (16.4) | 7 (43.8) |
| Reason for procedure | |||
| Isolated mediastinal lymphadenopathy | 6 (9.8) | 18 (30.5) | 12 (75.0) |
| Lymphadenopathy plus | |||
| Suspicious nodule/mass | 52 (85.3) | 2 (3.4) | 2 (12.5) |
| Parenchymal disease | 3 (4.9) | 39 (66.1) | 2 (12.5) |
| Underlying chronic medical disease | |||
| Coronary artery disease | 10 (16.4) | 5 (8.5) | 3 (18.8) |
| Congestive heart failure | 1 (1.6) | 3 (5.1) | 0 (0) |
| COPD | 26 (42.6) | 4 (6.8) | 1 (6.3) |
| Cerebrovascular accident | 3 (4.9) | 3 (5.1) | 0 (0) |
| Diabetes | 14 (23.0) | 6 (10.2) | 4 (25.0) |
| Prior malignancy | 33 (54.1) | 13 (22.0) | 10 (62.5) |
| Non-small cell lung cancer | 10 (16.4) | 1 (.17) | 0 (0) |
| Small cell lung cancer | 2 (3.3) | 0 (0) | 0 (0) |
| Lymphoma | 1 (1.6) | 3 (5.1) | 9 (56.3) |
| Other solid organ | 19 (31.2) | 8 (13.6) | 1 (6.3) |
| Other | 4 (6.6) | 4 (6.8) | 1 (6.3) |
| Sarcoidosis | 0 (0) | 3 (5.1) | 0 (0) |
| Tuberculosis | 2 (3.3) | 1 (1.7) | 0 (0) |
| Complications | |||
| Bleeding within 48 h | 0 (0) | 0 (0) | 0 (0) |
| Pneumomediastinum within 48 h | 0 (0) | 4 (6.8) | 0 (0) |
| Pneumonia within 10 d | 1 (1.6) | 0 (0) | 0 (0) |
| Pneumothorax within 48 h | 0 (0) | 4 (6.8) | 0 (0) |
| Respiratory failure | 0 (0) | 1 (1.7) | 0 (0) |
| Hemoptysis within 1 wk | 0 (0) | 1 (1.7) | 0 (0) |
Data are presented as n (%) unless otherwise indicated.
COPD, chronic obstructive pulmonary disease.
Among these 3 patient subgroups, the diagnostic yields of SOC and its supplementation with ca-TBFB were evaluated. For malignancy (n = 61), SOC resulted in a diagnostic yield of 91.8% (95% CI, 70.7%–100%) and its supplementation with ca-TBFB resulted in a diagnostic yield of 93.4% (95% CI, 72.1%–100%; P = .50). For sarcoidosis (n = 59), EBUS-TBNA resulted in a diagnostic yield of 62.7% (95% CI, 45.4%–86.6%), and its supplementation resulted in a diagnostic yield of 94.9% (95% CI, 73.0%–100%; P < .001). For lymphoma (n = 16), SOC resulted in a diagnostic yield of 62.5% (95% CI, 33.6%–100%), and its supplementation resulted in a diagnostic yield of 93.8% (95% CI, 56.5%–100%; P =.042).
Specimen quality was then assessed on a per-nodal analysis in patients diagnosed with solid-organ malignancy, sarcoidosis, or lymphoma. Because more than 1 lymph node could be biopsied from each patient, the total number of nodes analyzed exceeded the number of patients. Overall quality was evaluated by assigning an ordinal score of 0 to 2 using 4 distinct criteria: the amount of cellular material, background blood, degree of cellular degeneration and trauma, and retention of normal architecture where higher scores indicate better quality. Figure 2 presents and compares the means (95% CIs) of the aggregate pathology score (range, 0–8) from all lymph nodes sampled with SOC and ca-TBFB from patients diagnosed with malignancy, sarcoidosis, or lymphoma and lists the corresponding P values. For all 3 diseases the means (95% CIs) of the aggregate pathology scores from the nodes obtained by ca-TBFB were significantly higher than those sampled with SOC. For malignancy (n = 116), nodes sampled with SOC yielded a mean score of 5.4 (95% CI, 5.0–5.8), and nodes sampled with ca-TBFB yielded a mean score of 6.2 (95% CI, 5.9–6.6; P < .001). For sarcoidosis (n = 78), nodes sampled with SOC yielded a mean score of 4.3 (95% CI, 3.6–5.0), whereas nodes sampled with ca-TBFB yielded a mean score of 7.0 (95% CI, 6.5–7.5; P < .001). For lymphoma (n = 44), nodes sampled with SOC yielded a mean score of 5.4 (95% CI, 4.9–5.8), and nodes sampled with ca-TBFB yielded a mean score of 7.0 (95% CI, 6.6–7.5; P < .001). Figure 3 presents representative pathologic specimens for each condition.
Figure 2.
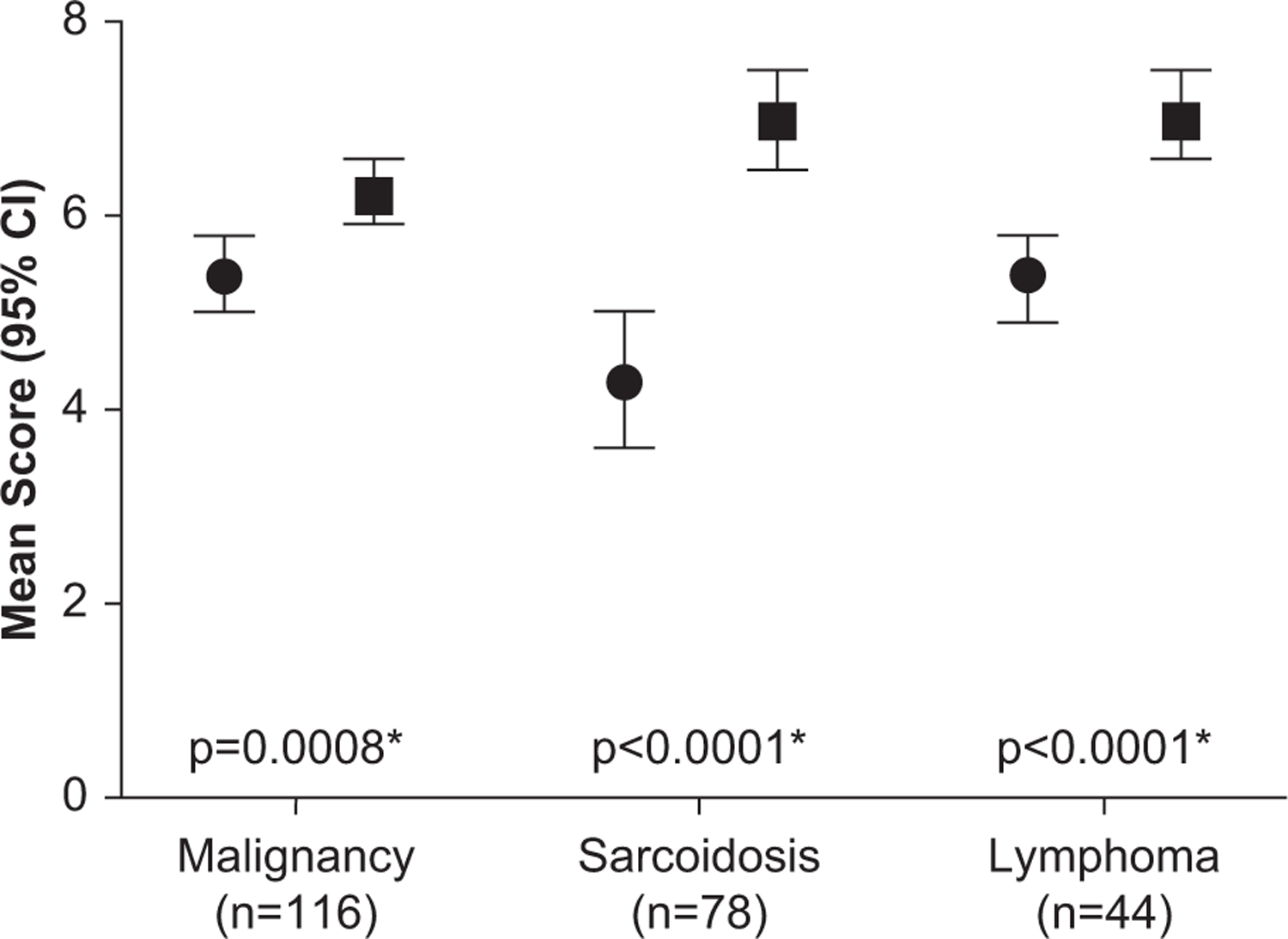
Per-nodal analysis in patients diagnosed with solid-organ malignancy, sarcoidosis, and lymphoma. For each patient, more than 1 lymph node was biopsied and thus the total nodes analyzed for specimen quality exceed the total number of patients. Each node was scored 0 to 2 using 4 criteria to determine quality: the amount of cellular material, background blood, degree of cellular degeneration and trauma, and the retention of normal architecture. Higher scores indicate higher specimen quality. The whiskers indicate the 95% confidence interval (CI). Circles and squares indicate TBNA and ca-TBFB, respectively. (ca-TBFB, cautery-assisted transbronchial nodal forceps biopsy; TBNA, transbronchial needle aspiration.)
Figure 3.
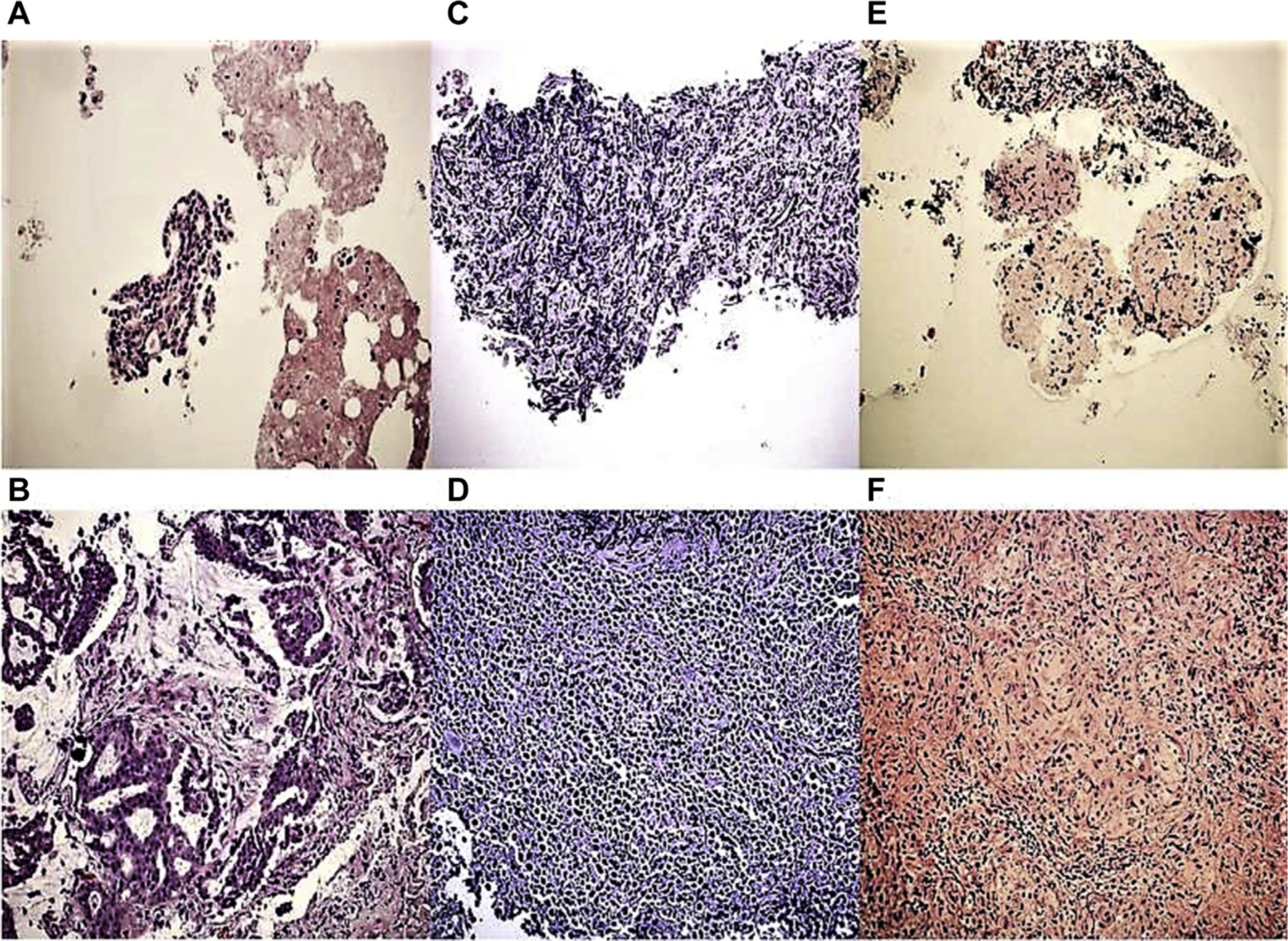
Representative images of specimens obtained by using (A, C, E) transbronchial needle aspiration (TBNA) and (B, D, F) cautery-assisted transbronchial nodal forceps biopsy (ca-TBFB) for (A, B) lung cancer, (C, D) lymphoma, and (E, F) sarcoidosis (hematoxylin and eosin stain, original magnification ×200). In the final analysis, specimen adequacy and cellularity, and therefore, quality, was determined to be superior in specimens obtained by ca-TBFB compared with TBNA.
As reported in Table 1, complications were extremely rare, with most attributed to transbronchial biopsies after careful review. Notably, because ca-TBFB was sequentially performed in the same setting with other diagnostic procedures, including TBNA, we could not assign causality to the complications. Pneumomediastinum is the only finding we have found to occur more frequently when ca-TBFB is performed, likely owing to the larger hole made in the airway. This condition is typically self-resolving and was safely managed on an outpatient basis.
Of the 40 patients excluded from analysis, 17 had an inflammatory condition, typically an interstitial lung disease with nonspecific mediastinal LAD, and 5 had infections. Of the 18 with no clear diagnosis, only 2 had follow-up imaging that was concerning for progression of malignancy, although this was not definitively established in either patient. The other 16 were alive at the conclusion of our analysis, suggesting they did not have a high-grade malignancy.
Comment
We found ca-TBFB increased the diagnostic sensitivity for both sarcoidosis and lymphoma relative to SOC alone. Owing to the excellent performance of SOC in malignancy, there was minimal gain in diagnostic yield. However, even in cases of malignancy, the specimens obtained with ca-TBFB were of higher quality. Our hypothesis that the larger specimens obtained with cautery enabled better preservation of tissue architecture is supported by the uniformly higher aggregate pathology scores exhibited across all 3 disease domains.
Strengths of this study include a novel approach to EBUS that individualizes the diagnostic strategy based on preprocedural radiographic findings. This study reports a large number of lymph node biopsies performed with EBUS-guided large biopsy forceps. We posit that a preprocedural determination of the best biopsy techniques can be based on radiographic imaging and the plausible etiology.
This study incorporates complementary clinical, radiographic, and pathologic findings in a robust fashion. In cases of isolated mediastinal LAD, we now routinely supplement SOC with ca-TBFB for its greater sensitivity to sarcoidosis and lymphoma. For sarcoidosis, we also send cultures to exclude infectious etiologies and routinely perform endobronchial biopsies to assess for granulomatous inflammation. When parenchymal abnormalities are minimal, we believe ca-TBFB can be performed without the need for transbronchial biopsies, essentially eliminating the risk of pneumothorax. When lymphoma is suspected, in addition to specimens obtained with TBNA and ca-TBFB, we perform flow cytometry. Our proposed protocol is depicted in Figure 4.
Figure 4.
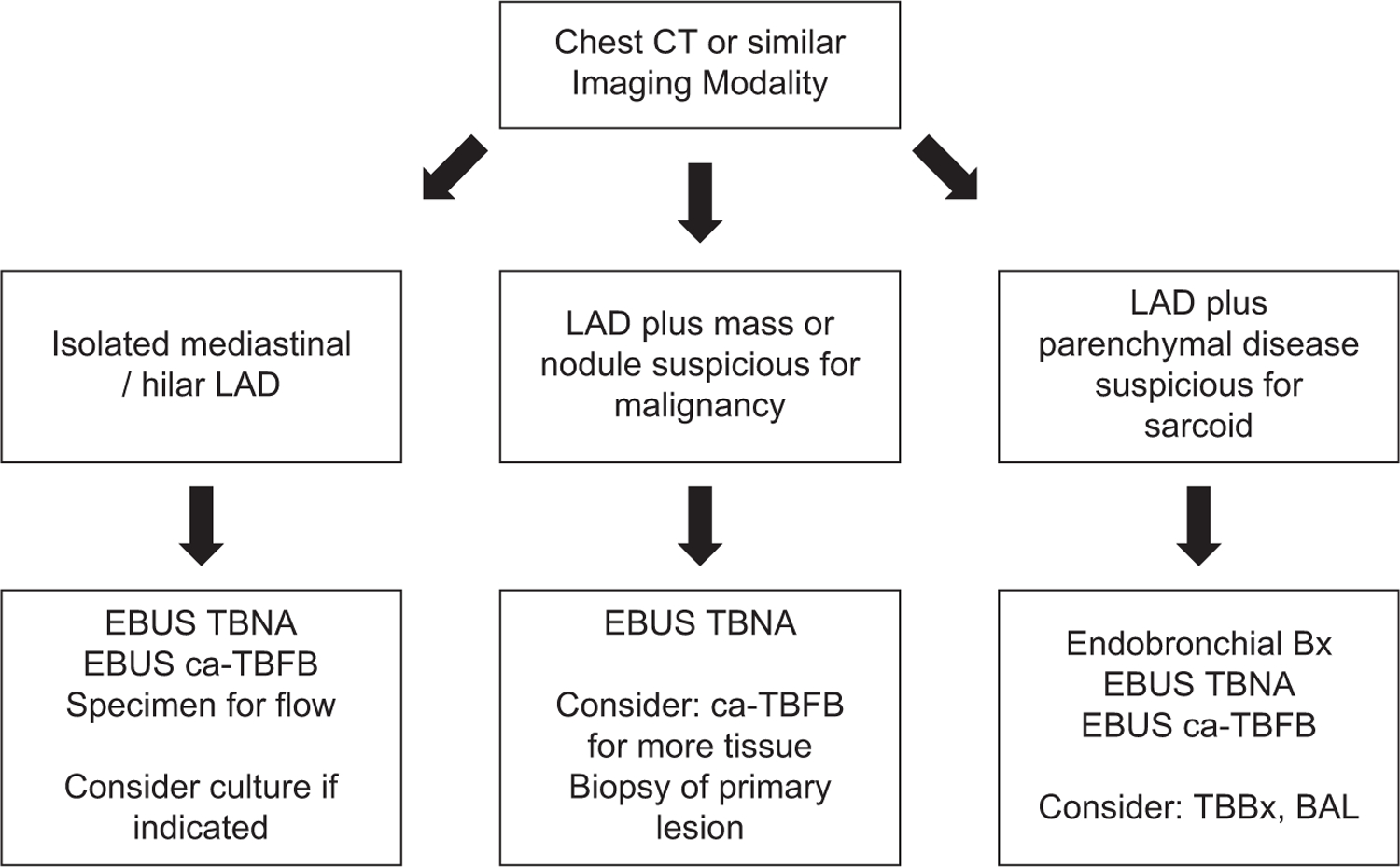
Proposed clinical algorithm for evaluating patients with mediastinal or hilar lymphadenopathy (LAD) when endobronchial ultrasound (EBUS) is performed. (BAL, bronchoalveolar lavage; ca-TBFB, cautery-assisted transbronchial nodal forceps biopsy; CT, computed tomography; TBBx, transbronchial biopsy; TBNA, transbronchial needle aspiration.)
In malignancy, next-generation sequencing is likely to become more routine, and the number of genes found that modify cancer cells will undoubtedly continue to expand. Although we routinely stage malignancy with SOC based on EBUS-TBNA, to improve specimen quality and quantity, we also incorporate at least 1 nodal station with samples obtained by ca-TBFB.
Limitations
Limitations of this study include its performance at a single site, its retrospective nature, and its reliance on a small number of people performing the biopsies. We did not use on-site cytology. The cautery knife creates visual artifacts that may obscure the image of the lymph node and thereby make maneuvering of the forceps more difficult. As such, this technique may not translate successfully to operators without EBUS expertise. Although we refer to this as a form of “advanced EBUS,” we believe it results in fewer complications than transbronchial biopsies, most notably the absence of pneumothorax and significant bleeding.
Although we attempted to review all nondiagnostic cases and all patients 6 months after the procedure, patients may have received postprocedural care elsewhere. We attempted to determine this by reviewing medical records in known patients and did not find anything to suggest a change in outcomes reported; however, a patient may have been missed during follow-up.
Lastly, this study does not specifically compare the diagnostic yield of SOC or ca-TBFB to the gold standard of mediastinoscopy. Our denominator therefore includes only those diagnoses made by bronchoscopic techniques and not by other methods. This analysis was limited to a comparison of the diagnostic yield of EBUS-TBNA–based SOC and its supplementation with ca-TBFB rather than when the motivation for using EBUS is nondiagnostic. We believe that the number of other procedures needed due to a nondiagnostic EBUS can be reduced by incorporating ca-TBFB when sarcoidosis and lymphoma are likely, but additional studies are needed.
Conclusions
We have developed a technique that improves on the diagnostic yield and specimen quality achievable with EBUS. Using an individualized patient approach based on preprocedural imaging, we propose an algorithm for use of EBUS-guided ca-TBFB that we believe to simultaneously optimize diagnostic yield and minimize serious complication. For isolated mediastinal LAD and conditions suggestive of lymphoma and sarcoidosis, we recommend use of ca-TBFB to improve yield. For solid-organ malignancy, we recommend ca-TBFB to improve the amount and quality of tissue obtained for immunohistochemistry and molecular/next-generation sequencing analysis. Additional prospective studies are warranted.
Supplementary Material
Acknowledgments
Ms Araujo and Dr Murphy were supported by the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center with a grant from the National Institute on Aging (P30-AG-021342).
Footnotes
The Video can be viewed in the online version of this article [https://doi.org/10.1016/j.athoracsur.2019.08.106] on http://www.annalsthoracicsurgery.org.
References
- 1.Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(Suppl):e211S–e250S. [DOI] [PubMed] [Google Scholar]
- 2.Guarize J, Casiraghi M, Donghi S, et al. Endobronchial ultrasound transbronchial needle aspiration in thoracic diseases: much more than mediastinal staging. Can Respir J 2018;2018:4269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyan CC, Machuca T, Czarnecka K, et al. Performance of endobronchial ultrasound-guided transbronchial needle aspiration for the diagnosis of isolated mediastinal and hilar lymphadenopathy. Respiration 2017;94:457–464. [DOI] [PubMed] [Google Scholar]
- 4.Grosu HB. EBUS-TBNA for the diagnosis of lymphoma: time to give in? J Bronchology Interv Pulmonol 2018;25:165–166. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy MP, Jimenez CA, Bruzzi JF, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. Thorax 2008;63:360–365. [DOI] [PubMed] [Google Scholar]
- 6.Moonim MT, Breen R, Fields PA, Santis G. Diagnosis and subtyping of de novo and relapsed mediastinal lymphomas by endobronchial ultrasound needle aspiration. Am J Respir Crit Care Med 2013;188:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin M, Wakely PE Jr. Endoscopic/endobronchial ultrasound-guided fine needle aspiration and ancillary techniques, particularly flow cytometry, in diagnosing deep-seated lymphomas. Acta Cytol 2016;60:326–335. [DOI] [PubMed] [Google Scholar]
- 8.Dong X, Qiu X, Liu Q, Jia J. Endobronchial ultrasound-guided transbronchial needle aspiration in the mediastinal staging of non-small cell lung cancer: a meta-analysis. Ann Thorac Surg 2013;96:1502–1507. [DOI] [PubMed] [Google Scholar]
- 9.Adams K, Shah PL, Edmonds L, Lim E. Test performance of endobronchial ultrasound and transbronchial needle aspiration biopsy for mediastinal staging in patients with lung cancer: systematic review and meta-analysis. Thorax 2009;64:757–762. [DOI] [PubMed] [Google Scholar]
- 10.Lerner AD, Feller-Kopman D. Bronchoscopic techniques used in the diagnosis and staging of lung cancer. J Natl Compr Canc Netw 2017;15:640–647. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Efficacy and safety of convex probe EBUS-TBNA in sarcoidosis: a systematic review and meta-analysis. Respir Med 2012;106:883–892. [DOI] [PubMed] [Google Scholar]
- 12.Sehgal IS, Dhooria S, Aggarwal AN, Behera D, Agarwal R. Endosonography versus mediastinoscopy in mediastinal staging of lung cancer: systematic review and meta-analysis. Ann Thorac Surg 2016;102:1747–1755. [DOI] [PubMed] [Google Scholar]
- 13.Labarca G, Folch E, Jantz M, Mehta HJ, Majid A, Fernandez-Bussy S. Adequacy of samples obtained by EBUS-TBNA for molecular analysis in patients with non-small cell lung cancer: systematic review and meta-analysis. Ann Am Thorac Soc 2018;15:1205–1216. [DOI] [PubMed] [Google Scholar]
- 14.Goyal A, Gupta D, Agarwal R, Bal A, Nijhawan R, Aggarwal AN. Value of different bronchoscopic sampling techniques in diagnosis of sarcoidosis: a prospective study of 151 patients. J Bronchology Interv Pulmonol 2014;21:220–226. [DOI] [PubMed] [Google Scholar]
- 15.Minami D, Ozeki T, Okawa S, et al. Comparing the clinical performance of the new 19-G ViziShot FLEX and 21-or 22-G ViziShot 2 endobronchial ultrasound-guided transbronchial needle aspiration needles. Intern Med 2018;57:3515–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrison G, Leclair T, Balla A, et al. Use of an additional 19-G EBUS-TBNA needle increases the diagnostic yield of EBUS-TBNA. J Bronchology Interv Pulmonol 2018;25:269–273. [DOI] [PubMed] [Google Scholar]
- 17.Biswas A, Wynne JP, Patel D, Weber M, Thakur S, Sriram PS. Comparison of the yield of 19-G eXcelon core needle to a 21-G EBUS needle during endobronchial ultrasound guided transbronchial needle aspiration of mediastinal lymph nodes for the detection of granulomas in cases of suspected sarcoidosis. J Thorac Dis 2017;9:E864–E866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chrissian A, Misselhorn D, Chen A. Endobronchial-ultrasound guided miniforceps biopsy of mediastinal and hilar lesions. Ann Thorac Surg 2011;92:284–288. [DOI] [PubMed] [Google Scholar]
- 19.Franke KJ, Bruckner C, Szyrach M, Ruhle KH, Nilius G, Theegarten D. The contribution of endobronchial ultrasound-guided forceps biopsy in the diagnostic workup of unexplained mediastinal and hilar lymphadenopathy. Lung 2012;190:227–232. [DOI] [PubMed] [Google Scholar]
- 20.Darwiche K, Freitag L, Nair A, et al. Evaluation of a novel endobronchial ultrasound-guided lymph node forceps in enlarged mediastinal lymph nodes. Respiration 2013;86:229–236. [DOI] [PubMed] [Google Scholar]
- 21.Gasparini S, Zuccatosta L, Sediari M, Mei F. Pilot feasibility study of transbronchial needle forceps: a new tool for obtaining histology samples from mediastinal subcarinal lymph nodes. J Bronchology Interv Pulmonol 2009;16:183–187. [DOI] [PubMed] [Google Scholar]
- 22.Xing J, Manos S, Monaco SE, Wilson DO, Pantanowitz L. Endobronchial ultrasound-guided transbronchial needle aspiration: a pilot study to evaluate the utility of the ProCore biopsy needle for lymph node sampling. Acta Cytol 2016;60: 254–259. [DOI] [PubMed] [Google Scholar]
- 23.Herath S, Cooper WA. The novel 19G endobronchial USS (EBUS) needle samples processed as tissue “core biopsies” facilitate PD-L1 and other biomarker testing in lung cancer specimens: case report and the view point from the respiratory physician and the pathologist. Respirol Case Rep 2017;5:e00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurya AK, Mehta A, Mani NS, Nijhawan VS, Batra R. Comparison of aspiration vs non-aspiration techniques in fine-needle cytology of thyroid lesions. J Cytol 2010;27:51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bramley K, Pisani MA, Murphy TE, Araujo KL, Homer RJ, Puchalski JT. Endobronchial ultrasound-guided cautery-assisted transbronchial forceps biopsies: safety and sensitivity relative to transbronchial needle aspiration. Ann Thorac Surg 2016;101:1870–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


