Abstract
Objective
It is easier for a listener to detect a brief tonal signal presented in a longer masking noise by increasing the delay between the signal and the masker. This phenomenon (overshoot) is influenced by a reduction in cochlear amplification and to date, there is no objective tool to investigate it. Therefore, a different paradigm of the auditory brainstem response (ABR) was utilized to measure auditory overshoot. It was assumed that increasing the delay onset time (DOT) between a signal and a masker reduces the latencies of waves I and III.
Materials & Methods
Sixteen normal young male guinea pigs were tested. A tone burst stimulus (signal: 16 kHz, 5ms in duration) and wide-band noise (masker: 0.1-8.0 kHz, 100ms in duration) at three DOTs were used. To diminish the effect of the noise on waves, waveforms were subtracted from those derived from the noise burst alone. The absolute latency of the waves I and III, inter-peak latency of the waves I-III, and amplitude ratio of the waves III/I were compared for the 0, 30, and 100ms DOTs and five signal-to-noise ratios.
Results
The latencies of increased from the 0 to 30ms DOT and then decreased from the 30 to 100ms DOT (p < 0.001). No significant changes were observed in the latency waves at the 100ms DOT compared to the 0ms DOT (p > 0.005). Moreover, there were no significant differences between the three DOTs regarding the inter-peak latency and amplitude ratio of the waves (p <0.005).
Conclusion
The study results showed an overshoot-like electrophysiological effect using ABR. Therefore, an objective test was used to investigate auditory cochlear gain.
Key Words: Auditory brainstem response, Overshoot, Temporal effect, Delay onset time, Latency
Introduction
In the auditory system, incoming acoustic stimuli propagate through different processing stages as they move from the external ear canal to the cortex that shapes perception, consciousness, and behavioral responses. Clinical practice depends on physiological and electrophysiological methods conducted on animal species. In the current study, the authors reported a different paradigm for the auditory brainstem response (ABR), which is related to the early stages of auditory processing, aiming to find an objective approach to measure overshoot in parallel with the known psycho-acoustical approach.
Overshoot is a phenomenon used to study the effects of background noise encountered in everyday hearing. The investigation of auditory overshoot may help to understand the ability to hear a brief sound in background noise. It is a temporal auditory effect (1-4)that depends on the timing between the onset of the signal and masker noise. When a listener must detect a brief tonal signal presented in a longer masking noise and the onset of the signal is slightly delayed from the onset of the masker, the detectability of the signal becomes easier by increasing the delay (5). The difference in the detectability threshold can be as much as 10-20dB, which is clinically important.
Overshoot has commonly been measured psycho-acoustically, which is time-consuming and cognitively demanding. Among the physiologic methods, nonlinear stimulus frequency otoacoustic emission (NSFOAE) and distortion product otoacoustic emission (DPOAE) have been frequently used. These methods have shown high variability and are affected by mild hearing loss (4, 6). It is necessary to find an objective tool that is more accurate and shows less variability.
The proposed physiological mechanisms of overshoot include classic firing rate adaptation, medial olivocochlear feedback, and higher stage effects. Researchers have stated that overshoot is the result of reduced cochlear amplifier gain and that the function may be due to efferent adaptation; therefore, the overshoot effect should be observable on the auditory nerve response (7, 8). Based on the effect of auditory cochlear function on overshoot, any changes in the detection of a signal in the presence of noise could be observed by the electrophysiological investigation of cochlear output in auditory nerves.
Electrocochleography and ABR are considered as tests to evaluate auditory cochlear outputs(9). Chatterjee et al. (1993) examined the overshoot phenomenon by studying the compound action potential. In their study, the competitive stimulus was the same as tone bursts, but at a different frequency from the signal. However, no study has been so far conducted on overshoot using ABR.
In psychoacoustic studies performed on overshoot, the competitive stimulus had a greater duration than the signal and a wider frequency band than the signal was recommended. Many other factors can affect the overshoot magnitude, including the use of a high-frequency tonal signal of short duration (8, 10) and wide-band noise at 60dB peak equivalent sound pressure level (peSPL) (7, 11, 12). A greater overshoot magnitude in humans has been achieved for mid-to-high frequency signals, especially at4 kHz. It is known that the mid-to-high frequency range in humans is almost equal to the low-frequency range in guinea pigs (13) and the maximal discharge of the auditory ipsilateral efferent nerve on the histogram curve is 20to 45ms after excitation(14).For this purpose, a 16 kHz tone burst stimulus and a wide-band noise burst of 100ms at 60dB peSPL with 30ms delay onset time (DOT)in addition to 0 and 100ms DOTs were used to investigate any potential trend of change in the recording parameters.
This study aimed to convey the effects of presenting ipsilateral noise stimuli on ABR waves and then to investigate the electro-physiologic representation of overshoot in guinea pigs. The assumption was that by increasing DOT, the latency would decrease, which relates to overshoot. Of interest was also to introduce an objective test of cochlear gain suitable for children not cooperating in psychoacoustic tasks.
Materials &Methods
Animals
The subjects were 16 young male guinea pigs weighing 250 to 350 g which were obtained from Karaj-Razi research Institute. Before starting the experiment, the animals were kept in a laboratory for three days to adapt to the new environment. The present study with the ethics code of, IR.IUMS.REC-1396.9311303001, the date of 01/20/2018, was conducted in the Animal Hearing Research Lab of the Iran University of Medical Sciences. The animals were placed in a double-walled soundproof booth throughout the experiment. The care and use of the animals were approved by the Institutional Animal Care and Use Committee at Iran University of Medical Sciences.
ABR recording
ABR was recorded using a Biologic Navigator Pro AEP (USA). The inverting needle electrode was set on the vertex(15),the non-inverting one on the right mastoid and the common one on the left mastoid. The impedance difference between electrodes was kept at less than 3Kohm. High- and low-pass filters and time window were set as 100-3000 Hz and 10ms, respectively. The animals were tested in an acoustic chamber for ABR, and the animals’ body temperature was measured using a digital thermometer and controlled with a heating pad. As the most stable ABR wave is the wave III in guinea pigs, it was used to determine the threshold. The threshold is the minimum level at which the wave III can be repeatedly detected and no other wave can be detected at less than 5dB from this value. Subjects with a threshold within ±10dB of the normal range were included for further analysis. The gain to ABR recordings was set as 100000, and a total of 250 stimuli were used for averaging waves (16).
Stimuli and recording parameters
Specific parameters were used to record overshoot electrophysiological. To this end, a 16 kHz tone burst stimulus and wide-band noise (0.1-8.0 kHz) were used for the signal and the noise, respectively. The noise with different DOTs was compared to the signal illustrated in Figure 1.The stimuli (the noise and the signal) were mixed using Cool Edit Pro (version 2.1), and the signal was 16 kHz that was presented (the ABR stimulus) simultaneously with the noise onset (a), 30ms after the noise onset (b), and 100ms after the noise onset (c).The signal included a complex of five tone bursts (5ms duration with 1ms rise/fall time, for each tone burst) with 11ms inter stimulus intervals (considering the minimal nerve recovery time). The noise had a duration of 100ms and an intensity level of 60dB peSPL. The noise level was fixed, and the signal level was modulated for five signal-to-noise ratios (SNRs; 0, +5, +10, +15, and +20dB) and the three DOTs (0, 30, and 100ms).
Fig 1.
Noise and tone burst stimuli and combinations. the signal was presented (the ABR stimulus) simultaneously with the noise onset (a) and 30ms after noise onset (b) and also immediately after the completion of the noise(c). The signal was included of five tone burst complex (5ms duration with 1ms rise/fall time, for each tone burst) with 11ms inter stimulus intervals. The duration of the noise was 100ms. The intensity level of the signal and noise was set at 60 dB PESPL. (dB PESPL: decibel Peak Equivalent Sound Pressure Level, ms: mili second, TB: Tone Burst, DOT: Delay Onset Time, WBN: Wide Band Noise, SNR: Signal to Noise Ratio)
(Fig.1)
Following the temporal parameters, the rate of the stimuli was kept at 5.81/sec. The polarity of the stimuli was alternate, and their intensity (the signal and the noise) was calibrated with a 2250 L sound level meter (B&K) at5cm from the speaker. The speaker covered a high-frequency sound of up to 20 kHz.
Procedure
The guinea pigs were anesthetized using (40 mg/kg) of ketamine 10% and (4 mg/kg) of 2%xylazine by intraperitoneal injection. For all the 16 animals, after determining the threshold of the wave III, ABR was recorded using the noise and tone burst with the described parameters. The speaker presented the stimuli to the right ear at 45° to the head of the animal. The animal’s left ear was closed by the blocker. After ABR measurement, the absolute latency, inter-peak latency, and amplitude ratio of the waves III/I were determined. The data were compared for the three DOTs for each SNR. To attenuate the neural response to the noise, the same procedure was repeated for all the data when ABR measurement of the noise and signal together was subtracted from that of the noise alone.
Statistical Analysis
Statistical analysis was performed in SPSS version 19.0 software package (SPSS Inc., Chicago, IL). All the data had a normal distribution (p>0.05) using the Kolmogorov-Smirnov test. The statistical significance was tested using global linear models. For each ABR, ANOVA was conducted to analyze the calculated latency, the inter-peak latency of the waves I-III, and the amplitude ratio of the waves III/I to the subtracted waves across the factors of time delay (three conditions) and SNR (five conditions) as within-subject variables. A pairwise comparison was conducted using Bonferroni analysis. The significance level was 0.05 for all statistical analyses.
Results
We first conducted data analysis on the original waves and then on the subtracted ones. The waveform of the noise and signal complex was subtracted from that of the noise alone at the three DOTs and different SNRs.
The absolute latency of the wave I
The analysis of the data (the absolute latency of the subtracted wave I) showed a significant main effect for DOT [F (2.0/30.00) =85.95; p<0.001; η2=0.851] and SNR [F (1.68/25.30) =12.61; p<0.05; η2=0.457]. Moreover, there was a significant interaction effect for DOT and SNR [F (2.27/34.09) =26.04; p<0.05; η2=0.616].
Bonferroni analysis showed that for 0 and 5dB SNRs, there were significant changes between the 0 and 30msDOTs and between the 30and 100ms DOTs (p<0.05). For the other SNRs (10, 15, and 20dB), there were significant changes between the 0ms DOT and the other DOTs (30 and100ms) and between the30and100ms DOTs (p<0.05).
The absolute latency of the wave III
The data analysis (the absolute latency of the subtracted wave III) showed that there was a significant main effect for DOT [F (2.0/30.00) =29.40, p<0.001, η2=0.662] and SNR [F (2.17/32.64) =27.20, p<0.001, η2=0.645]. Further, there was a significant interaction effect for DOT and SNR [F (2.72/40.81) =28.19; p<0.001; η2=0.653].
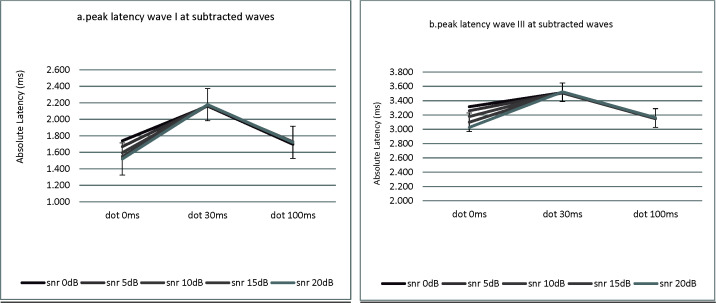
Bonferroni analysis also showed that for the 0dB SNR, there were significant changes between the 0ms DOT and the two other DOTs and between the 30 and 100ms DOTs (p<0.05). For the other SNRs (5, 10, 15, and 20dB), there were significant changes between the 0 and 30ms DOTs and between the 30 and 100ms DOTs (p<0.05). Figures 2(a) and 2(b) show the trend of change at peak latency for waves I and III, respectively. (Here, Figure2 (a-d)).
Fig 2.
(a, b) Mean values of latency wave I and III for five SNRs at three DOTs averaged over the 16 trials. (a. wave I, b. wave III). Noise was wide band noise and signal was at 16 kHz in all cases. Noise level was constant over time for each signal level (different SNRs) at 60 dB PESPL
Figure 2(a) shows that the trend of change differed with an increase in DOT in the subtracted waves. The latency of the wave I increased from the 0ms DOT to the 30ms DOT but decreased from the 30ms DOT to the 100ms DOT (p < 0.001). Figure 2(b) shows that the trend of change was similar to those for the latency of the wave I; that is, latency for the wave III increased from the 0ms DOT to the 30ms DOT but decreased from the 30ms DOT to the 100ms DOT (p < 0.001).
The inter-peak latency of the waves I-III
The analysis of the data (the inter-peak latency of the subtracted waves I-III) showed a significant main effect for DOT [F (2.0/30.00) =6.65; p<0.05; η2=0.307], but no significant main effect for SNR [F (2.35/35.25) =2.88; p>0.05; η2=0.161]. Moreover, there was no significant interaction effect between DOT and SNR [F (3.64/54.62) =2.18; p>0.05; η2=0.127].
Bonferroni analysis revealed that for the 0 and 5dB SNRs, there was a significant change between the0ms DOT and the two other DOTs (p<0.05) and between the 30 and 100ms DOTs (p<0.05). For the 10dB SNR, there was only a significant change between the 0 and 30ms DOTs (p<0.05). For the other SNRs, no significant differences were observed for the three DOTs.
The amplitude ratio of the waves III/I
The analysis of the data (the amplitude ratio of the subtracted waves III/I) showed no significant main effect for DOT [F (2.0/30.00) =1.76; p>0.05, η2=0.105], but a significant main effect for SNR [F (1.75/26.27) =4.18; p<0.05; η2=0.218]. Additionally, there was a significant interaction effect between DOT and SNR [F (8.00/36.29) =3.61; p<0.05; η2=0.194].
Bonferroni analysis also demonstrated no significant difference between any of the DOTs for all the SNRs(p>0.05). Figures 2(c) and 2(d) show the trend of change at the inter-peak latency and amplitude ratio waves III/I, respectively. Figure 2(c) shows that the trend of change of the subtracted wave differed with an increase in DOT. The inter-peak latency of the waves I-III decreased for the 0 and 30ms DOTs, but slightly increased for the 30 and 100ms DOTs.
Figure 2(d) shows an inverse trend of change for the subtracted waves. Accordingly, the amplitude ratio of the waves III/I changed irregularly for the 0 and 30ms DOTs, but did not change for the30to 100ms DOTs. (Here, Fig.3.)
Fig 3. (a, b).
Mean values of Inter peak latency between waves I-III and Amplitude ratio waves III / I for five SNRs at three DOTs averaged over the 16 trials respectively . Noise was wide band noise and signal was at 16 kHz in all cases. Noise level was constant over time for each signal level (different SNRs) at 60 dB PESPL
Figure 3. The grand average of the ABR waveforms (Left picture: the grand average of the ABR waveforms in response to the noise and the signal together with the three different DOTs under various stimulus conditions; Right picture: the subtraction of the waveforms of the noise and the signal together from the waveforms of the noise alone in the three DOTs at the 0dB SNR). The noise was wide-band (0.1-8.0 kHz, 100ms) at the 60dB peSPL. The signal was the five tone burst complex train at the 16 kHz frequency for the five different SNRs (n=16).
Discussion
To achieve electrophysiological overshoot, this study compared the absolute latency of the waves I&III, inter-peak latency of the waves I-III, and amplitude ratio of the waves III/I using ABR. ABR was measured for DOTs of 0, 30, and 100ms between the noise and the signal for five SNRs. These variables were measured once for the original waves and again after the subtraction of the waveform generated by the noise and the signal together from that generated by the noise alone. It was hypothesized that an increase in DOT would decrease the latencies of the waves. The results revealed a decrease in the ABR latencies, demonstrating overshoot in the subtracted waveforms. There was no evidence of overshoot in the original waveforms. It was concluded that overshoot can be measured objectively using ABR, especially when the noise effect is controlled.
This is the first study on overshoot using ABR. Chatterjee et al. (1993) demonstrated an overshoot-like effect using CAP, where the noise or the competitive stimulus was a tone burst at a different frequency from the signal(9). In overshoot, the duration of the competitive stimulus should be longer than the signal duration and a wider frequency band than the signal is advisable. Therefore, in the current study, wide-band noise was used as a competitive stimulus and a16 kHz tone burst as a signal at different DOTs. It can be considered that the ease of signal detectability reveals ABR at a lower threshold and lower latency.
It is accepted that decreasing the stimulus intensity increases the ABR latency. In the current study, the intensity of the stimuli was fixed for both the signal and the noise but DOT changed. It was expected that an increase in DOT would decrease the effect of the noise on the signal and improve the detectability of the signal. It was observed that the latency of the waves decreased at the 100ms DOT compared to the 0ms DOT, but there were no significant changes in the inter-peak latency and amplitude ratio of the waves. There was also an interesting trend in the response changes with an increase in DOT at different SNRs. Accordingly, the latency of the waves I and III from 0 to 30ms increased and from 30 to 100ms decreased. These changes showed a high effect size, especially for the initial waves (I).
A probable effective mechanism in overshoot is that of auditory efferent function (4, 7, 17-19). The time histograms of the auditory ipsilateral and contralateral efferent nerve function indicate a discharge or firing rate peak of 20ms to 45ms (14, 20). It is thought that at a 30ms DOT, the auditory efferent nerve function is at a maximum level for the ant masking effect. This means that the auditory efferent increases SNR when a signal is masked by noise, thereby enhancing the encoding of the signals in the noise (21). With a greater DOT, the effect of efferent nerves decreases, but the detectability of the ABR waves increases and decreases the latency, especially for earlier waves such as the wave I. It can be concluded that increasing the latency of the waves from the 0ms DOT to the 30ms DOT can be explained by auditory efferent nerve function.
The amplitude parameter was not investigated because of its high amount of variability. Instead, we investigated the amplitude ratio of the waves III/I, but the changes were not significant, probably because they occurred at the amplitude of the both waves. For the inter-peak latency of the waves I-III, no significant difference was found for the same reason. However, examining the absolute peak latency of the waves I&III revealed overshoot, especially for the wave I and, to a lesser extent, the wave III. We recommend to consider the absolute latency of the wave I in electrophysiological overshoot.
The overshoot magnitude in this study was 5 to 10dB. As increasing DOT will decrease the threshold for psychoacoustic overshoot, in which the noise effect on the signal decreases and signal detection becomes easier (1). The detectability of the signal in our study reflected the neural activity and also became much easier; however, the range of the observed phenomenon was not as salient as observed in psychoacoustic studies(4).As mentioned, the proposed factors or mechanisms that influence overshoot are the peripheral and central factors(4, 6, 22). The role of attention as a central factor and the efferent nerve function as a peripheral factor has been linked to overshoot(23). In psychoacoustic overshoot studies, the listener is alert and attends to the task. However, in our study, the guinea pigs were anesthetized. The efferent nerve function decreased, but did not disappear completely (24); therefore, the magnitude of electrophysiological overshoot was lower.
In the initial analysis of the original ABR waves, no sign of overshoot was observed. In an attempt to determine whether the response to the signal was affected by the noise, we subtracted the waveform generated by the noise and the signal together from that generated by the noise alone for the five SNRs and the different DOTs. After the subtraction of the waves, an overshoot-like effect was observed. This probably occurred because the fibers responding to the signal differed from those responding to the noise, considering the range of the used frequencies. The subtraction of the noise and signal waveforms from the noise waveform resulted in a waveform that was approximately identical in morphology, latency, and amplitude to the signal waveform alone. One can realize that the response to the noise and the signal is a summation of two overlapping waveforms in time, probably from two different neuronal populations.
In the current study, the noise was broadband, while the signal was a transient tone burst. It is known that the medial olivocochlear reflex acts to minimize the steady response to the noise by auditory nerve fibers, thereby maximizing the response to a transient signal and making it easier to detect the signal. Moreover, attention attenuates irrelevant auditory stimuli through the function of the caudal efferent system (8, 10). It is thought that anesthesia diminishes the effect of attention and the medial olivocochlear reflex. Accordingly, in our study, the subtraction of the waves lessened the effect of the noise on the signal and helped to extract major features.
Table 1.
The mean amount of absolute latency Wave I to three DOTs at five different SNRs subtracted waves
| Measured Parameters subtracted wave I | SNRs | P value | DOTs | P value | DOTs | P value | DOTs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 ms |
30 ms |
30 ms |
100 ms |
0 ms |
100 ms |
|||||
| Absolute Latency Wave I | 0dB | * | 1.739±.059 | 2.161±.043 | * | 2.161±.043 | 1.698±.038 | ** | 1.739±.059 | 1.698±.038 |
| 5dB | 1.669±.050 | 2.170±.044 | 2.170±.044 | 1.720±.038 | 1.669±.050 | 1.720±.038 | ||||
| 10dB | 1.596±.034 | 2.173±.040 | 2.173±.040 | 1.718±.037 | * | 1.596±.034 | 1.718±.037 | |||
| 15dB | 1.549±.031 | 2.168±.046 | 2.168±.046 | 1.728±.036 | 1.549±.031 | 1.728±.036 | ||||
| 20dB | 1.519±.027 | 2.178±.049 | 2.178±.049 | 1.721±.036 | 1.519±.027 | 1.721±.036 | ||||
* P<0.05, **P>0.05
Table 2.
The mean amount of absolute latency III wave to three DOTs at five SNRs for subtracted waves
| Measured Parameters subtracted wave III | SNRs | P value | DOTs | P value | DOTs | P value | DOTs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 ms |
30 ms |
30 ms |
100 ms |
0 ms |
100 ms |
|||||
| Absolute Latency Wave III | 0dB | * | 3.316±.052 | 3.513±.056 | * | 3.513±.056 | 3.158±.027 | * | 3.316±.052 | 3.158±.027 |
| 5dB | 3.259±.060 | 3.508±.055 | 3.508±.055 | 3.148±.027 | ** | 3.259±.060 | 3.148±.027 | |||
| 10dB | 3.179±.060 | 3.513±.057 | 3.513±.057 | 3.153±.027 | 3.179±.060 | 3.153±.027 | ||||
| 15dB | 3.099±.051 | 3.516±.053 | 3.516±.053 | 3.158±.029 | 3.099±.051 | 3.158±.029 | ||||
| 20dB | 3.027±.049 | 3.524±.054 | 3.524±.054 | 3.163±.029 | 3.027±.049 | 3.163±.029 | ||||
* P<0.05, **P>0.05
Table 3.
The mean amount of inter peak latency I - III waves to three DOTs at five SNRs for subtracted waves
| Measured Parameters subtracted waves | SNRs | P value | DOTs | P value | DOTs | P value | DOTs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 ms |
30 ms |
30 ms |
100 ms |
0 ms |
100 ms |
|||||
| Inter peak latency I-III | 0dB | * | 1.581±.040 | 1.391±.039 | * | 1.391±.039 | 1.464±.031 | * | 1.581±.040 | 1.464±.031 |
| 5dB | ** | 1.591±.038 | 1.380±.040 | ** | 1.380±.040 | 1.429±.032 | ** | 1.591±.038 | 1.429±.032 | |
| 10dB | * | 1.583±.041 | 1.383±.036 | 1.383±.036 | 1.437±.027 | 1.583±.041 | 1.437±.027 | |||
| 15dB | ** | 1.553±.046 | 1.380±.041 | 1.380±.041 | 1.432±.025 | 1.553±.046 | 1.432±.025 | |||
| 20dB | 1.514±.043 | 1.376±.043 | 1.376±.043 | 1.445±.024 | 1.514±.043 | 1.445±.024 | ||||
* P<0.05, **P>0.05
Table 4.
The mean amount of amplitude ratio III/I waves to three DOTs at five SNRs for subtracted waves P<0.05, **P>0.05
| Measured Parameters subtracted waves | SNRs | P value | DOTs | P value | DOTs | P value | DOTs | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 ms |
30 ms |
30 ms |
100 ms |
0 ms |
100 ms |
|||||
| Amplitude ratio III/I | 0dB | ** | 2.243±.262 | 1.864±.236 | ** | 1.864±.236 | 1.683±.164 | ** | 2.243±.262 | 1.683±.164 |
| 5dB | 2.819±.569 | 2.075±.389 | 2.075±.389 | 1.667±.155 | 2.819±.569 | 1.667±.155 | ||||
| 10dB | 1.759±.262 | 2.101±.294 | 2.101±.294 | 1.637±.134 | 1.759±.262 | 1.637±.134 | ||||
| 15dB | 1.583±.153 | 1.955±.239 | 1.955±.239 | 1.476±.103 | 1.583±.153 | 1.476±.103 | ||||
| 20dB | 1.532±.101 | 2.153±.263 | 2.153±.263 | 1.456±.103 | 1.532±.101 | 1.456±.103 | ||||
Fig 4.
The grand average of ABR waveforms (Left picture; The grand average of ABR waveforms in response to noise and signal together with three different DOTs under various stimulus conditions), (Right picture; subtractions of the waveforms of noise and signal together from waveforms of noise alone in three DOTs at 0 dB SNR). The noise was a Wide Band Noise (100ms) at 60 dB PESPL. The signal was five tone burst complex train at 16 kHz frequency for five SNRs
In Conclusion
Our study was the first to explore overshoot electro physiologically using ABR. The study indicated that the ABR overshoot was effective as an objective test of cochlear amplification. Of course, the findings of this study are specifically to the study itself and cannot be generalized. To find out more about the potential role of overshoot in the test battery of cochlear function assessment, especially in non-cooperative children, further clinical trials are recommended.
Authors Contribution
Authors had the main idea of the project, designed the study, cooperated in performing the study and collecting and interpreting the data, critically reviewed the manuscript, and approved the final manuscript as submitted.
Conflicts of interest
None of the authors declares any conflicts of interest.
Acknowledgment
This work was supported by a research grant from the Iran University of Medical Sciences. The authors conducted this and additional research on this topic while working on a PhD degree at The Faculty of rehabilitation sciences, IUMS, 2018 (IR.IUMS.REC-1396.9311303001, date of, 01/20/2018). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Iran University of Medical Sciences. We are grateful to Dr. Samer, Mohsen (MD, PhD of Audiology, Faculty of Health Sciences, Damascus University) for his generous contribution on the data collection.
References
- 1.Fletcher M, de Boer J, Krumbholz K. Is overshoot caused by an efferent reduction in cochlear gain? Adv Exp Med Biol. 2013;787:65–72. doi: 10.1007/978-1-4614-1590-9_8. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher M, de Boer J, Krumbholz K. Is off-frequency overshoot caused by adaptation of suppression? J Assoc Res Oto. 2015;16(2)::241–53. doi: 10.1007/s10162-014-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFadden D, Walsh KP, Pasanen EG, Grenwelge EM. Overshoot using very short signal delays. J Acoust Soc Am .2010;128(4)::1915–21. doi: 10.1121/1.3480568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh KP, Pasanen EG, McFadden D. Overshoot measured physiologically and psychophysically in the same human ears. Hear Res. 2010;268(1-2)::22–37. doi: 10.1016/j.heares.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jennings SG, Heinz MG, Strickland EA. Evaluating adaptation and olivocochlear efferent feedback as potential explanations of psychophysical overshoot. J Assoc Res Oto. 2011;12(3)::345–60. doi: 10.1007/s10162-011-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keefe DH, Schairer KS, Ellison JC, Fitzpatrick DF, Jesteadt W. Use of stimulus-frequency otoacoustic emissions to investigate efferent and cochlear contributions to temporal overshoot. J Acoust Soc Am. 2009;125(3)::1595–604. doi: 10.1121/1.3068443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Klitzing R, Kohlrausch A. Effect of masker level on overshoot in running- and frozen-noise maskers. J Acoust Soc Am. 1994;95(4)::2192–201. doi: 10.1121/1.408679. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt S, Zwicker E. The effect of masker spectral asymmetry on overshoot in simultaneous masking. J Acoust Soc Am. 1991;89(3)::1324–30. doi: 10.1121/1.400656. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee M, Smith RL. Physiological overshoot and the compound action potential. Hear Res. 1993;69(1-2)::45–54. doi: 10.1016/0378-5955(93)90092-f. [DOI] [PubMed] [Google Scholar]
- 10.Overson GJ, Bacon SP, Webb TM. The effect of level and relative frequency region on the recovery of overshoot. J Acoust Soc Am. 1996;99(2)::1059–65. doi: 10.1121/1.415232. [DOI] [PubMed] [Google Scholar]
- 11.Bacon SP. Effect of masker level on overshoot. J Acoust So Am. 1990;88(2)::698–702. doi: 10.1121/1.399773. [DOI] [PubMed] [Google Scholar]
- 12.Hicks ML, Bacon SP. The effect of pure-tone forward masking on overshoot. J Acoust Soc Am. 1991;90(1)::228–30. doi: 10.1121/1.401292. [DOI] [PubMed] [Google Scholar]
- 13.Heffner HE, Heffner RS. Hearing ranges of laboratory animals. J Am Assoc Lab Anim. 2007;46(1)::20–2. [PubMed] [Google Scholar]
- 14.Robertson D, Gummer M. Physiological and morphological characterization of efferent neurones in the guinea pig cochlea. Hear Res. 1985;20(1)::63–77. doi: 10.1016/0378-5955(85)90059-0. [DOI] [PubMed] [Google Scholar]
- 15.J H. New handbook of auditory evoked responses: pearson education. 2007. [Google Scholar]
- 16.Shi W, Ji F, Lan L, Liang SC, Ding HN, Wang H, et al. Characteristics of cochlear microphonics in infants and young children with auditory neuropathy. Acta Otolaryngol. 2012;132(2)::188–96. doi: 10.3109/00016489.2011.630016. [DOI] [PubMed] [Google Scholar]
- 17.de Boer J, Yasin I, Drga V, Plack CJ. Effect of human auditory efferent feedback on cochlear gain and compression. J Assoc Res Oto. 2014;34(46)::15319–26. doi: 10.1523/JNEUROSCI.1043-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eggermont JJ. Peripheral auditory adaptation and fatigue: a model oriented review. Hear Res. 1985;18(1)::57–71. doi: 10.1016/0378-5955(85)90110-8. [DOI] [PubMed] [Google Scholar]
- 19.Guinan JJ Jr, Cooper NP. Medial olivocochlear efferent inhibition of basilar-membrane responses to clicks: evidence for two modes of cochlear mechanical excitation. J Acoust Soc Am. 2008;124(2)::1080–92. doi: 10.1121/1.2949435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown MC. Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res. 1989;40(1-2)::93–109. doi: 10.1016/0378-5955(89)90103-2. [DOI] [PubMed] [Google Scholar]
- 21.Tomchik S, Lu Z. Modulation of Auditory Signal-to-Noise Ratios by Efferent Stimulation. J Neurophysiol. 2006;95(6)::3562–70. doi: 10.1152/jn.00063.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtenhan JT, Wilson US, Hancock KE, Guinan JJ Jr. Medial olivocochlear efferent reflex inhibition of human cochlear nerve responses. Hear Res. 2016;333:216–24. doi: 10.1016/j.heares.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guinan JJ Jr. Olivocochlear efferents: Their action, effects, measurement and uses, and the impact of the new conception of cochlear mechanical responses. Hear. Res. 2018;362:38–47. doi: 10.1016/j.heares.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers AR, Hancock KE, Maison SF, Liberman MC, Polley DB. Sound-evoked olivocochlear activation in unanesthetized mice. J Assoc Res Oto. 2012;13(2)::209–17. doi: 10.1007/s10162-011-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]