Abstract
The Coronavirus Disease 2019 (COVID-19) pandemic has transformed health systems worldwide. There is conflicting data regarding the degree of cardiovascular involvement following infection. A registry was designed to evaluate the prevalence of echocardiographic abnormalities in adults recovered from COVID-19. We prospectively evaluated 595 participants (mean age 45.5 ± 14.9 years; 50.8% female) from 10 institutions in Argentina and Brazil. Median time between infection and evaluation was two months, and 82.5% of participants were not hospitalized for their infection. Echocardiographic studies were conducted with General Electric equipment; 2DE imaging and global longitudinal strain (GLS) of both ventricles were performed. A total of 61.7% of the participants denied relevant cardiovascular history and 41.8% had prolonged symptoms after resolution of COVID-19 infection. Mean left ventricular ejection fraction (LVEF) was 61.0 ± 5.5% overall. In patients without prior comorbidities, 8.2% had some echocardiographic abnormality: 5.7% had reduced GLS, 3.0% had a LVEF below normal range, and 1.1% had wall motion abnormalities. The right ventricle (RV) was dilated in 1.6% of participants, 3.1% had a reduced GLS, and 0.27% had reduced RV function. Mild pericardial effusion was observed in 0.82% of participants. Male patients were more likely to have new echocardiographic abnormalities (OR 2.82, p = 0.002). Time elapsed since infection resolution (p = 0.245), presence of symptoms (p = 0.927), or history of hospitalization during infection (p = 0.671) did not have any correlation with echocardiographic abnormalities. Cardiovascular abnormalities after COVID-19 infection are rare and usually mild, especially following mild infection, being a low GLS of left and right ventricle, the most common ones in our registry. Post COVID cardiac abnormalities may be more frequent among males.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10554-022-02706-9.
Keywords: Echocardiography, COVID-19, Myocarditis, Diagnostic imaging, Ventricular remodeling, Humans
Introduction
In March 2020, a new infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic, and the disease spread globally [1]. The spectrum of the severity from the novel Coronavirus 19 Disease (COVID-19) ranges from asymptomatic to life-threatening infection where pulmonary involvement predominates, resulting in acute respiratory distress syndrome. However, as information about COVID-19 is further elucidated, cases of cardiovascular involvement have emerged, both during infection and following resolution [2–4]. Further, certain reports have suggested that the majority of patients following COVID-19 infections suffer some kind of cardiac sequelae, regardless of disease severity [5].
The SARS-CoV-2 virus binds to the angiotensin converting enzyme (ACE) II receptor, and it has been theorized that this is the gateway to enter the body [6]. ACE receptors are mainly expressed in the pulmonary alveoli, and the second organ with the highest number of ACE receptors is the heart. Notably, cardiac involvement during COVID-19 infection increases mortality and is correlated with worse outcomes [7–12].
In addition to the clinical manifestations which occur during the acute phase of COVID-19, a significant number of publications have described the persistence of symptoms and alterations after the microbiological recovery from COVID‐19 [13–15]. This phenomenon has been called post-COVID syndrome, with a wide prevalence across diverse cohorts [16, 17]. For example, it has been suggested that silent myocardial injury may occur after COVID-19, even among patients who remained asymptomatic during and after acute infection [5, 18].
To date, there are millions of individuals who have recovered from COVID-19 who may be evaluated for long-term cardiovascular sequelae. Additionally, echocardiogram is a widely available and relatively inexpensive method for assessing structural and functional characteristics of the heart. We aimed to explore the prevalence of echocardiographic cardiac abnormalities in ambulatory patients after recovery of a first documented COVID-19 infection.
Materials and methods
Study population
An observational registry was implemented at nine centers in Argentina and one in Brazil between November 2020 and February 2021, following the first wave of the COVID-19 pandemic in both countries. Adult patients (> 18 years old) who were scheduled to receive a Doppler echocardiogram after a confirmed COVID-19 infection were included, regardless of the reason for the test or the time elapsed since recovery, based on a non probability sampling. Patients with a suboptimal apical ultrasonic window where two or more myocardial segments were not correctly followed for global longitudinal strain (GLS) analysis were excluded from the registry. Similarly, those patients with irregular rhythms or high heart rate where reconstruction of a bull’s eye plot of the left ventricular GLS was not possible were also excluded. We prospectively and consecutively included 595 participants who recovered from a COVID-19 infection with an average age of 45.5 ± 14.9 years, of which 50.8% were female. The majority of patients (82.5%) had the disease at home or in an out-of-hospital center. Of the patients who required hospitalization, 15.3% were in a general ward, 1.9% in intensive care without requiring mechanical ventilation, and 0.3% required mechanical ventilation during the disease. The median time between infection and performance of the echocardiographic study was two months (interquartile range [IQR] 1–3 months). The minimum and maximum ranges between the epidemiological discharge of the patients and the echocardiographic examination was 15 days and nine months, respectively (see Appendix Figure S1).
Trial protocol
A case report form was developed in order to collect demographic data, pathological personal history, month in which COVID-19 infection was diagnosed, and severity of the disease. All the echocardiographic studies were carried out with General Electric echocardiography machines (see Appendix Table S1) with an established protocol. All examinations were performed by echocardiographers with at least two years of experience in the routine performance of GLS. In order to reduce the interobserver and the intervendor variability due to the software used, a single commercial brand was used, which previously demonstrated the greatest consistency between the measurements [19, 20].
Cardiovascular imaging
The diameters of the left ventricles were obtained in two dimensions (2D) in the left parasternal long axis [21]. The right ventricular (RV) diameter was obtained in the apical four-chamber view at the level of the tricuspid annulus. Pulsed Doppler was performed in the left ventricular inlet tract at the level of the mitral free edge to determine ventricular filling pressures, as well as pulsed tissue Doppler in the lateral mitral annulus to estimate the E/e’ ratio [22]. The size of the left atrium was determined through indexed atrial volume, performed in four and two apical chambers [21]. Analysis of myocardial deformation from the left ventricle was performed by automatic 2D GLS according to standard recommendations [23]. Ventricular-volume, left ventricular ejection fraction (LVEF), and GLS parameters were determined by automatic endomyocardial edge detection with the least possible intervention by the operator (automatic functional imaging; AFI). The RV function was assessed by visual inspection, the tricuspid annular plane systolic excursion (TAPSE), and the myocardial deformation (strain) which was measured in a RV-focused apical four-chamber view, excluding the interventricular septum [24, 25].
For the GLS analysis of both ventricles, a visual assessment of the 2D imaging tracking was made prior to the analysis. A manual correction of the endocardial borders was allowed to guarantee an appropriate monitoring of all analyzed segments. The software used was the one already installed in each ultrasound machine, which in all cases performed an analysis of the full myocardial wall [19, 20, 23, 25]. According to the brand and model of the equipment used, a normal GLS value was set for the left ventricle above − 18.0% and for the right ventricle above − 20.0% [19, 20, 25].
Statistical analysis
Continuous variables were reported as means with their standard deviations for normally distributed variables or median and interquartile range (IQR) for non-normally distributed variables. Normality was evaluated using graphic tools (histograms and normal probability plot) and Shapiro–Wilk test. Categorical variables were expressed by absolute values and percentages. For normally distributed variables, the analysis was performed using the Student's test. For non-normally distributed variables, the Wilcoxon rank sum test was used. Differences in proportions were evaluated by the Chi Square test or Fisher’s Exact test according to the frequency of expected values.
After excluding participants with previous cardiovascular disease or with any comorbidity, we manually performed a multiple logistic regression model in order to investigate which clinical variables were associated with presenting any abnormality on the echocardiogram. Once the final model was obtained, its predictive capacity was evaluated by constructing a Receiver Operating Characteristic (ROC) curve. A two-tail p-value < 0.05 was considered statistically significant in all cases. STATA version 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.) was used for the analyses.
Results
Study population and baseline clinical characteristics
The reasons for echocardiographic evaluation included: persisting or new symptoms after disease recovery (61.7%), patient fear of suffering from a cardiac disorder due to COVID (30.5%), and monitoring of pre-existing cardiovascular pathology (7.8%). At the time of the study, 58.2% of the participants were asymptomatic. Among patients who reported symptoms following COVID-19 recovery, the most frequently reported was dyspnea (47.4%), followed by mild symptoms such as asthenia, arterial hypertension or palpitations (32.9%), 12.9% referred chest pain, 6% of patients reported dyspnea and chest pain, and 0.8% reported various other symptoms.
The mean body mass index in the patients was 26.8 ± 4.8 kg/m2, and 61.7% of the participants denied any relevant medical history. The most frequent comorbidity was arterial hypertension (28.6%). Table 1 summarizes the characteristics of the included patients.
Table 1.
Characteristics of the study population, according to sex
| Characteristics | Overall (n = 595) | Female (n = 302) | Male (n = 293) | “p” |
|---|---|---|---|---|
| Body mass index (kg/m2) | 26.8 ± 4.8 | 26.3 ± 5.3 | 27.4 ± 4.1 | 0.005 |
| Arterial hypertension | 28.6% | 23.2% | 34.1% | 0.003 |
| Diabetes | 6.1% | 3.3% | 8.9% | 0.005 |
| Dyslipidemia | 3.5% | 2.0% | 5.1% | 0.046 |
| Smokers | 2.5% | 3.3% | 2.1% | 0.180 |
| Former smokers | 1.9% | 1.0% | 2.7% | 0.150 |
| Cardiovascular diseases | 3.0% | 1.7% | 4.8% | 0.036 |
| Valvular diseases | 2.0% | 2.0% | 2.1% | 0.593 |
| Other cardiovascular diseases | 3.5% | 4.3% | 2.7% | 0.376 |
| Non cardiovascular diseases | 3.9% | 4.3% | 3.4% | 0.573 |
Echocardiographic findings
Echocardiographic examination of participants demonstrated a mean left atrial volume of 33.1 ± 13.2 ml/m2, and 32.9% of the participants had some degree of left atrial enlargement. The mean LVEF was estimated at 61.0 ± 5.5%, and 3.7% of the participants had a LVEF below 50%. Table 2 summarizes relevant echocardiographic findings.
Table 2.
Echocardiographic findings according to sex
| Parameter | Overall | Female | Male | “p” |
|---|---|---|---|---|
| Left ventricular diastolic dimension (mm) | 46.1 ± 4.6 | 44.3 ± 4.1 | 48.0 ± 4.4 | < 0.001 |
| Septal thickness (mm) | 9.3 ± 2.8 | 8.6 ± 2.0 | 10.0 ± 3.2 | < 0.001 |
| Posterior wall thickness (mm) | 8.8 ± 1.5 | 8.1 ± 1.3 | 9.4 ± 1.4 | < 0.001 |
| Left ventricular systolic dimension (mm) | 29.8 ± 5.1 | 28.5 ± 4.2 | 31.1 ± 5.7 | < 0.001 |
| Left atrial volume | 33.1 ± 13.2 | 30.7 ± 11.1 | 35.5 ± 14.7 | < 0.001 |
| Left atrial: | ||||
| Mildly abnormal | 15.1% | 12.9% | 17.4% | 0.001 |
| Moderately abnormal | 6.9% | 7.0% | 6.8% | |
| Severely abnormal | 10.9% | 6.6% | 15.4% | |
| Left ventricular end-diastolic volume | 79.1 ± 26.9 | 68.7 ± 20.8 | 89.8 ± 28.4 | < 0.001 |
| Left ventricular end-systolic volume | 34.1 ± 15.6 | 29.4 ± 12.8 | 38.9 ± 16.8 | < 0.001 |
| Left ventricular ejection fraction | 61.0 ± 5.5 | 61.9 ± 5.7 | 60.2 ± 5.2 | < 0.001 |
| Left ventricular ejection fraction | ||||
| Mildly abnormal (LVEF 40–52 to 54%) | 3.0% | 2.7% | 3.4% | 0.541 |
| Moderately abnormal (LVEF 30–39%) | 0.7% | 1.0% | 0.3% | |
| Mitral E/e’ ratio | 7.2 ± 2.3 | 7.3 ± 2.3 | 7.2 ± 2.4 | 0.553 |
| Right ventricular basal diameter (mm) | 30.5 ± 6.3 | 28.6 ± 5.3 | 32.5 ± 6.5 | < 0.001 |
| TAPSE (mm) | 23.4 ± 3.4 | 23.2 ± 3.3 | 23.7 ± 3.4 | 0.113 |
| Pulmonary artery systolic pressure (mmHg) [n = 329] | 27.9 ± 5.2 | 27.6 ± 4.8 | 28.3 ± 5.5 | 0.210 |
| Global longitudinal strain (− %) | 20.6 ± 2.7 | 21.4 ± 2.6 | 19.9 ± 2.7 | < 0.001 |
| RV free wall GLS (− %) [n = 460] | 26.0 ± 4.4 | 26.1 ± 4.7 | 25.8 ± 4.2 | 0.462 |
mm millimeter, LVEF left ventricular ejection fraction, TAPSE tricuspid annular plane systolic 11excursion, RV right ventricle, GLS global longitudinal strain
Among patients with reduced LVEF (3.7%), 50.0% of them denied a relevant medical history and were unaware of this condition. In this subgroup, 90.1% had mildly abnormal LVEF (Table 2). The only patient with a moderately abnormal LVEF (0.3%) underwent cardiac magnetic resonance (CMR) which confirmed a myocarditis pattern. Abnormal wall motions by visual analysis were detected in 4.4% of the participants. After excluding those with a history of ischemic cardiomyopathy or depressed LVEF, 1.1% of the participants presented abnormal wall motions in the left ventricle. GLS had an average value of − 20.6 ± − 2.7%, and 12.1% of the participants had a value inferior to − 18.0%. After excluding participants with significant cardiovascular or clinical comorbidities, as well as those with ventricular dysfunction at the time of the examination, 5.7% of the participants had a reduced GLS. This finding was more frequent among male participants than female participants (11.1% versus 1.5%, p < 0.001).
The RV was dilated in 2.2% of the participants; after excluding patients with significant cardiovascular or clinical comorbidities, and two high-performance athletes, 1.6% of the patients did not have an alternative cause to explain this finding. The RV function was depressed in 0.8% of the participants; all of them had a TAPSE less than 17 mm (mm). It was feasible to analyze the RV free wall GLS from 77.3% of the participants: among them, 4.6% had a value less than − 20.0%. After excluding patients with significant cardiovascular or clinical comorbidities, and those with RV depressed function, 3.1% of the participants had a low RV free wall GLS with no other apparent cause. In this subgroup of patients, the mean TAPSE was 25.4 ± 4.2 mm. We did not find differences between sex in the frequency of abnormal RV free wall GLS (2.1% in female versus 1.8% in male, p = 0.527). Three patients with no relevant medical history (0.8%) had left ventricular GLS and RV free wall GLS below normal limits.
Pericardial effusion was observed in 1.2% of the participants, but was mild in all cases. No patient with pericardial effusion presented reduced GLS, while only one of them presented RV free wall GLS less than normal. We found no significant differences in LVEF between symptomatic and asymptomatic patients (61.4% versus 60.6% respectively, p = 0.104). Symptomatic patients showed slightly reduced GLS (− 20.3% versus − 20.9%, p = 0.012) with a trend in the same direction in the RV free wall GLS (− 25.6% versus − 26.3%, p = 0.103).
In Table 3 we summarized the echocardiographic abnormalities in patients with and without pre-existing comorbidities.
Table 3.
Echocardiographic abnormalities according to pre-existing comorbidities
| Echocardiographic finding | Non comorbidities (61.7%; n = 367) | With comorbidities (38.3%; n = 228) | “p” |
|---|---|---|---|
| Mid-range LVEF | 2.7% (10) | 3.6% (8) | 0.572 |
| LVEF below 40% | 0.3% (1) | 1.4% (3) | 0.128 |
| Abnormal wall motions in LV | 1.1% (4) | 8.8% (20) | < 0.001 |
| Reduced GLS (below −18.0%) | 5.7% (21) | 21.1% (48) | < 0.001 |
| Dilated RV | 1.6% (6) | 2.2% (5) | 0.630 |
| Reduced RV function | 0.27% (1) | 1.8% (4) | 0.074 |
| Reduced RV GLS (n = 460)* | 3.1% (9) | 7.2% (12) | 0.060 |
| Pericardial effusion | 0.82% (3) | 1.8% (4) | 0.437 |
| PASP (n = 329) | 27.3 ± 4.5 mmHg | 29.0 ± 5.9 mmHg | 0.003 |
*For RV a reduced GLS was considered with a value under − 20.0%
LVEF left ventricular ejection fraction, GLS global longitudinal strain, RV right ventricle, PASP Pulmonary artery systolic pressure
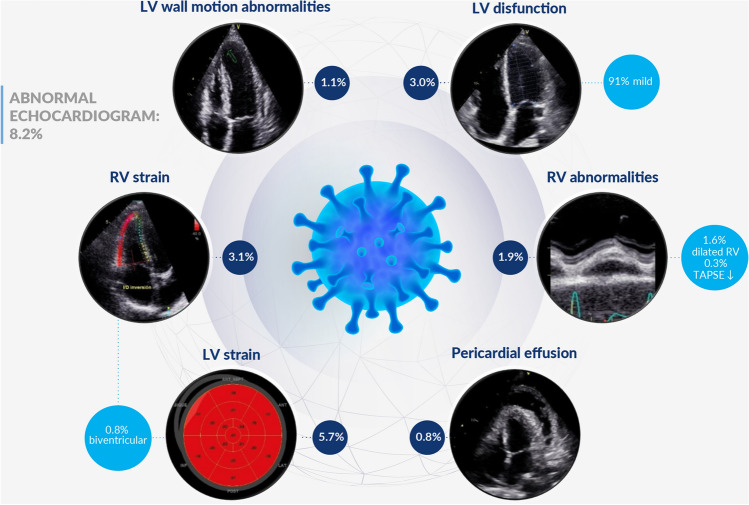
Globally, 8.2% of the participants without prior cardiovascular disease or significant comorbidities had some echocardiographic abnormality (Fig. 1). New echocardiographic abnormalities were more frequent in male patients than female patients (OR 2.82, 95% CI 1.46–5.45, p = 0.002). Conversely, we did not find any relationship between time elapsed from infection to study enrollment (OR 1.11 95% CI 0.93–1.33, p = 0.245), the presence of symptoms (OR 0.97 95% CI 0.51–1.83, p = 0.927), or requiring hospitalization during COVID-19 infection (OR 1.18 95% CI 0.55–2.51, p = 0.671). Through the construction of a ROC curve we were able to detect that age as a single variable had a low predictive value for new abnormalities in the echocardiogram (area under ROC curve of 0.65) (see Appendix Figure S2).
Fig. 1.
Summary of echocardiographic abnormalities after recovery from COVID-19 infection in patients without prior comorbidities
Due to the basal differences of the participants (Table 1), we developed a multivariable logistic regression model to explore the role of male sex to predict any echocardiographic cardiac abnormality irrespective of prior characteristics and comorbidities. Notably, the difference between sexes remains independent and statistically significant after adjusting for severity of the infection, time between COVID-19 disease and echocardiographic examination, presence of symptoms, age, hypertension, diabetes, dyslipidemia, smokes, cardiovascular diseases, left atrial volume and diastolic function. The estimated OR was 3.72 (95% CI 1.86–7.46, p < 0.001); age was the other independent variable that predicted echocardiographic abnormalities (OR 0.96 95% CI 0.94 – 0.99, p = 0.004).
Discussion
The main findings of our study were: (i) a relatively low rate of echocardiographic abnormalities after a mild COVID-19 infection; (ii) the most frequent alterations were a left ventricular GLS below the normal range, followed by an abnormal RV free wall GLS; (iii) the more severe abnormalities (a moderately reduced LVEF and pericardial effusion) were seen in less than 1% of participants; (iv) any echocardiographic abnormalities were more frequent in male patients irrespective of any other demographic or clinical characteristic.
The COVID-19 pandemic has created an enormous challenge for health systems around the world. The initial demand for the care of acute patients was then compounded by care of individuals affected by sequalae following recovery. Since the first literature emerged about COVID-19, possible cardiovascular involvement has been identified and linked to a worse prognosis [7, 9, 26]. Accordingly, an autopsy study from patients who died of COVID-19 reported that the virus was documented in the cardiac tissue of 61.5% of those studied [26]. Multiple publications have similarly identified the role of echocardiography in detecting cardiovascular abnormalities during the acute phase of the disease, and the prognostic implications of this finding [27–31]. Most of the data suggested that RV and LV strain measured by speckle-tracking were acceptable parameters to predict poor outcomes, including mortality [28–30].
Perhaps the more complex issue is determining the true degree of cardiac involvement in individuals recovered after a COVID-19 infection, and related clinical relevance. An initial study found that 78% of the individuals recently recovered after a COVID-19 infection presented some alteration in the CMR, and 60% presented signs of ongoing myocardial inflammation [5]. This study included only 100 participants and the main imaging findings were alterations in native T2 and T1 mapping. The CMR examination was performed on average 71 days (interquartile range [IQR] 64 to 92 days) after the initial positive test for COVID-19 [5]. In contrast, a case series which evaluated 145 young athletes with CMR on an average of 15 days after being diagnosed with COVID-19 infection found alterations compatible with myocarditis in 1.4% of them [32]. In this series, there were no participants with severe symptoms, and 16.6% of the participants had an asymptomatic infection [32]. A systematic review and meta-analysis of CMR also found a prevalence of myocarditis and late gadolinium enhancement of 14.0% and 20.5%, respectively, in those recovered from COVID-19 [33]. However, they included 890 patients from 16 studies with high heterogeneity, which limits the generalizability of this dataset [34, 35]. Conversely, participants in our registry have characteristics similar to most people recovering from COVID-19 infection.
Previous authors have investigated the cardiac impact of COVID-19 infection after recovery with echocardiography. Moody et al. performed an echocardiographic examination during COVID-19 disease and three months after the first transthoracic echocardiography (TTE) [36]. They included 79 patients, of whom all required hospitalization due to COVID infection, and 80% received mechanical ventilation, with the median hospitalization stay of 32 days. At follow-up, 29% of the participants presented some echocardiographic alteration, the most frequent being right ventricular dysfunction [36]. Similarly, Tangen et al. evaluated 92 adults who required hospitalization in Norwegian hospitals, and performed a TTE three months after infection [37]. Twenty percent of participants required admission to the intensive care unit, and three needed mechanical ventilation. All patients had preserved LVEF, but 6.5% of the participants had reduced left ventricle GLS, with no alternative explanation, similar to our findings [37]. The authors did not observe any right ventricle abnormality and could not find a relationship between echocardiographic abnormalities and the severity of the infection, as in our study [37]. Another study of Baruch et al. with 80 adult patients found that approximately three months after recovery 63% of the participants had some symptoms, but the LVEF of all participants was preserved. However, 25% had a decreased GLS, and 8% had an RV GLS below the normal value [38]. The findings of this late study are more similar to our study. Similar differences can be observed in more recent publications [39–42], with rates of subclinical ventricular in up to one in three hospitalized patients [40]. The most reasonable explanation for the discrepancies observed between series of post COVID-19 patients are the differences in the severity of the patients included in them. Accordingly, our registry is novel because a large proportion of included patients were not hospitalized for infection, allowing the assessment of the impact of a broad spectrum of disease severity. Since the beginning of the pandemic it has been described that around 70–80% of affected individuals have a mild or asymptomatic infection [43, 44]. This proportion is similar to the participants who suffered from a mild infection in our registry. An important issue is that despite the differences between the studies, all of them show that subclinical alterations are the leading form of cardiac post COVID-19 alterations, with overt manifestation being less frequent. Our registry reinforces this message, showing that these alterations are particularly infrequent after mild disease.
It is notable that although some authors have reported major cardiac abnormalities after recovery of COVID-19 infection, such as depressed LVEF [45], pericarditis, and pericardial effusion [46, 47], these findings have not been detected in most majority of publications to date [5, 36–42]. A possible explanation for these findings is that these are predominantly case reports or case series with small sample size. Our registry included a large number of patients, which has allowed us to document even low prevalence events and allows a more precise estimation of the occurrence of these complications. Regarding the lack of correlation between the presence of symptoms and the echocardiographic abnormalities in our study, these findings are in line with prior publications [36, 37] whereas other authors inform a positive association with subclinical alterations [38]. This last point raises questions about the possibility of using the presence of symptoms to select which patients will benefit the most from a more in-depth cardiovascular examination.
Finally, to the best of our knowledge, the differences in echocardiographic abnormalities by sex have not been previously reported. Although we cannot rule out that it is a spurious observation, or echocardiographic difference according to sexes, it is also possible that some hormonal or unknown factors influence this fact. As variations in COVID-19 infection between sexes has been previously reported [48, 49], the impact of these differences on long term follow-up is an interesting hypothesis to be explored in the future.
Limitations
Our study has important limitations that must to be considered when analyzing the results. First, we do not have cardiac echocardiographic of the patients prior to COVID-19 infection, or at the beginning of the disease. So, we were unable to confirm that all the abnormal findings were due to COVID-19 infection. Nevertheless, most people with mild COVID-19 infection haven’t had any other examination but the confirmation test of the disease. Second, we do not have cardiac echocardiographic studies during the acute period of infection, so we have not been able to compare the evolution of the abnormalities over time. However, most patients with COVID-19 do not receive an echocardiographic examination during its hospitalization, due to different reasons, especially due to the overload of health systems, with the consequent inability to carry out more complex studies on a massive scale. Unfortunately, this reality has been more noticeable in low- and middle-income countries, such as in our region. Third, as we only included people with an adequate ultrasonic window, this represents a potential selection bias, which is actually a common limitation to all studies based on echocardiographic findings. Furthermore, since most of the participants suffered from a mild viral infection, it is not possible to generalize these findings to all post COVID-19 patients, especially among patients with more severe disease. However, as we pointed out in previous paragraphs, the majority of people who have suffered from COVID-19 experienced a mild or asymptomatic infection. Additionally, we did not have a centralized core laboratory to analyze the biventricular global longitudinal strain. However, all analyses were performed by experienced operators and with the same brand of ultrasound machine (General Electric), which minimizes technical variation, as previously published [19, 20, 23, 25]. Even more, the brand ultrasound machine used in our registry has shown the higher interobserver agreement in prior publications [19, 20]. Another major limitation is the lack availability of confirmatory imaging modalities such as cardiac magnetic resonance. Though, due to the high burden of COVID-19 infection it is impracticable to perform complex imaging on a broad number of patients, particularly following mild disease. Finally, taking into account the relatively short time between the COVID-19 infection and the echocardiographic examination, it is also not possible to know the prevalence of long-term cardiovascular abnormalities. Nonetheless, there is no reason to suspect that new echocardiographic abnormalities will develop in a period of time greater than that of our record.
Despite the aforementioned, this registry had a considerable sample size, and the observations were made in different regions but with socio-demographic and technological equipment similarities, which reinforces their generalizability.
Conclusion
Our study suggests that cardiovascular abnormalities after a COVID-19 infection are infrequent and usually mild, even when they are evaluated during the first weeks after recovery from the disease. The most frequent findings were a decrease in the GLS of both the left and the right ventricle, followed by a mild reduction in the LVEF. In contrast, the most clinically significant abnormalities, such a pericardial effusion or a moderately depressed left ventricular ejection fraction were rare.
We found no relationship between echocardiographic abnormalities and the severity of the disease or the time elapsed since recovery. Interestingly, we also did not find a relationship between the presence of symptoms and the echocardiographic abnormalities. Our data also suggest that echocardiographic abnormalities may be more frequent among male patients. Further studies are needed to delineate which patients will develop cardiovascular abnormalities after COVID-19 infection.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
SGZ, JMP, NG and PMM designed the project SGZ, JMP and KB wrote the main manuscript text AKS, SOG and MA prepared tables 1-3 AJL, PMM and SOG prepared figure 1. All authors performed data collection & reviewed the manuscript.
Funding
The present study has not received any grants or financial support.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Atri D, Siddiqi HK, Lang JP, Nauffal V, Morrow DA, Bohula EA. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haseeb S, Gul EE, Cinier G, Bazoukis G, Alvarez-Garcia J, Garcia-Zamora S, et al. Value of electrocardiography in coronavirus disease 2019 (COVID-19) J Electrocardiol. 2020;62:39–45. doi: 10.1016/j.jelectrocard.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng L, Wang S, Cai J, Sun S, Wang S, Li J, et al. Clinical characteristics of COVID-19 with cardiac injury: a systematic review and meta-analysis. Epidemiol Infect. 2020;148:e266. doi: 10.1017/S0950268820002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker C, Deb S, Ling H, Wang Z. Assessing the elevation of cardiac biomarkers and the severity of COVID-19 infection: a meta-analysis. J Pharm Pharm Sci. 2020;23:396–405. doi: 10.18433/jpps31501. [DOI] [PubMed] [Google Scholar]
- 5.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Guan B, Su T, Liu W, Chen M, Bin Waleed K, et al. Impact of cardiovascular disease and cardiac injury on in-hospital mortality in patients with COVID-19: a systematic review and meta-analysis. Heart. 2020;106(15):1142–1147. doi: 10.1136/heartjnl-2020-317062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Zamora S, Lee S, Haseeb S, Bazoukis G, Tse G, Alvarez-Garcia J, et al. Arrhythmias and electrocardiographic findings in Coronavirus disease 2019: a systematic review and meta-analysis. Pacing Clin Electrophysiol. 2021;44(6):1062–1074. doi: 10.1111/pace.14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abate SM, Mantefardo B, Nega S, Chekole YA, Basu B, Ali SA, et al. Global burden of acute myocardial injury associated with COVID-19: a systematic review, meta-analysis, and meta-regression. Ann Med Surg (Lond) 2021;68:102594. doi: 10.1016/j.amsu.2021.102594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JW, Han TW, Woodward M, Anderson CS, Zhou H, Chen YD, et al. The impact of 2019 novel coronavirus on heart injury: a systematic review and meta-analysis. Prog Cardiovasc Dis. 2020;63(4):518–524. doi: 10.1016/j.pcad.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parohan M, Yaghoubi S, Seraji A. Cardiac injury is associated with severe outcome and death in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Eur Heart J Acute Cardiovasc Care. 2020;9(6):665–677. doi: 10.1177/2048872620937165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceban F, Ling S, Lui LMW, Lee Y, Gill H, Teopiz KM, et al. Fatigue and cognitive impairment in post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav Immun. 2021 doi: 10.1016/j.bbi.2021.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik P, Patel K, Pinto C, Jaiswal R, Tirupathi R, Pillai S, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)-A systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal FM, Lam K, Sounderajah V, Clarke JM, Ashrafian H, Darzi A. Characteristics and predictors of acute and chronic post-COVID syndrome: a systematic review and meta-analysis. EClinical Medicine. 2021;36:100899. doi: 10.1016/j.eclinm.2021.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568. doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasecka A, Pruc M, Kukula K, Gilis-Malinowska N, Filipiak KJ, Jaguszewski MJ, et al. Post-COVID-19 heart syndrome. Cardiol J. 2021;28(2):353–354. doi: 10.5603/CJ.a2021.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eiros R, Barreiro-Perez M, Martin-Garcia A, Almeida J, Villacorta E, Perez-Pons A, et al. Pericardial and myocardial involvement after SARS-CoV-2 infection: a cross-sectional descriptive study in healthcare workers. Rev Esp Cardiol (Engl Ed) 2021 doi: 10.1016/j.recesp.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farsalinos KE, Daraban AM, Unlu S, Thomas JD, Badano LP, Voigt JU. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: The EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr. 2015;28(10):1171–1181. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Nagata Y, Takeuchi M, Mizukoshi K, Wu VC, Lin FC, Negishi K, et al. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr. 2015;28(6):630–641. doi: 10.1016/j.echo.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. 2015;28(2):183–193. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Schneider M, Ran H, Aschauer S, Binder C, Mascherbauer J, Lang I, et al. Visual assessment of right ventricular function by echocardiography: how good are we? Int J Cardiovasc Imaging. 2019;35(11):2001–2008. doi: 10.1007/s10554-019-01653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19(6):591–600. doi: 10.1093/ehjci/jey042. [DOI] [PubMed] [Google Scholar]
- 26.Lindner D, Fitzek A, Brauninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5(11):1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain SS, Liu Q, Raikhelkar J, Fried J, Elias P, Poterucha TJ, et al. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr. 2020;33(10):1278–1284. doi: 10.1016/j.echo.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khani M, Tavana S, Tabary M, Naseri Kivi Z, Khaheshi I. Prognostic implications of biventricular strain measurement in COVID-19 patients by speckle-tracking echocardiography. Clin Cardiol. 2021;44(10):1475–1481. doi: 10.1002/clc.23708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothschild E, Baruch G, Szekely Y, Lichter Y, Kaplan A, Taieb P, et al. The predictive role of left and right ventricular speckle-tracking echocardiography in COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2471–2474. doi: 10.1016/j.jcmg.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnamoorthy P, Croft LB, Ro R, Anastasius M, Zhao W, Giustino G, et al. Biventricular strain by speckle tracking echocardiography in COVID-19: findings and possible prognostic implications. Future Cardiol. 2021;17(4):663–667. doi: 10.2217/fca-2020-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021;6(8):945–950. doi: 10.1001/jamacardio.2020.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JY, Han K, Suh YJ. Prevalence of abnormal cardiovascular magnetic resonance findings in recovered patients from COVID-19: a systematic review and meta-analysis. J Cardiovasc Magn Reson. 2021;23(1):100. doi: 10.1186/s12968-021-00792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334(7584):94–96. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–1415. doi: 10.1136/bmj.a117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moody WE, Liu B, Mahmoud-Elsayed HM, Senior J, Lalla SS, Khan-Kheil AM, et al. Persisting adverse ventricular remodeling in COVID-19 survivors: a longitudinal echocardiographic study. J Am Soc Echocardiogr. 2021;34(5):562–566. doi: 10.1016/j.echo.2021.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tangen J, Aukrust P, Barratt-Due A, Skulstad H, Edvardsen T. Reduced cardiac function by echocardiography in a minority of COVID-19 patients 3 months after hospitalization. J Am Soc Echocardiogr. 2022;35(2):243–244. doi: 10.1016/j.echo.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baruch G, Rothschild E, Sadon S, Szekely Y, Lichter Y, Kaplan A, et al. Evolution of right and left ventricle routine and speckle-tracking echocardiography in patients recovering from coronavirus diseasea longitudinal study. Eur Heart J Cardiovasc Imaging. 2019 doi: 10.1093/ehjci/jeab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tryfou ES, Kostakou PM, Chasikidis CG, Kostopoulos VS, Serafetinidis II, Ferdianaki EK, et al. Biventricular myocardial function in Covid-19 recovered patients assessed by speckle tracking echocardiography: a prospective cohort echocardiography study. Int J Cardiovasc Imaging. 2021;38(5):995–1003. doi: 10.1007/s10554-021-02498-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozer S, Candan L, Ozyildiz AG, Turan OE. Evaluation of left ventricular global functions with speckle tracking echocardiography in patients recovered from COVID-19. Int J Cardiovasc Imaging. 2021;37(7):2227–2233. doi: 10.1007/s10554-021-02211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Heuvel FMA, Vos JL, van Bakel B, Duijnhouwer AL, van Dijk APJ, Dimitriu-Leen AC, et al. Comparison between myocardial function assessed by echocardiography during hospitalization for COVID-19 and at 4 months follow-up. Int J Cardiovasc Imaging. 2021;37(12):3459–3467. doi: 10.1007/s10554-021-02346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baycan OF, Barman HA, Atici A, Tatlisu A, Bolen F, Ergen P, et al. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int J Cardiovasc Imaging. 2021;37(1):135–144. doi: 10.1007/s10554-020-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakurai A, Sasaki T, Kato S, Hayashi M, Tsuzuki SI, Ishihara T, et al. Natural history of asymptomatic SARS-CoV-2 infection. N Engl J Med. 2020;383(9):885–886. doi: 10.1056/NEJMc2013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmune Pharmacol. 2020;15(3):359–386. doi: 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Munoz D, Malik H, Eickenhorst D, Newman S, Varughese C, Ali F. Cardiac screening in a young adult male leading to discovery of post-COVID myocarditis with asymptomatic large apical left ventricular thrombus. CASE (Phila) 2021;5(5):309–312. doi: 10.1016/j.case.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carubbi F, Alunno A, Leone S, Di Gregorio N, Mancini B, Viscido A, et al. Pericarditis after SARS-CoV-2 infection: another pebble in the mosaic of long COVID? Viruses. 2021;13(10):1997. doi: 10.3390/v13101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaminski A, Albus M, Mohseni M, Mirzan H, Harrison MF. A delayed case of pericarditis following recovery from COVID-19 infection. Cureus. 2021;13(4):e14397. doi: 10.7759/cureus.14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohamed MS, Moulin TC, Schioth HB. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021;71(1):3–8. doi: 10.1007/s12020-020-02536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.