Abstract
The overall aroma is an important factor of the sensory quality of fruit wines, which attributed to hundreds of volatile compounds. However, the qualitative determination of trace volatile compounds is considered to be very challenging work. GC-Orbitrap-MS with high resolution and high sensitivity provided more possibilities for the determination of volatile compounds, but without the high-resolution mass spectral library. For accuracy of qualitative determination in fruit wines by GC-Orbitrap-MS, a high-resolution mass spectral library, including 76 volatile compounds, was developed in this study. Not only the HRMS spectrum but also the exact ion fragment, relative abundance, retention indices (RI), CAS number, chemical structure diagram, aroma description and aroma threshold (ortho-nasally) were provided and were shown in a database website (Food Flavor Laboratory, http://foodflavorlab.cn/). HRMS library was used to successfully identify the volatile compounds mentioned above in 16 fruit wines (5 blueberry wines, 6 goji berry wines and 5 hawthorn wines). The library was developed as an important basis for further understanding of trace volatile compounds in fruit wines.
Subject terms: Agriculture, Inorganic chemistry
| Measurement(s) | volatile compounds |
| Technology Type(s) | GC-Orbitrap-MS |
Background & Summary
Among the hundreds of volatile compounds detected in fruit wines, only a small percentage of them could play key roles in the contribution of characteristic aroma1. Currently, the gas chromatograph-mass spectrometer has been widely used for the identification and quantification of aroma compounds. The quadrupole mass spectrometer (qMS) could be the most common mass spectrometer for analysis2–6. However, some trace analytes were difficult to be detected using qMS due to their low resolution and sensitivity4,7–12. These trace compounds needed to be identified by other detectors. The aldehydes and ketones could be detected in Syrah wines13 and model wine solution by flame ionization detector (FID)14,15. The flame photometry (FPD) was used to identify sulfur compounds in Cabernet Sauvignon wines16,17. Besides, sulphur chemiluminescence (SCD)13,18,19 and pulsed flame photometry (PFPD)20,21 also could be used for the analysis of sulfur compounds in grape wines. The pyrazines could be identified in wines22 and oak woods23 by nitrogen-phosphorous detection (NPD). The triple-quadrupole mass spectrometer (QqQ-MS) in selected-reaction-monitoring (SRM) could identify lactones24, terpenes25 and sulfur compounds26 in wines. Thus, multiple methods had to be used for the detection of various aroma compounds14,16. Meanwhile, the use of multiple instruments is time-consuming and costly. And it is also difficult to have so many instruments in a same laboratory. And it is an urgent challenge to identify trace aroma volatile compounds mentioned above simply and effectively in fruit wines.
In recent years, high-resolution mass spectrometry, such as quadrupole-time-of-flight-MS (Q-TOF), could improve the accuracy of identification22,23,27. Since Orbitrap-MS technology invented by Alexander Makarov was first commercially available in 2005, this new technique of high resolution and high sensitivity mass spectrometry has been shown great advantages for qualitative and quantitative analysis of compounds28–30, and therefore many studies have been focused on metabolomics using liquid chromatography coupling31–34. After GC was coupled with Orbitrap-MS in 2015, its resolution could reach 60,000 (219 m/z, FWHM), mass accuracy could reach 1 ppm, and sensitivity could reach femtogram level, which provided more possibilities to advance the depth and breadth of GC-MS technology35,36. At present, GC-Orbitrap-MS began to be used to detect pesticide residues37, nitrosamines in children’s products38, persistent organic pollutants in the environment39, soluble and extractable substances in package materials40, stimulants and banned substances in urine41 and metabonomics42. GC-Orbitrap-MS can provide accurate qualitative quantification of benzene compounds in chili peppers43. In summary, the GC-Orbitrap-MS could be a potential technique for the determination of aroma volatile compounds in fruit wines due to its high resolution and high sensitivity.
At present, the NIST library is widely used for the identification of aroma volatile compounds analyzed by gas chromatography-mass spectrometry7,8,44,45. However, the mass spectrums in the NIST library were mostly obtained by low-resolution mass spectrometry. There were differences in ion fragments and ion abundance between high-resolution mass spectrums obtained by GC-Orbitrap-MS and low-resolution mass spectrums obtained by GC-Quadrupole-MS46, which led to the qualitative inaccuracy. The high-resolution mass spectrometry (HRMS) spectrums of aroma compounds analyzed by GC-Orbitrap-MS need to be established for accurate identification. In addition, the basic information of aroma compounds, such as CAS number, chemical structure diagram, aroma description and aroma threshold (ortho-nasally), need to be acquired by a large collection of literature. Thus, there is an urgent need to establish a library of HRMS spectrum and basic information to facilitate analyzing and consulting by scholars all over the world.
Methods
Overview of the experimental design
Materials and methods
Chemical and reagents
The information of standards was shown in Table 1. The individual stock solution of each standard is dissolved in ethanol and stored at −20 °C.
Table 1.
The information of standards used in this study.
| Compounds | CAS No. | Purity | Manufacturer | Formula | RI | Contenth/μg.L−1 |
|---|---|---|---|---|---|---|
| Ester | ||||||
| Ethyl butanoate | 105-54-4 | ≥99.5% | Aladdinb | C6H12O2 | 1065 | 10020 |
| Ethyl 2-methylbutanoate | 7452-79-1 | >98.0% | Aladdin | C7H14O2 | 1077 | 5030 |
| Ethyl isovalerate | 108-64-5 | >99.0% | Adamasc | C7H14O2 | 1093 | 11050 |
| Isoamyl acetate | 123-92-2 | ≥99.5% | Macklin | C7H14O2 | 1139 | 11390 |
| Methyl caproate | 106-70-7 | >99.0% | Macklin | C7H14O2 | 1200 | 5120 |
| Ethyl hexanoate | 123-66-0 | >99.0% | Aladdin | C8H16O2 | 1243 | 30300 |
| Ethyl heptanoate | 106-30-9 | ≥99.5% | Macklin | C9H18O2 | 1340 | 5050 |
| Ethyl lactate | 97-64-3 | ≥99.0% | Macklin | C5H10O3 | 1350 | 50810 |
| Heptyl acetate | 112-06-1 | ≥98.0% | TCI | C9H18O2 | 1380 | 3280 |
| Methyl octanoate | 111-11-5 | ≥99.0% | Adamas | C9H18O2 | 1394 | 2000 |
| Ethyl caprylate | 106-32-1 | >99.0% | Aladdin | C10H20O2 | 1439 | 29670 |
| Ethyl 3-hydroxybutyrate | 5405-41-4 | >99.0% | Macklin | C6H12O3 | 1511 | 15170 |
| Ethyl nonanoate | 123-29-5 | ≥95.0% | Macklin | C11H22O2 | 1521 | 7250 |
| Ethyl 2-hydroxy-4-methylpentanoate | 10348-47-7 | ≥98.0% | Aladdin | C8H16O3 | 1525 | 10180 |
| Ethyl caprate | 110-38-3 | >99.0% | Macklin | C12H24O2 | 1572 | 20980 |
| Ethyl succinate | 123-25-1 | ≥99.5% | Macklin | C8H14O4 | 1592 | 50360 |
| Methyl salicylate | 119-36-8 | ≥99.5% | Macklin | C8H8O3 | 1675 | 4760 |
| Ethyl benzeneacetate | 101-97-3 | ≥99.5% | Aladdin | C10H12O2 | 1689 | 1940 |
| Ethyl salicylate | 118-61-6 | >99.0% | Aladdin | C9H10O3 | 1710 | 5480 |
| Ethyl hydrocinnamate | 2021-28-5 | >98.0% | TCId | C11H14O2 | 1785 | 12040 |
| Ethyl cinnamate | 103-36-6 | >98.0% | Adamas | C11H12O2 | 2031 | 5040 |
| Monoethyl succinate | 1070-34-4 | >95.0% | Aladdin | C6H10O4 | 2308 | 11020 |
| Carbonyl compounds | ||||||
| (E)-2-Hexenal | 6728-26-3 | >98.0% | Aladdin | C6H10O | 1329 | 7120 |
| (E)-2-Heptenal | 18829-55-5 | >95.0% | Aladdin | C7H12O | 1362 | 6530 |
| (E)-2-Octenal | 2548-87-0 | >95.0% | Macklin | C8H14O | 1432 | 1900 |
| (E,E)-2,4-Heptadienal | 4313-03-5 | >90.0% | Macklin | C7H10O | 1498 | 10220 |
| (E,Z)-2,6-Nonadienal | 557-48-2 | ≥95.0% | Aladdin | C9H14O | 1545 | 4160 |
| Benzeneacetaldehyde | 122-78-1 | >95.0% | Macklin | C8H8O | 1574 | 5720 |
| High alcohols | ||||||
| Isobutanol | 78-83-1 | ≥99.5% | Aladdin | C4H10O | 1112 | 20620 |
| Isoamylol | 123-51-3 | ≥99.5% | Aladdin | C5H12O | 1217 | 78820 |
| 1-Pentanol | 71-41-0 | ≥99.5% | Macklin | C5H12O | 1259 | 6340 |
| 2-Heptanol | 543-49-7 | >98.0% | Aladdin | C7H16O | 1327 | 8700 |
| 3-Octenol | 3391-86-4 | >98.0% | Aladdin | C8H16O | 1456 | 3220 |
| 1-Heptanol | 111-70-6 | >95.0% | Macklin | C7H16O | 1460 | 5490 |
| 2-Nonanol | 628-99-9 | ≥98.0% | Aladdin | C9H20O | 1511 | 3240 |
| 1-Octanol | 111-87-5 | ≥99.5% | Macklin | C8H18O | 1532 | 9060 |
| 2-Phenylethanol | 60-12-8 | ≥99.5% | Aladdin | C8H10O | 1817 | 50690 |
| 2-Phenoxyethanol | 122-99-6 | ≥ 99.5% | Macklin | C8H10O2 | 2043 | 9900 |
| Lactone | ||||||
| γ-Octalactone | 104-50-7 | >98.0% | Sigma-Aldrich | C11H20O2 | 1814 | 4140 |
| δ-Octalactone | 698-76-0 | >98.0% | Sigma-Aldrich | C8H14O2 | 1862 | 3480 |
| γ-Nonalactone | 104-61-0 | >98.0% | Sigma-Aldrich | C8H14O2 | 1925 | 3260 |
| Pantolactone | 599-04-2 | >99.0% | Sigma-Aldrich | C6H10O3 | 1935 | 19860 |
| γ-Decalactone | 706-14-9 | >98.0% | Sigma-Aldrich | C10H18O2 | 2041 | 3700 |
| Sotolon | 28664-35-9 | >97.0% | Sigma-Aldrich | C6H8O3 | 2108 | 4980 |
| γ-Undecalactone | 104-67-6 | >98.0% | Sigma-Aldrich | C11H20O2 | 2161 | 3520 |
| Acid | ||||||
| Butanoic acid | 107-92-6 | ≥99.5% | Sigma-Aldrich | C4H8O2 | 1574 | 30960 |
| Hexanoic acid | 142-62-1 | ≥99.5% | Macklin | C6H12O2 | 1762 | 25780 |
| Ethylhexanoic acid | 149-57-5 | ≥99.9% | Aladdin | C8H16O2 | 1860 | 10090 |
| Octanoic acid | 124-07-2 | ≥99.5% | Aladdin | C8H16O2 | 1973 | 56880 |
| Decanoic acid | 334-48-5 | >99.0% | Aladdin | C10H20O2 | 2190 | 20170 |
| Benzoic acid | 65-85-0 | ≥99.9% | Aladdin | C7H6O2 | 2378 | 11630 |
| Pyrazine | ||||||
| 3-Isopropyl-2-methoxypyrazine | 25773-40-4 | >97.0% | Sigma-Aldrich | C8H12ON2 | 1435 | 1280 |
| 2-sec-Butyl-3-Methoxypyrazine | 24168-70-5 | >99.0% | Sigma-Aldrich | C9H14ON2 | 1453 | 980 |
| 5-Ethyl-2,3-dimethylpyrazine | 15707-34-3 | >98.0% | Sigma-Aldrich | C8H12N2 | 1459 | 2230 |
| 2-Isobutyl-3-methoxypyrazine | 24683-00-9 | >99.0% | Sigma-Aldrich | C9H14ON2 | 1513 | 1490 |
| Acetylpyrazine | 22047-25-2 | >97.0% | Sigma-Aldrich | C6H6N2O | 1565 | 2010 |
| Furan | ||||||
| Furfural | 98-01-1 | >99.0% | Sigma-Aldrich | C5H4O2 | 1472 | 5250 |
| Acetylfuran | 1192-62-7 | >99.0% | Sigma-Aldrich | C6H6O2 | 1505 | 9840 |
| 5-Methylfurfural | 620-02-0 | >99.0% | Sigma-Aldrich | C6H6O2 | 1540 | 1740 |
| Ethyl 2-furoate | 614-99-3 | >99.0% | Sigma-Aldrich | C7H8O3 | 1565 | 4250 |
| Furfuryl alcohol | 98-00-0 | >98.0% | Sigma-Aldrich | C5H6O2 | 1585 | 10820 |
| 5-Hydroxymethylfurfural | 67-47-0 | >99.0% | Sigma-Aldrich | C6H6O3 | 2415 | 20050 |
| Terpenes | ||||||
| D-Limonene | 5989-27-5 | ≥99.0% | TCI | C10H16 | 1203 | 1860 |
| Terpinolene | 586-62-9 | >90.0% | TCI | C10H16 | 1284 | 2330 |
| β-Linalool | 78-70-6 | >98.0% | Macklin | C10H18O | 1527 | 2410 |
| Citronellyl acetate | 150-84-5 | ≥95.0% | Aladdin | C12H22O2 | 1583 | 3180 |
| β-Ionone | 14901-07-6 | >97.0% | Aladdin | C13H20O | 1833 | 1560 |
| Benzene | ||||||
| o-Xylene | 95-47-6 | ≥99.0% | Macklin | C8H10 | 1192 | 1520 |
| Styrene | 100-42-5 | ≥99.5% | Macklin | C8H8 | 1264 | 2190 |
| p-Cymene | 99-87-6 | ≥99.5% | Macklin | C10H14 | 1273 | 2900 |
| Naphthalene | 91-20-3 | ≥ 99.5% | Macklin | C10H8 | 1635 | 2070 |
| Volatile phenol | ||||||
| 4-Methylguaiacol | 93-51-6 | >99.0% | Sigma-Aldrich | C8H10O2 | 1860 | 2820 |
| o-Cresol | 95-48-7 | >99.0% | Sigma-Aldrich | C7H7O | 1913 | 4980 |
| 4-Propylguaiacol | 2785-87-7 | >99.0% | Sigma-Aldrich | C10H14O2 | 2011 | 5370 |
| 4-Vinylphenol | 2628-17-3 | >95.0% | Sigma-Aldrich | C8H8O | 2306 | 2540 |
| Sulfide | ||||||
| 3-(Methylthio)propanol | 505-10-2 | ≥99.0% | Macklin | C4H10OS | 1618 | 6600 |
| Internal standard | ||||||
| 4-Methyl-2-pentanol | 108-11-2 | ≥98.0% | CNWf | C6H14O | 1065 | 1000 |
aShanghai Macklin Biochemical Co., Ltd (Shanghai, China).
bAladdin Bio-Chem Technology (Shanghai, China).
cAdamas Reagent, Co., Ltd. (Shanghai, China).
dTCI Development Co., Ltd. (Shanghai, China).
eSigma-Aldrich (St. Louis, MO, USA).
fCNW Technologies GmbH (Duesseldorf, Germany).
gBide Pharmatech Ltd. (Shanghai, China).
hThe contents of spiked standard mixtures used in direct liquid introduction method.
Wine Samples collection
Three kinds of commercial fruit wines (blueberry wine, B, goji berry wine, G and hawthorn wine, H) purchased from retail stores in China were used for the establishment of HRMS library. All blueberry samples were with an alcohol content of 12% v/v (percent by volume). Three blueberry wines were received from Beiyushidai, including blueberry dry wine produced in 2019 (B1) and 2017 (B2) and blueberry semi-dry wine produced in 2019 (B3). A blueberry dry wine (B4) was produced by Shenghua in 2019. Another blueberry dry wine (B5) produced in 2019 was provided by Yicunshanye. Goji berry semi-dry wine (G1) was produced by Ningxiahong in 2019, with an alcohol content of 7% v/v. Four batches of goji berry dry wine (G2-G5) produced by Senmiao in 2017 were with an alcohol content of 11% v/v. G6 was made by our laboratory in 2016 with an alcohol content of 11% v/v. All hawthorn wine samples were semi-dry wines from Shengbali. H1 and H2 produced in 2019 were with an alcohol content of 12% v/v. The other H3-H5 were produced in 2020 with an alcohol content of 13% v/v from Shengbali.
Preparation of the spiked mixture
The direct liquid introduction method was used to determine the mass spectral information of the target compound. The standard mixtures (Mixture 1 with 24 esters, Mixture 2 with 6 carbonyl compounds and 8 lactones and 6 acids, Mixture 3 with10 high alcohols and 6 furans and 5 pyrazines, Mixture 4 with 5 terpenes and 4 benzenes and 4 volatile phenols and 1 sulfide) were prepared to extract. The mother solution of each compound was dissolved in ethanol at higher concentration. Each standard mixtures were mixed by the mother solution of compounds according to the concentrations (Table 1).The standard mixtures were diluted with dichloromethane to volume in a 10-mL volumetric flask. 1 μL of each mixture was injected. The split mode was applied with a split ratio of 10:1. The liquid injection was performed using the TriPlus RSH autosampler (Thermo Fisher Scientific, Bremen, Germany).
Extraction of volatile compounds in wine samples
Headspace solid-phase microextraction (HS-SPME) was used to extract the volatile compounds from fruit wines. 5 mL of wine samples mixed with 1.00 g NaCl and 10 μL of internal standard (1.077 g/L 4-methyl-2-pentanol) were prepared in a 20 mL glass vial. The sample vials were stirred and heated at 60 °C for 30 min. Then the preconditioned fiber (50/30 μm Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS)) was used to absorb the volatile compounds in the headspace of the sample via for 30 min at 60 °C. After absorption, the fiber was inserted into the GC injection port for desorbing at 250 °C for 10 min. Two technical replicates were performed for each sample. Automatic headspace solid-phase microextraction was performed on the TriPlus RSH autosampler.
GC-Orbitrap-MS analysis
A Thermo Scientific Trace 1300 gas chromatography equipped with a Thermo Scientific Q-Exactive Orbitrap mass spectrometer (GC-Orbitrap MS, Thermo Scientific, Bremen, Germany) was used for detection. The spiked mixture was performed under the following GC-Orbitrap-MS conditions. A TG-WAXMS 30 m × 0.25 mm × 0.25 μm (Thermo Scientific, Bremen, Germany) was used to separate analytes. Helium was used as the carrier gas (1.2 mL/min). The oven temperature program was set as follows: 40 °C held for 5 min, then heated to 180 °C at 3 °C/min, finally increased from 180 °C to 240 °C at 30 °C/min and hold 15 min. The wine samples were performed under the following GC-Orbitrap-MS conditions. A DB-WAX 30 m × 0.25 mm × 0.25 μm (J&W Scientific, Folsom, CA, USA) was used to separate the volatile compounds under a 1.2 mL/min flow rate of helium (carrier gas).The oven temperature program was set as follows: 40°C held for 5 min, then heated to 180°C at 3 °C /min, finally increased from 180 °C to 250 °C at 30 °C/min and hold 10 min.
The Orbitrap-MS operated in full-scan MS acquisition mode (m/z 33–350). The ion source was maintained at 280 °C with an MSD transfer line temperature of 230 °C. Positive ion-electron ionization (EI) was used at 70 electron volts (eV) in Orbitrap-MS.
Identification of the compounds
Retention indices (RI) were calculated from the retention times of C6-C24 n-alkanes under the same chromatographic and mass spectrometric conditions. The high-solution mass spectrums of volatile compounds were collected in different standard mixtures. Then, the qualitative determination of target compounds in fruit wines was performed by the match of the retention time and ion fragments in samples and standards.The experimental design and analysis pipeline are shown in Fig. 1.
Fig. 1.
Flowchart of the experimental design.
Data Records
A total of 36 original data files were stored in MetaboLights47, including 4 standard mixtures and 32 wine samples (two technical replicates).
Technical Validation
Two technical replicates were performed on each wine sample. The qualitative determination of target volatile compounds in fruit wines was shown in Table 3.
Table 3.
The qualitative determination of target volatile compounds in goji berry wines, blueberry wines and hawthorn wines.
| Compounds | B1 | B2 | B3 | B4 | B5 | G1 | G2 | G3 | G4 | G5 | G6 | H1 | H2 | H3 | H4 | H5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ester | ||||||||||||||||
| Ethyl butanoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl 2-methylbutanoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl isovalerate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Isoamyl acetate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Methyl caproate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl hexanoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl heptanoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl lactate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Heptyl acetate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | nd | √ | nd | nd | nd | nd |
| Methyl octanoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl caprylate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl 3-hydroxybutyrate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl nonanoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl 2-hydroxy-4-methylpentanoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl caprate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl succinate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Methyl salicylate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | nd | √ |
| Ethyl benzeneacetate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl salicylate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl hydrocinnamate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl cinnamate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Monoethyl succinate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Carbonyl compounds | ||||||||||||||||
| (E)-2-Hexenal | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| (E)-2-Heptenal | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| (E)-2-Octenal | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| (E,E)-2,4-Heptadienal | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| (E,Z)-2,6-Nonadienal | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Benzeneacetaldehyde | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| High Alcohols | ||||||||||||||||
| Isobutanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Isoamylol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 1-Pentanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2-Heptanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 3-Octenol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 1-Heptanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2-Nonanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 1-Octanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2-Phenylethanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2-Phenoxyethanol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Lactone | ||||||||||||||||
| γ-Undecalactone | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| δ-Octalactone | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| γ-Octalactone | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Pantolactone | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| γ-Decalactone | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Sotolon | √ | √ | √ | √ | nd | nd | √ | nd | nd | √ | nd | nd | nd | nd | nd | nd |
| γ-Nonalactone | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Acid | ||||||||||||||||
| Butanoic acid | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Hexanoic acid | nd | √ | nd | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethylhexanoic acid | nd | √ | nd | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Octanoic acid | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Decanoic acid | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Benzoic acid | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Pyrazine | ||||||||||||||||
| 3-Isopropyl-2-methoxypyrazine | √ | √ | √ | √ | √ | √ | √ | √ | √ | nd | √ | √ | √ | √ | nd | √ |
| 2-sec-Butyl-3-Methoxypyrazine | √ | √ | √ | √ | nd | √ | √ | nd | √ | nd | nd | √ | √ | nd | √ | √ |
| 5-Ethyl-2,3-dimethylpyrazine | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 2-Isobutyl-3-methoxypyrazine | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Acetylpyrazine | nd | nd | √ | √ | nd | nd | √ | √ | √ | nd | nd | √ | √ | √ | √ | √ |
| Furan | ||||||||||||||||
| Furfural | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Acetylfuran | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5-Methylfurfural | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Ethyl 2-furoate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Furfuryl alcohol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 5-Hydroxymethylfurfural | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Terpenes | ||||||||||||||||
| D-Limonene | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Terpinolene | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | nd |
| β-Linalool | √ | √ | √ | √ | nd | nd | √ | √ | √ | √ | nd | nd | nd | nd | √ | nd |
| Citronellyl acetate | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| β-Ionone | √ | √ | √ | nd | √ | √ | √ | √ | √ | √ | √ | √ | nd | √ | nd | √ |
| Benzene | ||||||||||||||||
| o-Xylene | nd | nd | nd | nd | nd | nd | nd | nd | √ | nd | nd | nd | nd | √ | √ | √ |
| Styrene | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | nd | √ | √ | √ |
| p-Cymene | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Naphthalene | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Volatile phenol | ||||||||||||||||
| 4-Methylguaiacol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| o-Cresol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4-Propylguaiacol | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| 4-Vinylphenol | √ | √ | √ | √ | √ | nd | √ | √ | √ | √ | √ | nd | nd | nd | nd | nd |
| Sulfide | ||||||||||||||||
| 3-(Methylthio)propanol | √ | √ | √ | nd | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
‘B’ represent blueberry wine, ‘G’ represent goji berry wine, ‘H’ represent hawthorn wine.
Usage Notes
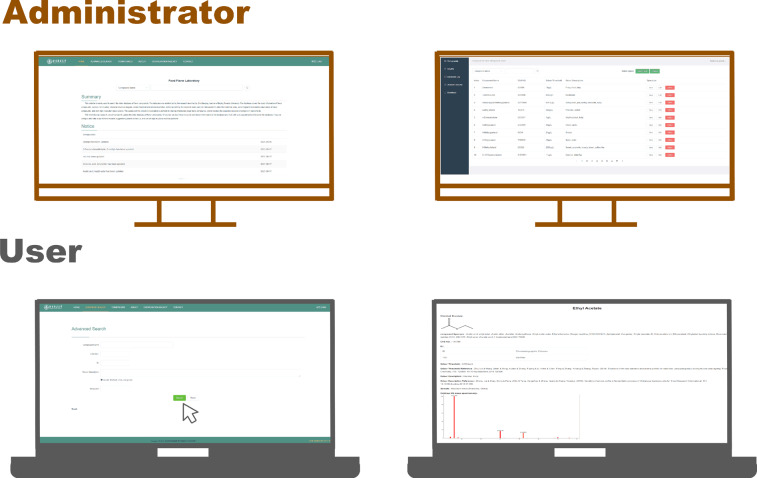
The HRMS library of volatile compounds was shown on the database website (http://foodflavorlab.cn/), including HRMS spectrum, exact ion fragment, relative abundance, RI, CAS number, chemical structure diagram, aroma description and aroma threshold (ortho-nasally). Table 1 showed CAS No., formula and RI of each target volatile compound. The information of standards and contents of spiked mixtures were shown in Table 1. Table 2 showed elemental composition judgments, exact ion fragments and error mass of each target volatile compound. Table 3 showed the qualitative determination of target volatile compounds in blueberry wine, goji berry wine and hawthorn wine. Figure 2 showed the web page of the database website (http://foodflavorlab.cn/) including the home page, upload page, search page and result page. Figure 3 showed the page view (PV) of the database website (http://foodflavorlab.cn/) from Nov. 2020 to May. 2022.
Table 2.
The qualitative and quantitative information of target volatile compounds.
| Compounds | Precursor ions | Quantifier ions | Qualifier ions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exact mass (m/z) | Molecular formula | Error mass (ppma) | Exact mass (m/z) | Molecular formula | Error mass (ppm) | Exact mass (m/z) | Molecular formula | Error mass (ppm) | |
| Ester | |||||||||
| Ethyl butanoate | 43.05422 | C3H7 | −0.99526 | 88.05202 | C4H8O2 | 0.7668 | |||
| Ethyl 2-methylbutanoate | 74.03639 | C3H6O2 | 0.2198 | 102.0677 | C5H10O2 | 0.41588 | |||
| Ethyl isovalerate | 57.06997 | C4H9 | 0.34743 | 61.0285 | C2H5O2 | 0.15943 | |||
| Isoamyl acetate | 43.01782 | C2H3O | −1.06298 | 55.05433 | C4H9 | 0.6148 | |||
| Methyl caproate | 43.01782 | C2H3O | −0.70828 | 74.03639 | C3H6O2 | 0.52895 | |||
| Ethyl hexanoate | 43.05422 | C3H7 | −0.99526 | 73.02851 | C3H5O2 | 0.70783 | |||
| Ethyl heptanoate | 73.02854 | C3H5O2 | 0.49889 | 88.05192 | C4H8O2 | 0.42009 | |||
| Ethyl lactate | 45.03354 | C2H5O | 1.30819 | 56.0621 | C4H8 | 0.9174 | |||
| Heptyl acetate | 43.01778 | C2H3O | −0.17621 | 70.07773 | C5H10 | 0.8118 | |||
| Methyl octanoate | 43.01782 | C2H3O | −0.70828 | 74.03639 | C3H6O2 | 0.73505 | |||
| Ethyl caprylate | 73.02845 | C3H5O2 | −0.44136 | 101.05977 | C5H9O2 | −0.43741 | |||
| Ethyl 3-hydroxybutyrate | 43.01778 | C2H3O | −0.6196 | 71.01285 | C3H3O2 | 1.29569 | |||
| Ethyl nonanoate | 73.02845 | C3H5O2 | −0.54583 | 101.05977 | C5H9O2 | −0.51291 | |||
| Ethyl 2-hydroxy-4-methylpentanoate | 69.06999 | C5H9 | 0.12138 | 45.03355 | C2H5O | 1.22348 | |||
| Ethyl caprate | 73.02853 | C3H5O2 | 0.39441 | 61.0285 | C2H5O2 | 0.28445 | |||
| Ethyl succinate | 101.02348 | C4H5O3 | 0.02484 | 73.02853 | C3H5O2 | 0.60336 | |||
| Methyl salicylate | 152.04683 | C8H8O3 | 0.22088 | 120.02077 | C7H4O2 | 0.28082 | 92.02578 | C6H4O | 0.15454 |
| Ethyl benzeneacetate | 164.08322 | C10H12O2 | 0.24546 | 91.05439 | C7H7 | −0.13544 | 136.05219 | C8H8O2 | 0.66442 |
| Ethyl salicylate | 166.06245 | C9H10O3 | 0.05133 | 120.02077 | C7H4O2 | 0.40795 | 92.02578 | C6H4O | 0.15454 |
| Ethyl hydrocinnamate | 178.09898 | C11H14O2 | 0.85652 | 104.06216 | C8H8 | 0.34761 | 105.06997 | C8H9 | 0.15241 |
| Ethyl cinnamate | 176.08331 | C11H12O2 | 0.96579 | 131.04938 | C9H7O | 0.98604 | 103.05436 | C8H7 | 0.62066 |
| Monoethyl succinate | 101.02348 | C4H5O3 | 0.10036 | 73.02853 | C3H5O2 | 0.60336 | |||
| Carbonyl compounds | |||||||||
| (E)-2-Hexenal | 83.04919 | C5H7O | 0.17795 | 69.03339 | C6H9O | 0.46658 | |||
| (E)-2-Heptenal | 83.04919 | C5H7O | 0.54542 | 41.03839 | C3H5 | −3.22212 | |||
| (E)-2-Octenal | 83.04919 | C5H7O | 0.17795 | 41.03839 | C3H5 | −4.80235 | |||
| (E,E)-2,4-Heptadienal | 81.03347 | C5H5O | −0.26157 | 109.0647 | C7H9O | 0.11559 | |||
| (E,Z)-2,6-Nonadienal | 41.03839 | C3H5 | −4.33758 | 70.04136 | C4H6O | −0.48152 | |||
| Benzeneacetaldehyde | 120.05711 | C8H8O | 1.14129 | 91.05439 | C7H7 | 0.61866 | 92.06208 | C7H8 | 0.31004 |
| High alcohols | |||||||||
| Isobutanol | 41.0384 | C3H5 | −4.52349 | 45.0336 | C2H5O | 2.32468 | |||
| Isoamylol | 57.0699 | C4H9 | 0.41428 | 70.07784 | C5H10 | 0.37632 | |||
| 1-Pentanol | 57.06991 | C4H9 | 0.54796 | 70.07784 | C5H10 | 0.59406 | |||
| 2-Heptanol | 45.03354 | C2H5O | 0.88465 | 83.08566 | C6H11 | 0.5339 | |||
| 3-Octenol | 57.03355 | C3H5O | 0.49786 | 85.06478 | C5H9O | 0.50696 | |||
| 1-Heptanol | 43.05422 | C2H3O | −1.06298 | 70.07338 | C5H10 | 0.48519 | |||
| 2-Nonanol | 105.03364 | C7H5O | 0.74249 | 122.03642 | C7H6O2 | 0.75852 | |||
| 1-Octanol | 69.06999 | C5H9 | 0.23184 | 55.05433 | C4H7 | 0.3996 | |||
| 2-Phenylethanol | 122.07275 | C8H10O | 1.06311 | 91.05439 | C7H7 | 0.95382 | 92.06208 | C7H8 | −0.51868 |
| 2-Phenoxyethanol | 108.05687 | C7H8O | −0.89706 | 94.04132 | C6H6O | 0.04701 | |||
| Lactone | |||||||||
| γ-Undecalactone | 85.02853 | C4H5O2 | 0.69766 | 95.0493 | C6H7O | 0.95817 | |||
| δ-Octalactone | 99.04407 | C5H7O2 | −0.11627 | 71.04915 | C4H7O | 0.74492 | |||
| γ-Octalactone | 85.02851 | C4H5O2 | −0.10989 | 57.03359 | C3H5O | 0.49786 | |||
| Pantolactone | 71.04915 | C4H7O | 0.10063 | 43.05414 | C3H7 | −2.23569 | |||
| γ-Decalactone | 85.02853 | C4H5O2 | 0.1593 | 95.0493 | C6H7O | 0.47656 | |||
| Sotolon | 128.04693 | C6H8O3 | 0.18604 | 83.04919 | C5H7O | 0.08357 | 55.05427 | C4H7 | 0.81287 |
| γ-Nonalactone | 85.02851 | C4H5O2 | −0.02016 | 57.03359 | C3H5O | 0.36409 | |||
| Acid | |||||||||
| Butanoic acid | 60.02063 | C2H4O2 | 0.56154 | 73.02845 | C3H5O2 | 0.39441 | |||
| Hexanoic acid | 73.02853 | C3H5O2 | 0.18547 | 60.02069 | C2H4O2 | 0.39361 | |||
| Ethylhexanoic acid | 73.02853 | C3H5O2 | 0.18547 | 87.04422 | C4H7O2 | 0.48125 | |||
| Octanoic acid | 73.02853 | C3H5O2 | 0.18547 | 101.05988 | C5H9O2 | 0.6195 | |||
| Decanoic acid | 73.02844 | C3H5O2 | 0.49889 | 101.05976 | C5H9O2 | 0.54401 | |||
| Benzoic acid | 122.03632 | C7H6O2 | 0.75852 | 105.03364 | C7H5O | 0.66985 | 122.03642 | C7H6O2 | 0.75852 |
| Pyrazine | |||||||||
| 3-Isopropyl-2-methoxypyrazine | 152.09455 | C8H12ON2 | 0.50811 | 137.071 | C7H9ON2 | 0.50114 | 124.06324 | C6H8ON2 | 0.86187 |
| 2-sec-Butyl-3-Methoxypyrazine | 166.10973 | C9H14ON2 | −1.99624 | 138.07886 | C7H10ON2 | 0.56568 | 124.06321 | C6H8ON2 | 0.75882 |
| 5-Ethyl-2,3-dimethylpyrazine | 136.0996 | C8H12N2 | 0.64728 | 135.0918 | C8H11N2 | 0.714 | 121.07612 | C7H9N2 | −0.02603 |
| 2-Isobutyl-3-methoxypyrazine | 166.11008 | C9H14ON2 | 0.08705 | 124.0632 | C6H8ON2 | 0.58057 | 95.06044 | C5H7N2 | −0.08289 |
| Acetylpyrazine | 122.04759 | C6H6ON2 | 0.53454 | 94.0526 | C5H6N2 | 0.35341 | 80.03695 | C4H4N2 | 0.43185 |
| Furan | |||||||||
| Furfural | 96.02053 | C5H4O2 | −0.84083 | 95.01279 | C5H3O2 | 0.16541 | 39.02277 | C3H3 | −3.43066 |
| Acetylfuran | 110.03637 | C6H6O2 | 0.42523 | 95.01281 | C5H3O2 | 0.64721 | 43.01782 | C2H3O | −0.41717 |
| 5-Methylfurfural | 110.03625 | C6H6O2 | −0.47613 | 109.02855 | C6H5O2 | 0.68404 | 53.03864 | C4H5 | 1.24689 |
| Ethyl 2-furoate | 140.04697 | C7H8O3 | 0.56667 | 95.01279 | C5H3O2 | −0.07548 | 112.01554 | C5H4O3 | −0.07007 |
| Furfuryl alcohol | 98.03629 | C5H6O2 | 0.01035 | 97.02851 | C5H5O2 | 0.29686 | 81.0336 | C5H5O | 0.11503 |
| 5-Hydroxymethylfurfural | 126.03131 | C6H6O3 | 0.34424 | 97.02849 | C5H5O2 | 0.29686 | 69.03357 | C4H5O | 0.5771 |
| Terpenes | |||||||||
| D-Limonene | 136.1252 | C10H16 | 1.65914 | 93.07005 | C7H9 | 1.89353 | 121.10146 | C9H13 | 1.60836 |
| Terpinolene | 136.12471 | C10H16 | 0.4261 | 121.10132 | C9H13 | 0.22236 | 93.06999 | C7H9 | 0.25403 |
| β-Linalool | 93.07005 | C7H9 | 0.41798 | 69.03339 | C5H9 | 0.3423 | |||
| Citronellyl acetate | 81.06996 | C6H9 | 0.00931 | 95.08559 | C7H11 | −0.17538 | |||
| β-Ionone | 177.12753 | C12H17O | 0.28057 | 178.13091 | C12H17O | −2.13518 | |||
| Benzene | |||||||||
| o-Xylene | 106.07779 | C8H10 | −0.11101 | 91.05439 | C7H7 | 0.03214 | 103.05429 | C8H7 | 0.62066 |
| Styrene | 104.0621 | C8H8 | −0.09229 | 104.0621 | C8H8 | −0.09229 | 78.04652 | C6H6 | 0.78457 |
| p-Cymene | 134.10954 | C10H14 | 0.39181 | 119.0857 | C9H11 | 0.05216 | 115.0543 | C9H7 | 0.68855 |
| Naphthalene | 128.06218 | C10H8 | 0.04416 | 128.06218 | C10H8 | 0.04416 | 129.06557 | C10H8 | −3.16989 |
| Volatile phenol | |||||||||
| 4-Methylguaiacol | 138.06754 | C8H10O2 | 0.04814 | 138.06754 | C8H10O2 | 0.04814 | 123.04407 | C7H7O2 | −0.00386 |
| o-Cresol | 107.04918 | C7H7O | 0.20933 | 107.04918 | C7H7O | 0.20933 | 79.05427 | C6H7 | 0.42305 |
| 4-Propylguaiacol | 166.09877 | C10H14O2 | −0.18399 | 137.05968 | C8H9O2 | 0.01147 | 122.03631 | C7H6O2 | 0.19587 |
| 4-Vinylphenol | 120.057 | C8H8O | 0.14583 | 120.057 | C8H8O | 0.14583 | 91.05425 | C7H7 | 0.19972 |
| Sulfide | |||||||||
| 3-(Methylthio)propanol | 106.04483 | C7H6O | 3.52157 | 106.04483 | C7H6O | 3.52157 | 88.03425 | C7H4 | 3.50426 |
| Internal standard | |||||||||
| 4-Methyl-2-pentanol | 45.03355 | C2H5O | 0.79994 | ||||||
appm means parts per million mass error.
Fig. 2.
The web page of the database website (http://foodflavorlab.cn/) including the home page, upload page, search page and result page.
Fig. 3.

The page view (PV) of database website (http://foodflavorlab.cn/).
Acknowledgements
The authors express gratitude to Shan Zengguang for his help to the database. This work was financially supported by the Fundamental Research Funds for the Central Universities (2021ZY65), Beijing Municipal Natural Science Foundation (6192017), Key R&D projects in China People’s Police University (ZDX202101) and R&D projects in Hebei Province (19275416D).
Author contributions
Y.R.L. designed the experiments and wrote the manuscript. N.L. collected the basic information of volatile compounds. X.Y.L. build the database website. W.C.Q. and J.N.L. analyzed the data of GC-Orbitrap-MS. Q.Y.S. and Y.X.C. performed the GC-Orbitrap-MS experiment. B.L.Z., B.Q.Z. and J.X.C. review the manuscript. B.Q.Z. and J.X.C. supported the funding acquisition. B.Q.Z. designed the experiments. J.X.C. supervised the study.
Code availability
The Processing setup, Quan browser and Qual browser (Thermo Fisher Scientific, Les Ulis, France) in Xcalibur version 4.1 and Thermo Scientific TraceFinder (version 4.1) were used for collecting the HRMS library of volatile compounds. The structures of the volatile compounds were drawn using ChemDraw Professional 17.0 (Cambridgesoft, USA). High-resolution mass spectrums are plotted using Python (version 3.7).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yaran Liu, Na Li, Xiaoyao Li.
Contributor Information
Baoqing Zhu, Email: zhubaoqing@bjfu.edu.cn.
Jinxin Cheng, Email: chengjx063@163.com.
References
- 1.Ferreira, V. in Managing Wine Quality (ed A. G., Reynolds) 3–28 (Woodhead Publishing, 2010).
- 2.Ouyang X-Y, et al. Comparison of volatile composition and color attributes of mulberry wine fermented by different commercial yeasts. Journal of Food Processing and Preservation. 2017;42:e13432. doi: 10.1111/jfpp.13432. [DOI] [Google Scholar]
- 3.Wei M, et al. Comparison of physicochemical indexes, amino acids, phenolic compounds and volatile compounds in bog bilberry juice fermented by Lactobacillus plantarum under different pH conditions. Journal of Food Science and Technology. 2018;55:2240–2250. doi: 10.1007/s13197-018-3141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan G-S, et al. Effect of Raw Material, Pressing and Glycosidase on the Volatile Compound Composition of Wine Made From Goji Berries. Molecules. 2016;21:1324. doi: 10.3390/molecules21101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alegre Y, Sáenz-Navajas M-P, Hernández-Orte P, Ferreira V. Sensory, olfactometric and chemical characterization of the aroma potential of Garnacha and Tempranillo winemaking grapes. Food Chemistry. 2020;331:127207. doi: 10.1016/j.foodchem.2020.127207. [DOI] [PubMed] [Google Scholar]
- 6.Lan, Y. et al. Characterization of key odor-active compounds in sweet Petit Manseng (Vitis vinifera L.) wine by gas chromatography–olfactometry, aroma reconstitution, and omission tests. Journal of Food Sciencen/a, 10.1111/1750-3841.15670 (2021). [DOI] [PubMed]
- 7.Cai W, et al. Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS-SPME–GC–MS. Food Chemistry. 2020;330:127330. doi: 10.1016/j.foodchem.2020.127330. [DOI] [PubMed] [Google Scholar]
- 8.Niu Y, et al. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC–O, odor threshold and sensory analysis: An insight at the molecular level. Food Chemistry. 2019;275:143–153. doi: 10.1016/j.foodchem.2018.09.102. [DOI] [PubMed] [Google Scholar]
- 9.Tian T, Sun J, Wu D, Xiao J, Lu J. Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles. Food Chemistry. 2021;340:128179. doi: 10.1016/j.foodchem.2020.128179. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Xie T, Xie J, Ai L, Tian H. Characterization of key aroma compounds in Chinese rice wine using gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chemistry. 2019;293:8–14. doi: 10.1016/j.foodchem.2019.03.071. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y. et al. Effect of Lactobacillus acidophilus, Oenococcus oeni, and Lactobacillus brevis on Composition of Bog Bilberry Juice. Foods8, 10.3390/foods8100430 (2019). [DOI] [PMC free article] [PubMed]
- 12.Yin L, et al. A multi-step screening approach of suitable non-Saccharomyces yeast for the fermentation of hawthorn wine. LWT. 2020;127:109432. doi: 10.1016/j.lwt.2020.109432. [DOI] [Google Scholar]
- 13.Ontañón I, Vela E, Hernández-Orte P, Ferreira V. Gas chromatographic-sulfur chemiluminescent detector procedures for the simultaneous determination of free forms of volatile sulfur compounds including sulfur dioxide and for the determination of their metal-complexed forms. Journal of Chromatography A. 2019;1596:152–160. doi: 10.1016/j.chroma.2019.02.052. [DOI] [PubMed] [Google Scholar]
- 14.Aith Barbará J, et al. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chemistry. 2020;308:125552. doi: 10.1016/j.foodchem.2019.125552. [DOI] [PubMed] [Google Scholar]
- 15.Mitropoulou A, Hatzidimitriou E, Paraskevopoulou A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva. Food Research International. 2011;44:1561–1570. doi: 10.1016/j.foodres.2011.04.023. [DOI] [Google Scholar]
- 16.Falcão LD, de Revel G, Rosier JP, Bordignon-Luiz MT. Aroma impact components of Brazilian Cabernet Sauvignon wines using detection frequency analysis (GC–olfactometry) Food Chemistry. 2008;107:497–505. doi: 10.1016/j.foodchem.2007.07.069. [DOI] [Google Scholar]
- 17.Mestres M, Busto O, Guasch J. Headspace solid-phase microextraction analysis of volatile sulphides and disulphides in wine aroma. Journal of Chromatography A. 1998;808:211–218. doi: 10.1016/S0021-9673(98)00100-9. [DOI] [PubMed] [Google Scholar]
- 18.Schoenauer S, Schieberle P. Screening for novel mercaptans in 26 fruits and 20 wines using a thiol-selective isolation procedure in combination with three detection methods. Journal of Agricultural and Food Chemistry. 2019;67:4553–4559. doi: 10.1021/acs.jafc.9b01242. [DOI] [PubMed] [Google Scholar]
- 19.Siebert TE, Solomon MR, Pollnitz AP, Jeffery DW. Selective determination of volatile sulfur compounds in wine by gas chromatography with sulfur chemiluminescence detection. Journal of Agricultural and Food Chemistry. 2010;58:9454–9462. doi: 10.1021/jf102008r. [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Sha S, Qian M, Xu Y. Characterization of volatile sulfur compounds in moutai liquors by headspace solid-phase microextraction Gas Chromatography-Pulsed Flame Photometric detection and odor activity value. Journal of Food Science. 2017;82:2816–2822. doi: 10.1111/1750-3841.13969. [DOI] [PubMed] [Google Scholar]
- 21.Chenot C, Briffoz L, Lomartire A, Collin S. Occurrence of ehrlich-derived and varietal polyfunctional thiols in Belgian white wines made from Chardonnay and Solaris grapes. Journal of Agricultural and Food Chemistry. 2020;68:10310–10317. doi: 10.1021/acs.jafc.9b05478. [DOI] [PubMed] [Google Scholar]
- 22.Lyu, J., Ma, Y., Xu, Y., Nie, Y. & Tang, K. Characterization of the key aroma compounds in Marselan wine by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission tests. Molecules24, 10.3390/molecules24162978 (2019). [DOI] [PMC free article] [PubMed]
- 23.Romano A, et al. Wine analysis by FastGC proton-transfer reaction-time-of-flight-mass spectrometry. International Journal of Mass Spectrometry. 2014;369:81–86. doi: 10.1016/j.ijms.2014.06.006. [DOI] [Google Scholar]
- 24.Qian X, et al. Comprehensive investigation of lactones and furanones in icewines and dry wines using gas chromatography-triple quadrupole mass spectrometry. Food Research International. 2020;137:109650. doi: 10.1016/j.foodres.2020.109650. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Li S, Gao J, Cheng K, Yuan F. Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. LWT. 2019;109:233–240. doi: 10.1016/j.lwt.2019.03.100. [DOI] [Google Scholar]
- 26.Slaghenaufi D, Tonidandel L, Moser S, Román Villegas T, Larcher R. Rapid Analysis of 27 volatile sulfur compounds in wine by headspace solid-phase microextraction gas chromatography tandem mass spectrometry. Food Analytical Methods. 2017;10:3706–3715. doi: 10.1007/s12161-017-0930-2. [DOI] [Google Scholar]
- 27.Bordiga M, Piana G, Coïsson JD, Travaglia F, Arlorio M. Headspace solid-phase micro extraction coupled to comprehensive two-dimensional with time-of-flight mass spectrometry applied to the evaluation of Nebbiolo-based wine volatile aroma during ageing. International Journal of Food Science & Technology. 2014;49:787–796. doi: 10.1111/ijfs.12366. [DOI] [Google Scholar]
- 28.Peterson AC, et al. Development of a GC/Quadrupole-Orbitrap mass spectrometer, Part I: design and characterization. Analytical Chemistry. 2014;86:10036–10043. doi: 10.1021/ac5014767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson AC, Balloon AJ, Westphall MS, Coon JJ. Development of a GC/Quadrupole-Orbitrap mass spectrometer, Part II: New approaches for discovery metabolomics. Analytical Chemistry. 2014;86:10044–10051. doi: 10.1021/ac5014755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eliuk S, Makarov A. Evolution of Orbitrap mass spectrometry instrumentation. Annual Review of Analytical Chemistry. 2015;8:61–80. doi: 10.1146/annurev-anchem-071114-040325. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, et al. LC-Orbitrap MS analysis of the glycation modification effects of ovalbumin during freeze-drying with three reducing sugar additives. Food Chemistry. 2018;268:171–178. doi: 10.1016/j.foodchem.2018.06.092. [DOI] [PubMed] [Google Scholar]
- 32.Gómez-Ramos MM, Ferrer C, Malato O, Agüera A, Fernández-Alba AR. Liquid chromatography-high-resolution mass spectrometry for pesticide residue analysis in fruit and vegetables: Screening and quantitative studies. Journal of Chromatography A. 2013;1287:24–37. doi: 10.1016/j.chroma.2013.02.065. [DOI] [PubMed] [Google Scholar]
- 33.Pimpão RC, et al. Urinary metabolite profiling identifies novel colonic metabolites and conjugates of phenolics in healthy volunteers. Molecular Nutrition & Food Research. 2014;58:1414–1425. doi: 10.1002/mnfr.201300822. [DOI] [PubMed] [Google Scholar]
- 34.Savic S, et al. Enzymatic oxidation of rutin by horseradish peroxidase: Kinetic mechanism and identification of a dimeric product by LC–Orbitrap mass spectrometry. Food Chemistry. 2013;141:4194–4199. doi: 10.1016/j.foodchem.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Peterson AC, McAlister GC, Quarmby ST, Griep-Raming J, Coon JJ. Development and Characterization of a GC-Enabled QLT-Orbitrap for High-Resolution and High-Mass Accuracy GC/MS. Analytical Chemistry. 2010;82:8618–8628. doi: 10.1021/ac101757m. [DOI] [PubMed] [Google Scholar]
- 36.Mol HGJ, Tienstra M, Zomer P. Evaluation of gas chromatography-electron ionization-full scan high resolution Orbitrap mass spectrometry for pesticide residue analysis. Analytica Chimica Acta. 2016;935:161–172. doi: 10.1016/j.aca.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Lozano A, Uclés S, Uclés A, Ferrer C, Fernández-Alba AR. Pesticide residue analysis in fruit- and vegetable-based baby foods using GC-Orbitrap MS. Journal of AOAC INTERNATIONAL. 2018;101:374–382. doi: 10.5740/jaoacint.17-0413. [DOI] [PubMed] [Google Scholar]
- 38.Li R, et al. High resolution GC–Orbitrap MS for nitrosamines analysis: Method performance, exploration of solid phase extraction regularity, and screening of children’s products. Microchemical Journal. 2021;162:105878. doi: 10.1016/j.microc.2020.105878. [DOI] [Google Scholar]
- 39.Gómez-Ramos MM, Ucles S, Ferrer C, Fernández-Alba AR, Hernando MD. Exploration of environmental contaminants in honeybees using GC-TOF-MS and GC-Orbitrap-MS. Science of The Total Environment. 2019;647:232–244. doi: 10.1016/j.scitotenv.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Dorival-García N, et al. Large-Scale assessment of extractables and leachables in single-use bags for biomanufacturing. Analytical Chemistry. 2018;90:9006–9015. doi: 10.1021/acs.analchem.8b01208. [DOI] [PubMed] [Google Scholar]
- 41.de Albuquerque Cavalcanti G, et al. Non-targeted acquisition strategy for screening doping compounds based on GC-EI-hybrid quadrupole-Orbitrap mass spectrometry: A focus on exogenous anabolic steroids. Drug Testing and Analysis. 2018;10:507–517. doi: 10.1002/dta.2227. [DOI] [PubMed] [Google Scholar]
- 42.Weidt S, et al. A novel targeted/untargeted GC-Orbitrap metabolomics methodology applied to Candida albicans and Staphylococcus aureus biofilms. Metabolomics. 2016;12:189. doi: 10.1007/s11306-016-1134-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera-Pérez A, López-Ruiz R, Romero-González R, Garrido Frenich A. A new strategy based on gas chromatography–high resolution mass spectrometry (GC–HRMS-Q-Orbitrap) for the determination of alkenylbenzenes in pepper and its varieties. Food Chemistry. 2020;321:126727. doi: 10.1016/j.foodchem.2020.126727. [DOI] [PubMed] [Google Scholar]
- 44.Lan Y-B, et al. Characterization and differentiation of key odor-active compounds of ‘Beibinghong’ icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution. Food Chemistry. 2019;287:186–196. doi: 10.1016/j.foodchem.2019.02.074. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, et al. Chemical profiles and aroma contribution of terpene compounds in Meili (Vitis vinifera L.) grape and wine. Food Chemistry. 2019;284:155–161. doi: 10.1016/j.foodchem.2019.01.106. [DOI] [PubMed] [Google Scholar]
- 46.Belarbi S, et al. Comparison of new approach of GC-HRMS (Q-Orbitrap) to GC–MS/MS (triple-quadrupole) in analyzing the pesticide residues and contaminants in complex food matrices. Food Chemistry. 2021;359:129932. doi: 10.1016/j.foodchem.2021.129932. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, 2021. A high-resolution Orbitrap Mass spectral library for trace volatile compounds in fruit wines. MetaboLights. MTBLS3840 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Liu Y, 2021. A high-resolution Orbitrap Mass spectral library for trace volatile compounds in fruit wines. MetaboLights. MTBLS3840 [DOI] [PMC free article] [PubMed]
Data Availability Statement
The Processing setup, Quan browser and Qual browser (Thermo Fisher Scientific, Les Ulis, France) in Xcalibur version 4.1 and Thermo Scientific TraceFinder (version 4.1) were used for collecting the HRMS library of volatile compounds. The structures of the volatile compounds were drawn using ChemDraw Professional 17.0 (Cambridgesoft, USA). High-resolution mass spectrums are plotted using Python (version 3.7).