Abstract
Objectives
Recently, a number of studies have explored the possible attenuation of the immune response by disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis (RA). Our study objective was to investigate the presumed attenuated humoral response to vaccination against SARS-CoV-2 in patients with RA treated with Janus kinase (JAK) inhibitors with or without methotrexate (MTX). The immune responses were compared with controls without RA.
Method
The humoral vaccination response was evaluated by determining titres of neutralising antibodies against the S1 antigen of SARS-CoV-2. One hundred and thirteen fully vaccinated individuals were included at 6 ± 1 weeks after second vaccination (BioNTech/Pfizer (69.9%), AstraZeneca (21.2%), and Moderna (8.9%)). In a cross-sectional and single-centre study design, we compared titres of neutralising antibodies between patients with (n = 51) and without (n = 62) medication with JAK inhibitors.
Results
Treatment with JAK inhibitors led to a significantly reduced humoral response to vaccination (P = 0.004). A maximum immune response was seen in 77.4% of control patients, whereas this percentage was reduced to 54.9% in study participants on medication with JAK inhibitors (effect size d = 0.270). Further subanalyses revealed that patients on combination treatment (JAK inhibitors and MTX, 9 of 51 subjects) demonstrated an even significantly impaired immune response as compared to patients on monotherapy with JAK inhibitors (P = 0.028; d = 0.267).
Conclusions
JAK inhibitors significantly reduce the humoral response following dual vaccination against SARS-CoV-2. The combination with MTX causes an additional, significant reduction in neutralising IgG titres. Our data suggest cessation of JAK inhibitors in patients with RA in the context of vaccination against SARS-CoV-2.
|
Key Points • It was shown that DMARD therapy with JAK inhibitors in patients with rheumatoid arthritis leads to an attenuation of the humoral vaccination response against SARS-CoV-2. • The effect under medication with JAK inhibitors was significant compared to the control group and overall moderate. • The combination of JAK inhibitors with MTX led to an additive and significant attenuation of the humoral response. |
Keywords: Arthritis, rheumatoid; COVID-19; Janus kinase inhibitor; Methotrexate; SARS-CoV-2; Vaccination
Introduction
Although vaccination against SARS-CoV-2 has been available for more than a year now, there is still a relevant degree of uncertainty regarding the effectiveness of SARS-CoV-2 vaccination in patients with inflammatory systemic diseases and immunomodulatory therapy with disease-modifying antirheumatic drugs (DMARDs). Data on the immunogenicity of COVID-19 vaccines in patients with immune-mediated inflammatory diseases (IMIDs) are lacking from the original vaccine trials leading to vaccination approval. This is because these patients were excluded to a large extent from the trials [1, 2].
Initial studies on this topic have shown a strong dependence of the vaccination response on the underlying DMARD therapy [3]. Glucocorticoids, mycophenolate, abatacept, and especially rituximab seem to be associated with a particularly strong attenuation of the humoral response [4]. In addition, age, comorbidities, and the underlying inflammatory disease itself have been identified as additional factors influencing the vaccination response to SARS-CoV-2 [5–10].
Janus kinase (JAK) inhibitors are considered an integral part of rheumatological care. Despite the increasingly common use of the group of JAK inhibitors, for example, in rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis, there has been relatively little work to date on this group of targeted synthetic DMARDs (tsDMARDs) with regard to the effectiveness of vaccination against SARS-CoV-2 [11–13]. In this respect, the topic of the effectiveness of vaccination under JAK inhibitor therapy is of great importance in the clinical routine. Scientific associations such as the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) gave recommendations for vaccination in patients with IMIDs at an early stage based on the available data and adapted them on an ongoing basis [14, 15]. Interestingly, JAK inhibitors, especially baricitinib and tofacitinib, are even used in the treatment of severe SARS-CoV-2 infection [16].
Our study aimed to compare the extent of the humoral response to a two-dose vaccination against SARS-CoV-2 between patients with rheumatoid arthritis (RA) under JAK inhibitor therapy with or without concomitant therapy with methotrexate and a reference group including subjects not suffering from RA or receiving JAK inhibitors or MTX. The immune response was measured by the titres of neutralising antibodies to the spike protein under routine clinical conditions.
Materials and methods
Study participants
For the present study, 113 fully vaccinated individuals were consecutively enrolled in a routine care setting between April 12, 2021, and September 14, 2021. For each participant, the respective evaluation time point was 6 ± 1 weeks after the second vaccination. Study participants were recruited in a single-centre and cross-sectional study design from the Rheumatological Outpatient Clinic of MED|BAYERN OST Medizinische Versorgungszentren Altötting Burghausen, Burghausen, Germany. The total study sample consisted of two independent subgroups:
Fifty-one patients were in the treatment group with rheumatoid arthritis who received JAK inhibitors as disease-modifying antirheumatic medication. Of these, 42 patients (82.4%) were given the drug as monotherapy. The remaining 9 study participants in the treatment group additionally received MTX (17.6%). In addition, 10 of 51 (19.6%) treatment patients received prednisolone with a mean dosage of 4.30 mg per day.
The control group without DMARD therapy consisted of 62 patients who were diagnosed with osteoarthritis of the hands or other peripheral joints and who did not undergo treatment with immunomodulatory medication.
The primary study goal was to compare the humoral vaccination responses between independent subgroups of participants with and without medication with JAK inhibitors when vaccinated against SARS-CoV-2. Primary outcome measures were the levels of neutralising antibody titres 6 ± 1 weeks after the second vaccination with specific vaccines from BioNTech/Pfizer, Moderna, and AstraZeneca.
The process of organising and conducting the study was in accordance with the principles and formal criteria of “Good Clinical Practice” [17, 18]. Accordingly, all patients gave written informed consent to participate in the study and agreed to publication of the generated scientific data. The present study was approved by the ethics committee of the University Hospital of Würzburg, Würzburg, Germany.
A confirmed diagnosis of RA according to ACR-EULAR 2010 criteria represented the primary inclusion criterion for the JAK inhibitor treatment subgroup. Additional criteria for recruitment were at least 18 years of age and written informed consent.
A relative or absolute contraindication for therapy with JAK inhibitors, a previously known intolerance of JAK inhibitors, prior use of rituximab, use of other conventional synthetic DMARDs (csDMARDs) than methotrexate in combination with JAK inhibitors, and a history of SARS-CoV-2 infection were exclusion criteria.
The control group was made up of study participants without any inflammatory rheumatic disease and without antirheumatic or any kind of immunomodulatory therapy. These were patients with osteoarthritis of the hands or other peripheral joints.
Measurements of immune response
The most important outcome variable in this study was the immune response as evaluated by the titres of neutralising antibodies against SARS-CoV-2. For the determination of these titres, we used a quantitative ELISA test for IgG antibodies against the S1 antigen of SARS-CoV-2: Anti-SARS-CoV-2-QuantiVac ELISA (IgG); manufacturer: EUROIMMUN Medizinische Labordiagnostika AG, Lübeck, Germany.
Statistical analysis
The sample size calculations were based on the primary study analysis (comparison of two independent subgroups with and without JAK medication). With these considerations in mind, we assumed two independent samples, a significance level of 5% and a statistical power of at least 80%, to detect a medium effect size. Based on this background, the optimal sample size for one-sided testing was calculated to be a total of 102 subjects (i.e., at least 51 individuals per independent study subgroup: patients with JAK inhibitors vs. controls).
For data handling and processing as well as statistical analyses, we used Microsoft Excel or SPSS (German version 17.0.0) software, where appropriate [19]. Inferential tests were considered statistically significant at P < 0.05. We applied Pearson chi-square tests to compare the frequencies of categorical variables between independent subgroups. Additionally, an analysis of variance (one-way ANOVA) was performed to test for mean differences in continuous variables between independent patient subgroups with and without medication with JAK inhibitors. With respect to the assessed antibody titres, we used Mann–Whitney U tests to detect differences in the central tendency between independent patient groups with and without treatment with JAK inhibitors. This was considered necessary because the distributions of titres of neutralising antibodies were analysed, and we found that this variable only reached ordinal data levels due to laboratory-related ceiling effects. Consequently, we applied a 4-point Likert scale that was largely determined by the laboratory test used: nonresponse (< 34 BAU/mL), low (34 to 175 BAU/mL), moderate (176 to 383 BAU/mL), and maximum vaccine response (≥ 384 BAU/mL).
Results
The total study sample consisted of N = 113 patients. It was composed of a treatment subgroup with medication with JAK inhibitors (51 patients, 45.1%) and a corresponding reference group without immunomodulatory treatment (62 patients, 54.9%). The treatment subgroup included a total of 42 patients (82.4%) treated with a JAK inhibitor as monotherapy. Nine study participants in the treatment group additionally received MTX medication (17.6%). For all included study subjects, blood sampling and evaluation of antibody titres were performed at week 6 ± 1 after the second vaccination.
The main characteristics of both subgroups are displayed in Table 1. Most patient characteristics and the vast majority of medical data were in the same range for both patient subgroups. The most remarkable group difference was the significantly reduced renal function in patients treated with JAK inhibitors (mean GFR values 72.61 mL/min vs. 81.26 mL/min in control patients; P = 0.016).
Table 1.
Patient characteristics, including relevant medical data (stratified by patient subgroups with and without medication with JAK inhibitors)
| RA patients with JAK inhibitors (n = 51) |
Reference group without JAK inhibitors (n = 62) |
P value | |
|---|---|---|---|
| Age (years; mean ± SD) | 62.5 ± 11.8 | 64.3 ± 9.2 | 0.345 |
| Female sex (%) | 68.6 | 79.0 | |
| Male sex (%) | 31.4 | 21.0 | 0.279 |
| Mean RA disease duration (years) | 11.49 | 0 | n.a |
| Seropositivity (%) | 78.4 | 0 | n.a |
| Prednisolone use (%) | 19.6 | 0 | n.a |
| Mean dose prednisolone (mg/day) | 4.30 | 0 | n.a |
| Diabetes (%) | 15.7 | 12.9 | 0.673 |
| Mean GFR values (mL/min; mean ± SD) | 72.61 ± 21.18 | 81.26 ± 14.96 | 0.016 |
| Mean RR syst. (mm Hg; mean ± SD) | 142.59 ± 25.82 | 143.00 ± 16.69 | 0.964 |
| Mean RR diast. (mm Hg; mean ± SD) | 86.76 ± 11.30 | 83.44 ± 13.21 | 0.453 |
| Overall tolerability (sec. vacc.; mean ± SD) | 1.76 ± 0.89 | 1.79 ± 0.93 | 0.882 |
| BioNTech/Pfizer (%) | 72.5 | 67.7 | |
| AstraZeneca (%) | 19.6 | 22.6 | |
| Moderna (%) | 7.9 | 9.7 | 0.854 |
| SARS-CoV-2 IgG (BAU/mL; mean ± SD) | 282.2 ± 139.1 | 345.1 ± 83.8 | 0.006 |
| Maximum response (≥ 384 BAU/mL) (%) | 54.9 | 77.4 | |
| Moderate response (176 to 383 BAU/mL) (%) | 15.7 | 14.5 | |
| Low response (34 to 175 BAU/mL) (%) | 21.6 | 8.1 | |
| Nonresponse (< 34 BAU/mL) (%) | 7.8 | 0 | 0.004 |
Importantly, the frequencies of the types of vaccines did not differ significantly (P = 0.854) between the study cohorts (see Table 1). The vaccines used were distributed as follows in the total sample: BioNTech/Pfizer (69.9%), AstraZeneca (21.2%), and Moderna (8.9%).
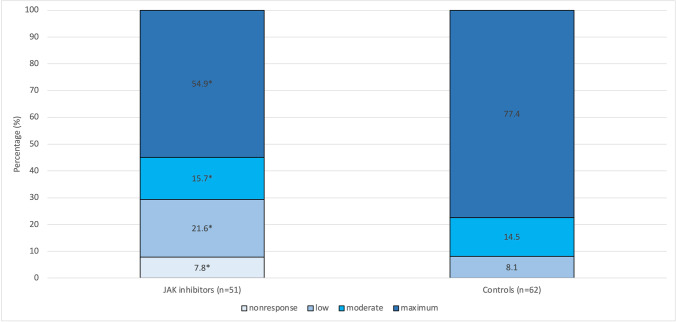
It was an important finding that laboratory data referring to levels of neutralising SARS-CoV-2 antibodies showed marked ceiling effects in a large proportion of participating individuals: Seventy-six out of 113 study participants (67.3%) had a maximum antibody response of ≥ 384.0 binding antibody units (BAU) per mL. Above this upper limit, the test could not differentiate any further. Therefore, due to the demonstrated lack of normally distributed values, we chose to statistically analyse the antibody response using nonparametric procedures. Consequently, we performed Mann–Whitney U tests to compare vaccination responses between independent subgroups. According to our analyses, individuals treated with JAK inhibitors showed a significantly lower antibody response to SARS-CoV-2 vaccination (P = 0.004; Mann–Whitney U test, Table 1); only 54.9% of patients with JAK inhibitor treatment were maximum responders, whereas 77.4% of controls responded completely. This JAK-inhibitor-induced reduction in the immune response was statistically significant (see above) and showed a considerable and therefore probably clinically relevant effect size of d = 0.270. The associated results are displayed in Fig. 1.
Fig. 1.
Humoral immune response measured by titres of neutralising IgG antibodies against the S1 antigen of SARS-CoV-2 depending on the use of JAK inhibitors. Immunoresponse in terms of titres of neutralising antibodies differs significantly between patients with JAK inhibitors (n = 51; 54.9% with maximum response) and control patients (n = 62; 77.4% with maximum response; P = 0.004*; d = 0.270)
The putative effect of the sociodemographic variables age and sex on the immunoresponse is well controlled in our study (i.e., comparable distributions in both subgroups; see Table 1). Nevertheless, we additionally and explicitly evaluated the contribution of these covariates in a further multivariate analysis. This additionally performed ordinal logistic regression analysis confirmed the significant reduction in vaccination response by the use of JAK inhibitors (P = 0.002). Moreover, also the simultaneously included independent variables age (older age; P = 0.002) and sex (male sex; P = 0.030) were significantly associated with a reduced vaccination response.
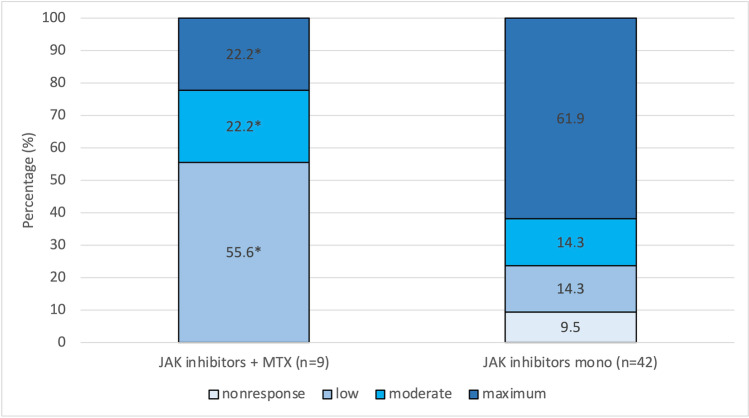
Some of the patients in the treatment group underwent combination treatment with JAK inhibitors and MTX (9 of 51 patients, 17.6%). We evaluated whether this combination treatment also influenced the extent of the vaccination response. Indeed, this additional analysis revealed that combination treatment with both JAK inhibitors and MTX significantly impaired the immune response (22.9% with maximum response) compared with medication with JAK inhibitors only (61.9% with maximum response; P = 0.028; one-sided testing; d = 0.267). The corresponding results are presented graphically in Fig. 2.
Fig. 2.
Humoral immune response measured by titres of neutralising IgG antibodies against the S1 antigen of SARS-CoV-2 depending on the use of JAK inhibitors. Immunoresponse in terms of titres of neutralising antibodies differed significantly between patients on combination treatment (JAK inhibitors and MTX, 9 of 51 subjects; 22.9% with a maximum response) and patients on monotherapy with JAK inhibitors (61.9% with a maximum response; P = 0.028*; d = 0.267)
Glucocorticoid use did not significantly affect the vaccine response in our cohort. This was due to the relatively low mean daily dose of 4.30 mg prednisolone. A total of 80.4% of patients treated with JAK inhibitors had no therapy with glucocorticoids at all.
Discussion
Vaccination against SARS-CoV-2 is widely regarded as a crucial measure in the fight against the SARS-CoV-2 pandemic. Congenital or acquired immunodeficiency, underlying malignancies, advanced age, and comorbidities such as diabetes mellitus and renal insufficiency not only increase the risk of a severe disease course but also lead to a reduced immune response after immunisation against SARS-CoV-2, according to current data [5–10, 20]. JAK inhibitors affect both the innate and the adaptive immune system, including inhibition of type 1 interferon production by dendritic cells, alteration of T-cell stimulation and the TH1 response, and B-cell-specific changes such as differentiation of B-cells into plasmablasts [21–24]. Thus, due to the numerous cellular and immunological effects of JAK inhibitors, significant impacts on the immunogenicity of vaccination against SARS-CoV-2 under JAK inhibitors must be assumed.
Several studies in recent months have addressed the potential attenuation of the immune response by DMARDs in patients with IMIDs. The first data on this were provided by Furer et al. [4]. In this observational multicentre study, the immunogenicity and safety of two doses of the BioNTech/Pfizer vaccine in adult patients with IMIDs (N = 686) were compared with the healthy normal population (N = 121). IgG titres to the SARS-CoV-2 spike S1/S2 protein were measured 2–6 weeks after the second dose of vaccine.
Interestingly, the mere presence of inflammatory systemic disease led to a significantly reduced rate of seropositive patients. Furthermore, older age and treatment with glucocorticoids, rituximab, mycophenolate mofetil, or abatacept were associated with a reduced humoral immune response.
In contrast to our work, Furer et al. included a heterogeneous group of patients with psoriatic arthritis, axial spondyloarthritis, systemic lupus erythematosus, inflammatory myopathies, and vasculitis in addition to rheumatoid arthritis. This limits to some extent the comparability and thus the significance of the results regarding the aspect described by Furer et al. that medication with IMIDs per se represents a risk factor. In the cited study, a total of 45 patients were treated with JAK inhibitors, 21 of them received monotherapy. The rate of patients with IgG > 15 BAU/mL was 90% with JAK inhibitors and 92% in combination with methotrexate (P = 0.02 and P = 0.03, respectively). The authors consider the attenuating effects of JAK inhibitors concerning the humoral vaccination response to be only modest overall [4]. A comparatively mild attenuation of the humoral immune response under JAK inhibitors could also be shown for vaccination against pneumococci and influenza [25, 26].
In line with the results presented by Furer et al., in our study cohort, we found a numerically moderate, yet significant attenuation of the humoral vaccination response under JAK inhibitors compared to controls. According to our data — and in contrast to the work presented by Furer et al. — this attenuating effect regarding the immune response was significantly more pronounced in patients under comedication with both JAK inhibitors and methotrexate. Iancovici et al. [27] reported comparable findings regarding both monotherapy with JAK inhibitors and the role of methotrexate as concomitant therapy. In the cited study, exclusively patients with rheumatoid arthritis under therapy with JAK inhibitors with or without MTX comedication were examined — this was analogous to our study design. However, their study cohort included only twelve patients with rheumatoid arthritis and 26 healthy controls. In addition, the evaluation time points after the second vaccination with the BioNTech/Pfizer vaccine ranged considerably widely from 3 to 11 weeks. Moreover, a closer look at the study sample revealed that it was rather heterogeneous regarding the combination therapies used (e.g., tofacitinib/hydroxychloroquine, tofacitinib/leflunomide, or baricitinib/leflunomide). Taken together, these factors limit its validity and informative value compared to our work. Nevertheless, the results of Iancovici et al. [27] are largely congruent with the findings and conclusions of our study.
The main limitations of our study are the lack of data on the cellular vaccine response and the use of different vaccines. Furthermore, the results were not controlled for impaired renal function, which is among the factors mentioned above with a potential attenuating effect on humoral immunogenicity. According to our data, the glomerular filtration rate was significantly reduced in our JAK inhibitor group compared to the control group. Overall, the reduction was quite small (72.61 mL/min vs. 81.26 mL/min). Consequently, we do not assume a clinically relevant influence on SARS-CoV-2 titres compared to that of JAK inhibitor therapy.
In our study as well as in the work of Iancovici et al., mainly nonselective JAK inhibitors, such as baricitinib and tofacitinib, were used [28–30]. There are no data thus far on differential effects in humoral vaccination response to SARS-CoV-2 depending on the selectivity of JAK inhibitors. For statistical reasons, the number of patients in our cohort unfortunately does not allow for any further subanalyses and respective conclusions to be drawn in this regard. In a recent review on the influence of DMARDs on the vaccination response in patients with inflammatory rheumatic systemic diseases, Friedmann et al. also pointed out the currently poor and partly contradictory data available on JAK inhibitors [31]. For instance, while in the cohort of Depaak et al. [32], a total of eleven patients with JAK inhibitors showed a markedly reduced vaccine response measured by antibody titres compared to healthy controls, only a minimal attenuation of the vaccine response could be measured by Furer et al. as mentioned above [4]. Overall, the data on JAK inhibitors remain very limited compared to other DMARDs, such as methotrexate, TNFalpha inhibitors, and rituximab.
The American College of Rheumatology, specifically the ACR COVID-19 Vaccine Clinical Guidance Taskforce, recommends withholding JAK inhibitors for 1 to 2 weeks (as disease activity allows) after each COVID vaccine dose [14]. This recommendation was based partly on the abovementioned data on the immunogenicity of vaccination against pneumococci [25, 33]. The main message of these data on vaccination against pneumococci is consistent with our findings on JAK inhibitors regarding monotherapy and on the additive attenuation of the humoral immune response by the additional use of methotrexate. At this point, the American College of Rheumatology explicitly points out the difficulty of extrapolating data from vaccination studies, for example, against influenza, pneumococci, or tetanus, to the current recommendations for immunisation against SARS-CoV-2 [34].
The safety of the SARS-CoV-2 vaccination in our study population of patients with JAK inhibitors with or without methotrexate did not differ from that of the control group (data not shown). This is in accordance with findings from Sattui et al. [35].
Through a systematic literature search, Kroon et al. investigated the risk of infection, the prognosis in the case of SARS-CoV-2 infection, and the immunogenicity of vaccination against SARS-CoV-2 in IMIDs [36]. JAK inhibitors were identified as risk factors for a serious prognosis in cases of infection with SARS-CoV-2. This observation is initially contradictory to the fact that tofacitinib and baricitinib are currently used therapeutically in severe courses of SARS-CoV-2 infection. However, this only appears to be a contradiction at first glance when the different phases of the disease are considered: it is indeed possible that JAK inhibitors do possess a risk-increasing property in the phase leading up to an infection regarding the acquisition of the infection but beneficial effects later during the phase of the cytokine storm through inhibition of the JAK/STAT signalling pathway [37].
In summary, the available literature and our data suggest cessation of JAK inhibitors in the context of vaccination against SARS-CoV-2. This seems to be feasible in routine clinical practice due to their very short pharmacological halftimes compared to conventional or biological DMARDs and their rapid onset of action after restart. However, based on the available data, it is not possible to determine whether pausing is necessary before or after vaccination and for what length of time. Additional studies are needed for this purpose, also considering clinical endpoints such as an increased rate of actual infections or severe disease as a potential result of the attenuation of the humoral vaccination response against SARS-CoV-2 by JAK inhibitors.
Declarations
Ethics approval and consent to participate
All patients provided written informed consent for study participation and publication of the scientific data obtained. This study was approved by the ethics committee of the University Hospital of Würzburg, Würzburg, Germany. The process of organising and conducting the study followed the principles of “Good Clinical Practice” [17, 18].
Conflict of interest
Dr. Schäfer reports personal fees from Amgen, AstraZeneca, and Novo Nordisk Pharma GmbH outside of the submitted work. Dr. Kovacs has nothing to disclose. Dr. Eder has nothing to disclose. Dr. Nigg has nothing to disclose. Dr. Feuchtenberger reports personal fees from AbbVie, Novartis, Roche, and UCB outside of the submitted work. All authors disclosed financial or nonfinancial interests that are directly or indirectly related to the work submitted for publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arne Schäfer, Email: arne.schaefer@me.com.
Magdolna Szilvia Kovacs, Email: magdolna.kovacs@med-bayern-ost.de.
Anna Eder, Email: anna.eder@med-bayern-ost.de.
Axel Nigg, Email: a.nigg@innklinikum.de.
Martin Feuchtenberger, Email: m.feuchtenberger@innklinikum.de.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KAs, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Group CCT Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, Group CS Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sood AM, Murthy V, Gonzalez, E. (2021) Efficacy of SARS-CoV-2 vaccine in patients with rheumatic diseases: a systematic review and meta-analysis. In: ACR convergence 2021. All Virtual
- 4.Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, Broyde A, Polachek A, Wollman J, Litinsky I, Meridor K, Nochomovitz H, Silberman A, Rosenberg D, Feld J, Haddad A, Gazzit T, Elias M, Higazi N, Kharouf F, Shefer G, Sharon O, Pel S, Nevo S, Elkayam O. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80(10):1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 5.Boekel L, Steenhuis M, Hooijberg F, Besten YR, van Kempen ZLE, Kummer LY, van Dam KPJ, Stalman EW, Vogelzang EH, Cristianawati O, Keijzer S, Vidarsson G, Voskuyl AE, Wieske L, Eftimov F, van Vollenhoven R, Kuijpers TW, van Ham SM, Tas SW, Killestein J, Boers M, Nurmohamed MT, Rispens T, Wolbink G. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol. 2021;3(11):e778–e788. doi: 10.1016/S2665-9913(21)00222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manolache NG, Ursachi V, Scohy A, Desmet C, Yombi JC, Nzeusseu Toukap A, Stoenoiu MS. Attenuated anti-SARS-CoV-2 antibody response to vaccination in patients with rheumatic diseases. J Infect. 2021 doi: 10.1016/j.jinf.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simon D, Tascilar K, Fagni F, Kronke G, Kleyer A, Meder C, Atreya R, Leppkes M, Kremer AE, Ramming A, Pachowsky ML, Schuch F, Ronneberger M, Kleinert S, Hueber AJ, Manger K, Manger B, Berking C, Sticherling M, Neurath MF, Schett G. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021;80(10):1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raptis C, Andrey D, Berger C, Finckh A, Lescuyer P, Ciurea A, Maletic T, Polysopoulos C, Riek M, Scherer A, Lauper K, Moeller B, Safford J, Schweizer S, von Loga I, Vuilleumier N, Rubbert-Roth A. Immunogenicity of mRNA COVID-19 vaccines at 4 and 12 weeks post full vaccination in patients with inflammatory rheumatic diseases [abstract] Arthritis Rheumatol. 2021;73(suppl 10):L01. [Google Scholar]
- 9.Jena A, Mishra S, Deepak P, Kumar MP, Sharma A, Patel YI, Kennedy NA, Kim AHJ, Sharma V, Sebastian S. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2022;21(1):102927. doi: 10.1016/j.autrev.2021.102927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feuchtenberger M, Kovacs MS, Eder A, Nigg A, Schäfer A (2022) Methotrexate significantly reduces the humoral vaccination response against SARS-CoV-2 in older but not younger patients with rheumatoid arthritis. Int J Rheumatol 42. 10.1007/s00296-022-05123-2 [DOI] [PMC free article] [PubMed]
- 11.Smolen JS, Landewe RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Muller-Ladner U, Mysler EF, da Silva JAP, Poor G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- 12.Coates LC, Corp N, van der Windt DA, O’Sullivan D, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: 2021 update. J Rheumatol. 2022 doi: 10.3899/jrheum.211331. [DOI] [PubMed] [Google Scholar]
- 13.Danve A, Deodhar A. Treatment of axial spondyloarthritis: an update. Nat Rev Rheumatol. 2022;18(4):205–216. doi: 10.1038/s41584-022-00761-z. [DOI] [PubMed] [Google Scholar]
- 14.Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, Calabrese C, Gravallese EM, Harpaz R, Kroger A, Sadun RE, Turner AS, Williams EA, Mikuls TR (2022) American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 5. Available from: https://www.rheumatology.org/Portals/0/Files/COVID-19-Vaccine-Clinical-Guidance-Rheumatic-Diseases-Summary.pdf [Accessed 11 July 2022]
- 15.Landewe RBM, Kroon FPB, Alunno A, Najm A, Bijlsma JW, Burmester GR, et al. EULAR recommendations for the management and vaccination of people with rheumatic and musculoskeletal diseases in the context of SARS-CoV-2: the November 2021 update. Ann Rheum Dis. 2022 doi: 10.1136/annrheumdis-2021-222006. [DOI] [PubMed] [Google Scholar]
- 16.Alunno A, Najm A, Machado PM, Bertheussen H, Burmester GR, Carubbi F, De Marco G, Giacomelli R, Hermine O, Isaacs JD, Kone-Paut I, Magro-Checa C, McInnes IB, Meroni PL, Quartuccio L, Ramanan AV, Ramos-Casals M, Rodriguez Carrio J, Schulze-Koops H, Stamm TA, Tas SW, Terrier B, McGonagle DG, Mariette X. 2021 update of the EULAR points to consider on the use of immunomodulatory therapies in COVID-19. Ann Rheum Dis. 2022;81(1):34–40. doi: 10.1136/annrheumdis-2021-221366. [DOI] [PubMed] [Google Scholar]
- 17.(2001) ICH Harmonised Tripartite Guideline: guideline for good clinical practice. J Postgrad Med 47(3):199–203 [PubMed]
- 18.ICH (2016) Guideline for good clinical practice E6 (R2). ICH Harmonised Tripartite Guideline. Version of December 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-e-6-r2-guideline-good-clinical-practice-step-5_en.pdf. Accessed 18 Sep, 2020
- 19.SPSS (2008) SPSS for windows release 17.0. In Armonk, NY: IBM; 2008
- 20.Geisen UM, Berner DK, Tran F, Sumbul M, Vullriede L, Ciripoi M, Reid HM, Schaffarzyk A, Longardt AC, Franzenburg J, Hoff P, Schirmer JH, Zeuner R, Friedrichs A, Steinbach A, Knies C, Markewitz RD, Morrison PJ, Gerdes S, Schreiber S, Hoyer BF. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis. 2021;80(10):1306–1311. doi: 10.1136/annrheumdis-2021-220272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moura RA, Fonseca JE. JAK inhibitors and modulation of B cell immune responses in rheumatoid arthritis. Front Med (Lausanne) 2020;7:607725. doi: 10.3389/fmed.2020.607725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo S, Nakayamada S, Sakata K, Kitanaga Y, Ma X, Lee S, Ishii A, Yamagata K, Nakano K, Tanaka Y. Janus kinase inhibitor baricitinib modulates human innate and adaptive immune system. Front Immunol. 2018;9:1510. doi: 10.3389/fimmu.2018.01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks) Genome Biol. 2004;5(12):253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:111–115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winthrop KL, Silverfield J, Racewicz A, Neal J, Lee EB, Hrycaj P, Gomez-Reino J, Soma K, Mebus C, Wilkinson B, Hodge J, Fan H, Wang T, Bingham CO., 3rd The effect of tofacitinib on pneumococcal and influenza vaccine responses in rheumatoid arthritis. Ann Rheum Dis. 2016;75(4):687–695. doi: 10.1136/annrheumdis-2014-207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winthrop KL, Bingham CO, 3rd, Komocsar WJ, Bradley J, Issa M, Klar R, Kartman CE. Evaluation of pneumococcal and tetanus vaccine responses in patients with rheumatoid arthritis receiving baricitinib: results from a long-term extension trial substudy. Arthritis Res Ther. 2019;21(1):102. doi: 10.1186/s13075-019-1883-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iancovici L, Khateeb D, Harel O, Peri R, Slobodin G, Hazan Y, Melamed D, Kessel A, Bar-On Y. Rheumatoid arthritis patients treated with Janus kinase inhibitors show reduced humoral immune responses following BNT162b2 vaccination. Rheumatology (Oxford) 2021 doi: 10.1093/rheumatology/keab879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riese RJ, Krishnaswami S, Kremer J. Inhibition of JAK kinases in patients with rheumatoid arthritis: scientific rationale and clinical outcomes. Best Pract Res Clin Rheumatol. 2010;24(4):513–526. doi: 10.1016/j.berh.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 29.O’Sullivan LA, Liongue C, Lewis RS, Stephenson SE, Ward AC. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Mol Immunol. 2007;44(10):2497–2506. doi: 10.1016/j.molimm.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178(5):2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 31.Friedman MA, Curtis JR, Winthrop KL. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(10):1255–1265. doi: 10.1136/annrheumdis-2021-221244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deepak P, Kim W, Paley MA, Yang M, Carvidi AB, Demissie EG, El-Qunni AA, Haile A, Huang K, Kinnett B, Liebeskind MJ, Liu Z, McMorrow LE, Paez D, Pawar N, Perantie DC, Schriefer RE, Sides SE, Thapa M, Gergely M, Abushamma S, Akuse S, Klebert M, Mitchell L, Nix D, Graf J, Taylor KE, Chahin S, Ciorba MA, Katz P, Matloubian M, O’Halloran JA, Presti RM, Wu GF, Whelan SPJ, Buchser WJ, Gensler LS, Nakamura MC, Ellebedy AH, Kim AHJ. Effect of immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2: a prospective cohort study. Ann Intern Med. 2021;174(11):1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winthrop KL, Lebwohl M, Cohen AD, Weinberg JM, Tyring SK, Rottinghaus ST, Gupta P, Ito K, Tan H, Kaur M, Egeberg A, Mallbris L, Valdez H. Herpes zoster in psoriasis patients treated with tofacitinib. J Am Acad Dermatol. 2017;77(2):302–309. doi: 10.1016/j.jaad.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 34.Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Gravallese EM, Bass AR, Calabrese C, Harpaz R, Kroger A, Sadun RE, Turner AS, Williams EA, Mikuls TR. Reply. Arthritis. Rheumatol. 2021;73(9):1769–1770. doi: 10.1002/art.41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sattui SE, Liew JW, Kennedy K, Sirotich E, Putman M, Moni TT, Akpabio A, Alpizar-Rodriguez D, Berenbaum F, Bulina I, Conway R, Singh AD, Duff E, Durrant KL, Gheita TA, Hill CL, Howard RA, Hoyer BF, Hsieh E, El Kibbi L, Kilian A, Kim AH, Liew DFL, Lo C, Miller B, Mingolla S, Nudel M, Palmerlee CA, Singh JA, Singh N, Ugarte-Gil MF, Wallace J, Young KJ, Bhana S, Costello W, Grainger R, Machado PM, Robinson PC, Sufka P, Wallace ZS, Yazdany J, Harrison C, Larche M, Levine M, Foster G, Thabane L, Rider LG, Hausmann JS, Simard JF, Sparks JA (2021) Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open 7(3). 10.1136/rmdopen-2021-001814 [DOI] [PMC free article] [PubMed]
- 36.Kroon FPB, Najm A, Alunno A, Schoones JW, Landewe RBM, Machado PM, Navarro-Compan V. Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-221575. [DOI] [PubMed] [Google Scholar]
- 37.Saeedi-Boroujeni A, Asadi-Samani M. JAK inhibitors as a barrier to the destructive cytokine storm in COVID-19. Curr Drug Res Rev. 2022 doi: 10.2174/2589977514666220304203816. [DOI] [PubMed] [Google Scholar]