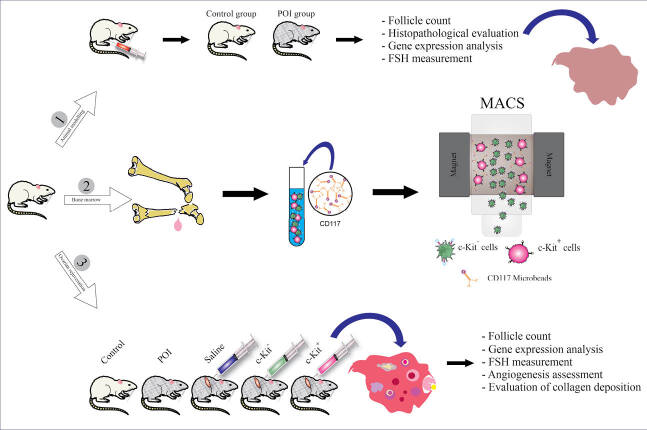
Abstract
Introduction: Cell-based therapies with certain cell types are touted as novel and hopeful therapeutic intervention in the clinical setting. Here, we aimed to assess the regenerative potential of c-Kit+ cells in the rejuvenation of ovarian tissue and fertility rate in rat model of premature ovarian failure (POF).
Methods: Rats were treated with 160 mg/kg/BW of 4-vinylcyclohexene dioxide for 15 days. Freshly enriched rat bone marrow-derived c-Kit+ (MACS) and c-Kit- cells (4×105 cells/10 µL) were transplanted into the ovaries of treatment and control animals. Prior to transplantation as well as 2, 4, 6, and 8 weeks post-transplantation, randomly-selected rats were euthanized and ovarian tissues were subjected to pathophysiological examinations and real-time PCR analyses.
Results: POF status was confirmed by the presence of pathological features and a decreased number of immature and mature follicles compared with the control group (P < 0.05). Histological examination revealed a substantial reduction of atretic follicles in POF rats receiving c-Kit+ cells in comparison with POF rats that did not receive these cells (P < 0.05). Compared with the control samples, angiogenesis-related genes, Angpt2 and KDR, showed increased and decreased expressions in POF ovaries, respectively (P < 0.05). c-Kit+ cells had potential to restore angiogenesis in the ovarian tissue within normal ranges. Systemic levels of FSH did not significantly change in pre- or post-transplantation time points for any group (P > 0.05). Notable reduction of collagen deposition was found in c-Kit-treated rats. Transplantation of c-Kit+ cells also restored the reduced fertility rate (P < 0.05).
Conclusion: The administration of c-Kit+ cells can modulate angiogenesis and pathological changes, leading to the rejuvenation of ovarian function of a rat model of premature menopause.
Keywords: Premature ovarian failure, Bone marrow c-Kit+ cells , Follicular competence, Angiogenesis, Fertility
Introduction
Premature ovarian failure (POF) is a condition caused by ovarian insufficiency during the reproductive age that affects ~1% of women globally. Diagnostically, POF is defined as the cessation of ovarian germinative and hormonal functions prior to age 40 with concurrent presentation of oligomenorrhea or sudden amenorrhea (for >4 months), elevated serum follicle-stimulating hormone levels (FSH, >40 U/L), and decreased levels of estradiol (E2, <20 pg/mL). 1,2 Women diagnosed with POF are prone to suffering from infertility, osteoporosis, endothelial dysfunction, cardiovascular disease, various autoimmune diseases, and/or other complications such as Parkinson's and Alzheimer's diseases. 2-5 The etiology of POF is not well understood, although some cases can be caused by gonadotoxic cancer treatments, concomitant autoimmune diseases, environmental toxicants, lifestyle choices, and/or genetic predispositions affecting the ovaries. 6,7
Various strategies have been proposed for treating POF; suggestions include clinical interventions such as hormonal replacement or immunomodulation therapy and several clinical or experimental-assisted reproduction modalities. 8-11 Advances in the cryopreservation of oocytes, embryos, and ovarian tissue are promising in the preservation of the reproductive potential, especially when combined with restoration of endocrine function. 6,12-15 However, these latter strategies should only be considered when a large ovarian reserve is present, and this is rare in circumstance. Cryopreservation of ovarian tissue may be an option for prepubertal girls undergoing gonadotoxic treatments with a high risk of POF, 16 but this procedure is both inefficient and associated with legal and ethical limitations. 17 As such, current treatment strategies do not always produce a satisfactory outcome and alternative options are still being explored.
One such option is stem cell therapy, an emerging therapeutic approach for treating patients undergoing an early decline in their fertility status. In various studies, several stem cell lineages, including adipose, bone marrow-derived, umbilical cord, and endothelial and/or mesenchymal skin stem cells, have been successfully used to ameliorate ovarian insufficiency. 17-21 These novel developments offer renewed hope for cancer survivors diagnosed with early menopause. So far, the use of mesenchymal stem cells derived from bone medulla is particularly promising. 22 Its success is likely due to the secretory milieu of bone marrow cells which contains many cytokines including the stem cell factor (SCF, also known as the kit ligand, steel factor, or mast cell growth factor). The SCF protein can be found in both soluble and transmembrane forms which bind to the c-Kit receptor (CD117). These cytokines are essential in promoting cell proliferation and survival, as well as angiogenesis. 23,24 It is also known that stem cells derived from the bone marrow niche have the potential to give rise to different cellular lineages. 25 The subpopulations of bone marrow-derived cells harboring c-Kit receptors (CD117+ cells) include hematopoietic, endothelial, and mesenchymal lineages. 26 Interactions between SCF and the cell-membrane bound c-Kit receptor contribute to the regulation of the stem cell niche. 27 The SFC/c-Kit axis is also important in regenerative potency during hematopoiesis, gametogenesis, and melanogenesis, where the presence of c-Kit receptor in target cells is shown to be integral to their multipotentiality and differentiation capacity. 23 Therefore, we hypothesized that cells harboring the c-Kit receptor provide an appropriate candidate for the restoration of injured ovarian tissue. To our knowledge, the therapeutic effects of enriched populations of c-Kit+ cells on the rejuvenation of ovarian tissue have not yet been reported or described. Thus, in the present study, we aimed to investigate the recovery of ovarian function following transplantation of c-Kit+ cells using a rat model of POF. On this basis, we used conventional POF induction protocol induced by VCD (4-vinylcyclohexene dioxide) which is touted as a typical gonadotoxic chemical targeting primary follicles. 28
Materials and Methods
Materials
Chemicals and materials were prepared for the current experiment as follows; VCD (Cat no: 94956; Sigma-Aldrich); Bovine serum albumin (BSA; Cat no: A2153; Sigma-Aldrich); Anti-CD117 microbeads (Cat no: 130-091-224; Miltenyi Biotec) and LS column (Cat no: 130-042-401; Miltenyi Biotec); PE-conjugated anti-mouse secondary antibody (Cat no: 12-4015-82; eBioscience); and Anti-rat α-SMA antibody (Cat no: PM 001 AA; Biocare Medical; HRP-conjugated secondary antibody (Cat no: ab97046; Abcam).
Animal care and handling
A total of 91 female and 10 male albino Wistar rats were used (Med Zist Institute, Karaj, Iran). This included 10 female rats as bone marrow cell donors, 6 female rats for collection of blood and ovarian tissue samples prior to intra-ovarian injections, 75 female rats in different treatment/control groups, and 10 male rats for breeding trials. At arrival, the rats were 8-10 weeks old and weighed ~200 ± 20 g. Female and male rats were group-housed separately in random orders (up to 5 rats per cage) and maintained under standard animal care conditions including 12-hour dark-light cycles, a temperature of 22 ± 2˚C, and ad libitum access to water and pelleted food. All rats were allowed to acclimate for one week before any procedures. For surgeries, ketamine (87 mg/kg) and Xylazine (13 mg/kg) were intraperitoneally (IP) administered for anesthesia, 29 and euthanasia was induced using an overdose of anesthesia, followed by exsanguishing and bilateral thoracotomy to ensure death.
Induction of the rat model of menopause
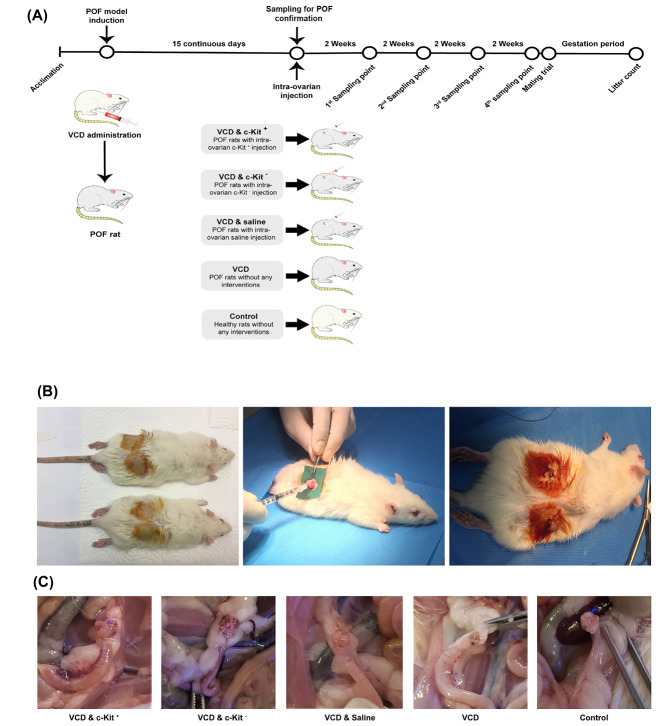
Rats were weighed every 3 days throughout the study. To induce the rat model of menopause, we used VCD at 160 mg/kg/BW dissolved in 200 µL of saline, injected IP every day for 15 days. 30 Vehicle control rats received 200 μL of saline IP for 15 days (Fig. 1A).
Fig. 1.
Schematic representation of the experimental design and timeline (A); Intra-ovarian cell transplantation during the surgical intervention (B); and ovarian tissue sampling (C).
Isolation of bone marrow c-Kit+ cells using magnetic-activated cell sorting
Ten female rats were randomly selected to serve as bone marrow donors for the isolation of marrow-derived c-Kit+ cells. For this purpose, rats were euthanized and the humerus and femur bones were obtained under sterile conditions. The bones were then cut by sterile scissors and marrow contents were flushed with sterile phosphate-buffered saline (PBS) using a 26-gauge needle attached to a 1-mL syringe. Samples were minced and gently triturated to yield a single cell suspension then rinsed in PBS and centrifuged at 400 ×g for 5 minutes. Freshly isolated bone marrow mononuclear cells (MNCs) were obtained using the Ficoll-Paque Plus density gradient medium (Cat no: 17-1440-02; GE Healthcare) according to the manufacturer’s instruction. To enrich c-Kit+ cells using magnetic-activated cell sorting (MACS), MNCs were blocked with 1% BSA for 20 minutes at room temperature, and anti-CD117 microbeads were added to the cell suspension and maintained at 4°C for 1 hour. Cells were then passed through the LS column and both c-Kit positive and negative cells were separated into different conical tubes.
Purity assessment of c-Kit+ cells by flow cytometry
c-Kit positive samples were fixed in ice-cold paraformaldehyde (4% v/v), washed twice with PBS, and incubated with PE-conjugated anti-mouse secondary antibody at 4°C for 30 minutes. The percent of c-Kit+cells was calculated using a cell analyzer system (BD FACSCalibur) and the FlowJo software (version 7.6.1).
Transplantation of cells into the ovarian tissue
Fig. 1A summarizes the study design, assignment of groups, and timelines. To evaluate the effect of c-Kit+ cells on the restoration of ovarian function in the rat model of menopause, 75 female rats were randomly divided into five groups (n = 15 rats/group) as follows; I) VCD & intra-ovarian c-Kit+ cells, II) VCD & intra-ovarian c-Kit - cells, III) VCD & intra-ovarian saline (sham), VI) VCD without intra-ovarian injections, and V) vehicle control without intra-ovarian injections. On the day after the last treatment (Day 16), rats of groups I-III were anesthetized and the skin of the supra flank region was shaved and prepared for surgery. A small skin incision was then made on both sides, followed by the opening of the abdominal wall and blunt dissection to exteriorize the ovaries. The intra-ovarian injections were made using a 26-gauge needle and the cells (4×105 cells for groups I and II) were suspended in 10 μL of saline, hence group III also received 10 μL of saline in each of their ovaries (Fig. 1B). During the intra-ovarian injections, the ovarian tissues were carefully monitored and no leakage was observed after transplantation. After the surgical procedure and cell transplantations were completed, 250 mg/L Neomycin was added to water bottles for 3 days as a prophylactic antibiotic.
Tissue and blood sampling
One day after the final VCD/vehicle administration (i.e., Day 16) and prior to any intra-ovarian cell transplantations, three randomly selected rats from each of the VCD and vehicle-injected groups were euthanized to confirm the POF model status (i.e., pre-transplantation time point). To assess the effect of cell transplantation, we also euthanized three randomly selected rats from each of the five (I-V) groups at 2, 4, 6, and 8 weeks post-transplantation (Fig. 1C). Ovarian samples were obtained at all time points for various analyses, and blood samples were taken at pre-transplantation and 8 weeks post-transplantation for hormone measurements. Ovaries were rinsed in PBS; right ovaries fixed in 4% formaldehyde for subsequent histopathological and immunohistochemistry (IHC) analyses, while left ovaries were transferred to cryovials, snap-frozen in liquid nitrogen (LN2), and maintained in LN2 for mRNA extraction at a later date.
Histopathological evaluation of follicles and fibrotic changes in the ovaries
Formalin-fixed samples were dehydrated in alcohol series, embedded in paraffin blocks, and sectioned at 5 μM thickness. Sections were stained with hematoxylin and eosin (H&E) for routine histopathological evaluations, including the number and quality of follicles and corpus lutea. Sections from the 8 weeks post-transplantation samples were also stained with Masson’s trichrome to assess the possibility of aberrant fibrotic changes in terms of collagen deposition. Histomorphological features and the presence of blue-colored collagen fibers were monitored under light microscopy and documented using a digital camera.
Detection of microvascular density using immunohistochemistry
The extent of vascularization was also assessed in ovarian tissue sections using the IHC detection of alpha-smooth muscle actin (α-SMA). The sections were pre-treated with 3% H2O2 for 10 minutes to inhibit peroxidase activity, followed by incubation with an anti-rat α-SMA antibody overnight at 4◦C. Thereafter, the slides were treated with an HRP-conjugated secondary antibody for 30 minutes. For background staining, cells were stained with hematoxylin. To calculate the microvascular density, the number of α-SMA positive vascular units were counted in 10 random fields of view using a 20X objective.
Real-time PCR analysis
The left ovaries snap-frozen at euthanasia were removed from LN2 and smashed using a mortar and pestle. Total RNA was isolated from these frozen-thawed samples using a Blood/Cultured Cell Total RNA Purification Kit (Cat no: FABRK 001; Yekta Tajhiz, Iran). Absorbance measurements were performed at 260/280 nM to determine the purity of isolated RNAs. cDNA synthesis was performed using a cDNA Synthesis Kit (Cat no: YT4500; Yekta Tajhiz,). Angiogenesis was evaluated by assessing transcripts for the angiopoietin-2 (Angpt2) and vascular endothelial growth factor receptor-2 (VEGFR2, also known as the kinase insert domain receptor – KDR), using quantitative real-time PCR (qRT-PCR; Roche Light Cycler 96) and an SYBR Green Real-Time PCR Master Mix (Cat no: YT2551; Yekta Tajhiz). qRT-PCR was performed over 40 cycles of three-step amplification with denaturation at 95°C for 10 seconds, annealing at 60°C for 10 seconds, and temperature extension at 72°C for 10 seconds. Melting curves were evaluated to verify single product amplification. Primers were designed using NCBI online program (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Standard curves were also prepared using PCR products in 10-fold dilutions to confirm the specificity of the primer pairs. In the end, expression levels were normalized with the reference gene. The list of primers is summarized in Table 1.
Table 1. List of primers used for qRT-PCR .
| Gene | Sequence | Annealing (°C) |
| Angpt-2 |
Forward 5’-GCAGCGTTGACTTCCAGAGA-3’ Reverse 5’-ATACAGAGAGTGTGCCTCGC-3’ |
60 |
| KDR |
Forward 5’-AGATGCGGGAAACTACACGG-3’ Reverse 5’-GGGAGGGTTGGCATAGACTG-3’ |
60 |
| β -Actin |
Forward 5’-TCACCCACACTGTGCCCATCTACGA-3’ Reverse 5’-GGATGCCACAGGATTCCATACCC-3’ |
60 |
Measurement of FSH using ELISA assays
Blood samples were obtained at pre-transplantation and 8-week post-transplantation from each of the VCD and vehicle-injected groups of rats (as described above). The samples were taken from the heart under anesthesia and prior to euthanasia. The blood samples were allowed to clot in glass tubes, centrifuged at 400 × g for 20 minutes, the sera carefully aspirated, and stored at -80°C for subsequent analyses. FSH levels were quantified using a commercial kit (425-300 FSH AccuBind ELISA Rev3- STAT-Fax, DRG) as per manufacturer’s instructions.
Post-transplantation evaluation of fertility
The ovarian function and fertility status of rats in various groups were assessed at 8 weeks post-transplantation. To this end, the remaining three female rats from each group were housed together with one male rat for five days. The presence of a vaginal plug was used as a determinant sign that mating had occurred. Subsequently, each female rat was caged individually for an average of 22 days, the expected length of gestation. During their pregnancy period, rats were weighed every day. The fertility rate, litter size (number of offspring), and birth weights of pups were recorded.
Statistical analysis
GraphPad Prism v8.0.2.263 (GraphPad Software Inc., San Diego CA) was used to compare data amongst the groups. The Kruskal-Wallis test was used to determine the normality of samples. One-way ANOVA followed by a least significant difference (LSD) test was conducted for statistical comparisons among groups. Repeated measures ANOVA test was also used to compare changes in the number of follicles, gene expression levels, and weight variations over time. Values are expressed as mean ± standard deviation (SD). A P value ≤0.05 was considered statistically significant.
Results
VCD administration induced menopause
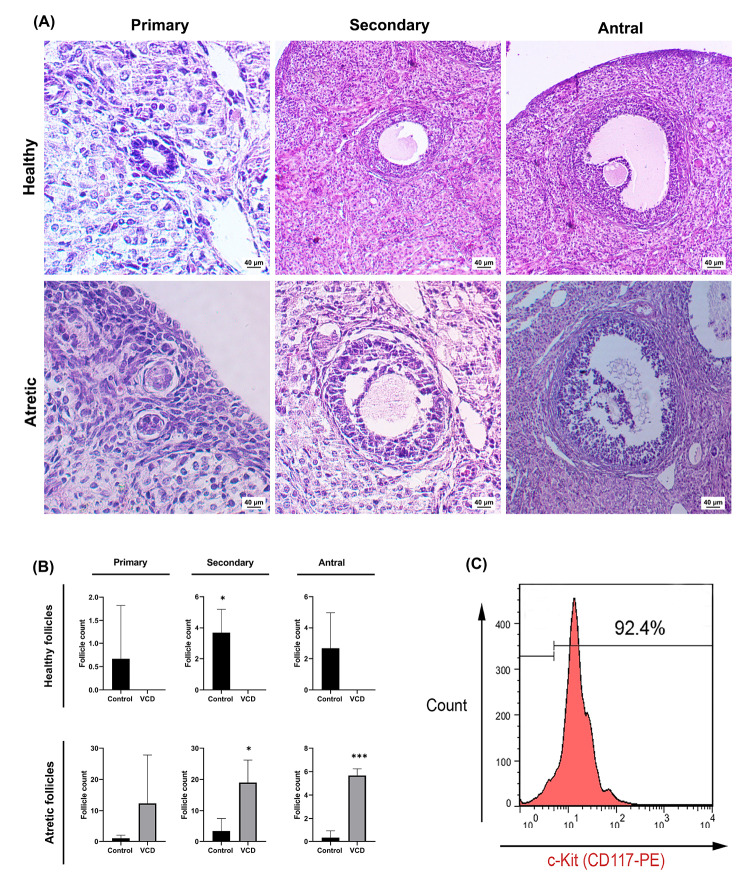
To induce the rat model of menopause/POF, we administered VCD for 15 consecutive days. Besides, we assessed the putative effects of VCD on ovarian tissues one day after the final injection. Histopathological examinations revealed multiple changes in the ovaries of VCD-treated rats compared with those of the control (Fig. 2A). Along with the reduction in number of all follicle types, the pathological changes and ultrastructural remodeling included a widespread follicular atresia (indicated with morphologically degenerative changes) as evidenced by a lack of healthy follicles in the VCD group (Fig. 2A-B). Cells furnishing the primary (composed of single-layered of granulosa cells with typical cuboidal structure) and secondary follicles (with multi-layered cuboidal granulosa cells) in the VCD group underwent degeneration and detached from the basement membrane. These changes coincided with the retention of follicular fluid (Fig. 2A). Other pathological observations included the presence of hyperemia, necrotic changes, and the formation of collagen fibers in ovarian stroma of VCD treated rats (data not shown).
Fig. 2.
Histological evaluation of primary, secondary, and antral follicles after VCD injection (H&E staining A); Mean follicular number following POF induction (B); Flow cytometric analysis of CD-117+ cells post-MACS technique (C). Asterisks represent significant differences (P≤0.05) between experimental groups.
Evaluation of c-Kit+ populations confirmed purity
To assess the purity of the c-Kit+ cells following MACS enrichment, we used flow cytometry analysis. The results showed that the proportion of c-Kit+ cells among isolated cells exceeded 90% (Fig. 2C).
Injection of c-Kit+ increased the population of follicles
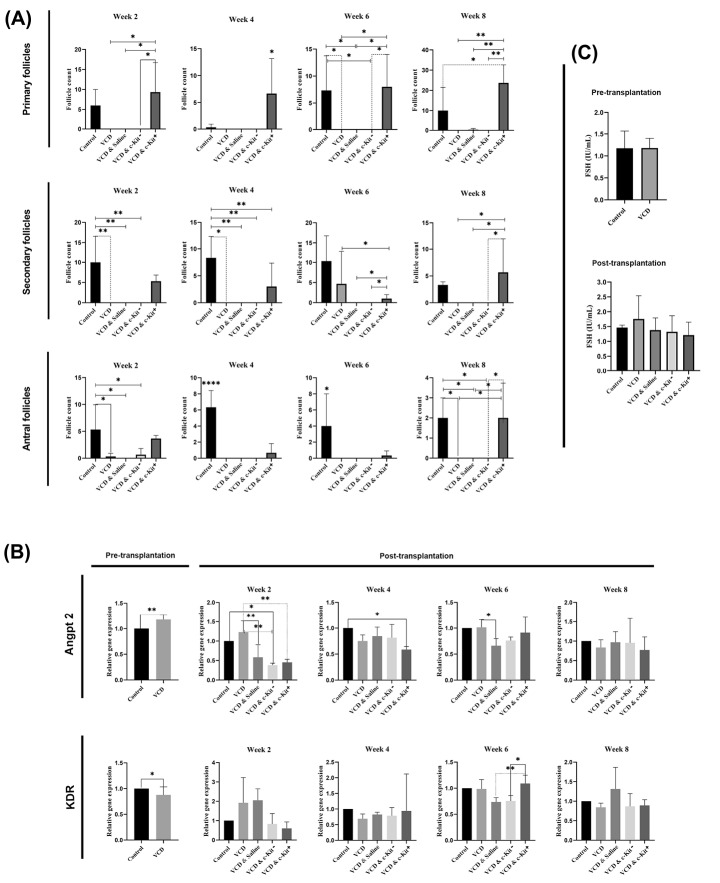
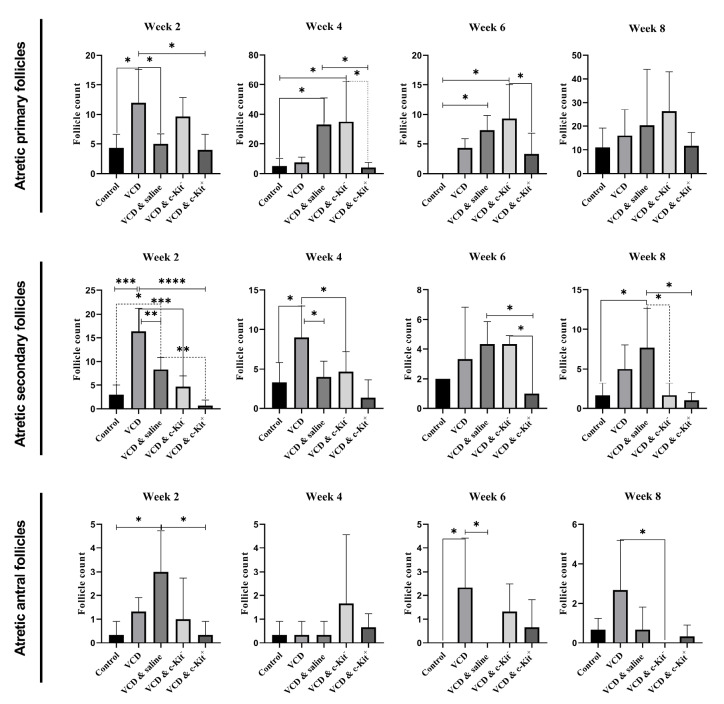
Pre-transplantation, a low number of normal follicles and a high number of immature atretic follicles was observed in the VCD group (Fig. 2B). This continued post-transplantation in groups II-V which did not receive intra-ovarian c-Kit+ cell injections (P < 0.05; Fig. 3A). Conversely, transplantation of c-Kit+ cells was found to increase the number of primary, secondary, and antral follicles (contained fluid-filled antrum with immature oocyte) present in the ovarian tissue compared to other VCD groups at all time points (P < 0.05; Fig. 3A). The number of immature follicles in VCD + c-Kit+ rats were comparable to the control group. We also found that the administration of c-Kit+ cells into ovarian tissue led to increased numbers of secondary follicles over time, with a maximum number counted 8 weeks post-transplantation. Comparatively, administration of c-Kit - cells did not change the number of primary and secondary follicles compared with the VCD alone or normal saline + VCD groups. Histological examinations revealed that antral follicles in VCD + c-Kit+ rats were present at numbers comparable to the control group and greater than all other groups (P < 0.05; Fig. 3A). In contrast, the number of atretic follicles of all stages declined over time in the VCD + c-Kit+ group (Fig. 4). Quantification of corpus lutea did not show differences among the groups (data not shown).
Fig. 3.
Number of healthy follicle counts after 2, 4, 6, and 8 weeks post-stem cell transplantation (A). Relative expression of Angpt2 and KDR transcripts pre- and post-transplantation (B). Serum levels of FSH pre-transplantation and 8 weeks post-transplantation (C). Asterisks represent significant differences (P ≤ 0.05) between experimental groups.
Fig. 4.
Atretic follicle counts (primary, secondary and antral follicles) observed 2, 4, 6, and 8 weeks post- stem cell transplantation. Asterisks represent significant differences (P ≤ 0.05) between experimental groups.
c-Kit cell injections decreased the rate of collagen deposition in VCD ovaries
Masson’s trichrome staining was used to evaluate the presence of collagen fibers as an indication of fibrotic changes in ovarian tissue samples. Under bright-field imaging, blue-colored fibers were extensively present in samples from VCD treated rats. Following the intra-ovarian administration of c-Kit positive or negative cells, rates of tissue remodeling and collagen fiber deposition were reduced; however, this was most prominently evident in ovaries receiving c-Kit+ cells (Fig. 5A).
Fig. 5.
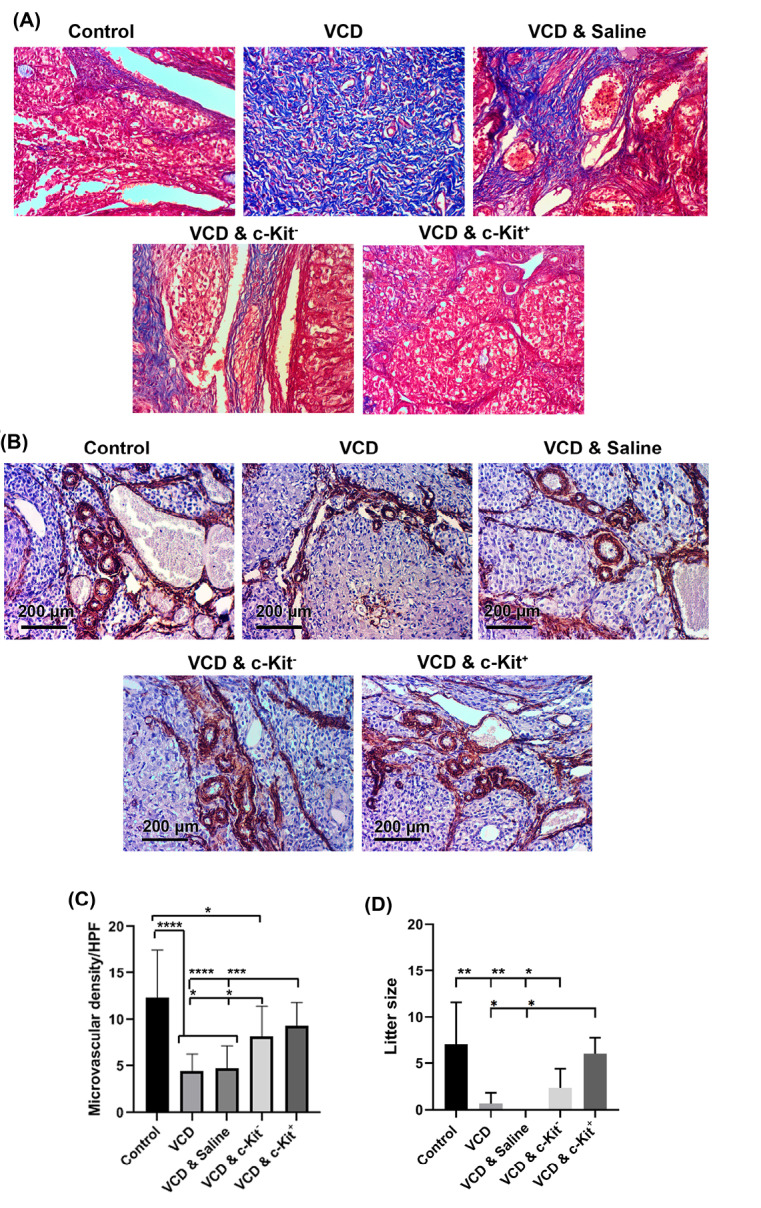
Masson’s Trichrome staining 8 weeks after administration of c-Kit cells in VCD-treated rats (A). Measuring angiogenic potential of c-Kit+ cells after IHC staining against α-SMA (B and C); Litter size following the cell transplantation 8 weeks after intervention (D); The increasing number of asterisks represents increasing significant differences (P ≤0.05, P ≤0.01, P ≤0.001, P ≤0.0001, respectively).
c-Kit lineage cells improved the vascularization of ovaries
IHC was used to assess the angiogenic effect of c-Kit cell transplantation in VCD-treated rats, as indicated by the levels of α-SMA positive vessels. This analysis showed a significant reduction of vascular density in ovarian samples taken from rats receiving VCD without c-Kit cell transplantations in comparison with the control group (P < 0.0001; Fig. 5B-C). However, after intra-ovarian injections of bone marrow-derived cells (i.e., either c-Kit positive or negative), vascular density increased (P < 0.05; Fig. 5B, C). Although transplantation of c-Kit - cells also improved angiogenesis of the ovaries, the extent of vascularization was less than those of the control tissue. Conversely, the injection of c-Kit+cells increased the number of α-SMA positive vessels to near-control values (P > 0.05; Fig. 5B-C).
Expressions of Angpt2 and KDR were altered
Quantitative real-time PCR was performed to examine the transcription levels of angiogenesis-related genes, with results summarized in Fig. 3B. In pre-transplantation samples, expression of Angpt2 increased while that of KDR decreased in VCD-treated rats compared with the control rats (P < 0.05). Expression of Angpt2 was higher in VCD rats receiving no ovarian injections than that in all other VCD rats at 2 weeks (P < 0.05), as well as those receiving saline at 6 weeks post-transplantation (P < 0.05). We also found that the expression of KDR increased in VCD rats 6 weeks after intra-ovarian injection of c-Kit+ cells when compared with VCD rats receiving c-Kit - cells or saline (P < 0.05). By 8 weeks post-transplantation, Angpt2 and KDR transcripts for all treatment groups were in the range of control animals.
Serum levels of FSH did not change
In pre-transplantation samples, serum levels of FSH were not altered by VCD treatments. FSH levels at 8 weeks post-transplantation also did not differ among groups (Fig. 3C).
c-Kit+ cells restored fertility rate in menopausal rats
To assess the fertility outcome of various treatments at 8 weeks post-transplantation, three female rats per group were allowed to mate with male rats. Breeding trials resulted in a birth-giving rate of 3 out of 3 rats for the control and VCD & c-Kit+ groups. A total of 19 and 18 offsprings were recorded for the control and VCD & c-Kit+ groups, respectively. In the c-Kit - group, 2 out of 3 rats gave birth to a total of 7 pups. No pregnancy and parturition were observed in the VCD & saline group, whereas only 1 of 3 rats became pregnant and gave birth (to a single pup) in the VCD group (Fig. 5D). No abnormalities were observed in any of the pup born during this study.
Weight gain after cell transplantation
To monitor the potential effect of treatments on body weight, all rats were weighed every 3 days throughout the study, including before and after transplantations. Prior to cell transplantation, weight loss was observed over one week following the onset of the intraperitoneal VCD injection, with a gradual pattern of weight gain noticed thereafter. Rats in the control group also exhibited weight gain and intra-ovarian saline injection did not affect their total performance. Following transplantation, significant weight gain occurred, as reported in our weekly based weighing schedule (Fig. S1, Supplementary file 1).
Discussion
Given the clinical significance of POF in women, this study was designed to investigate the efficacy of using intra-ovarian transplantation of bone marrow-derived c-Kit+ cells in restoring ovarian function in rats experiencing experimentally-induced POF. To test this hypothesis, a combination of histopathological, IHC, gene expression, hormonal, and fertility assessments were used. Taken together, the results are in support of our hypothesis. To our knowledge, we are first to report positive therapeutic effects of enriched populations of c-Kit+ bone marrow-derived cells on the rejuvenation of ovarian tissue in a rat model of POF.
As a first step, we set out to generate a reliable rat model of POF. This was achieved through daily administrations of VCD, a chemical that is known to induce premature ovarian insufficiency in animal models. 31,32 In a study by Mayer and colleagues, various doses of VCD were used to induce the menopausal model in mice (80-320 mg/kg/d for 15 days), where 160 mg/kg/d for 15 days was reported as an optimal dose for the induction of ovarian failure. 30,33 In the present study, we used this latter dose to achieve the depletion of follicles in rats. Our findings confirm that VCD injections can induce ovarian fibrosis and cause atresia of follicles at all stages of their development in rats. It has been suggested that this of VCD action occurs through its effect on the KIT-KITLG and apoptotic signaling pathways, leading to ovarian deficiency. 33,34 Our results are consistent with previous studies that reported VCD as being capable of reducing the number of ovarian follicles, especially in their earlier and later stages. 31,33,35
As a next step, we used MACS to isolate bone marrow-derived which were then confirmed to be highly enriched for c-Kit+ cells. These cells along with c-Kit - cells and saline were used for intra-ovarian injections in different groups of VCD-treated rats.
In the present study, the administration of c-Kit+ cells, but not c-Kit - cells or normal saline, restored ovarian competence compared with the untreatedVCD rats. In our rat model of menopause, restoration of ovarian competence was defined as an increased relative number of primary, secondary, and antral follicles. Our results also showed that bone marrow-derived cells, including c-Kit - but especially c-Kit+, have the potential to slow down aberrant remodeling in VCD-treated ovarian tissue. Similarly, although intra-ovarian injection of c-Kit - cells improved the VCD-induced angiogenesis deficiency, transplantation of c-Kit+ cells restored the vascularization levels to a greater degree, which were ultimately comparable to the control values.
In line with these observations, our results confirmed that the expression of Angpt2 and KDR genes were altered in VCD groups; Angpt2 expression was likely elevated in response to the VCD-induced inflammatory and degenerative changes occurring within the ovarian tissues, 36,37 but it is not clear why KDR expression had decreased. It may be reasonable to hypothesize that the severe inflammation had promoted angiogenic signaling through the up-regulation of the pro-angiogenic factor Angpt2, which then led to a compensatory down-regulation of its relevant receptor (KDR) gene. In either case, levels of Angpt2 and KDR expression returned to their normal range by 8 weeks post-transplantation, which would correlate with the reduction of inflammation responses by this time. We also noted that VCD injections contributed to the weight loss recorded during the first 5 days, which was then followed by a gradual pattern of weight gain that continued until the end of the experiment. We assume this to be caused by the effects of VCD being triggered during drug administration, which would later have been metabolized via renal and hepatic routes. Consistent with our study, bone marrow-derived c-kit/Sca-1 cells have been shown to restore oocyte numbers in mice sterilized by chemotherapy, further demonstrating their restorative potential. In their study, Johnson and co-workers highlighted the up-regulation of genes associated with germline markers such as Dazl, Fragilis, Stella, Nobox, etc. 38 In support for the regenerative role of bone marrow c-kit cells, we showed that the reduction of fibrosis and induction of angiogenesis could be an effective strategy in the restoration of follicles.
The number of offsprings born from the VCD & c-Kit+ rats was significantly greater than those in the VCD & c-Kit - group and was in the range of control rats. In support of this finding, Takehara and colleagues used adipose-derived stem cells, bone marrow mesenchymal stem cells, and tail fibroblast cells in their treatment of rats with cyclophosphamide-induced POF. According to their results, rats treated with bone marrow stem cells and tail fibroblasts exhibited significantly greater offspring numbers compared with POF rats left untreated. 18 Similarly, Leia and colleagues reported that skin-derived stem cells have the potential to increase the number of offspring born to mice experiencing busulfan-induced menopause. 19
Finally, we monitored the serum levels of FSH in rats on the first day after receiving their final dose of VCD, as well as 8 weeks after cell transplantation. Neither pre- nor post-transplantation FSH data differed among the groups, which was rather unexpected given that VCD treated rats showed a significant reduction of antral follicles, the only follicle which produces enough estradiol to regulate FSH through a feedback system. The reasons for this lack of change are unclear, but could result from the low number of animals (three) tested per time point, which may not have been sufficient to allow detection of changes in a pulsatile hormone such as FSH. It could also be due to the short experimental period of our study, which lasted only 8 weeks, since it was previously reported that hormonal alterations reach significant values at 240 days post-induction of POF. 31 In another study, however, VCD injection in mice resulted in the elevation of FSH hormone only 37 days after induction, with significant differences compared with the control. 30 Therefore, according to our hormonal and histological findings, it is conceivable that hormonal examination would require a greater number of samples per time point, in addition to longer intervals than the time allotted in the present study. Future studies will be necessary to determine whether FSH should serve as a viable biomarker for this disease model and/or its therapies, since our results suggest that it should not be used to measure the restoration of ovarian tissue following c-Kit+ cells transplantation.
Research Highlights
What is the current knowledge?
√c-Kit+ cells are a fraction of bone marrow cells with regenerative outcomes.
√ The application of this lineage open hopes in the alleviation of different pathologies.
What is new here?
√ The promotion of angiogenesis and reduction of fibrotic changes in POF after injection of c-Kit+ cells.
√ The decrease of follicular atresia with improved fertility upon the injection of c-Kit+ cells
Conclusion
Our findings clearly illustrate the potential of c-Kit+ bone marrow-derived cells modulating angiogenesis signaling, pathological changes, and fertility rate leading to the rejuvenation of ovarian function after exposure to VCD as a rat model for POF and menopause.
Acknowledgments
We appreciate the personnel of Stem Cell Research Center and Drug Applied Research Center for help and guidance. We also thank Ms. Savannah Goldstein for proof-reading the manuscript.
Funding sources
This manuscript was supported by a grant from Tabriz University of Medical Sciences (Grant No. 59694).
Ethical statement
Experimental procedures involving animals were conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publication No. 85-23, revised 1996) and were reviewed and approved by the Animal Ethical Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1396.1197).
Competing interests
The authors declare that they have no competing interests.
Authors’ contribution
SS and SA conducted the experiments, performed the analysis and co- wrote the initial draft of the manuscript. MMA, and RB, participated in the surgical procedures, MS, MN, RR, and AH reviewed and revised the initial draft of the manuscript. MM designed the whole project and conceptualized the manuscript.
Supplementary Materials
Supplementary file 1 contains Fig. S1.
References
- 1.Nelson LM. Nelson LMCllinical practicePrimary ovarian insufficiency. N Engl J Medicine. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. 2010;65:161–6. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen L, Song L, Liu B, Li H, Zheng X, Zhang L, et al. Effects of early age at natural menopause on coronary heart disease and stroke in Chinese women. Int J Cardiol. 2017;241:6–11. doi: 10.1016/j.ijcard.2017.03.127. [DOI] [PubMed] [Google Scholar]
- 4.Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, et al. Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (Lond) 2009;5:39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agaba P, Meloni S, Sule H, Ocheke A, Agaba E, Idoko J, et al. Factors associated with early menopause among women in Nigeria. J Virus Erad. 2017;3:145–51. doi: 10.1016/S2055-6640(20)30333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gracia CR, Sammel MD, Freeman E, Prewitt M, Carlson C, Ray A, et al. Impact of cancer therapies on ovarian reserve. FertilSteril 2012; 97: 134-40.e1. 10.1016/j.fertnstert.2011.10.040 [DOI] [PMC free article] [PubMed]
- 7.Jankowska K. Premature ovarian failure. Przegladmenopauzalny= Menopause review. 2017;16:51. doi: 10.5114/pm.2017.68592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhupathiraju SN, Grodstein F, Stampfer MJ, Willett WC, Hu FB, Manson JE. Exogenous Hormone Use: Oral Contraceptives, Postmenopausal Hormone Therapy, and Health Outcomes in the Nurses' Health Study. Am J Public Health. 2016;106:1631–7. doi: 10.2105/ajph.2016.303349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao T, Del Valle A. Human oocyte and ovarian tissue cryopreservation and its application. J Assist Reprod Genet. 2008;25:287–96. doi: 10.1007/s10815-008-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenfeld Z. GnRH-agonists in fertility preservation. CurrOpin Endocrinol Diabetes Obes. 2008;15:523–8. doi: 10.1097/MED.0b013e32831a46e9. [DOI] [PubMed] [Google Scholar]
- 11.Rienzi LF, Iussig B, Dovere L, Fabozzi G, Cimadomo D, Ubaldi FM. Perspectives in Gamete and Embryo Cryopreservation. Semin Reprod Med. 2018;36:253–64. doi: 10.1055/s-0038-1677463. [DOI] [PubMed] [Google Scholar]
- 12.al-Shawaf T, Dave R, Harper J, Linehan D, Riley P, Craft I. Transfer of embryos into the uterus: how much do technical factors affect pregnancy rates? J Assist Reprod Genet. 1993;10:31–6. doi: 10.1007/BF01204437. [DOI] [PubMed] [Google Scholar]
- 13.Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. FertilSteril. 2003;79:1323–6. doi: 10.1016/s0015-0282(03)00258-9. [DOI] [PubMed] [Google Scholar]
- 14. Kondapalli LA. Ovarian tissue cryopreservation and transplantation. Oncofertility Medical Practice: Springer; 2012. p. 63-75.
- 15.Sheshpari S, Shahnazi M, Mobarak H, Ahmadian S, Bedate AM, Nariman-Saleh-Fam Z, et al. Ovarian function and reproductive outcome after ovarian tissue transplantation: a systematic review. J Transl Med. 2019;17:396. doi: 10.1186/s12967-019-02149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadoul P, Dolmans M-M, Donnez J. Fertility preservation in girls during childhood: is it feasible, efficient and safe and to whom should it be proposed? Hum Reprod Update. 2010;16:617–30. doi: 10.1093/humupd/dmq010. [DOI] [PubMed] [Google Scholar]
- 17.Zhu SF, Hu HB, Xu HY, Fu XF, Peng DX, Su WY, et al. Human umbilical cord mesenchymal stem cell transplantation restores damaged ovaries. J Cell Mol Med. 2015;19:2108–17. doi: 10.1111/jcmm.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, Sano C, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93:181–93. doi: 10.1038/labinvest.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai D, Wang F, Dong Z, Zhang Q. Skin-derived mesenchymal stem cells help restore function to ovaries in a premature ovarian failure mouse model. PLoS One. 2014;9:e98749. doi: 10.1371/journal.pone.0098749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao R, Xu P, Wang Y, Wang J, Xiao L, Li G, et al. Bone marrow derived mesenchymal stem cells transplantation rescues premature ovarian insufficiency induced by chemotherapy. Gynecol Endocrinol. 2018;34:320–6. doi: 10.1080/09513590.2017.1393661. [DOI] [PubMed] [Google Scholar]
- 21.Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. doi: 10.1186/s12967-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 23.Gagari E, Rand MK, Tayari L, Vastardis H, Sharma P, Hauschka PV, et al. Expression of stem cell factor and its receptor, c‐kit, in human oral mesenchymal cells. European journal of oral sciences. 2006;114:409–15. doi: 10.1111/j.1600-0722.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim JY, Choi JS, Song SH, Im JE, Kim JM, Kim K, et al. Stem cell factor is a potent endothelial permeability factor. ArteriosclerThrombVasc Biol. 2014;34:1459–67. doi: 10.1161/atvbaha.114.303575. [DOI] [PubMed] [Google Scholar]
- 25.Matluobi D, Araghi A, Maragheh BFA, Rezabakhsh A, Soltani S, Khaksar M, et al. Carvacrol promotes angiogenic paracrine potential and endothelial differentiation of human mesenchymal stem cells at low concentrations. Microvascular Research. 2018;115:20–7. doi: 10.1016/j.mvr.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Escribano L, Ocqueteaub M, Almeida J, Orfao A, Migue JFS. Expression of the c-kit (CD117) molecule in normal and malignant hematopoiesis. Leuk Lymphoma. 1998;30:459–66. doi: 10.3109/10428199809057558. [DOI] [PubMed] [Google Scholar]
- 27.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–64. [PubMed] [Google Scholar]
- 28.Ahmadian S, Sheshpari S, Pazhang M, Bedate AM, Beheshti R, Abbasi MM, et al. Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reproductive Biology and Endocrinology. 2020;18:78. doi: 10.1186/s12958-020-00638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Pelt LF. Ketamine and xylazine for surgical anesthesia in rats. J Am Vet Med Assoc. 1977;171:842–4. [PubMed] [Google Scholar]
- 30.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. The follicle-deplete mouse ovary produces androgen. Biol Reprod. 2004;71:130–8. doi: 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 31.Kappeler CJ, Hoyer PB. 4-vinylcyclohexene diepoxide: a model chemical for ovotoxicity. Syst Biol Reprod Med. 2012;58:57–62. doi: 10.3109/19396368.2011.648820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmadian S, Sheshpari S, Mahdipour M, Pazhang M, Tsai PJ, Nouri M, et al. Toxic effects of VCD on kidneys and liver tissues: a histopathological and biochemical study. BMC Res Notes. 2019;12:446. doi: 10.1186/s13104-019-4490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol. 1996;139:394–401. doi: 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Wang LY, Xing XX, Fan GW. Conditions and possible mechanisms of VCD-induced ovarian failure. Altern Lab Anim. 2015;43:385–92. doi: 10.1177/026119291504300606. [DOI] [PubMed] [Google Scholar]
- 35.Laviolette LA, Ethier JF, Senterman MK, Devine PJ, Vanderhyden BC. Induction of a menopausal state alters the growth and histology of ovarian tumors in a mouse model of ovarian cancer. Menopause. 2011;18:549–57. doi: 10.1097/gme.0b013e3181fca1b6. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu K, Oku N. Cancer anti-angiogenic therapy. Biol Pharm Bull. 2004;27:599–605. doi: 10.1248/bpb.27.599. [DOI] [PubMed] [Google Scholar]
- 37.Roozbahani M, Jamshidian H, Mahmoudi E, Arshi A. Angiogenesis: A Review of Molecular Mechanism. The Scientific Journal of Iranian Blood Transfusion Organization. 2018;15:59–70. [Google Scholar]
- 38.Johnson J, Bagley J, Skaznik-Wikiel M, Lee H-J, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122:303–15. doi: 10.1016/j.cell.2005.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1 contains Fig. S1.