Abstract
Background
Etoposide plus cisplatin (EP) combined with concurrent accelerated hyperfractionated thoracic radiotherapy (AHTRT) is the standard treatment strategy for unresectable limited‐disease (LD) small cell lung cancer (SCLC), which has remained unchanged for over two decades. Based on a previous study that confirmed the non‐inferiority of amrubicin (AMR) plus cisplatin (AP) when compared with EP for extensive‐disease (ED) SCLC, we have previously conducted a phase I study assessing AP with concurrent TRT (2 Gy/time, once daily, 50 Gy in total) for LD‐SCLC therapy. Our findings revealed that AP with concurrent TRT could prolong overall survival to 39.5 months with manageable toxicities. Therefore, we plan to conduct a phase I study to investigate and determine the effect of AP combined with AHTRT, recommended dose (RD), maximum tolerated dose (MTD), and dose‐limiting toxicity (DLT) of AP in patients with LD‐SCLC.
Methods
Treatment‐naive patients with LD‐SCLC, age between 20 and 75 years, who had a performance status of 0 or 1 and adequate organ functions will be enrolled. For chemotherapy, cisplatin 60 mg/m2/day (day 1) and AMR (day 1 to 3) will be administered with AHTRT (1.5 Gy/time, twice daily, 45 Gy in total). The initial AMR dose is set to 25 mg/m2/day. RD and MTD will be determined by evaluating toxicities.
Discussion
Based on our previous study, the initial dose of AMR 25 mg/m2 is expected to be tolerated and acceptable. Here, we aim to determine whether treatment with AP and concurrent AHTRT would be an optimal choice with manageable toxicities for LD‐SCLC.
Keywords: amrubicin, chemotherapy, radiotherapy, small cell lung cancer
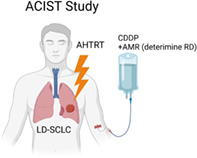
Schema of ACIST study; phase I study to evaluate the efficacy and toxicity of CDDP + AMR + AHTRT for the patients with LD‐SCLC.
INTRODUCTION
Small cell lung cancer (SCLC) accounts for ~13% of all lung cancers and is characterized by rapid growth, early metastasis, and high rates of relapse. 1 Approximately one‐third of patients with SCLC are diagnosed with limited‐disease (LD)‐SCLC, and the treatment for patients with LD‐SCLC involves concurrent chemotherapy and radiotherapy, as <5% of these patients are suitable to undergo radical surgery. 2 , 3 Etoposide plus cisplatin (EP) combined with concurrent accelerated hyperfractionated thoracic radiotherapy (AHTRT) is considered the standard treatment for unresectable LD‐SCLC 4 , 5 , 6 ; however, a high proportion of patients with LD‐SCLC experience disease relapse, accompanied by an insufficient long‐term survival rate. Accordingly, novel strategies are needed to achieve long‐term response and cure in patients with LD‐SCLC.
Amrubicin (AMR), or its active metabolite amurubicinol, exerts an antitumor effect by stabilizing the cleavable complex via topoisomerase II, which results in DNA cleavage and suppresses tumor growth. 7 In treatment‐naive patients with extensive‐disease (ED)‐SCLC, AMR monotherapy has shown a remarkable improvement in overall response rate (ORR) (75.8%) and median overall survival (OS) (11.7 months). 8 In addition, a clinical study has revealed the non‐inferiority of AMR plus cisplatin (AP) when compared with EP for treatment‐naive patients with ED‐SCLC in a Chinese population. 9 These findings suggest the promising antitumor effect of AMR in treatment‐naive ED‐SCLC, which could also be used in LD‐SCLC. We previously conducted a phase I study to assess AP with concurrent TRT (2 Gy/time, once daily, 50 Gy in total) in patients with LD‐SCLC. 10 Although the obtained results were from a limited patient cohort in a phase I study, AP with concurrent TRT markedly improved OS to 39.5 months. Dose‐limiting toxicities (DLTs) included neutropenia and leukopenia; however, all toxicities were manageable. Although this study indicated the promising results of AP with once‐daily concurrent TRT, no patient could achieve complete response, suggesting that further study will be required to “cure” the patients with LD‐SCLC.
Twice‐daily accelerated hyperfractionated irradiation (ie, 1.5 Gy/time, 5 days a week, 45 Gy in total) could significantly improve OS when compared with once‐daily irradiation (ie, 1.8 Gy/time, once daily, 5 days a week, 45 Gy in total) 4 ; hence, the twice‐daily irradiation schedule could be tolerated and potentially prolong survival when combined with AP. Based on the previous studies, we plan to conduct a phase I study of AP combined with AHTRT (1.5 Gy/time, twice daily, 45 Gy in total) and aim to investigate and determine the effect, recommended dose (RD), maximum tolerated dose (MTD), and DLT of AP in patients with LD‐SCLC.
METHODS
Study design
This is a phase I, multi‐institutional, single‐arm, interventional study. The study design is shown in Figure 1. We initially plan to enroll three patients and evaluate the emergence of AMR‐related DLTs during the first cycle; the AMR dose is set at 25 mg/m2 for the first cycle of chemotherapy. DLTs will be confirmed if any of the following events occur: (i) grade 4 neutropenia or leukopenia for more than 4 days; (ii) grade 4 neutropenia or leukopenia with a fever of >38°C, suggesting an infection; (iii) grade 4 thrombocytopenia; and (iv) grade 3 or higher non‐hematologic toxicities. Adverse events will be assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
FIGURE 1.
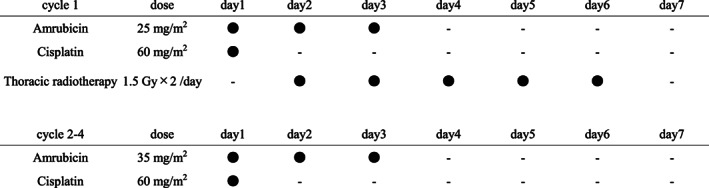
In the first cycle, AMR 25 (or 20, if reduced) mg/m2/day (from day 1 to day 3) and cisplatin 60 mg/m2/day (day 1) will be administered. In the following three cycles, AMR 35 mg/m2/day (from day 1 to day 3) and cisplatin 60 mg/m2/day (day 1) will be administered. The accelerated hyperfractionated thoracic radiotherapy, as 1.5 Gy/time, twice daily, 5 days a week, 45 Gy in total, will be administered concurrently from day 2 of the first chemotherapy cycle
MTD and RD will be determined as shown in Figure 2. (1) If DLTs are not observed in the initial three patients, we plan to add six patients as the extension study. The 25 mg/m2 of AMR will be determined as MTD and RD. (2) If DLTs are confirmed in one of the first three patients enrolled, we will add three additional patients at 25 mg/m2 of AMR to evaluate DLTs further. (2)‐1: If DLTs are confirmed in none of the additional three patients, we will add six additional patients as the extension study. The 25 mg/m2 of AMR will be determined as MTD and RD. (2)‐2: If DLTs are confirmed in more than one of the additional three patients, the study will be finished. The 25 mg/m2 of AMR will be determined as MTD and the 20 mg/m2 of AMR will be determined as RD. (3) If DLTs are confirmed in more than two of the first three patients, we plan to add three patients to receive a reduced dose of 20 mg/m2. (3)‐1: If DLTs are confirmed in none of the additional three patients, the 20 mg/m2 of AMR will be determined as MTD and RD. (3)‐2: If DLTs are confirmed in more than one of the additional three patients, the study will be considered to terminate.
FIGURE 2.
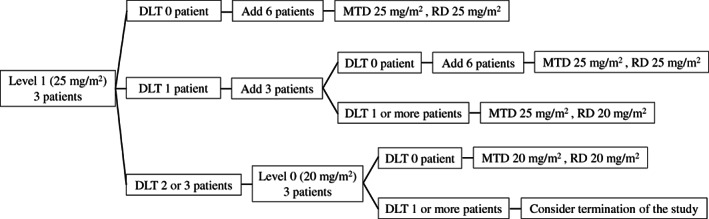
The schema of the method to determine RD and MTD in ACIST study. If DLTs are not observed in the initial three patients, we plan to add six patients. The 25 mg/m2 of AMR will be determined as MTD and RD. If DLTs are confirmed in one of the first three patients enrolled, we will add three additional patients at 25 mg/m2. If DLTs are confirmed in none of the additional three patients, we will add six additional patients. The 25 mg/m2 of AMR will be determined as MTD and RD. If DLTs are confirmed in more than one of the additional three patients, the 25 mg/m2 of AMR will be determined as MTD and the 20 mg/ml of AMR will be determined as RD. If DLTs are confirmed in more than two of the first three patients, we plan to add three patients to receive a reduced dose of 20 mg/m2. If DLTs are confirmed in none of the additional three patients, the 20 mg/m2 of AMR will be determined as MTD and RD. If DLTs are confirmed in more than one of the additional three patients, the study will be considered to terminate
All patients enrolled in the study will provide written informed consent, and the study will be conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocol was reviewed and approved by the clinical review board of Nagasaki University (CRB7180001). This study was registered in the Japan Registry of Clinical Trials (no. jRCT1071200033) on September 30, 2020.
Inclusion criteria
(i) Histologically or cytologically confirmed SCLC (combined SCLC is acceptable); (ii) LD; (iii) age between 20 to 75 years; (iv) Eastern Cooperative Oncology Group Performance Status of 0 or 1 (v) regardless of measurable lesions; (vi) no prior chemotherapy; (vii) no multiple active cancers; (viii) latest clinical laboratory test within 14 days before registration meets the following criteria: (a) neutrophil count ≥1500 /μL, (b) platelet count ≥1.0 × 105 /μL, (c) total‐bilirubin ≤1.5 mg/dL, (d) aspartate aminotransferase ≤100 U/L and alanine aminotransferase ≤100 U/L, (e) serum creatine ≤1.2 mg/dL, (f) SpO2 ≥ 92% (room air); (ix) radiologists expect sufficient preservation of respiratory function after chemoradiotherapy; and (x) written informed consent.
LD is defined as lesions limited to the ipsilateral thorax, contralateral mediastinum, and supraclavicular lymph nodes without malignant pleural or pericardial effusions.
Exclusion criteria
(i) Resectable stage IA; (ii) presence of symptoms because of pericardial effusion; (iii) urgent radiation therapy is necessary because of superior vena cava syndrome; (iv) persistence of heart disease; (v) uncontrolled diabetes; (vi) uncontrolled hypertension; (vii) active systemic infection necessitating treatment; (viii) pregnant, possibly pregnant, postpartum within 28 days, breastfeeding, and males considering children; (ix) fever of 38.0°C; (x) mental disorder or symptoms interfering with daily life and inappropriate for enrollment; (xi) continuous intravenous or oral administration of steroids or immunosuppressants; (xii) hepatitis B surface antigen‐positive or hepatitis C virus antibody‐positive; (xiii) human immunodeficiency virus antibody‐positive (not necessarily examined); (xiv) interstitial pneumonia, pulmonary fibrosis, or emphysema confirmed by computed tomography; and (xv) other inappropriate complications.
Interventions
Chemotherapy
In the first cycle, AMR 25 (or 20, if reduced) mg/m2/day (from day 1 to day 3) and cisplatin 60 mg/m2/day (day 1) will be administered. In the following three cycles, AMR 35 mg/m2/day (from day 1 to day 3) and cisplatin 60 mg/m2/day (day 1) will be administered (Figure 1).
Radiation therapy
AHTRT, as 1.5 Gy/time, twice daily, 5 days a week, 45 Gy in total, will be administered concurrently from day 2 of the first chemotherapy cycle (Figure 1). In addition to the three‐dimensional conformal radiation therapy, intensity‐modulated radiation therapy is acceptable. Calculating the actual value of V20 (volume of normal lung irradiated over 20 Gy compared to all lung volumes) or proportion of irradiation field for one lung is unnecessary before registration, and estimation by chest X‐ray or computed tomography is acceptable. To determine whether the desired respiratory function is preserved after radiotherapy, the target criterion of V20 is under 30%.
Endpoints
The primary endpoint of the present study is to determine RD, and secondary endpoints are evaluating the response rate (RR), OS, progression‐free survival (PFS), MTD, DLTs and adverse effects. Efficacy will be assessed according to the Response Evaluation Criteria in Solid Tumors ver.1.1. PFS is defined as the time from the start date of chemotherapy to disease progression or death from any cause, whereas OS is defined as the time from the start date of chemotherapy to the last day confirmed to be alive or dead from any cause.
The Kaplan–Meier method will be used to estimate PFS and OS. Survival analysis will be conducted at the end of the follow‐up period. STATA SE version 16.1 (Stata Corp) will be used to perform analyses.
Monitoring and follow‐up
Patients will be monitored twice yearly to ensure the accuracy and quality of the study. The principal investigator is responsible for compliance with the study protocol. The first three patients will be carefully monitored to determine whether the treatment was precisely conducted, data were collected, and serious nonconformities or adverse events occurred. Efficacy and toxicity will be followed up until the end of the follow‐up period.
DISCUSSION
Several clinical trials have demonstrated the effects and toxicities of EP combined with AHTRT, exhibiting a median PFS between 15.4 and 18.8 months, median OS between 23.0 and 30.0 months, and manageable toxicities. 4 , 5 , 6 , 11 Although EP has been the standard regimen for patients with LD‐SCLC for two decades, novel therapeutic options are needed to further improve patient prognosis.
Regarding the combination of drugs other than EP with AHTRT, a phase III clinical study has compared EP and irinotecan plus cisplatin (IP) after induction of EP with AHTRT as consolidation therapy; however, three cycles of IP failed to improve OS when compared with three cycles of EP after one cycle of EP with concurrent AHTRT. 12
In another study examining the consolidation of three cycles of AP after one cycle of EP with AHTRT, the authors documented an impressive 5‐year OS rate of 57.8%, suggesting the therapeutic potential of AP combined with AHTRT. The incidence of grade 3/4 neutropenia was 100%, whereas that of febrile neutropenia was 43%; however, all observed adverse effects were manageable with granulocyte‐colony stimulating factor support. 13 It should be noted that the dose of AMR was set at 40 mg/m2 according to the RD from the phase I‐II study of AP for treatment‐naive ED‐SCLC, 14 however, dose reduction was required in 33% of patients, implying that dose of 40 mg/m2 AMR might be too toxic for the patients with LD‐SCLC who will receive AP combined with AHTRT. Considering the toxicities associated with AMR, the RD of AMR with once‐daily TRT was evaluated from 20 mg/m2 in a dose‐escalation manner, and the RD of AMR was determined as 25 mg/m2 in our previous study. 7 Following the introduction of twice‐daily irradiation, we plan to undertake a dose de‐escalation approach from 25 mg/m2 to determine the RD of AMR with twice‐daily AHTRT, based on our previous phase I study, which showed that doses of 25 mg/m2 AMR and 60 mg/m2 cisplatin are recommended for AP combined with TRT (2 Gy/day, once‐daily). 10 We also set to raise the dose of AMR to 35 mg/m2 from second to fourth cycles of AP because AHTRT will finish during the first cycle of AP in this protocol. In a phase III study, which compared the efficacy of AP and that of IP in treatment naive Japanese patients with ED‐SCLC, the dose of AMR was reduced from 40 mg/m2 to 35 mg/m2 because of the high incidence of severe hematological toxicities. 15 Furthermore, our retrospective study revealed that the real‐world incidence rate of febrile neutropenia among patients with thoracic malignancies that were treated with single‐agent AMR chemotherapy was 30%. 16 Based on these findings for Japanese patients, the dose of 35 mg/m2 AMR from second cycle to fourth is predicted as the suitable dose in this protocol.
Sequential treatment with AMR or amurubicinol before ionizing radiation induces an additive radio‐enhancement effect in vitro. 17 In addition, fractionation of a customary once‐daily dose into two treatments each day affords biological advantages, along with a high antitumor effect, resulting in the same total doses. 3 The phase III CONVERT trial failed to demonstrate a survival advantage with high‐dose TRT when compared with standard twice‐daily TRT. 5 Based on these findings, AP combined with twice‐daily AHTRT is expected to be a potential candidate for optimal treatment of LD‐SCLC.
Immunotherapy using immune checkpoint inhibitors (ICIs), such as programmed cell death 1 (PD‐1), programmed death‐ligand 1 (PD‐L1), and cytotoxic T‐lymphocyte‐associated protein 4, either as single agents or combined with cytotoxic chemotherapy, has revolutionized the treatment of several solid tumors, including SCLC. 18 A recent study has shown that the addition of an anti‐PD‐1 antibody, pembrolizumab, to AMR for relapsed SCLC could afford a remarkable ORR of 52% and PFS rate of 14.4% at one year, 19 indicating the promising effect of AMR when combined with ICIs. For some patients with LD‐SCLC who would benefit from ICIs, a combination with AP may be an optimal strategy in the future.
In summary, the present study is the first to evaluate AP combined with AHTRT for LD‐SCLC from the first chemotherapy cycle. This study will reveal whether AP combined with twice‐daily AHTRT could be tolerated and is an optimal regimen for LD‐SCLC.
REGISTRATION DETAILS
This study was registered in the Japan Registry of Clinical Trials (no. jRCT1071200033) on September 30, 2020.
AUTHOR CONTRIBUTIONS
All authors had full access to the protocol of the study and take responsibility for the integrity of the protocol. Conceptualization, M.F. Investigation, K.A., H.T. (Taniguchi), M.F., T.Y., H.T. (Tomono), S.O., T.S, M.S., H.G, S.T., H.Y., D.Y., H.S. (Senju), H.S. (Soda), T.M., Y.M., T.I., S.N., Y.T, D.N., K.K., K.N., E.S., K.H. Methodology, M. F. Project Administration, H.T. (Taniguchi) and M. F. Supervision, H. M. Visualization, K. A. Writing‐ Original Draft Preparation, K. A. and H.T. (Taniguchi). Writing‐ Review and editing, H.T. (Taniguchi), M.F., T.Y., H.T. (Tomono), S.O., T.S, M.S., H.G, S.T., H.Y., D.Y., H.S. (Senju), H.S. (Soda), T.M., Y.M., T.I., S.N., D.N., K.K., K.N., E.S., K.H., and H.M.
DISCLOSURE
All the authors declare no potential conflicts of interest related to this work.
ACKNOWLEDGMENT
We are grateful to all patients, oncologists, allied healthcare professionals, research concierges, and research assistants who participated in this study.
Akagi K, Taniguchi H, Fukuda M, Yamazaki T, Ono S, Tomono H, et al. Phase I study of amrubicin plus cisplatin and concurrent accelerated hyperfractionated thoracic radiotherapy for limited‐disease small cell lung cancer: protocol of ACIST study. Thorac Cancer. 2022;13(16):2404–2409. 10.1111/1759-7714.14555
REFERENCES
- 1. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The effect of advances in lung‐cancer treatment on population mortality. N Engl J Med. 2020;383:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhong L, Suo J, Wang Y, Han J, Zhou H, Wei H, et al. Prognosis of limited‐stage small cell lung cancer with comprehensive treatment including radical resection. World J Surg Onc. 2020;18:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. [DOI] [PubMed] [Google Scholar]
- 4. Turrisi AT 3rd, Kim K, Blum R, et al. Twice‐daily compared with once‐daily thoracic radiotherapy in limited small‐cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;28(340):265–71. [DOI] [PubMed] [Google Scholar]
- 5. Faivre‐Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once‐daily versus twice‐daily chemoradiotherapy in patients with limited‐stage small‐cell lung cancer (CONVERT): an open‐label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited‐stage small‐cell lung cancer: results of the Japan clinical oncology group study 9104. J Clin Oncol. 2002;20:3054–60. [DOI] [PubMed] [Google Scholar]
- 7. Hanada M, Mizuno S, Fukushima A, Saito Y, Noguchi T, Yamaoka T. A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II‐DNA complex. Jpn J Cancer Res. 1998;89:1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yana T, Negoro S, Takada M, Yokota S, Takada Y, Sugiura T, et al. Phase II study of amrubicin in previously untreated patients with extensive‐disease small cell lung cancer: West Japan thoracic oncology group (WJTOG) study. Invest New Drugs. 2007;25:253–8. [DOI] [PubMed] [Google Scholar]
- 9. Sun Y, Cheng Y, Hao X, Wang J, Hu C, Han B, et al. Randomized phase III trial of amrubicin/cisplatin versus etoposide/cisplatin as first‐line treatment for extensive small‐cell lung cancer. BMC Cancer. 2016;16:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shimada M, Yamaguchi H, Fukuda M, Tomono H, Honda N, Dotsu Y, et al. Dose escalation study of amrubicin and cisplatin with concurrent thoracic radiotherapy for limited‐disease small cell lung cancer. Cancer Chemother Pharmacol. 2019;84:1059–64. [DOI] [PubMed] [Google Scholar]
- 11. Bonner JA, Sloan JA, Shanahan TG, Brooks BJ, Marks RS, Krook JE, et al. Phase III comparison of twice‐daily split‐course irradiation versus once‐daily irradiation for patients with limited stage small‐cell lung carcinoma. J Clin Oncol. 1999;17:2681–91. [DOI] [PubMed] [Google Scholar]
- 12. Kubota K, Hida T, Ishikura S, Mizusawa J, Nishio M, Kawahara M, et al. Etoposide and cisplatin versus irinotecan and cisplatin in patients with limited‐stage small‐cell lung cancer treated with etoposide and cisplatin plus concurrent accelerated hyperfractionated thoracic radiotherapy (JCOG0202): a randomised phase 3 study. Lancet Oncol. 2014;15:106–13. [DOI] [PubMed] [Google Scholar]
- 13. Sekine I, Sumi M, Satouchi M, Tsujino K, Nishio M, Kozuka T, et al. Feasibility study of chemoradiotherapy followed by amrubicin and cisplatin for limited‐disease small cell lung cancer. Cancer Sci. 2016;107:315–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohe Y, Negoro S, Matsui K, Nakagawa K, Sugiura T, Takada Y, et al. Phase I‐II study of amrubicin and cisplatin in previously untreated patients with extensive‐stage small‐cell lung cancer. Ann Oncol. 2005;16:430–6. [DOI] [PubMed] [Google Scholar]
- 15. Satouchi M, Kotani Y, Shibata T, Ando M, Nakagawa K, Yamamoto N, et al. Phase III study comparing amrubicin plus cisplatin with irinotecan plus cisplatin in the treatment of extensive‐disease small‐cell lung cancer: JCOG 0509. J Clin Oncol. 2014;32:1262–8. [DOI] [PubMed] [Google Scholar]
- 16. Dotsu Y, Yamaguchi H, Fukuda M, Suyama T, Honda N, Umeyama Y, et al. Real‐world incidence of febrile neutropenia among patients treated with single‐agent Amrubicin: necessity of the primary prophylactic Administration of Granulocyte Colony‐Stimulating Factor. J Clin Med. 2021;10:4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayashi S, Hatashita M, Matsumoto H, Shioura H, Kitai R, Kano E. Enhancement of radiosensitivity by topoisomerase II inhibitor, amrubicin and amrubicinol, in human lung adenocarcinoma A549 cells and kinetics of apoptosis and necrosis induction. Int J Mol Med. 2006;18:909–15. [PubMed] [Google Scholar]
- 18. Taniguchi H, Sen T, Rudin CM. Targeted therapies and biomarkers in small cell lung cancer. Front Oncol. 2020;10:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akamatsu H, Teraoka S, Hayashi H, Fujimoto D, Hayata A, Haratani K, et al. Pembrolizumab plus Amrubicin in patients with relapsed SCLC: multi‐institutional, single‐arm phase 2 study. JTO Clin Res Rep. 2021;2:100184. 10.1016/j.jtocrr.2021.100184 [DOI] [PMC free article] [PubMed] [Google Scholar]


