Abstract
It has been shown previously that inactivation of the cshA gene, encoding a major cell surface polypeptide (259 kDa) in the oral bacterium Streptococcus gordonii, generates mutants that are markedly reduced in hydrophobicity, deficient in binding to oral Actinomyces species and to human fibronectin, and unable to colonize the oral cavities of mice. We now show further that surface fibrils 60.7 ± 14.5 nm long, which are present on wild-type S. gordonii DL1 (Challis) cells, bind CshA-specific antibodies and are absent from the cell surfaces of cshA mutants. To more precisely determine the structural and functional properties of CshA, already inferred from insertional-mutagenesis experiments, we have cloned the entire cshA gene into the replicative plasmid pAM401 and expressed full-length CshA polypeptide on the cell surface of heterologous Enterococcus faecalis JH2-2. Enterococci expressing CshA exhibited a 30-fold increase in cell surface hydrophobicity over E. faecalis JH2-2 carrying the pAM401 vector alone and 2.4-fold-increased adhesion to human fibronectin. CshA expression in E. faecalis also promoted cell-cell aggregation and increased the ability of enterococci to bind Actinomyces naeslundii cells. Electron micrographs of negatively stained E. faecalis cells expressing CshA showed peritrichous surface fibrils 70.3 ± 9.1 nm long that were absent from control E. faecalis JH2-2(pAM401) cells. The fibrils bound CshA-specific antibodies, as detected by immunoelectron microscopy, and the antibodies inhibited the adhesion of E. faecalis cells to fibronectin. The results demonstrate that the CshA polypeptide is the structural and functional component of S. gordonii adhesive fibrils, and they provide a molecular basis for past correlations of surface fibril production, cell surface hydrophobicity, and adhesion in species of oral “sanguis-like” streptococci.
Streptococci are found at most sites in the oral cavity (9) and are among the predominant bacteria in early dental plaque (34). The growth and survival of streptococci within the oral cavity are dependent, at least in part, on the adhesion of bacteria to oral surfaces coated with salivary proteins and glycoproteins, to host epithelial cells, and to other adherent bacteria such as Actinomyces species (24). The list of identified streptococcal cell surface molecules that serve as adhesins continues to grow (24), which emphasizes both the multiplicity of bacterium-host interactions and the molecular complexity of the streptococcal cell surface.
Several species of oral streptococci elaborate cell surface structures such as fibrils and fimbriae (12). Most strains of Streptococcus gordonii and Streptococcus sanguis produce fibrils between 50 and 80 nm long (12, 43). Fibrils are nearly always peritrichous and vary from being sparsely to densely distributed according to strain (12). Some strains of Streptococcus oralis and S. sanguis also produce tufts of fibrils (12, 20), which may appear as longer (up to 200-nm) fibrils projecting through a fringe of shorter fibrils (12). The presence of fibrillar structures on the surfaces of S. gordonii and S. sanguis has been linked to their cell surface hydrophobicities (8, 10), abilities to coaggregate with other oral bacteria (12), and adhesion to saliva-coated hydroxylapatite (5, 10, 14, 33). However, it has proved difficult to ascribe adhesive functions to specific surface structures on “sanguis-like” streptococci, and the protein components of these adhesive fibrils remain uncharacterized.
In Streptococcus salivarius HB, four distinct classes of fibrils that differ in length and composition are present (40). Two of these fibril classes have been isolated from cells, and their structural and adhesive properties have been investigated. Short fibrils, 91 nm long, are comprised of a 320-kDa polypeptide designated antigen B (AgB) and mediate interbacterial coaggregation with Veillonella species and other gram-negative oral bacteria. Shorter fibrils, 72 nm long, contain a glycoprotein with an apparent molecular mass of 220 to 280 kDa and are involved in the adhesion of bacteria to host surfaces (40, 41). To investigate the structural and functional properties of long fibrils present in tufts on a related bacterium, S. oralis CN3410, Jameson et al. (20) identified several fibril protein components with molecular masses ranging from 260 to 290 kDa. However, no evidence was obtained to demonstrate that these proteins had an adhesive function, suggesting that the structural components of the long fibrils may not themselves contain the adhesive epitopes (20).
Recently, genes that encode proteins believed to be intimately linked with the production of surface structures have been identified in Streptococcus parasanguis and S. gordonii. Expression of fap1, encoding a polypeptide with a molecular mass of approximately 200 kDa, is required for the production of surface fimbriae in S. parasanguis FW213 (46). Afimbrial isogenic fap1 mutants are deficient in adhesion to saliva-coated hydroxylapatite but are unaffected in cell surface hydrophobicity (46). In S. gordonii, expression of the cshA gene, encoding a 259-kDa wall-anchored polypeptide, is associated with the presence of surface fibrillar material (28). Isogenic cshA mutants are deficient in binding to human fibronectin (31) and also to Actinomyces naeslundii and S. oralis cells (31, 32), although the nature of the receptor(s) on these cells has not yet been determined. In contrast to the observations for isogenic fap1 mutants of S. parasanguis, cshA mutants of S. gordonii show much-reduced surface hydrophobicity, while their ability to adhere to saliva-coated hydroxylapatite is unaltered (31, 32). It seems likely that fap1 and cshA genes are unrelated, based on sequence comparisons and on the observations that CshA antibodies do not react with cells or cell extracts of S. parasanguis (30).
Previous evidence therefore has implicated CshA as a surface protein associated with fibril production in S. gordonii. To confirm a structural role for the CshA polypeptide in fibrils, and to better characterize the functional properties of the polypeptide, we have expressed CshA on the surfaces of Enterococcus faecalis cells. In this article we show that the CshA polypeptide assembles to form fibrils on the enterococcal cell surface. These fibrils are morphologically identical to S. gordonii surface fibrils, and they confer on E. faecalis the hydrophobic properties and adhesive functions previously attributed to CshA based on gene inactivation and antibody inhibition experiments in S. gordonii.
MATERIALS AND METHODS
Bacteria and media.
The bacterial strains and plasmids used are listed in Table 1. Streptococci, enterococci, and A. naeslundii T14V were grown at 37°C on Trypticase soy broth-yeast extract (TSBY) agar (25) in a GasPak System (BBL Microbiology Systems, Cockeysville, Md.). Liquid cultures were grown without shaking in screw-cap tubes or bottles at 37°C in TSBY or tryptone-yeast extract (TY)-glucose medium (25). Escherichia coli HB101 (1) was grown aerobically at 37°C in Luria-Bertani (LB) medium (37) or on LB medium supplemented with 15 g of agar per liter. Erythromycin (1 μg/ml, for S. gordonii) or chloramphenicol (5 μg/ml for S. gordonii or E. faecalis; 20 μg/ml for E. coli) was incorporated into the growth medium where required.
TABLE 1.
Streptococcal and enterococcal strains and plasmids used
| Plasmid or bacterial strain | Genotype and/or phenotype | Reference or source |
|---|---|---|
| Plasmids | ||
| pAM401 | 10.4 kb; Tcr Cmr p15Aori pAMβ-1ori | 45 |
| pAMCshA | 18.5-kb pAM401 carrying cshA | This study |
| Strains | ||
| S. gordonii | ||
| DL1 (Challis) | Wild type | 36 |
| OB235 | cshA3::ermAM | 32 |
| OB271 | cshB2::ermAM | 32 |
| OB277 | cshA31::cat cshB2::ermAM | 32 |
| E. faecalis | ||
| JH2-2 | Wild type | 19 |
| OB513 | Strain JH2-2 harboring pAM401 | This study |
| OB516 | Strain JH2-2 harboring pAMCshA | This study |
DNA manipulations.
Routine molecular biology techniques were performed as specified by Sambrook et al. (37). Plasmid DNA was isolated, by using a commercial kit (Qiagen, Inc., Chatsworth, Calif.), from spheroplasts of E. faecalis generated by digestion of cell walls with mutanolysin (4) and lysozyme (0.2 mg/ml) in an osmotically supported buffer (4). Chromosomal DNA was isolated from S. gordonii as described previously (21). The generation of electrocompetent E. faecalis cells, and their transformation with plasmid DNA, was performed by the method of Cruz-Rodz and Gilmore (2). Briefly, enterococcal cells were grown at 37°C for 18 h in M17 medium (Difco Laboratories, Detroit, Mich.) containing 0.5 M sucrose and 5% (wt/vol) glycine. Bacteria were harvested, washed, and suspended in electroporation buffer (1 ml) as described previously (2). Portions (0.04 ml) of the cell suspension were subjected to an electric pulse (peak voltage, 2.5 kV; capacitance, 25 μF; resistance, 200 Ω) with up to 0.1 μg of DNA. Following incubation at 37°C for 2 h, recovered cells were plated onto SR agar (2) containing chloramphenicol, and plates were incubated at 37°C for 2 days. Restriction and modifying enzymes (from New England Biolabs Inc., Beverly, Mass.) were used under the conditions recommended by the manufacturer.
PCR amplification.
The entire cshA gene and upstream sequences were amplified by PCR with synthetic oligonucleotides (DNA Express, Colorado State University, Fort Collins, Colo.) derived from the nucleotide sequence of cshA (GenBank accession no. X65164). To facilitate cloning of the cshA PCR product into pAM401, the nucleotide sequence at the 5′ end of each primer was modified to introduce a unique restriction site (underlined below) not present in the amplified DNA. The primer pair that comprised SMAP1 (nucleotides 240 to 266, cshA locus), 5′CTGCCCGGGATCGTGACTATCTATTTG, and CTERM2 (complementary to nucleotides 8298 to 8324), 5′TTGTCTAGAATACAGGACAGAAAACCC, generated a product of 8,084 bp following PCR with Taq and Pwo polymerases (Expand High Fidelity System; Boehringer, Mannheim, Germany) under the following cycle conditions: 94°C for 2 min (1 cycle); 94°C for 15 s, 64°C for 30 s, and 68°C for 8 min (10 cycles); 94°C for 15 s, 64°C for 30 s, and 68°C for 8 min, plus 20 s of ramping per cycle (20 cycles); and 68°C for 20 min (1 cycle). The PCR product was purified by using a Qiaquick PCR purification kit (Qiagen), digested with SmaI and XbaI, and ligated with the compatible ends of pAM401 produced by digestion with a combination of EcoRV and XbaI.
Electron microscopy.
Early-stationary-phase cells from cultures in TSBY medium were harvested by centrifugation (10,000 × g, 2 min) and washed twice in sodium cacodylate buffer (0.2 M [pH 7.3]). Cells were fixed and stained with ruthenium red (RR) and osmium tetroxide as described elsewhere (13). Samples were then embedded in Procure 812 resin, and sections were cut on a Reichert-Jung Ultracut E microtome and examined by using an Akashi EM-002A transmission electron microscope (TEM). For negative staining, bacterial cells were harvested by centrifugation as before, washed three times by suspension in distilled water and centrifugation, and suspended in 1/100 volume of distilled water. A drop of bacterial-cell suspension was applied to a Formvar-coated copper grid (400 mesh; Agar Scientific, Stansted, United Kingdom) that had been carbon coated in a coating unit (Nanotech, Manchester, United Kingdom) and plasma glowed in a plasma barrel etcher (model PT 7150; Fisher Scientific UK, Loughborough, United Kingdom) to render the grid surface hydrophilic (12). Bacteria were then negatively stained with 1% (wt/vol) methylamine tungstate (Sigma Chemical Co., Poole, United Kingdom) and viewed in a Philips 201 TEM.
Bacterial cells were prepared for immunogold labeling by using a modification of the method of Willcox et al. (44). Cells from early-stationary-phase cultures were harvested by centrifugation, washed three times by suspension in TB buffer (0.05 M Tris hydrochloride [pH 8.0] containing 1.0% [wt/vol] ovalbumin, 0.1% [wt/vol] gelatin, and 0.05% [vol/vol] Tween 20), and suspended to 1/100 volume in TB solution. Single drops of cell suspension were placed onto carbon- and Formvar-coated nickel grids, excess moisture was removed by absorption, and the grids were inverted onto drops (25 μl) of rabbit antiserum (31) diluted 1:10 in TB buffer and incubated at the ambient temperature for 30 min. Grids were then inverted onto drops of TB buffer, sequentially washed five times, and then applied to drops of 10-nm-diameter gold–anti-rabbit immunoglobulin G (IgG) conjugate (Sigma) diluted 1:10 in TB buffer and incubated at the ambient temperature for 30 min. Grids were washed as before, and cells were negatively stained with 1% (wt/vol) methylamine tungstate and then viewed in a Philips 201 TEM. Control grids were prepared for each strain: these were taken through the entire process, except for the incubation with primary antibodies, to test for nonspecific binding of the gold probe to the cell surface.
Hydrophobicity, adhesion, and aggregation assays.
Cell surface hydrophobicity was measured by hexadecane partition assay (21). A bacterial-cell suspension (2.5 ml; 4 × 108 cells/ml) was vortex-mixed with hexadecane (0.25 ml) for 30 s, the suspension was allowed to stand for 10 min, and hydrophobicity was expressed as the percentage of total cells removed from the aqueous phase. The extent of autoaggregation of cells in early-stationary-phase cultures in TY-glucose medium was estimated as described previously (28). Bacterial adhesion to human fibronectin immobilized onto microwells was determined as previously described (25, 31). Bacterial-cell coaggregation with A. naeslundii was estimated by using a modification of the visual assay of Kolenbrander and Andersen (26). Bacterial cells from early-stationary-phase cultures in TSBY medium were harvested by centrifugation (6,000 × g, 4°C, 10 min), suspended in TBSC buffer (10 mM Tris hydrochloride [pH 7.6] containing 0.15 M NaCl and 5 mM CaCl2), washed twice, and suspended in TBSC buffer at an A600 of 5.0 (approximately 1 × 1010 streptococcal cells/ml or 5 × 109 Actinomyces cells/ml). Equal volumes of suspensions of each cell type were vortex-mixed in a glass tube for 20 s, the tube was allowed to stand at the ambient temperature, and the formation of coaggregates was assessed visually after 10 min. Coaggregation scores were assigned as follows: 4 (maximum coaggregation), formation of large clumps that settled immediately, leaving a clear supernatant; 3, formation of large coaggregates that settled but left a slightly turbid supernatant; 2, formation of definite coaggregates that did not settle immediately; 1, finely dispersed aggregates in a turbid background; 0, no change in turbidity and no visible coaggregation.
ELISA and antibody inhibition of adhesion.
The immunoreactivity of CshA on intact cells was determined by enzyme-linked immunosorbent assay (ELISA) as previously described (17) by using antibodies raised to a recombinant fragment of CshA polypeptide purified from E. coli and comprising the N-terminal 844 amino acid residues of mature CshA polypeptide (31). Inhibition by antibodies of enterococcal- or streptococcal-cell attachment to fibronectin was measured as previously described (31).
Analysis of bacterial proteins.
Cell wall-associated polypeptides were extracted from early-stationary-phase streptococcal cells following mutanolysin treatment in the presence of osmotic stabilizing buffer (4). Lysozyme (0.2 μg/ml) in addition to mutanolysin was used for the extraction of wall-associated proteins from intact enterococcal cells. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 8% (wt/vol) acrylamide gels and were transferred to Hybond-C nitrocellulose membranes (Amersham Corp., Arlington Heights, Ill.) by electroblotting (27). Nitrocellulose blots were incubated with antiserum diluted 1:500, and antibody binding was detected by using peroxidase-conjugated swine immunoglobulins to rabbit IgG as described elsewhere (23).
RESULTS
Surface structures on S. gordonii.
Electron microscopic analysis of negatively stained cells of S. gordonii DL1 (Challis) indicated that these carried sparsely distributed fibrils, present on approximately 35% of cells within a population from early-stationary-phase cultures. The fibrils had no accurately measurable width but projected 60.7 ± 14.5 nm away from the cell surface (Fig. 1A). Fibrils were peritrichous and were usually more visible on the half of the dividing cell farther away from the septum. Previous work has suggested that CshA comprises, at least in part, the fibrillar material that is present on the surfaces of S. gordonii DL1 cells and that is visualized by electron microscopy of sections stained with RR and osmium tetroxide (27). In support of this suggestion, peritrichous fibrils were observed on negatively stained preparations of S. gordonii OB271 cshB2 cells, in which expression of the structurally related but functionally independent cshB gene is inactivated (31, 32) (data not shown). On the other hand, cells of isogenic S. gordonii OB277 cshA31 cshB2, which express neither CshA nor CshB proteins, were devoid of cell surface fibrils (Fig. 1B) and exhibited completely smooth surfaces.
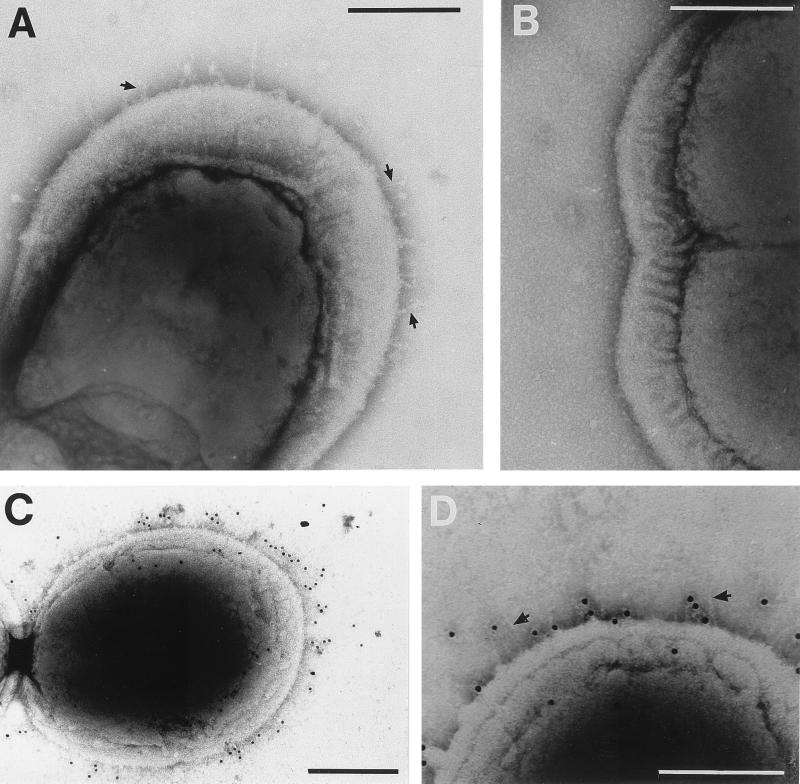
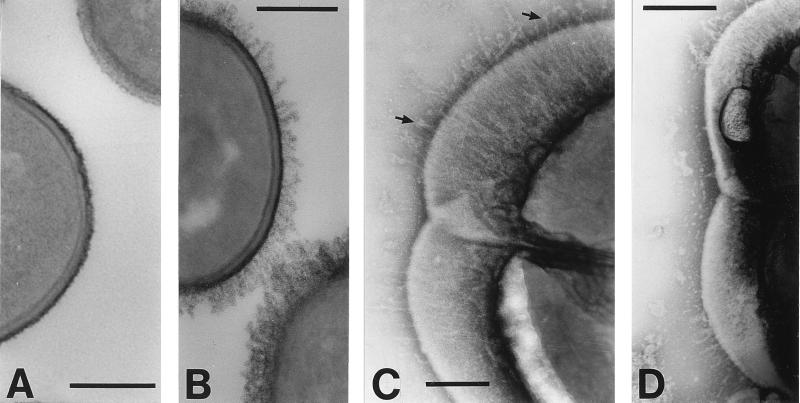
FIG. 1.
Electron micrographs of S. gordonii CshA fibrils negatively stained with 1% (wt/vol) methylamine tungstate (A and B) or reacted with CshA-specific antibodies and 10-nm gold particle-conjugated secondary antibody (C and D). Fibrils of 60.7 ± 14.5 nm in length are present on the wild-type strain DL1 (indicated by arrows in panel A) but are absent from the surfaces of OB277 cshA31 cshB2 mutant cells (B). (C and D) S. gordonii DL1 cells labeled with immunogold. Gold particles demonstrate a bias for “older wall” (see Discussion) and are located up to 61.3 ± 19.2 nm from the cell surface. In panel D, fibrils may be seen at one end of the cell (arrows). In all images of negatively stained cells, some plasmolysis was observed, with the cytoplasmic membrane pulled away to some degree from the cell wall. Bars, 200 nm.
To show that the fibril structures were comprised of CshA, S. gordonii DL1 cells were reacted with antibodies specific to the N-terminal region of recombinant CshA polypeptide, and antibody binding was detected with gold-conjugated secondary antibody. Gold particles were found to be distributed over the entire cell surface and often showed a higher density on one half of the dividing cell (Fig. 1C). Gold particles extended away from the cell surface to an average distance, measured around the cell circumference, of 61.0 ± 19.2 nm, a measurement correlating well with that calculated for the average length of fibrils (see above). Fibril structures could not be discerned underneath the gold particles (Fig. 1C), possibly because bound antibodies obscured the very thin fibrils. Occasionally it was possible to see discrete fibrils on immunogold-labeled cell preparations (Fig. 1D) to which gold particles had not bound.
Expression of CshA on the surface of E. faecalis.
To corroborate the evidence suggesting that S. gordonii DL1 fibrils are composed of CshA polypeptide, and to determine the adhesive functions of CshA ex vivo, the entire cshA gene was cloned and expressed in E. faecalis JH2-2. This enterococcal strain is readily electrotransformable, exhibits minimal cell surface hydrophobicity and coaggregation with A. naeslundii cells, and shows low-level binding to fibronectin. A DNA fragment (8,084 bp) containing the entire cshA open reading frame (7,527 bp) was amplified by high-fidelity PCR. The sequence 5′ to the start of the cshA coding sequence comprised the entire intergenic region between the upstream aldB gene and the start of cshA, and it included the aldB transcriptional terminator and cshA promoter with conventional −10 and −35 sequences (29). The sequence 3′ to the cshA stop codon (240 bp) carried the cshA transcriptional terminator (Fig. 2). PCR products were cloned into the E. coli-E. faecalis high-copy-number shuttle vector pAM401 (45), amplified in E. coli by one passage only because of product toxicity, and then introduced into E. faecalis JH2-2 by electroporation with selection for chloramphenicol resistance. Transformants were screened for plasmids of the predicted size, and plasmids from a number of transformants were isolated and restriction enzyme mapped. One representative transformant, designated strain OB516, was selected for further characterization.
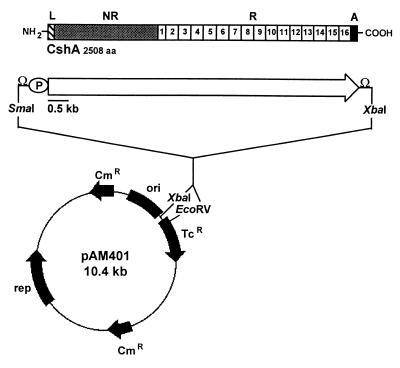
FIG. 2.
Construction of plasmid pAMCshA. The cshA gene and flanking regions were PCR amplified from S. gordonii DL1 DNA, and the SmaI-XbaI segment (8.1 kb) was ligated into the E. coli-E. faecalis shuttle vector pAM401 digested with EcoRV and XbaI as detailed in Materials and Methods. The structural features of the CshA polypeptide are shown as follows: L, leader peptide (41 amino acid residues); NR, nonrepetitive region (residues 42 to 878); R, amino acid repeat block region (residues 879 to 2417); A, cell wall anchor (residues 2418 to 2508); aa, amino acids; P, promoter; Ω, rho-independent transcriptional terminator.
To quantify the relative level of CshA cell surface expression by E. faecalis OB516, intact cells were applied to microtiter plate wells and reacted with N-terminal CshA-specific antibodies in an ELISA. The reactivity of E. faecalis JH2-2 cells carrying pAM401 vector alone with these antibodies was negligible, while E. faecalis OB516 cells reacted strongly with the antibodies, demonstrating cell surface expression and exposure of N-terminal CshA epitopes (Table 2). The ELISA value for E. faecalis OB516 cells was 20% greater than that for an equivalent number of S. gordonii DL1 cells (Table 2), while S. gordonii OB235 cshA3 cells demonstrated <3% of wild-type reactivity (Table 2). Western blots of cell wall protein extracts prepared from enterococcal strains and reacted with CshA-specific antibodies revealed that E. faecalis OB516 cells carrying pAMCshA expressed a major protein band with an apparent molecular mass of 250 kDa that was absent from extracts of vector-alone controls (Fig. 3). This material was extracted from E. faecalis OB516 cells in significant amounts only following the incubation of cells with murolytic enzymes, implying covalent linkage of the CshA polypeptide to cell wall peptidoglycan. The major CshA antibody-reactive band in E. faecalis extracts migrated similarly in SDS-PAGE to CshA polypeptide from S. gordonii DL1 (Fig. 3). However, heterologously expressed CshA appeared to be subject to more proteolytic degradation within enterococcal cell wall protein extracts than in the corresponding streptococcal extracts (Fig. 3).
TABLE 2.
CshA expression, hydrophobicity, and adhesion properties of E. faecalis and S. gordonii strains
| Bacterium and strain | ELISA (OD492) reactivitya | Hydrophobicity (%) | Bacterial cell autoaggregation (%) | Adhesion to fibronectin (% of input cells)b | Coaggregationc with A. naeslundii T14V |
|---|---|---|---|---|---|
| E. faecalis | |||||
| OB513 pAM401 | 0.008 ± 0.002d | 1.3 ± 0.5 | 3.5 ± 0.7 | 7.7 ± 1.1 | 0 |
| OB516 pAMCshA | 0.520 ± 0.036 | 35.5 ± 2.3 | 64.0 ± 2.7 | 18.0 ± 1.5 | 1 |
| S. gordonii | |||||
| DL1 (wild type) | 0.406 ± 0.033 | 65.0 ± 4.8 | 92.6 ± 4.4 | 30.4 ± 3.0 | 4 |
| OB235 cshA3 | 0.012 ± 0.004 | 31.5 ± 5.3 | 21.4 ± 0.4 | 15.5 ± 2.5 | 3 |
Cells (2 × 107 per well) were immobilized, and primary antiserum to N-terminal CshA was diluted 1:1,000. ELISA values were corrected for respective values obtained with preimmune serum (diluted 1:1,000). OD492, optical density at 492 nm.
Fibronectin (1 μg per well) was applied to microtiter plate wells, and the numbers of radioactively labeled cells bound (input of 2 × 107) were determined.
Visual coaggregation score; 0 = no coaggregation, 4 = maximum coaggregation.
Standard deviation; n = 4.
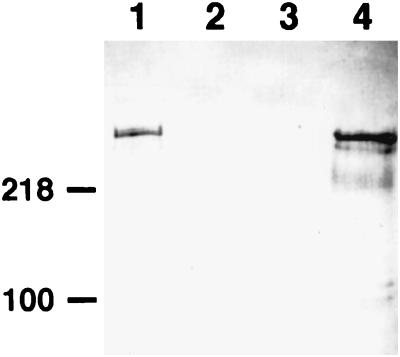
FIG. 3.
Western immunoblot detection of CshA expression in S. gordonii or E. faecalis strains. Surface proteins were extracted from cells in the early-stationary phase of growth following incubation with the murolytic enzyme mutanolysin or lysozyme (see Materials and Methods). Extracts were subjected to SDS-PAGE, proteins were electroblotted onto nitrocellulose membranes, and blots were probed with polyclonal antibodies raised to the N-terminal region of recombinant CshA diluted 1:1,000. Lanes: 1, S. gordonii DL1 (wild type); 2, S. gordonii OB235 cshA3; 3, E. faecalis OB513(pAM401); 4, E. faecalis OB516(pAMCshA). Note the lack of reactivity of antibodies in lane 2, demonstrating their specificity for CshA polypeptide. Protein loadings were equalized (10 μg per lane). The numbers on the left indicate the positions of molecular mass markers (in kilodaltons).
Phenotypic properties of E. faecalis expressing CshA.
S. gordonii DL1 cells autoaggregate extensively in TY-glucose-grown cultures, while S. gordonii OB235 cshA3 mutant cells show much-reduced autoaggregation (Table 2). This CshA-associated autoaggregative property was conferred upon E. faecalis JH-2 cells expressing CshA, with 18-fold-increased autoaggregation of E. faecalis OB516 over control E. faecalis OB513 cells carrying pAM401 (Table 2).
Other phenotypic properties attributed to cshA gene expression in S. gordonii DL1 include cell surface hydrophobicity, binding to oral Actinomyces, and adhesion to fibronectin (see Introduction). Accordingly, the hydrophobic and adhesive properties of E. faecalis OB516 cells expressing CshA were compared with those of E. faecalis OB513 controls. In a hexadecane partition assay, approximately 1% of E. faecalis OB513 cells adsorbed to the organic phase, compared with 35% of E. faecalis OB516 cells, showing the latter to be much more hydrophobic (Table 2). CshA expression by E. faecalis OB516 also promoted fibronectin binding, with a 2.4-fold increase in the numbers of E. faecalis OB516 cells adhering to human fibronectin compared with E. faecalis OB513 control cells (Table 2). Antibodies reactive with the N-terminal region of CshA specifically inhibited the adhesion of E. faecalis OB516 cells to fibronectin in a dose-dependent manner (Fig. 4), from 58.3% inhibition (at a 1:30 antibody dilution) to 21.2% inhibition (at a 1:120 dilution). The antibody inhibition profile for E. faecalis OB516 cells was almost identical to the profile obtained for inhibition by the same antibody of S. gordonii DL1 binding to fibronectin (Fig. 4) (31).
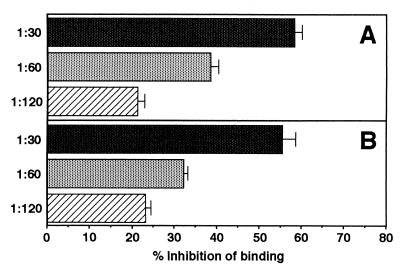
FIG. 4.
Inhibition by antibodies to N-terminal CshA of bacterial-cell adhesion to fibronectin. Radioactively labeled cells of E. faecalis OB516 (A) or S. gordonii DL1 (B) were mixed with immune or preimmune antiserum diluted appropriately, and portions (2 × 107 per well) were applied to microtiter plate wells coated with human fibronectin (1 μg per well) or to wells blocked with bovine albumin. The numbers of cells that adhered were measured as described elsewhere (31). Data are presented as percent inhibition of adhesion in the presence of immune serum compared with adhesion in the presence of preimmune serum. The results for CshA antibody inhibition of S. gordonii cells binding to fibronectin are as previously reported (31).
Further, expression of CshA by E. faecalis OB516 imparted to enterococci the ability to coaggregate with A. naeslundii T14V cells (Table 2). Inactivation of cshA in S. gordonii leads to only a partial reduction in the coaggregation of streptococci with Actinomyces (Table 2) because the reaction is multimodal (31). These coaggregation assays were not affected by autoaggregation properties of the streptococci or enterococci, since buffer-washed cells suspended in coaggregation buffer did not autoaggregate significantly over the course of the experiments.
Surface structures on E. faecalis.
Electron micrographs of thin sections of E. faecalis JH2-2 cells, or of OB513 cells carrying pAM401, showed the presence of a dense and compact surface layer, approximately 20 nm thick, that stained with RR and osmium tetroxide (Fig. 5A). Cells of E. faecalis OB516 expressing CshA also produced this densely staining layer, but in addition a peritrichous fringe of fibrillar material, emanating approximately 50 nm from the cell wall, was visible (Fig. 5B).
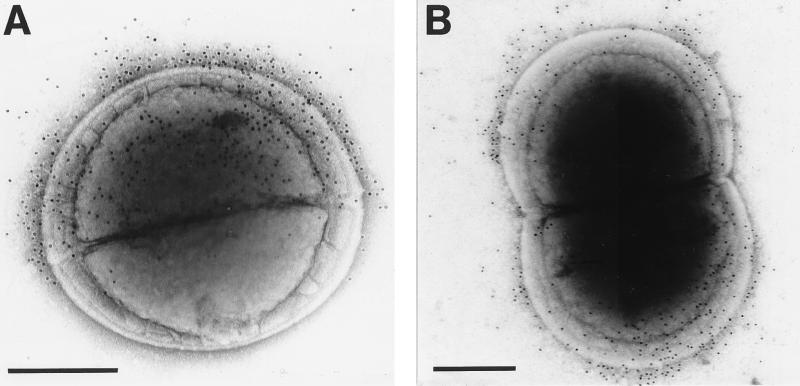
FIG. 5.
Electron microscopy of E. faecalis JH2-2 cells carrying pAM401 or pAMCshA. (A and B) Thin-section micrographs stained with RR and osmium tetroxide; (C and D) negatively stained cells. (A) E. faecalis OB513(pAM401) control cells are surrounded by a compact and densely stained layer covering a less-densely stained cell wall layer. (B) E. faecalis OB516(pAMCshA) cells expressing CshA polypeptide show a densely stained fibrillar fringe. (C) E. faecalis OB516 cells show peritrichous fibrils 70.3 ± 9.1 nm long that are more sparsely located in the region of the septum, and some fibrils have globular ends (arrows). (D) E. faecalis OB516 cells showing the absence of fibrils from the septal region and their expression restricted to the older ends of the cells. Bars, 200 nm.
Negatively stained E. faecalis JH2-2 cells do not show the presence of surface fibrils, although 1 to 2% of cells in a population carry long fimbriae 3 to 4 nm wide (11). By contrast, when viewed following negative staining, 60% of E. faecalis OB516 cells from early-stationary-phase cultures carried surface fibrils (Fig. 5C and D). The mean length of these fibrils was 70.3 ± 9.1 nm, and there was no statistically significant difference between this length and that of S. gordonii fibrils (P < 0.05). Fibrils were carried by a greater proportion of E. faecalis OB516 cells than of S. gordonii DL1 cells, and the fibrils were of greater surface density on the enterococci (compare Fig. 5C and 1A). For E. faecalis OB516 cells undergoing cell division, fibrils were more densely located towards the ends of the cells and few could be visualized in the growing equatorial region of new cell wall (Fig. 5D).
When E. faecalis OB516 cells expressing CshA were reacted with CshA-specific antibodies, a range of distribution patterns of gold labeling was observed. For example, newly divided cells were labeled on one side only of the nascent septum (Fig. 6A). By contrast, on dividing cells that had started to show elongation, gold labeling was restricted towards the ends of the dividing cells (Fig. 6B). Gold particles were evenly distributed over the surfaces of cells that were not apparently undergoing wall growth (data not shown). Gold-conjugated antibodies bound material to a distance of 61.2 ± 12.2 nm from the cell surface; this distance was not statistically different (P < 0.01) from the distance measured for gold-labeled CshA fibrils on S. gordonii DL1 cells. It was difficult to discern surface fibril structures on E. faecalis cells that had been incubated with antibodies, and as in S. gordonii (Fig. 1C), fibrils could not be resolved when gold particles were bound to them (Fig. 6).
FIG. 6.
Immunogold-labeled E. faecalis OB516(pAMCshA) cells. Cells from the early-stationary phase of growth were incubated with N-terminus-specific CshA antibodies and a gold-conjugated secondary antibody. (A) A newly-divided cell binds gold label that is restricted to one-half of the cell as defined by the nascent septum; (B) sparse deposition of gold particles in the septal region of the cell in the process of cell wall synthesis prior to cell division. Gold particles were located 61.7 ± 12.2 nm from the cell surface. Bars, 200 nm.
DISCUSSION
Oral streptococci express multiple adhesins that enable them to adhere to the complex macromolecular surfaces present in the human oral cavity and to colonize ecologically diverse sites. A number of the adhesive properties of S. gordonii have been attributed to expression of the antigen I/II polypeptides designated SspA and SspB which bind salivary glycoproteins, collagen, and other oral bacterial cells, including A. naeslundii and Porphyromonas gingivalis (3, 4, 18, 24). The SspB polypeptide exhibits salivary-protein binding properties when expressed on the surface of E. faecalis (3), while presentation of the SspA and SspB polypeptides in active form on the surface of Lactococcus lactis has recently enabled a more detailed description of their relative binding affinities for common ligands (18). Conversely, CshA, which has been identified as an important adhesin in S. gordonii mediating binding to fibronectin and to oral bacteria, including A. naeslundii and S. oralis (31, 32), has not previously been purified or expressed heterologously in active form. Recombinant fragments of CshA comprising the N-terminal nonrepetitive region or the C-terminal amino acid repeat block region have been expressed and purified from E. coli but do not exhibit binding properties in vitro (31). This has led to the suggestion that the adhesion properties of CshA depend on correct folding of the native protein into an active conformation (31). This assumption is borne out by the results now presented in this article, which demonstrate that CshA polypeptide indeed contains the complete structural information for fibril formation, as well as for the functional properties of adhesion.
CshA has been demonstrated to be a cell wall-anchored protein and to be linked to cell wall peptidoglycan via a C-terminal anchor region (27). Although there are a number of potential N-glycosylation sites within the primary sequence (32), it is unlikely that native CshA is extensively glycosylated, because it migrates upon SDS-PAGE at an apparent molecular mass close to that predicted from the amino acid sequence. The N-terminal nonrepetitive sequence region (93 kDa) of CshA, which is surface exposed and bound by N-terminal CshA-specific antibodies, is predicted to adopt a mainly α-helical structure (30, 32). By comparison, the M6 (Emm6.1) protein of Streptococcus pyogenes has a molecular mass of approximately 45 kDa and is expressed as an α-helical coiled-coil dimer that extends 50 to 60 nm from the streptococcal-cell surface (6). Thus the measured external fibril length of 61 nm could be easily accounted for by the 259-kDa CshA polypeptide. However, while the N-terminal region of CshA is predicted to be predominantly α-helical, it does not demonstrate the periodic distribution of hydrophobic amino acid residues consistent with the formation of a coiled coil. The extensive C-terminal amino acid repeat block region of CshA, comprising 13 repeats of 101 amino acid residues rich in proline and glycine, may, on the other hand, adopt an extended and elastic conformation (32). This C-terminal region, though, has not yet been shown to carry any adhesive function, aside from being implicated in conferring surface hydrophobicity (30). It seems likely, therefore, that the C-terminal region is necessary for presentation of the CshA adhesive functions which have been localized to the N-terminal region (31).
Streptococcal fibrils have an ill-defined width that is much less than that of 10-nm-diameter gold particles. Thus, a sufficiently detailed analysis by immunogold-labeling techniques of the CshA domains that are cell surface accessible may be precluded by steric considerations. It is interesting, however, that for streptococci and enterococci expressing CshA, N-terminal reactive antibodies bound by the gold-conjugated secondary antibodies were visualized at a distance of 60 to 70 nm from the surface. This distance from the surface correlates with the ends or tips of the fibrils calculated from fibril measurements on negatively stained micrographs of cells. Closer examination of negatively stained fibrils on E. faecalis OB516 cells expressing CshA suggests that many fibrils have a globular region at their tips (Fig. 5C and D). Similar globular ends were noted in micrographs of the purified fibrillar proteins AgB and AgC of S. salivarius (41). We speculate, therefore, that the globular ends comprise the N-terminal nonrepetitive region of CshA containing the primary adhesion-mediating sequences held distal from the cell surface. We are attempting currently to generate recombinant N-terminally truncated CshA molecules to test this hypothesis.
CshA fibrils expressed on the surface of S. gordonii or E. faecalis usually exhibited asymmetric distribution over the cell surface. Fibrils, and bound antibodies to CshA localized by gold labeling, tended to be more densely associated with the ends of cells (corresponding to older wall) and were sparsely distributed in the region of the septum. This localization pattern for CshA mirrors the distribution patterns for the E. faecalis cell wall-linked aggregation substance proteins Asc10 and Sec10 (35, 39). In gram-positive cocci, new wall synthesis is proposed to occur at the septal region (16), so that older wall is present towards the ends of the dividing cells. Upon separation, daughter cell hemispheres comprise one-half new wall and one-half old wall, separated by the nascent cross wall. The asymmetric distribution pattern of CshA on streptococcal and enterococcal cells, with diplococci often being gold labeled on one-half of the cell only (Fig. 6A), indicates that CshA is inserted into older wall. The cshA gene is expressed maximally in late-exponential-phase cultures of S. gordonii (29), so as cultures reach stationary phase and cell division slows, CshA protein may become more evenly distributed over the cell surface. Not only are fibrils found distributed asymmetrically on cells, but they are observed on only about 35% of cells in early-stationary-phase cultures of S. gordonii DL1, and those cells that express fibrils demonstrate a range of fibril densities. These observations highlight the heterogeneity within populations of gram-positive cocci with respect to cell surface protein presentation (35) and surface structure production (12). The frequency of S. gordonii cells within the population carrying fibrils, and individual cell surface fibril densities, must be regulated by a complex control network involving intrinsic as well as environmental factors, some of which modulate cshA gene transcription (29). Indeed, the greater percentage (60%) of E. faecalis OB516 cells producing fibrils, and the somewhat higher densities of fibrils on these cells, could be accounted for by increased levels of CshA expressed from the plasmid-encoded cshA gene in enterococci (see Table 2).
CshA-like proteins are produced by S. gordonii, S. oralis, and S. sanguis but not by mutans group streptococci (30), and surface expression levels of CshA correlate well with streptococcal cell surface hydrophobicity. A spontaneously derived mutant of S. gordonii DL1 with increased cell surface hydrophobicity (22) showed increased expression of CshA (30) and an increased ability to coaggregate with oral Actinomyces (22). Moreover, nonhydrophobic variants of S. sanguis have been shown to lack surface fibrils (10). Our results therefore provide a molecular explanation for the many previous correlations of hydrophobicity, coaggregation, adhesion, and fibril production in S. sanguis and related streptococci (10, 12, 15, 21, 22, 33, 43, 44) and suggest that levels of cshA gene expression could determine surface fibril density. The data further imply a function for CshA in fibril production and adhesion that is independent of expression of the closely related surface protein CshB in S. gordonii (32). It is possible that the surface fibrils of S. gordonii to which gold particles did not bind (Fig. 1D) were comprised of CshB. However, there is no evidence that CshB has an adhesive function, and the impaired ability of cshB mutants to bind fibronectin results apparently from reduced expression of surface CshA by these cells (31).
Heterologous protein expression on the surfaces of gram-positive bacteria has been used in the development of these organisms as vaccine delivery agents (reviewed in references 7 and 42) and as a means of anchoring biologically active enzymes on surfaces for biotechnological applications (38). Recently, the binding properties of two related oral streptococcal adhesins expressed in native conformation on the surface of L. lactis have been compared (18). In this paper, we have now extended the utility of heterologous expression to provide a morphological, as well as a more-detailed functional, analysis of the CshA adhesin. In summary, this high-molecular-mass wall-anchored polypeptide constitutes adhesive fibrils, providing a molecular basis for previous correlations of fibril production, cell surface hydrophobicity, and adhesion amongst the “sanguis-like” streptococci, including S. gordonii and S. sanguis.
ACKNOWLEDGMENTS
We thank D. B. Clewell and J. O. Cisar for providing strains and R. A. Baker for technical assistance.
This work was supported by the Health Research Council of New Zealand.
REFERENCES
- 1.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 2.Cruz-Rodz A L, Gilmore M S. High-efficiency introduction of plasmid DNA into glycine-treated Enterococcus faecalis by electroporation. Mol Gen Genet. 1990;224:152–154. doi: 10.1007/BF00259462. [DOI] [PubMed] [Google Scholar]
- 3.Demuth D R, Berthold P, Leboy P S, Golub E E, Davis C A, Malamud D. Saliva-mediated aggregation of Enterococcus faecalis transformed with a Streptococcus sanguis gene encoding the SSP-5 surface antigen. Infect Immun. 1989;57:1470–1475. doi: 10.1128/iai.57.5.1470-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demuth D R, Duan Y, Brooks W, Holmes A R, McNab R, Jenkinson H F. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordonii to human and bacterial receptors. Mol Microbiol. 1996;20:403–413. doi: 10.1111/j.1365-2958.1996.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 5.Fachon-Kalweit S, Elder B L, Fives-Taylor P. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect Immun. 1985;48:617–624. doi: 10.1128/iai.48.3.617-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischetti V A, Medaglini D, Pozzi G. Gram-positive commensal bacteria for mucosal vaccine delivery. Curr Opin Biotechnol. 1996;7:659–666. doi: 10.1016/s0958-1669(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 8.Fives-Taylor P M, Thompson D W. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect Immun. 1985;47:752–759. doi: 10.1128/iai.47.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frandsen E V G, Pedrazzoli V, Kilian M. Ecology of viridans streptococci in the oral cavity and pharynx. Oral Microbiol Immunol. 1991;6:129–133. doi: 10.1111/j.1399-302x.1991.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons R J, Etherden I, Skobe Z. Association of fimbriae with the hydrophobicity of Streptococcus sanguis FC-1 and adherence to salivary pellicles. Infect Immun. 1983;41:414–417. doi: 10.1128/iai.41.1.414-417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handley P S, Jacob A E. Some structural and physiological properties of fimbriae of Streptococcus faecalis. J Gen Microbiol. 1981;127:289–293. doi: 10.1099/00221287-127-2-289. [DOI] [PubMed] [Google Scholar]
- 12.Handley P S, Carter P L, Wyatt J E, Hesketh L M. Surface structures (peritrichous fibrils and tufts of fibrils) found on Streptococcus sanguis strains may be related to their ability to coaggregate with other oral genera. Infect Immun. 1985;47:217–227. doi: 10.1128/iai.47.1.217-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harty D W S, Handley P S. Expression of the cell-surface properties of the fibrillar Streptococcus salivarius HB and its adhesion-deficient mutants grown in continuous culture under glucose limitation. J Gen Microbiol. 1989;135:2611–2621. doi: 10.1099/00221287-135-10-2611. [DOI] [PubMed] [Google Scholar]
- 14.Harty D W S, Willcox M D P, Wyatt J E, Oyston P C F, Handley P S. The surface ultrastructure and adhesive properties of a fimbriate Streptococcus sanguis strain and six non-fimbriate mutants. Biofouling. 1990;2:75–86. [Google Scholar]
- 15.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins M L, Shockman G D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970;101:643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes A R, Gopal P K, Jenkinson H F. Adherence of Candida albicans to a cell surface polysaccharide receptor on Streptococcus gordonii. Infect Immun. 1995;63:1827–1834. doi: 10.1128/iai.63.5.1827-1834.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes A R, Gilbert C, Wells J M, Jenkinson H F. Binding properties of Streptococcus gordonii SspA and SspB (antigen I/II family) polypeptides expressed on the cell surface of Lactococcus lactis MG1363. Infect Immun. 1998;66:4633–4639. doi: 10.1128/iai.66.10.4633-4639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jameson M W, Jenkinson H F, Parnell K, Handley P S. Polypeptides associated with tufts of fibrils in an oral Streptococcus. Microbiology. 1995;141:2729–2738. doi: 10.1099/13500872-141-10-2729. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson H F. Novobiocin-resistant mutants of Streptococcus sanguis with reduced cell hydrophobicity and defective in coaggregation. J Gen Microbiol. 1987;133:1909–1918. doi: 10.1099/00221287-133-7-1909. [DOI] [PubMed] [Google Scholar]
- 22.Jenkinson H F, Carter D A. Cell surface mutants of Streptococcus sanguis with altered adherence properties. Oral Microbiol Immunol. 1988;3:53–57. doi: 10.1111/j.1399-302x.1988.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 23.Jenkinson H F, Easingwood R A. Insertional inactivation of the gene encoding a 76-kilodalton cell surface polypeptide in Streptococcus gordonii Challis has a pleiotropic effect on cell surface composition and properties. Infect Immun. 1990;58:3689–3697. doi: 10.1128/iai.58.11.3689-3697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkinson H F, Lamont R J. Streptococcal adhesion and colonization. Crit Rev Oral Biol Med. 1997;8:175–200. doi: 10.1177/10454411970080020601. [DOI] [PubMed] [Google Scholar]
- 25.Jenkinson H F, Terry S D, McNab R, Tannock G W. Inactivation of the gene encoding surface protein SspA in Streptococcus gordonii DL1 affects cell interactions with human salivary agglutinin and oral actinomyces. Infect Immun. 1993;61:3199–3208. doi: 10.1128/iai.61.8.3199-3208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolenbrander P E, Andersen R N. Characterization of Streptococcus gordonii (S. sanguis) PK488 adhesin-mediated coaggregation with Actinomyces naeslundii PK606. Infect Immun. 1990;58:3064–3072. doi: 10.1128/iai.58.9.3064-3072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNab R, Jenkinson H F. Gene disruption identifies a 290-kDa cell-surface polypeptide conferring hydrophobicity and coaggregation properties in Streptococcus gordonii. Mol Microbiol. 1992;6:2939–2949. doi: 10.1111/j.1365-2958.1992.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 28.McNab R, Jenkinson H F. Aggregation-deficient mutants of Streptococcus gordonii Channon altered in production of cell-surface polysaccharides and proteins. Microb Ecol Health Dis. 1992;5:277–289. [Google Scholar]
- 29.McNab R, Jenkinson H F. Altered adherence properties of a Streptococcus gordonii hppA (oligopeptide permease) mutant result from transcriptional effects on cshA adhesin gene expression. Microbiology. 1998;144:127–136. doi: 10.1099/00221287-144-1-127. [DOI] [PubMed] [Google Scholar]
- 30.McNab R, Holmes A R, Jenkinson H F. Cell-surface polypeptides as determinants of hydrophobicity in Streptococcus gordonii and Streptococcus sanguis. Colloids Surf B. 1995;5:135–142. [Google Scholar]
- 31.McNab R, Holmes A R, Clarke J M, Tannock G W, Jenkinson H F. Cell surface polypeptide CshA mediates binding of Streptococcus gordonii to other oral bacteria and to immobilized fibronectin. Infect Immun. 1996;64:4204–4210. doi: 10.1128/iai.64.10.4204-4210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNab R, Jenkinson H F, Loach D M, Tannock G W. Cell-surface-associated polypeptides CshA and CshB of high molecular mass are colonization determinants in the oral bacterium Streptococcus gordonii. Mol Microbiol. 1994;14:743–753. doi: 10.1111/j.1365-2958.1994.tb01311.x. [DOI] [PubMed] [Google Scholar]
- 33.Morris J E, Ganeshkumar N, Song M, McBride B C. Identification and preliminary characterization of a Streptococcus sanguis fibrillar glycoprotein. J Bacteriol. 1987;169:164–171. doi: 10.1128/jb.169.1.164-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nyvad B, Kilian M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:369–380. doi: 10.1111/j.1600-0722.1987.tb01627.x. [DOI] [PubMed] [Google Scholar]
- 35.Olmsted S B, Erlandsen S L, Dunny G M, Wells C L. High-resolution visualization by field emission scanning electron microscopy of Enterococcus faecalis surface proteins encoded by the pheromone-inducible conjugative plasmid pCF10. J Bacteriol. 1993;175:6229–6237. doi: 10.1128/jb.175.19.6229-6237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pakula R, Walczak W. On the nature of competence of transformable streptococci. J Gen Microbiol. 1963;31:125–133. doi: 10.1099/00221287-31-1-125. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Strauss A, Gotz F. In vivo immobilization of enzymatically active peptides on the cell surface of Staphylococcus carnosus. Mol Microbiol. 1996;21:491–500. doi: 10.1111/j.1365-2958.1996.tb02558.x. [DOI] [PubMed] [Google Scholar]
- 39.Wanner G, Formanek H, Galli D, Wirth R. Localization of aggregation substance of Enterococcus faecalis after induction by sex pheromones. An ultrastructural comparison using immunolabelling, transmission and high resolution scanning electron microscopic techniques. Arch Microbiol. 1989;151:491–497. doi: 10.1007/BF00454864. [DOI] [PubMed] [Google Scholar]
- 40.Weerkamp A H, Handley P S, Baars A, Slot J W. Negative staining and immunoelectron microscopy of adhesion-deficient mutants of Streptococcus salivarius reveal that the adhesive proteins are separate classes of cell surface fibril. J Bacteriol. 1986;165:746–755. doi: 10.1128/jb.165.3.746-755.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weerkamp A H, van der Mei H C, Liem R S B. Structural properties of fibrillar proteins isolated from the cell surface and cytoplasm of Streptococcus salivarius (K+) cells and nonadhesive mutants. J Bacteriol. 1986;165:756–762. doi: 10.1128/jb.165.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells J M, Robinson K, Chamberlain L M, Schofield K M, Le Page R W F. Lactic acid bacteria as vaccine delivery vehicles. Antonie Leeuwenhoek. 1996;70:317–330. doi: 10.1007/BF00395939. [DOI] [PubMed] [Google Scholar]
- 43.Willcox M D P, Drucker D B. Surface structures, co-aggregation and adherence phenomena of Streptococcus oralis and related species. Microbios. 1989;59:19–29. [PubMed] [Google Scholar]
- 44.Willcox M D P, Wyatt J E, Handley P S. A comparison of the adhesive properties and surface ultrastructure of the fibrillar Streptococcus sanguis 12 and an adhesion-deficient nonfibrillar mutant 12na. J Appl Bacteriol. 1989;66:291–299. doi: 10.1111/j.1365-2672.1989.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 45.Wirth R, An F Y, Clewell D B. Highly efficient protoplast transformation system for Streptococcus faecalis and a new Escherichia coli-S. faecalis shuttle vector. J Bacteriol. 1986;165:831–836. doi: 10.1128/jb.165.3.831-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Mintz K P, Ladha M, Fives-Taylor P M. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol Microbiol. 1998;28:487–500. doi: 10.1046/j.1365-2958.1998.00805.x. [DOI] [PubMed] [Google Scholar]