Abstract
Objectives
This study aimed to describe the burden of illness and impact on health and working situation among former intensive care patients treated for COVID-19.
Methods
A prospective cohort study was performed at one intensive care unit of a university hospital in Sweden during the first wave of COVID-19 in spring 2020. The burden of illness in health status, cognitive, physical, and psychological outcomes, and working situation were assessed at four and 12 months after discharge from intensive care, using nine validated instruments.
Results
Forty-six participants treated for COVID-19 participated in both follow-ups and were included in this study. General fatigue was reported by 37 of 46 participants (82%) at both follow-ups (p = 1.000). For overall health status 28 (61%) participants at the first follow-up and 26 (57%) (p = 0.414) at the second reported lower values than the general population. Cognitive impairment was seen in 22 (52%) participants at four months and in 13 (31%) at 12 months (p = 0.029). The proportion of participants on sick-leave decreased between the first and second follow-up (24% vs 13%, p = 0.025), but the proportion of participants working full-time was almost the same at both follow-ups (35% vs 37%, p = 0.317).
Conclusions
The burden of illness of patients treated in intensive care due to COVID-19 included cognitive, physical, and psychological impacts. Cognitive functions were improved after 12 months, but no clear improvements could be distinguished in the physical or psychological outcome. Higher burden of illness was associated with inability to return to work.
Keywords: COVID-19, Follow-up, Intensive care, Long-term outcome
Implications for clinical practice.
-
•
When resuming life after intensive care and COVID-19, it is important to seriously consider the remaining symptoms.
-
•
Recovery measurements should be considered after intensive care and COVID-19 which include health status, cognitive, physical, and psychological outcomes.
-
•
To increase patients’ possibility to return to previous work and life there is a need to highlight the importance of personalized rehabilitation for former intensive care treated COVID-19 patients.
Background
The severe acute respiratory syndrome corona virus 2 caused the Coronavirus disease (COVID-19) pandemic, with over 422 million people worldwide confirmed as infected by February 2022 (WHO, 2022). The first wave of the COVID-19 pandemic, in the spring of 2020, called for an increased number of intensive care unit (ICU) beds. The rate of admission to intensive care among those infected varied between 1.6% and 6.7% in the Nordic countries (Chew et al., 2021). In Sweden, 3472 COVID-19-infected people were treated in intensive care during the first wave. Most had longer ICU stays, a more extended time on mechanical ventilation often in prone position and therefore received sedation for longer than ICU patients before the pandemic (SIR (The Swedish Intensive Care Registry), 2020).
Before the pandemic, ICU patients followed-up after discharge described both positive emotions (Hashem et al., 2016) and a wide range of mental and physical impacts which affected daily life (Geense et al., 2021, Hashem et al., 2016). Female patients tend to report higher frailty and fatigue scores one year after ICU discharge than male patients (Geense et al., 2021). Long-term symptoms and their effects on health status after COVID-19 are now beginning to be reported (Evans et al., 2021, Huang et al., 2021b, Schandl et al., 2021, Sigfrid et al., 2021, Wallin et al., 2021). However, knowledge about the process of recovery after severe COVID-19 with intensive care and especially about the health consequences of long-term symptoms, is lacking. Therefore, this study aimed to describe the burden of illness and its impact on health and working situation among former ICU patients treated for COVID-19.
Methods
Study design and patients
A prospective, longitudinal cohort study design.
The study was performed at a general ICU at a university hospital in Sweden. The treatment and care of COVID-19 patients during the first wave of the pandemic were based on international and national recommendations and continuously updated guidelines. This ICU had eight beds before the pandemic, but temporary ICU beds were set up to manage the increased number of patients. At the peak of the first wave, 23 ICU beds were in use for COVID-19 patients. The criteria for admittance to ICU during the first wave were based on the concept of potential benefit, in accordance with the usual criteria for intensive care in Sweden and included patients in need of high flow nasal oxygen, non-invasive ventilation or invasive ventilation (Swedish Society of Anaesthesiology and Intensive Care (SFAI), 2015).
Patients were eligible to participate in this follow-up study if they had previously been included in an ongoing research project investigating the acute effects of COVID-19 (ClinicalTrials ID NCT04316884). This study had two follow-ups, the first at 3–6 months and the second at 12 months after discharge from the ICU. On both occasions, patients were contacted by telephone and asked to participate. The participants in this follow-up study were admitted to the ICU during the period 13th March–14th July 2020, i.e. the first wave of COVID-19.
Ethical approval
The study was approved by the Swedish Ethical Review Authority (EPM-2020-02697 with revisions 2020-03629, 2020-05758, and 2021-02205) and was performed in accordance with the Declaration of Helsinki as adopted in 1964, and its subsequent revisions. Participants were included after giving informed consent. The study was registered à priori (ClinicalTrials ID: NCT04474249).
Data collection
Participants were contacted and informed about the study at 3–6 months after the discharge from the ICU by telephone by the first or last author. At 12 months the participants were contacted again by telephone by the first or last author. They were invited to individual face-to-face meetings at the hospital within three weeks of these phone calls. Before these meetings, forms for self-reporting outcome assessments were sent by post to the participants (The Multidimensional Fatigue Inventory (MFI20), Generalised Anxiety Disorder 7 (GAD-7), Patient Health Questionnaire 9 (PHQ-9), who were asked to fill them out and bring them to their meeting. At the face-to-face meetings, participants were clinically assessed and evaluated for cognitive and physical performance using clinician reporting outcome assessments (modified Rankin Scale (mRS), Post-COVID-19 Functional Status Scale (PCFS), Clinical Frailty Scale (CFS), self-reporting outcome assessments (EQ-5D-5L, EQ-VAS), and performance outcome assessment (Montreal Cognitive Assessment (MoCA), 6-minute walk test [6MWT]).
Outcomes
Our main outcomes were burden of illness and working situation at four and 12 months after ICU discharge and secondary outcomes were changes in burden of illness and working situation between four and 12 months after ICU discharge. The following outcome measures were assessed.
Living condition
Return to the previous occupational status and place of residence.
Functional status
The modified Rankin Scale (mRS) is a robust, clinician-reported measure; the structured interview was used to monitor the functional status of each patient (Rankin, 1957). Dichotomized mRS scores of 0–2 were defined as no health consequences, with scores of 3–6 defined as remaining health consequences.
The Post-COVID-19 Functional Status Scale (PCFS) was used to quantify current functional outcomes. This clinician-reported scale was adopted for COVID-19 at the beginning of the first wave of the pandemic (Klok et al., 2020). The PCFS is a simple tool to monitor the course of symptoms and their impact on the functional status of patients treated for COVID-19. The scale focuses on relevant aspects of health consequences and everyday life after a COVID-19 infection. The scale has five steps, from 0 (“no limitations in everyday life”) to 4 (“extensive limitations in everyday life”). Dichotomized PCFS scores of 0–2 were defined as no health consequences limiting everyday life, with scores of 3–4 defined as remaining health consequences limiting everyday life.
Frailty
To measure frailty, the Clinical Frailty Scale (CFS) was used (Muscedere et al., 2017). The CFS was used as a clinician-reported tool, comprising nine classes from very fit to terminally ill. A simple visual description was used for categorization. When dichotomized, a CFS score of 5 and above was used as a frailty cut-off.
Health status
The EQ-5D-5L questionnaire was used to measure health status (Rabin and De Charro, 2001). It includes two descriptive sections. The first is a five-question component that explores five dimensions: Mobility, Self-care, Usual activities, Pain/discomfort, and Anxiety/depression. Each dimension is rated on a scale from 1 to 5: no problems, slight problems, moderate problems, severe problems, and extreme problems. A score of ≥ 2 indicates problems, with higher scores indicating greater severity.
The second section is a visual analogue scale (EQ-VAS) and is a measure of self-rated overall health status, ranging from “The best health you can imagine” (100) to “The worst health you can imagine” (0). A mean score of 79.5 in the Swedish population in EQ-VAS was used as cut-off score to define good self-related overall health status (Burström et al., 2020).
Cognitive function
To examine global cognitive function, the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) was used. It encompasses visuospatial ability, executive function, attention/working memory, episodic memory, and language. The score range is 0–30. A low score indicates worse cognitive performance and a cut-off of 26 was used to define cognitive dysfunction.
Fatigue
The Multidimensional Fatigue Inventory (MFI20) was used to assess fatigue (Smets et al., 1995). This is a 20-item self-report questionnaire covering fatigue and its intensity and nature. It encompasses five different dimensions of fatigue: General fatigue, Physical fatigue, Mental fatigue, Reduced motivation, and Reduced activity. Each subscale contains two positively formulated items (e.g., “I feel very active”) and two negatively formulated items (e.g., “I get tired easily”). Items are rated on a 5-point Likert scale (range 1 “Yes, that is true” to 5 “No, that is not true”) which are summed up to a simple total score for each subscale with a minimum value of 4 (absence of fatigue) and a maximum value of 20. Higher scores indicate higher levels of fatigue. The dimension General fatigue can be used to identify fatigue more generally. Dichotomized MFI20 scores of 4–8 (absence or mild) were defined as no fatigue, with scores of 9–20 (moderate, severe, and very severe) defined as fatigue symptoms.
Anxiety and depression
The self-assessment form Generalised Anxiety Disorder 7-item scale (GAD-7) (Spitzer et al., 2006) was assessed to measure anxiety. The GAD-7 includes seven items rated on a 4-point Likert scale ranging from 0 (“not at all”) to 3 (“nearly every day”). A total score of 0–21 is calculated by summing up all the items. The total score is categorized into minimal (0–4), mild (5–9), moderate (10–14), or severe (15–21) anxiety symptoms. In this study, the GAD-7 score was dichotomized (≥10) as occurrence of generalized anxiety.
The Patient Health Questionnaire 9 (PHQ-9) (Kroenke et al., 2001) was used to measure depression. The PHQ-9 is a self-report scale and includes nine items on a 4-point Likert scale ranging from 0 (“not at all”) to 3 (“nearly every day”). A total score of 0–27 is obtained by summing up all items. The depression symptom severity is categorized into minimal (0–4), mild (5–9), moderate (10–14), moderately severe (15–19), or severe (20–27). In this study, the PHQ-9 score was dichotomized (≥10) as occurrence of depression.
Physical capacity
Physical capacity was assessed using the 6-minute walk test (6MWT) (Crapo et al., 2002). It measures the distance that a person can walk in 6 min on a hard flat surface. A predicted value was calculated for each participant based on age, gender, weight, and height. A lower limit of normal was used as the cut-off point when 6MWT results were dichotomized (Jay and Enright, 2000).
For all instruments except for PCFS, Swedish validated versions were used. PCFS was constructed for COVID-19 patients during the first wave of COVID-19 and was not validated in Swedish when conducting this study.
Statistical analysis
Descriptive statistics and results are presented as frequencies (n), percentages (%), and medians with interquartile ranges (IQRs). The Wilcoxon signed-rank test was used to describe differences over time and the Mann-Whitney U test to analyze differences between groups. Statistical significance was defined as a p-value < 0.05 (two-sided). Statistical analyses were performed using SPSS version 27 (SPSS Inc. Chicago, Illinois, USA). The MFI20, had two missing responses in the domain general fatigue and one missing response in the other domains. The PHQ-9, had six questionnaires with missing responses and the GAD-7 had one questionnaire with a missing response, and questionnaires with missing response were not included in the results.
Results
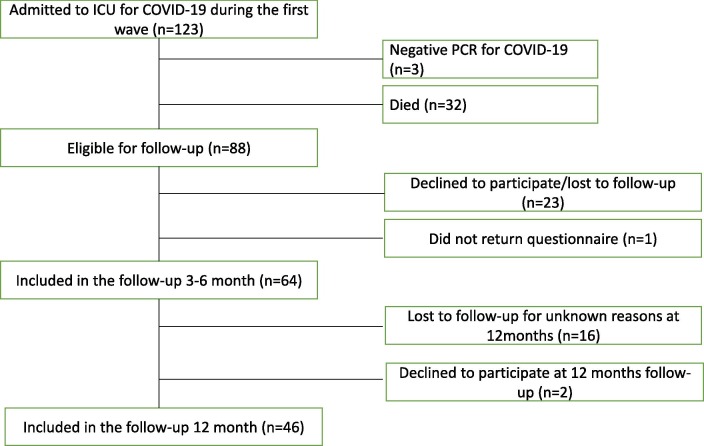
During the first wave of COVID-19, 123 patients were included. Out of them, three were not diagnosed with COVID-19 and thus excluded. At the time of the first follow-up, 32 had died, 23 declined to participate or were lost to follow-up and one did not return the questionnaire. This resulted in 64 participants in the first follow-up. Eighteen patients were lost to follow-up or declined to participate in the second follow-up (Fig. 1 ). The patients lost to follow-up had less hypertension and more malignancy than those who participated in both follow-ups (Table 1 ). The median time to the first follow-up after discharge from ICU was four months, and 12 months to the second follow-up (Table 2 ). Importantly, no differences were seen in outcomes at four months between those who participated in or were lost to follow-up at 12 months. Those who participated in both follow-ups (n = 46) were included in the final analysis (Fig. 1).
Fig. 1.
Flowchart of COVID-19 patients treated in ICU during the first wave of the pandemic and was included in the follow-up study. ICU: intensive care unit.
Table 1.
Patient’s demographics and clinical characteristics on follow-up at two occasions and patients lost in follow-up.
| n = 46 (follow-up at two occasions) | n = 18 (lost in follow-up) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age | 59 (53–69) | 55 (39–73) | 0.285 |
| Sex (female) | 12 (26%) | 5 (28%) | 0.891 |
| BMI at admission (n = 44) | 29 (27–33) | 29 (27–33) | 0.629 |
| Comorbidities at admission | |||
| Lung disease | 13 (28%) | 4 (22%) | 0.626 |
| Hypertension | 26 (56%) | 4 (22%) | 0.014 |
| Heart failure | 0 | 0 | 1.000 |
| Ischemic heart disease | 3 (6%) | 0 | 0.271 |
| Vascular disease | 5 (11%) | 1 (6%) | 0.515 |
| Malignancy | 0 | 3 (17%) | 0.005 |
| Diabetes mellitus | 9 (20%) | 3 (17%) | 0.791 |
| Neurological disease | 2 (4%) | 0 | 0.373 |
| Psychiatric disease | 3 (6%) | 0 | 0.271 |
| ICU characteristics | |||
| SAPS3 | 52 (46–55) | 50 (39–59) | 0.515 |
| Length of ICU stay (days) | 10 (6–17) | 8 (5–12) | 0.320 |
| Invasive ventilation therapy (Days with invasive ventilation therapy) |
25 (54%) (9 (4–16)) |
11 (61%) (2 (0–8)) |
0.627 0.969 |
| Mild ARDS | 1 (2%) | 2 (11%) | 1.000 |
| Moderate ARDS | 20 (43%) | 7 (39%) | 1.000 |
| Severe ARDS | 18 (39%) | 6 (33%) | 1.000 |
| Vasopressor | 24 (52%) | 9 (50%) | 0.877 |
| Renal replacement therapy | 6 (13%) | 2 (11%) | 0.835 |
| Delirium | 6 (13%) | 1 (6%) | 0.392 |
| Critical illness weakness | 5 (11%) | 2 (11%) | 0.978 |
| Length of hospital stay (days) | 22 (13–38) | 23 (12–32) | 0.523 |
| Tertiary education (≥12 years schooling) | 26 (56%) | 10 (56%) | 0.945 |
| Marital status | |||
| Living together | 36 (78%) | 14 (78%) | 0.845 |
| Living alone | 9 (20%) | 4 (22%) | |
| Missing data | 1 (2%) | ||
Categorical data presented as number of total, n (percentage) and compared using Wilcoxon signed-rank test. Continuous data presented as median with interquartile range (IQR) and compared with Mann-Whitney U test.
ARDS: Acute respiratory distress syndrome, ICU: intensive care unit, IQR: interquartile range, SAPS3: Simplified acute physiology score-3.
Table 2.
Baseline and outcome data at follow-up in patients who participated in both follow-ups (n = 46).
| Before COVID-19 (n = 46) | 4 months (n = 46) | 12 months (n = 46) |
p value (4 vs 12 months) |
|
|---|---|---|---|---|
| Time to follow-up (months) | 4 (3.8–4.3) | 12 (11.6–12.3) | ||
| BMI | 29 (27–33) | 30 (26–33) | 32 (27–35) | <0.001 |
| Place of residence: | ||||
| Home | 46 (100%) | 44 (96%) | 46 (100%) | 0.157 |
| Convalescent/rehabilitation home | 2 (4%) | |||
| Occupational status: | ||||
| Working full-time | 29 (63%) | 16 (35%) | 17 (37%) | 0.317 |
| Working part-time | 3 (6%) | 4 (9%) | 5 (11%) | 0.317 |
| Unemployed | 0 | 1 (2%) | 3 (6%) | 0.157 |
| Retired | 11 (24%) | 14 (30%) | 15 (33%) | 0.317 |
| On sick leave | 3 (6%) | 11 (24%) | 6 (13%) | 0.025 |
| Outcome | p value | |||
| modified Rankin Scale (mRS) (n = 46) | 1 (1–2) | 1 (1–2) | 1.000 | |
| Post-COVID-19 Functional Status Scale (PCFS) (n = 46) | 1 (1–2) | 1 (1–3) | 0.323 | |
| Clinical Frailty Scale (CFS) (n = 46) | 2 (2–3) | 3 (2–4) | 0.002 | |
| EQ-5D-5L (n = 46) | ||||
| Mobility | 1.5 (1–2) | 1 (1–2) | 0.819 | |
| Self-Care | 1 (1–1) | 1 (1–1) | 0.414 | |
| Usual Activity | 2 (1–3) | 2 (1–3) | 0.201 | |
| Pain/discomfort | 2 (1–3) | 2 (1–3) | 0.860 | |
| Anxiety/Depression | 1.5 (1–2) | 2 (1–2) | 1.000 | |
| EQ-VAS | 70 (59–85) | 75 (50–85) | 0.893 | |
| Montreal Cognitive Assessment (MoCa) (n = 42) | 26 (24–28) | 27 (26–29) | 0.039 | |
| Multidimensional Fatigue Inventory (MFI 20) | ||||
| General fatigue (n = 44) | 14 (10–18) | 14.5 (10–17) | 0.796 | |
| Physical fatigue (n = 45) | 14 (8–18) | 14 (9–16) | 0.539 | |
| Mental fatigue (n = 45) | 10 (6–14) | 9 (6–13) | 0.084 | |
| Reduced motivation (n = 45) | 9 (6–11) | 8 (6–12) | 0.974 | |
| Reduced activity (n = 45) | 12 (8–17) | 10 (8–15) | 0.170 | |
| Generalised Anxiety Disorder 7-item scale (GAD-7) (n = 45) | 2 (0–7) | 2 (0–6) | 0.112 |
|
| Patient Depression Questionnaire 9 (PHQ-9) (n = 40) | 4 (1–11) | 3 (1–11) | 0.211 | |
| 6-minute walk test (6MWT) (n = 45) | 522 (396–597) | 525 (415–594) | 0.802 | |
Data are presented as number (percentage) or median with interquartile range (IQR).
Categorical data presented as number of total, n (percentage) and compared using Wilcoxon signed-rank test. Continuous data presented as median with interquartile range (IQR) and compared with Mann-Whitney U test.
Demographic and clinical characteristics for the included participants are shown in Table 1. The median age was 59 years and the majority of the participants were men (n = 34, 74%). Twenty-five (54%) had received invasive ventilation and the median length of ICU stay was 10 days (IQR 6–17) (Table 1). Before getting COVID-19, 29 (63%) participants worked full-time and 3 (6%) part-time (Table 2).
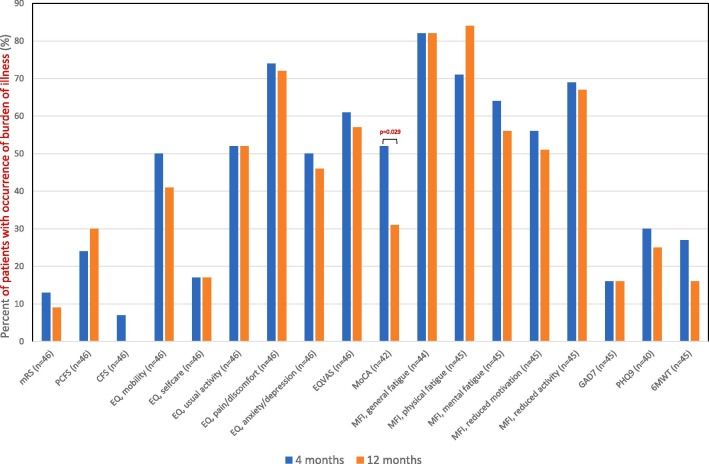
The results showed no improvements between the first and second follow-up, except for cognitive function, measured with MoCA, which improved from 26 (24–28) to 27 (26–29), p = 0.039. Impairment increased in frailty from 2 (2–3) to 3 (2–4), p = 0.002 (Table 2). The most common symptom was fatigue, which occurred in 51% (n = 23) to 84% (n = 38) (Fig. 2 ).
Fig. 2.
Dichotomized results of occurrence of burden of illness in health status, cognitive, physical and psychological outcomes at four months and 12 months after discharge from ICU. Presented as precent of number of patients with occurrence of burden of illness. No differences were seen between 4 and 12 months, except for MoCA (p = 0.029). 6MWT: 6-minute walk test, CFS: Clinical Frailty Scale, GAD-7: Generalised Anxiety Disorder 7-item scale, ICU: intensive care unit, MoCA: Montreal Cognitive Assessment, MFI 20: Multidimensional Fatigue Inventory, mRS: modified Rankin Scale, PCFS: Post-COVID-19 Functional Status Scale, PHQ-9: Patient Depression Questionnaire 9. Categorical data presented percentage and compared using Wilcoxon signed-rank test.
No differences were seen between male and female in any of the outcome measures (Supplement Table 1). At the first follow-up, those treated with invasive ventilation scored higher on functional status than those treated non-invasive ventilation, on both the mRS (median 2 (1–2.5) vs 1 (0.5–2), p = 0.011) and the PCFS (median 2 (1–3) vs 1 (0.5–1), p = 0.025). At the second follow-up, the participants who had been treated with invasive ventilation showed reduced activity on the MFI20 (median 14 (9–16) vs 9 (6–14), p = 0.031). For the other outcome measures, no differences were seen (Supplement Table 2).
The proportion of participants on sick leave decreased between the first and second follow-up (24% vs 13%, p = 0.025), but the proportion of participants working full-time was almost the same at both follow-ups (35% vs 37%, p = 0.317) (Table 2). Among the participants who worked before getting COVID-19 and not returned to work at the first follow-up, had longer stays in ICU (p = 0.005) and several of them had been on invasive ventilation (p < 0.001). At the second follow-up, these differences were not seen, but those who had not returned to work were more affected in health status, cognitive function, fatigue, anxiety and depression (Table 3 ).
Table 3.
Outcome related to working situation among patients who worked before COVID-19.
| Working before COVID-19, n = 32 |
Working before COVID-19, n = 32 |
|||||
|---|---|---|---|---|---|---|
| 3–6 month | 3–6 month | 12 month | 12 month | |||
| Returned to work, n = 20 | Not returned to work, n = 12 | p-value | Returned to work, n = 22 | Not returned to work, n = 10 | p-value | |
| Age | 56 (53–61) | 58 (50–67) | 0.640 | 56 (52–61) | 59 (53–65) | 0.370 |
| Sex (female) | 5 (25) | 1 (8) | 0.250 | 5 (23) | 1 (10) | 0.400 |
| SAPS3 | 50 (46–53) | 53 (46–57) | 0.159 | 51 (46–56) | 51 (46–53) | 0.759 |
| Invasiv ventilation therapy (Days with invasive ventilation therapy) |
5 (25) 0 (0–1.5) |
11 (92) 10.5 (6–22) |
0.000 0.000 |
10 (46) 0 (0–8.5) |
6 (60) 7 (0–28) |
0.453 0.215 |
| Days in ICU |
7 (4–12) | 21 (9–37) | 0.005 | 8 (6–14) | 15 (3–40) | 0.541 |
| Education (≥12 years) | 12 (60) | 5 (42) | 0.322 | 12 (55) | 5 (50) | 0.814 |
| Outcome | ||||||
| EQ-5D-5L | ||||||
| Mobility | 1 (1–2) | 2 (1–2) | 0.209 | 1 (1–1) | 2 (2–3) | 0.001 |
| Self-Care | 1 (1–1) | 1 (1–2) | 0.477 | 1 (1–1) | 1 (1–2) | 0.182 |
| Usual Activity | 1 (1–2) | 3 (2–5) | 0.003 | 1 (1–2) | 3 (2–5) | 0.004 |
| Pain/discomfort | 2 (1–3) | 3 (2–3) | 0.307 | 2 (1–3) | 3 (2–3) | 0.659 |
| Anxiety/Depression | 1 (1–2) | 1 (1–3) | 0.058 | 1 (1–2) | 2 (2–3) | 0.136 |
| EQ-VAS | 75 (66–90) | 68 (51–80) | 0.170 | 80 (69–93) | 53 (48–76) | 0.003 |
| MoCA | 28 (26–29) | 25 (22–28) | 0.019 | 29 (27–29) | 28 (26–28) | 0.046 |
| MFI General fatigue | 10 (6–12) | 17 (8–19) | 0.104 | 12 (8–15) | 16 (15–18) | 0.014 |
| MFI Physical fatigue | 12 (8–17) | 15 (10–18) | 0.407 | 11 (8–15) | 16 (11–19) | 0.013 |
| MFI Mental fatigue | 12 (6–15) | 17 (8–20) | 0.082 | 8 (6–10) | 11 (6–18) | 0.121 |
| MFI reduced motivation | 10 (6–12) | 12 (8–16) | 0.279 | 8 (6–11) | 8 (6–11) | 1.000 |
| MFI reduced activity | 8 (6–11) | 9 (4–11) | 0.967 | 9 (6–14) | 14 (10–17) | 0.022 |
| PHQ9 | 3 (1–8) | 7 (1–11) | 0.338 | 2 (0–5) | 9 (4–15) | 0.012 |
| GAD7 | 3 (0–4) | 4 (0–7) | 0.270 | 1 (0–2) | 5 (3–10) | 0.002 |
| 6MWT | 560 (522–629) | 410 (363–539) | 0.004 | 558 (505–613) | 461 (390–578) | 0.132 |
Categorical data presented as number of total, n (percentage) and compared using Wilcoxon signed-rank test. Continuous data presented as median with interquartile range (IQR) and compared with Mann-Whitney U test. 6MWT: 6-minute walk test, CFS: Clinical Frailty Scale, GAD-7: Generalised Anxiety Disorder 7-item scale, ICU: intensive care unit, MoCA: Montreal Cognitive Assessment, MFI 20: Multidimensional Fatigue Inventory, mRS: modified Rankin Scale, PCFS: Post-COVID-19 Functional Status Scale, PHQ-9: Patient Depression Questionnaire.
Discussion
The main finding of the present study is that the majority of patients reported persistent long-term symptoms that did not improve substantially between 3–6 months and one year after discharge from ICU. In addition, although 87% of patients returned to work during the first year after intensive care for COVID-19, only 35–37% returned to full-time work. The participants were affected in most of the outcome measures, with fatigue being the most common symptom. Improvements over time were only seen in cognitive outcome.
Return to work is a key factor for individuals to return to daily life (Kamdar et al., 2020). In the present study, the number of participants on sick-leave decreased between the first and second follow-up, indicating the ability to return to daily life. Notably, the number of participants back to working full-time was almost the same at both follow-ups. Many participants reported remaining symptoms and a burden of illness in health status, physical, and psychological outcomes at both follow-ups. This may be why the number of participants back in full-time work at 12 months was not larger. Symptoms and burden of illness affect both the well-being of the individual and the general economy and need to be investigated further.
Fatigue after critical illness is shown to have an impact on recovery and the quality of life and may lead to difficulties in daily life as well as economic consequences for the individual (Bench et al., 2021). In our study fatigue was the most common remaining symptom and occurred in all dimensions of fatigue, measured using the MFI20. Furthermore, fatigue seemed to be more common after severe COVID-19 and intensive care than in other ICU patients (Bench et al., 2021). However, how fatigue affects the participants overall health status have not been tested in our study.
The distance of walking among participants during the 6MWT was longer than shown in previous studies in critically ill patients (Parry et al., 2021), but similar to studies of severe COVID-19 sufferers (Huang et al., 2021b, Schandl et al., 2021). This is an interesting finding which needs to be studied further, since the fatigue described by the participants does not correlate well with their actual physical capacity measured with the 6MWT.
The level of frailty measured with the CFS was low. Only a few participants were above the cut-off point for frailty at the first follow-up and none at the second. However, the prevalence of frailty increased over time. Frailty often occurs following critical illness, and also among patients with no frailty before a critical illness, persistent illness may lead to increased frailty over time (Brummel et al., 2020). In this study, frailty was not measured before ICU admission and we can only assume that the majority of participants had no frailty before their COVID-19 infection.
Cognitive impairment occurs in patients treated in intensive care for critical illness, regardless of age or diagnosis (Pandharipande et al., 2013, Rousseau et al., 2021), but we found cognitive improvements over time.
Health status measured using the EQ-VAS revealed lower values than the general population (Burström et al., 2020), but similar values as a population given care for critical illness (Gerth et al., 2019).
One third of patients report presence of anxiety and depression after critical illness and intensive care, which is persistent at a 12-month follow-up (Nikayin et al., 2016, Rabiee et al., 2016). We found similar results, with anxiety reported marginally less often than depression, and a tendency for both anxiety and depression to have decreased at 12 months.
As far as we know, no studies have compared outcome over time for patients treated in intensive care for COVID-19. However, our findings are similar to those of other studies reporting outcomes among patients treated for COVID-19, showing patients to have a burden of illness in several areas (Heesakkers et al., 2022, Huang et al., 2021a, Huang et al., 2021b, Schandl et al., 2021, Seeßle et al., 2021, Zhang et al., 2021, Zhao et al., 2021). The variation in and occurrence of deficits may depend on when follow-up is performed and what instrument is used. We used MoCA, which has been recommended as a core outcome measurement for assessing cognitive function (Needham et al 2017). To assess symptoms and functional status in former ICU patients in a simple way we used the mRS scale (Rankin, 1957). We also used GAD-7 and PHQ-9 to measure anxiety and depression to get a psychiatric perspective. However, mRS, GAD-7 and PHQ-9 have not been widely used in the COVID-19 ICU population, and further investigations would may be useful to fully interpret the results. The overall analysis strategy in this study was not to validate mRS, GAD-7 or PHQ-9 in patients treated for COVID-19, but to be descriptive and generating hypothesis for future studies. There is no consensus on most suitable test for fatigue or at what timepoint the tests should be performed after critical illness and intensive care. These are factors to take into account when interpreting results from different studies. However, by using MFI-20 we gained an increased knowledge of what dimension the patients were most affected in. This study was performed among patients from the first wave of COVID-19, if our results also apply to patients from later waves of COVID-19 needs to be further investigated. However, patients surviving other critical illnesses, show similar burdens of illness after intensive care (Bench et al., 2021, Brummel et al., 2020, Elliott et al., 2014, Geense et al., 2021, Gerth et al., 2019, Hashem et al., 2016, Nikayin et al., 2016, Pandharipande et al., 2013, Parry et al., 2021, Rabiee et al., 2016, Yao et al., 2021) which may indicate that COVID-19 patients do not differ from other ICU-treated patients in that regard. This highlights the importance of personalized rehabilitation and follow-up, to increase the possibility to return to previous work and life, calling for more intervention studies among ICU patients.
Strength and limitations
This study’s strengths included former ICU patients being prospectively followed up over time on two occasions, to examine the development of their burden of illness on health status and cognitive, physical, and psychological outcomes. To reduce the risk of information bias, the study was strengthened through the use of well-validated outcome questionnaires and tests.
We also acknowledge some limitations. The study was performed among patients from the first wave of COVID-19 at a single hospital and thus reflected only the perceptions of patients treated there at that time. However, our COVID-19 population showed similar demographic characteristics as the entire Swedish ICU-treated COVID-19 population during the first wave of COVID-19 (SIR (The Swedish Intensive Care Registry), 2022, Zettersten et al., 2021), suggesting that our cohort was representative for the whole population. A further weakness is that patients were lost between the first and second follow-up, but in this setting a response rate of 67% is relatively high for a population of ICU survivors. Importantly, only minor differences in comorbidities and outcomes at the first follow-up between those who participated in or were lost to the second follow-up indicate that loss to follow-up is not likely to bias the results. In this relatively small population where a wide array of instruments have been used both type 1 and type 2 errors may be expected for any single instrument. However, the picture of limited recovery between four months and 12 months after treatment in ICU is consistent across the board suggesting some measure of reliability of the results.
Conclusions
Former ICU patients had a burden of illness on health status and cognitive, physical, and psychological outcomes at both 3–6 and 12 months after being treated in ICU for severe COVID-19. Most patients were able to return to work within one year of ICU discharge, but only a minority returned to full-time work. No clear improvement in symptoms could be distinguished between four months and one year after ICU discharge, with the exception of a slight improvement in cognitive function. A higher burden of illness was more common in participants who had not returned to work after 12 months.
Data availability
Data is available from the corresponding author on reasonable request pending appropriate permissions and data access agreements (https://doi.org/10.17044/scilifelab.14229410).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors would like to thank the participants for sharing their experiences for this study, and research nurses Joanna Wessbergh and Elin Söderman for help with project and data organization.
Funding
The study was funded by the SciLifeLab/Knut and Alice Wallenberg national COVID-19 research program (M.H.: KAW 2020.0182, KAW2020.0241), the Swedish Heart-Lung Foundation (M.H.: 20210089, 20190639, 20190637), the Swedish Research Council (R.F.: 2014-02569, 2014-07606), the Swedish Kidney Foundation (R.F.: F2020-0054) and the Swedish Society of Medicine (M.H.: SLS-938101). Funding bodies had no role in the design of the study, data collection, interpretation, or in writing of the manuscript.
Authors’ contributions
All authors contributed to the study design. IML and EW characterized participants, summarized data, interpreted data, performed statistical analyses and wrote the first draft. MH, RF, ML and SR interpreted data and revised early versions of the manuscript. All authors read and approved the final manuscript.
Trial registration NCT04474249.
Footnotes
This study was performed at Department of Surgical Sciences Anaesthesiology & Intensive Care, Uppsala University, Sweden
Supplementary data to this article can be found online at https://doi.org/10.1016/j.iccn.2022.103311.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bench S., Stayt L., Shah A., Dhiman P., Czuber-Dochan W. Prevalence and experience of fatigue in survivors of critical illness: a mixed-methods systematic review. Anaesthesia. 2021;76(9):1233–1244. doi: 10.1111/anae.15441. [DOI] [PubMed] [Google Scholar]
- Brummel N.E., Girard T.D., Pandharipande P.P., Thompson J.L., Jarrett R.T., Raman R., Hughes C.G., Patel M.B., Morandi A., Gill T.M., Ely E.W. Prevalence and course of frailty in survivors of critical illness. Crit. Care Med. 2020;48(10):1419–1426. doi: 10.1097/CCM.0000000000004444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burström K., Teni F.S., Gerdtham U.G., Leidl R., Helgesson G., Rolfson O., Henriksson M. Experience-based Swedish TTO and VAS value sets for EQ-5D-5L health states. Pharmacoeconomics. 2020;38(8):839–856. doi: 10.1007/s40273-020-00905-7. [DOI] [PubMed] [Google Scholar]
- Chew M.S., Kattainen S., Haase N., Buanes E.A., Kristinsdottir L.B., Hofsø K., Laake J.H., Kvåle R., Hästbacka J., Reinikainen M., Bendel S., Varpula T., Walther S., Perner A., Flaatten H.K., Sigurdsson M.I. A descriptive study of the surge response and outcomes of ICU patients with COVID-19 during first wave in Nordic countries. Acta Anaesthesiol. Scand. 2021;1–9 doi: 10.1111/aas.13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo R.O., Casaburi R., Coates A.L., Enright P.L., MacIntyre N.R., McKay R.T., Johnson D., Wanger J.S., Zeballos R.J., Bittner V., Mottram C. ATS Committee on Proficiency Standards for clinical pulmonary function laboratories. Am. J. Respir. Crit. Care Med. 2002;166(1):111–117. doi: 10.1164/rccm.166/1/111. [DOI] [Google Scholar]
- Elliott D., Davidson J.E., Harvey M.A., Bemis-Dougherty A., Hopkins R.O., Iwashyna T.J., Wagner J., Weinert C., Wunsch H., Bienvenu O.J., Black G., Brady S., Brodsky M.B., Deutschman C., Doepp D., Flatley C., Fosnight S., Gittler M., Gomez B.T., Needham D.M. Exploring the scope of post-intensive care syndrome therapy and care. Crit. Care Med. 2014;42(12):2518–2526. doi: 10.1097/ccm.0000000000000525. [DOI] [PubMed] [Google Scholar]
- Evans R.A., McAuley H., Harrison E.M., Shikotra A., Singapuri A., Sereno M., Elneima O., Docherty A.B., Lone N.I., Leavy O.C., Daines L., Baillie J.K., Brown J.S., Chalder T., De Soyza A., Diar B.N., Easom N., Geddes J.R., Greening N.J., Brightling C.E. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir. Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geense W.W., Zegers M., Peters M.A.A., Ewalds E., Simons K.S., Vermeulen H., Van Der Hoeven J.G., Van Den Boogaard M. New physical, mental, and cognitive problems 1 year after ICU admission: A prospective multicenter study. Am. J. Respir. Crit. Care Med. 2021;203:1512–1521. doi: 10.1164/rccm.202009-3381OC. [DOI] [PubMed] [Google Scholar]
- Gerth A.M.J., Hatch R.A., Young J.D., Watkinson P.J. Changes in health-related quality of life after discharge from an intensive care unit: a systematic review. Anaesthesia. 2019;74(1):100–108. doi: 10.1111/anae.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem M.D., Nallagangula A., Nalamalapu S., Nunna K., Nausran U., Robinson K.A., Dinglas V.D., Needham D.M., Eakin M.N. Patient outcomes after critical illness: A systematic review of qualitative studies following hospital discharge. Crit. Care. 2016;20(1):1–10. doi: 10.1186/s13054-016-1516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesakkers H., van der Hoeven J.G., Corsten S., Janssen I., Ewalds E., Simons K.S., Westerhof B., Rettig T.C.D., Jacobs C., van Santen S., Slooter A.J.C., van der Woude M.C.E., van den Boogaard M., Zegers M. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327(6):559–565. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., Hu P., Guo L., Liu M., Xu J., Zhang X., Qu Y., Fan Y., Li X., Li C., Yu T., Xia J., Wei M., Chen L., Cao B. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay S.J., Enright P. Reference equations for the six-minute walk in healthy adults [1] (multiple letters) Am. J. Respir. Crit. Care Med. 2000;161(4 I):1396. doi: 10.1164/ajrccm.161.4.16147a. [DOI] [PubMed] [Google Scholar]
- Kamdar, B.B., Suri, R., Suchyta, M.R., Digrande, K.F., Sherwood, K.D., Colantuoni, E., Dinglas, V.D., Needham, D.M., Hopkins, R.O., 2020. Return to work after critical illness: A systematic review and meta-analysis. Thorax 75(1), 17-27. 10.1136/thoraxjnl-2019-213803. [DOI] [PMC free article] [PubMed]
- Klok F.A., Boon G.J.A.M., Barco S., Endres M., Miranda Geelhoed J.J., Knauss S., Rezek S.A., Spruit M.A., Vehreschild J., Siegerink B. The post-COVID-19 functional status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020;56(1) doi: 10.1183/13993003.01494-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K., Spitzer, R.L., Williams, J.B.W. 2001. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 16(9), 606-13. [DOI] [PMC free article] [PubMed]
- Muscedere J., Waters B., Varambally A., Bagshaw S.M., Boyd J.G., Maslove D., Sibley S., Rockwood K. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Needham D.M., Sepulveda K.A., Dinglas V.D., Chessare C.M., Friedman L.A., Bingham C.O. 3rd, Turnbull A.E. 2017. Core outcome measures for clinical research in acute respiratory failure survivors. An international modified Delphi Consensus Study. Am. J. Respir. Crit. Care Med. 196(9):1122-1130. doi: 10.1164/rccm.201702-0372OC. PMID: 28537429; PMCID: PMC5694837. [DOI] [PMC free article] [PubMed]
- Nikayin S., Rabiee A., Hashem M.D., Huang M., Bienvenu O.J., Turnbull A.E., Needham D.M. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen. Hosp. Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandharipande P.P., Girard T.D., Jackson J.C., Morandi A., Thompson J.L., Pun B.T., Brummel N.E., Hughes C.G., Vasilevskis E.E., Shintani A.K., Moons K.G., Geevarghese S.K., Canonico A., Hopkins R.O., Bernard G.R., Dittus R.S., Ely E.W. Long-term cognitive impairment after critical illness. N. Engl. J. Med. 2013;369(14):1306–1316. doi: 10.1056/nejmoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry S.M., Nalamalapu S.R., Nunna K., Rabiee A., Friedman L.A., Colantuoni E., Needham D.M., Dinglas V.D. Six-minute walk distance after critical illness: A systematic review and meta-analysis. J. Intensive Care Med. 2021;36(3):343–351. doi: 10.1177/0885066619885838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiee, A., Nikayin, S., Hashem, M.D., Huang, M., DInglas, V.D., Bienvenu, O.J., Turnbull, A.E., Needham, D.M., 2016. Depressive symptoms after critical illness: A systematic review and meta-analysis. Crit. Care Med. 44(9), 1744-53. 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed]
- Rabin R., De Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann. Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- Rankin J. Cerebral vascular accidents in patients over the age of 60: II. Prognosis. Scott. Med. J. 1957;2(5):200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- Rousseau A.F., Minguet P., Colson C., Kellens I., Chaabane S., Delanaye P., Cavalier E., Chase J.G., Lambermont B., Misset B. Post-intensive care syndrome after a critical COVID-19: cohort study from a Belgian follow-up clinic. Ann. Intensive Care. 2021;11(1) doi: 10.1186/s13613-021-00910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schandl A., Hedman A., Lyngå P., Fathi Tachinabad S., Svefors J., Roël M., Geborek A., Andersson Franko M., Söderberg M., Joelsson-Alm E., Darlington P. Long-term consequences in critically ill COVID-19 patients: A prospective cohort study. Acta Anaesthesiol. Scand. 2021;65(9):1285–1292. doi: 10.1111/aas.13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeßle J., Waterboer T., Hippchen T., Simon J., Kirchner M., Lim A., Müller B., Merle U. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): A prospective cohort study. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigfrid, L., Drake, T.M., Pauley, E., Jesudason, E.C., Olliaro, P., Lim, W.S., Gillesen, A., Berry, C., Lowe, D.J., McPeake, J., Lone, N., Munblit, D., Cevik, M., Casey, A., Bannister, P., Russell, C.D., Goodwin, L., Ho, A., Turtle, L., Scott, J.T., ISARIC4C investigators, 2021. Long Covid in adults discharged from UK hospitals after Covid-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. Lancet Reg. Heal. Eur. 8, 100186. 10.1016/j.lanepe.2021.100186. [DOI] [PMC free article] [PubMed]
- SIR (The Swedish Intensive Care Registry), 2022. Retrived from https://www.icuregswe.org/data--resultat/utdataportalen/ Assessed 13-02-2022.
- Smets E.M.A., Garssen B., Bonke B., De Haes J.C.J.M. The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B.W., Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Swedish Society of Anaesthesiology and Intensive Care (SFAI), 2015. Guidelines for Intensive Care Sweden. Retrived from https://sfai.se/sfai/om-sfai/. Assessed 11-12-2021.
- Wallin E., Hultström M., Lipcsey M., Frithiof R., Rubertsson S., Larsson I.-M. Intensive care-treated COVID-19 patients’ perception of their illness and remaining symptoms. Acta Anaesthesiol. Scand. 2021;66(2):240–247. doi: 10.1111/aas.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2022). WHO Coronavirus Disease (COVID-19) Dashboard. s. Retrived from https://covid19.who.int. Accesed 08-02-2022.
- Yao L., Li Y., Yin R., Yang L., Ding N., Li B., Shen X., Zhang Z. Incidence and influencing factors of post-intensive care cognitive impairment. Intensive Crit. Care Nurs. 2021;67:103106. doi: 10.1016/j.iccn.2021.103106. [DOI] [PubMed] [Google Scholar]
- Zettersten E., Engerström L., Bell M., Jäderling G., Mårtensson J., Block L., Larsson E. Long-term outcome after intensive care for COVID-19: differences between men and women—a nationwide cohort study. Crit. Care. 2021;25(1):86. doi: 10.1186/s13054-021-03511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Wang F., Shen Y., Zhang X., Cen Y., Wang B., Zhao S., Zhou Y., Hu B., Wang M., Liu Y., Miao H., Jones P., Ma X., He Y., Cao G., Cheng L., Li L. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw. Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yang C., An X., Xiong Y., Shang Y., He J., Qiu Y., Zhang N., Huang L., Jia J., Xu Q., Zhang L., Zhao J., Pei G., Luo H., Wang J., Li Q., Gao Y., Xu A. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int. J. Infect. Dis. 2021;327(6):559–565. doi: 10.1016/j.ijid.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the corresponding author on reasonable request pending appropriate permissions and data access agreements (https://doi.org/10.17044/scilifelab.14229410).