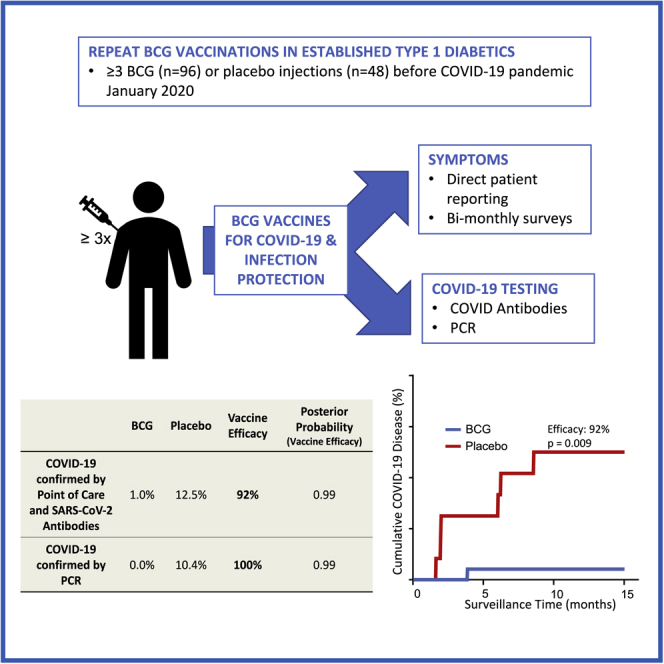
Summary
There is a need for safe and effective platform vaccines to protect against coronavirus disease 2019 (COVID-19) and other infectious diseases. In this randomized, double-blinded, placebo-controlled phase 2/3 trial, we evaluate the safety and efficacy of a multi-dose Bacillus Calmette-Guérin (BCG) vaccine for the prevention of COVID-19 and other infectious disease in a COVID-19-unvaccinated, at-risk-community-based cohort. The at-risk population is made of up of adults with type 1 diabetes. We enrolled 144 subjects and randomized 96 to BCG and 48 to placebo. There were no dropouts over the 15-month trial. A cumulative incidence of 12.5% of placebo-treated and 1% of BCG-treated participants meets criteria for confirmed COVID-19, yielding an efficacy of 92%. The BCG group also displayed fewer infectious disease symptoms and lesser severity and fewer infectious disease events per patient, including COVID-19. There were no BCG-related systemic adverse events. BCG’s broad-based infection protection suggests that it may provide platform protection against new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and other pathogens.
Key words: Bacillus Calmette-Guérin, BCG, COVID-19, infectious diseases, phase 2/3 trial, host microbe interactions, hygiene hypothesis, type 1 diabetes, autoimmune, randomized double blinded clinical trial, vaccine, Clinicaltrials.gov: NCT02081326
Graphical abstract
Highlights
-
•
We report on our randomized trial of multi-dose BCG for protection against COVID-19
-
•
BCG is safe and has 92% efficacy versus placebo against COVID-19
-
•
Findings also suggest platform protection against additional infectious diseases
-
•
Efficacy takes 1–2 years to manifest, but the protection may last decades
In a randomized, double-blinded, placebo-controlled phase 2/3 trial, Faustman et al. investigate the safety and efficacy of multiple doses of the more than 100-year-old Bacillus Calmette-Guérin (BCG) vaccine against COVID-19. They also determine vaccine efficacy against other infectious diseases, which, if present, would suggest platform protection against new SARS-CoV-2 variants.
Introduction
The Bacillus Calmette-Guérin (BCG) vaccine is a more than 100-year-old vaccine originally developed for tuberculosis protection. It is heralded as the safest vaccine ever developed, with 3–4 billion people already vaccinated, and an annual 120 million newborns vaccinated.1 It is highly affordable at about 10–75 cents/dose. Over the last 17 years, randomized clinical trials and epidemiology studies have shown that the BCG vaccine protects humans from a multitude of infections, including upper respiratory tract infections, leprosy, malaria, viral, and bacterial infections.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 The first indication of the broad infectious disease protective abilities of this vaccine came 100 years ago when Albert Calmette, the vaccine’s co-inventor, noted a 4-fold decline in child mortality (unrelated to tuberculosis) in vaccinated children, presumably from broad infectious disease protection.17 These protective effects appear also when adolescents are re-vaccinated with BCG after the typical newborn dose.10 The BCG vaccine may also protect humans from immune diseases such as type 1 diabetes and multiple sclerosis.18, 19, 20, 21 The mechanisms behind these wide-ranging benefits are a topic of active scientific discovery.
With the onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, some epidemiology studies began to uncover an association between neonatal BCG vaccination and reduced morbidity and mortality from COVID-19, even in older adults decades after the typical neonatal vaccinations, on a country-by-country basis.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 In some other global populations with varying neonatal exposures, different BCG strains, and other populations, benefits were not observed.24,41, 42, 43, 44, 45, 46, 47 Because the United States has never vaccinated newborns or adults with BCG, a randomized trial of BCG for possible protection from SARS-CoV-2 infection offers a clean comparison in a vaccine-naive adult United States population. This parallel trial was adapted from an ongoing double-blinded, randomized trial of BCG for treatment of long-standing adult type 1 diabetes, so all cohorts were fully vaccinated with 3 BCG or placebo vaccinations at the start of the coronavirus 2019 (COVID-19) pandemic in the United States on January 1, 2020. Those with type 1 diabetes represent one of the most vulnerable populations in the United States for morbidity and mortality from COVID-19.48 Patients with type 1 diabetes also have well-known increased infectious disease risk, including COVID-19.49
As the COVID-19 pandemic worsens, vaccine development assumes center stage. But antigen-specific vaccines, the focus of most clinical programs, are struggling to keep pace with new viral variants. The ideal vaccine should be safe, efficacious, affordable, and offer durable protection against ever-changing viral variants and future infectious disease pandemics. We conducted a randomized, double-blinded, placebo-controlled platform trial over 15 months (January 1, 2020 to April 2021) to determine whether the BCG vaccine might offer a platform vaccine technology for broad-based infectious disease protection, including protection against COVID-19 in the vulnerable type 1 diabetic population.
Results
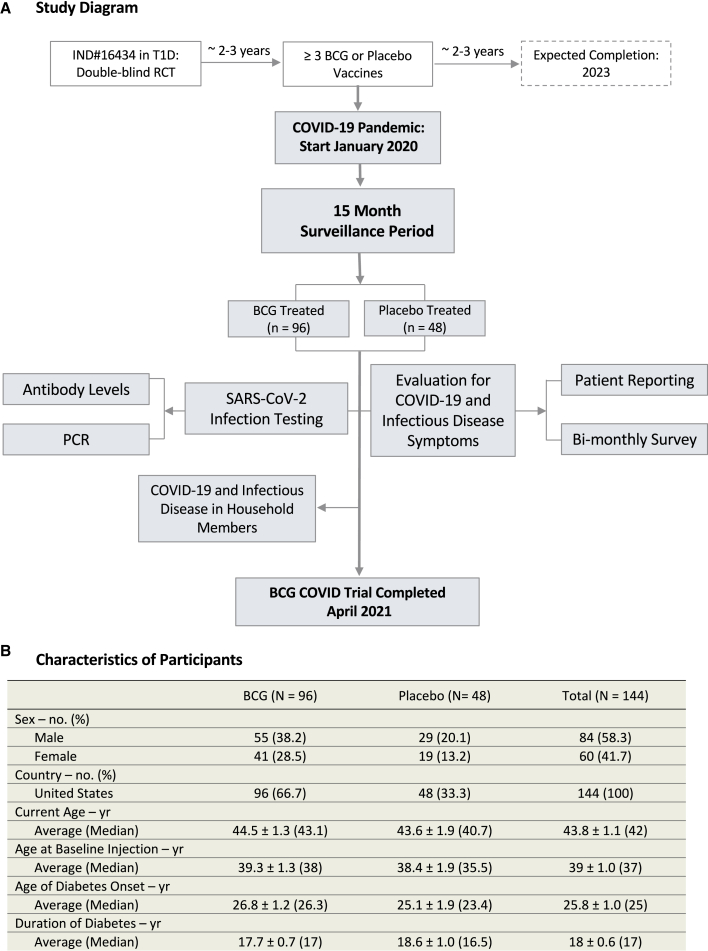
We assessed the impact of multi-dose BCG vaccinations for protection from SARS-CoV-2 infections and other infections (Figure 1A). All participants at the start of this parallel study had 3 BCG vaccinations or 3 placebo vaccinations over a 2-year time period preceding the pandemic.
Figure 1.
Flow diagram and characteristics of participants
(A) Flow diagram representing all enrolled participants from January 1, 2020 to April, 2021 for this double-blinded, randomized clinical trial testing repeat Tokyo-172 BCG vaccination versus placebo for COVID-19 protection. All 144 subjects were followed for 15 months with a 2:1 randomization and no dropouts. Data collection for this trial ended on April 2, 2021, the date when subjects started to receive provisionally approved COVID-19-specific vaccines. The geographical locations of the participants within the United States are shown in Figure S5.
(B) Table of participant characteristics.
The trial, which had no dropouts, found that multi-dose BCG was safe
All 144 participants were United States citizens not previously vaccinated with BCG and had long-standing type 1 diabetes, a co-morbid condition for worse COVID-19 disease and symptoms (Figure 1B).49 None of the participants was lost to follow up or dropped out during the current 15-month parallel study. A total of 150 participants were enrolled for the first study on type 1 diabetes; at the start of this parallel clinical trial (January 2020) evaluating the effects of BCG multi-dosing on overall infections and COVID-19-specific disease, there were 96 participants treated with BCG and 48 treated with placebo. These recipients were all vaccinated with BCG or placebo in the 2 years prior. Unlike antigen-specific vaccines, no BCG-related systemic adverse events occurred in any of the participants during the vaccination time period. BCG vaccines do cause local skin reactions that usually appear at 2–4 weeks. No excess local reactions were reported as adverse events. During the time period of this trial, other COVID-19 vaccines were not yet available and therefore had no influence on this study.
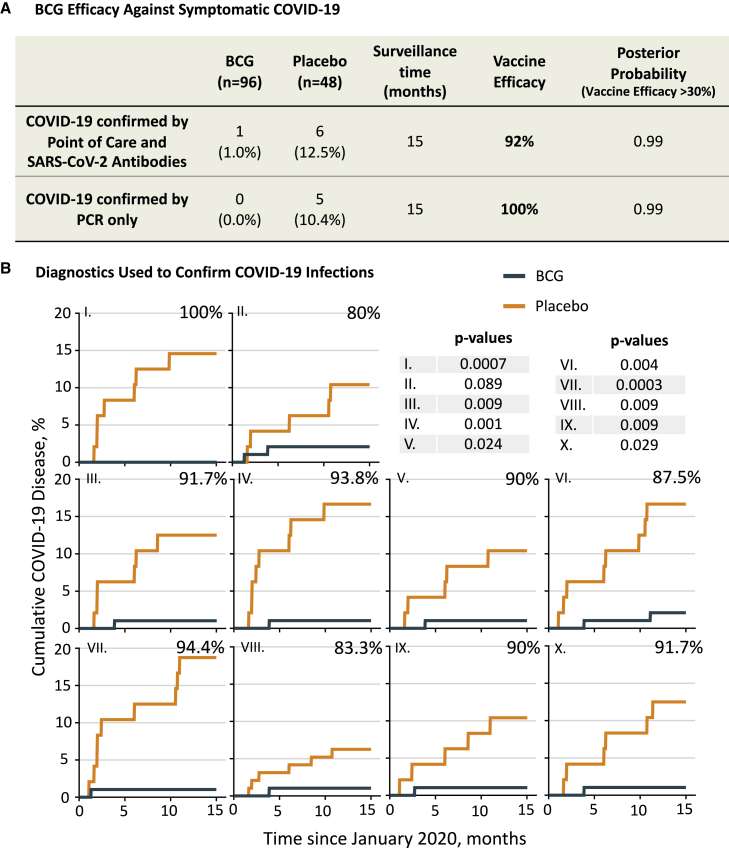
The BCG group had fewer cases of confirmed COVID-19 than the placebo group, with vaccine efficacy at 92%
The cumulative incidence of confirmed COVID-19 in the BCG cohort was 1 out of 96 (1%), whereas it was 6 out of 48 (12.5%) in the placebo group, at the end of the 15-month COVID-19 trial. The criteria for a confirmed case of COVID-19 were developed early in the pandemic prior to widespread availability of PCR. Therefore, the criteria required (see STAR Methods and Figure S1) were reporting at least 1 FDA-defined symptom of COVID-19 plus positive findings on ≥5 out of 10 assays (9 SARS-CoV-2 immunoglobulin G [IgG] antibody binding assays and a 10th assay that included PCR testing for viral RNA). Because of the limited PCR availability, several subjects were diagnosed on the basis of symptoms plus multiple positive serologies only. The efficacy of BCG (versus placebo) for preventing a confirmed case of COVID-19 was 92% (Figure 2). Monte Carlo statistics resulted in a posterior probability (with vaccine efficacy >30%) of 0.99. Because the current standard of diagnosis for COVID-19 is based on PCR, we also calculated BCG efficacy based on at least one FDA-defined symptom of COVID-19 plus a positive PCR alone. We found no symptomatic subjects that were PCR positive in the BCG group (0%), whereas 5 symptomatic and PCR-positive subjects were in the placebo group (10.4%). These results indicate an efficacy of 100% at 0.99 posterior probability if only PCR results are considered. This can also be readily visualized in the PCR cumulative graph (Figure 3B), which shows the first detected COVID-19 case based on PCR at 7 months into the trial, whereas serology testing for SARS-CoV-2 antibodies revealed the first positive cases as early as 2 months (Figure 2B).
Figure 2.
BCG vaccine efficacy and diagnostic confirmation of COVID-19
(A) Shown is the cumulative incidence of confirmed COVID-19 as a primary endpoint. During the 15-month surveillance time, one BCG recipient out of 96 (1.0%) met our criteria for molecularly confirmed COVID-19. In contrast, 6 out of 48 placebo recipients met the criteria (12.5%). Fisher’s exact testing showed a significant difference (two-tailed p = 0.006). Calculated vaccine efficacy was 92%, and the posterior probability (vaccine efficacy >30%) was 0.99. This was calculated using the Monte Carlo method. Vaccine efficacy was defined as (p1 – p2)/p1 × 100, where p1 is the percentage of COVID-positive subjects in the placebo group and p2 is the percentage of COVID-positive subjects in the BCG group. Our criteria for confirmed COVID-19 (see main text) required a combination of COVID-19 symptom(s) and ≥5 of 10 positive antibody assays including PCR, if available. Since many current clinical trials only define confirmed COVID-19 by symptom(s) and positive PCR testing, the PCR-only group was studied separately for vaccine efficacy (A and B).
(B) Cumulative findings from each of the 10 diagnostic tests used to confirm COVID-19 (along with positive symptoms). These tests included the presence of COVID-19-specific antibodies to various SARS-CoV-2 virus epitopes through protein display (I–VIII), antibodies to the receptor-binding domain with an ELISA test (IX), and point-of-care testing (X) that included PCR. Our criteria for having confirmed COVID-19 required at least 5 of 10 detection methods to be positive, along with symptom(s). For the antibody assays, a patient was considered positive when the test resulted in a Z score of ≥3. In the cumulative graphs, the x axis data show the time period of the 15-month trial. The y axis shows the cumulative percentage of positive subjects. Except for the point-of-care graph (X), all other graphs represent the percentage of BCG and placebo patients with a Z score of ≥3 for the anti-SARS-CoV-2 antibody binding to a given protein region of the virus, i.e., the average antibody level during the COVID trial period was at least 3 standard deviations greater than the average level in the period preceding the COVID trial (baseline). The percentiles at the top right of each graph represent the calculated vaccine efficacy if this test alone was used to diagnose COVID-19 disease. Respective efficacy and Fisher’s exact p values for each COVID-19 antibody test were as follows: (I) 100%, p = 0.0007; (II) 80%, p = 0.089; (III) 91.7%, p = 0.009; (IV) 93.8%, p = 0.001; (V) 90%, p = 0.024; (VI) 87.5%, p = 0.004; (VII) 94.4%, p = 0.0003; (VIII) 83.3%, p = 0.009, (IX) 90%, p = 0.009; and (X) 91.7%, p = 0.029. For all graphs, BCG n = 96, placebo n = 48.
Figure S1 shows the viral protein regions for each anti-COVID antibody tested. Number at risk data for each cumulative graph are shown in Figure S4.
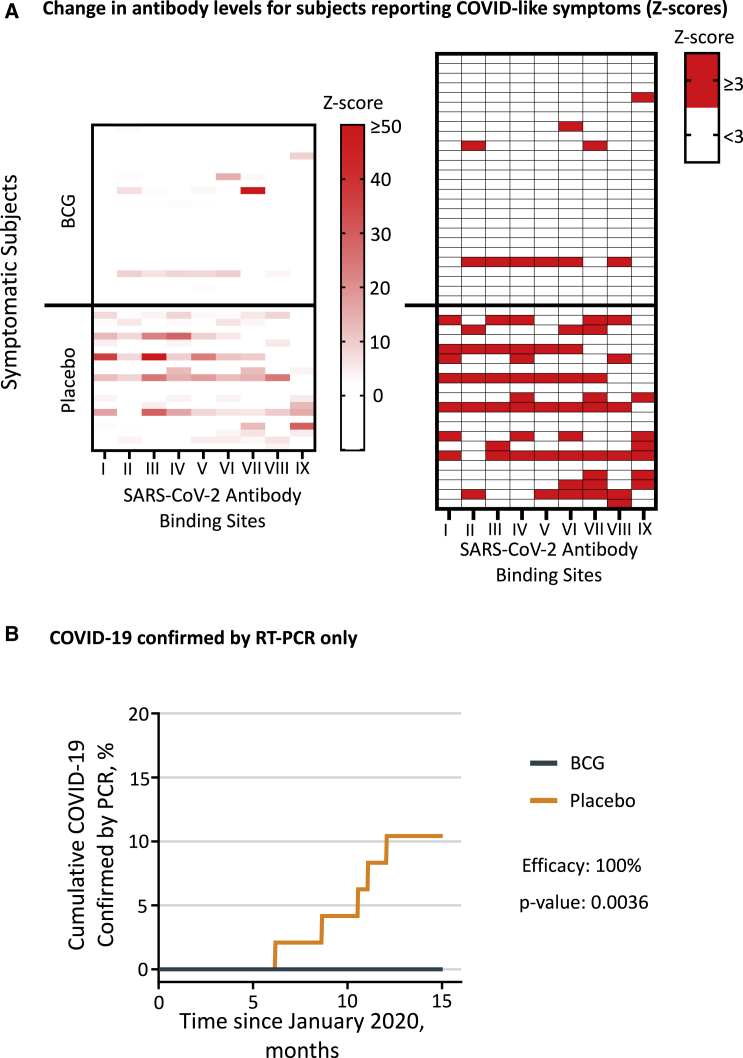
Figure 3.
Reduced COVID-19 disease markers after BCG vaccination
(A) The heatmaps show Z scores for antibodies against SARS-CoV-2 in BCG- and placebo-treated symptomatic subjects (BCG n = 26, placebo n = 21). Since some of the Z scores in the left map are faint, the right map shows which Z scores are ≥3. The Roman numerals at the bottom of each lane denote antibodies against SARS-CoV-2 protein regions as listed in Figure S1. The maximum Z score in the left heatmap was 126.
(B) Cumulative incidence of confirmed COVID-19 defined by symptomatic subjects testing positive solely by PCR. The BCG group (n = 96) had no symptomatic subjects who were PCR positive (0%), whereas the placebo group (n = 48) had 5 symptomatic and PCR-positive subjects (10.4%). This difference was significant (Fisher’s exact p = 0.0036) and translated to a vaccine efficacy of 100% with 0.99 posterior probability. There was low availability of positive PCR tests at point-of-care locations during the first 7 months of the trial. This, together with the need to perform the PCR test in a narrow window of about 2 weeks to be positive, is the reason why we designed the 9 non-PCR methods shown in Figures 2A, 2B, and 3A.
There were no deaths from COVID-19 in either the BCG group or the placebo group.
Heatmap comparison shows higher SARS-CoV-2 antibody-level presence and intensity in the placebo group over the BCG group
Heatmap comparisons of SARS-CoV-2 antibody-level presence and intensity (Z scores versus pre-COVID baseline) show a significant increase in infections and the intensity of the antibody response in symptomatic subjects in the placebo over the BCG group (Figure 3A). These data are in symptomatic subjects. For completeness, we also present the COVID-19 antibodies in all trial subjects, with and without symptoms. The heatmap of all trial participants again shows the presence of COVID-19 infections almost exclusively in the placebo subjects (Figure S2).
Cumulative graphs for the anti-COVID-19 antibody tests to different regions of the virus are shown in Figure 2B. Number at risk data are shown in Figure S4. The identity of the SARS-CoV-2 protein region eliciting an antibody response, mostly the spike protein, is denoted by the Roman numerals listed in Figure S1. Six protein regions eliciting an antibody response were from viral spike protein 1, two were located in spike protein 2, and one viral region was specific for antibodies against viral RNA polymerase. Almost all COVID-19 viral regions included in these tests show significantly increased accumulation of antibody reactivity in the placebo group compared with the BCG group, except for COVID-19 viral epitope II.
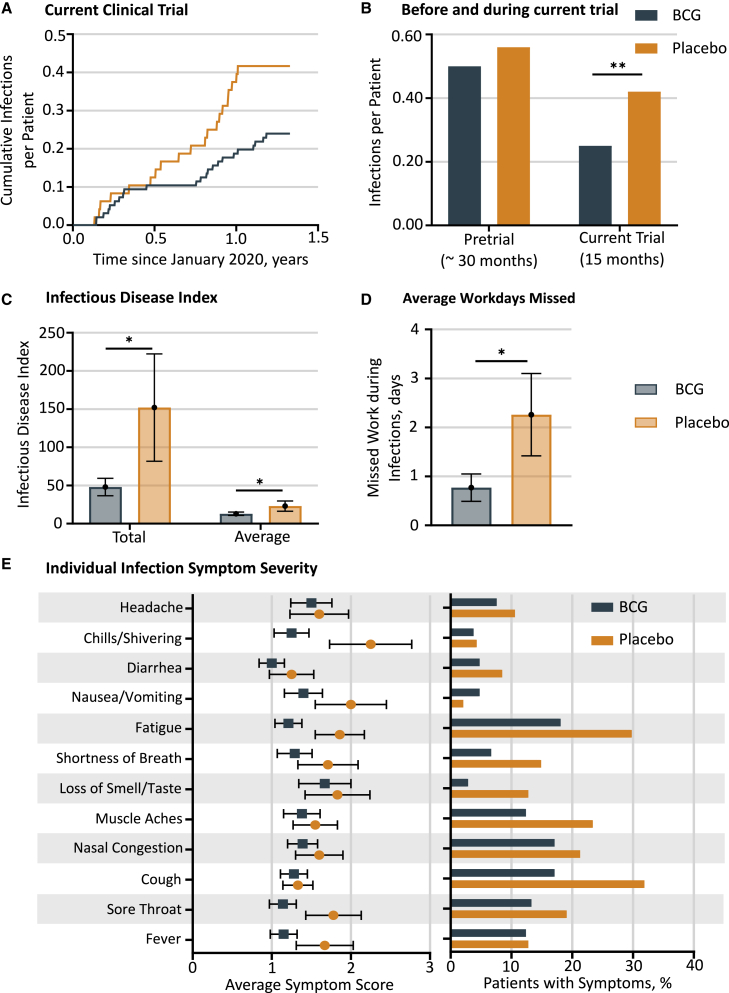
The BCG group (versus placebo group) exhibited fewer infections per patient and fewer infectious disease symptoms and severity assessed by the total and average infectious disease index
We analyzed infectious disease events collected as adverse events by the Medical Dictionary for Regulatory Activities (MedDRA) classification coding (Figure S3B). Prior clinical trial data suggest that BCG vaccination exhibits “off-target” effects, i.e., viral and bacterial infectious disease prevention (and, in this study, prevention of SARS-CoV-2 viral infections) beyond its intended use in tuberculosis protection.22, 23, 24, 25,27,37, 38, 39,50 Using the time period of the current COVID-19 trial, we analyzed infectious disease symptoms including COVID-19 and other infections. The cumulative infectious diseases reported by the subjects and represented as cumulative infections per patient were significantly fewer in the BCG versus placebo group (Poisson model comparing adverse events rates p = 0.004; Figures 4A and S3B). These findings are likely to represent the minimum protection. Some clinical trial data for other off-target effects of the BCG vaccine suggest that the time period for BCG clinical effectiveness is often in the 2- to 3-year range after vaccinations begin.19,20 To look at this time period of BCG onset of vaccine effectiveness but in the setting of infection protection, the clinical trial infectious disease adverse events were broken into the pre-COVID trial period, during which the three BCG vaccines were administered, versus the subsequent 15-month time period of this current clinical trial (Figure 4B). While comparison of infectious adverse events in BCG versus placebo groups during the current trial had a significant Poisson distribution (p = 0.004), there was no significant difference in all infections during the pre-COVID trial period (Poisson p = 0.46). These data suggest that, just like other reported off-target events of the BCG vaccine, it might similarly take about 2 years after the first vaccine for maximal effectiveness for the platform infectious disease protection.
Figure 4.
Cumulative infections and infection severity
(A) Cumulative total infectious diseases during the 15 months of surveillance. This cumulative figure shows all infections per patient, including all COVID-19 events, within the BCG group (blue) compared with the placebo group (red). Included are the infections for which multiple subject events were documented in both BCG and placebo groups. Comparison by means of a Poisson model yields a significant difference with p = 0.004 (BCG n = 24 out of 96 T1D total, placebo n = 20 out of 48 T1D total).
(B) Cumulative infections for two different time periods: pre-COVID-19 pandemic (the 2.5-year period prior to this trial, i.e., the pretrial) and during the COVID-19 pandemic (the current trial period of 15 months). The pretrial time period was when all clinical trial subjects received their ≥3 BCG vaccines or placebo vaccines; the current trial was when the subjects were monitored during the 15-month observation during the COVID-19 pandemic (total patients BCG n = 96, placebo n = 48). The lack of a statistical difference in the number of infections between BCG and placebo groups in the pretrial period suggests that a longer length of time than 2.5 years is necessary to realize BCG’s maximal infectious disease protection. It appears that during the entire period of 15 months, prior BCG vaccinations were protecting from COVID-19 and other infections. ∗∗p < 0.01.
(C) Infectious disease index for symptomatic patients. Symptomatic patients in the BCG-treated group had significantly reduced total infectious disease symptom index (placebo 152 ± 70 [n = 20] versus BCG 48 ± 11 [n = 31], p = 0.04, single tail and unpaired) as well as average infectious disease symptom index (placebo 23 ± 7 [n = 20] and BCG 13 ± 2 [n = 31], p = 0.04, single tail and unpaired). We first calculated total and average symptom scores per patient and then calculated average and SEM of each of these for BCG and placebo cohorts separately (∗Student’s t test p < 0.05, one-tailed, unpaired).
(D) Patients in the BCG cohort reported significantly fewer days of missed work during infections compared with the placebo group (∗p = 0.02). Missed work was reported by 7 out of 32 BCG patients and by 8 out of 17 placebo patients.
(E) The number of patients that reported at least one symptom were 20 out of 48 in the placebo group and 33 out of 96 in the BCG group. The placebo group had more severe average symptoms compared with the BCG group for 12 out of 12 symptoms (left panel). The number of patients in BCG and placebo groups that reported each symptom was then expressed (right panel). For 11 out of 12 symptoms, there was a higher percentage of symptomatic patients in the placebo group compared with the BCG group. Statistical analysis by Student’s t test (one-tailed, unpaired, ∗p < 0.05).
Our intent was to evaluate the severity of COVID-19 symptoms with BCG treatment compared with placebo in confirmed COVID-19 patients, but this was not possible because only one subject in the BCG group fit our criteria for confirmed COVID-19. We therefore analyzed the symptoms in symptomatic BCG- and placebo-treated participants—regardless of whether they were confirmed COVID positive—to understand the impact of BCG on overall infectious disease severity.
Using data from symptom surveys based on FDA guidelines51 that were completed every 2 months, we calculated a total and an average infectious disease index (Figure S3A). Indexes for each individual patient were calculated separately and then averaged across all subjects in the BCG and placebo cohorts (Figure 4C). Comparing only symptomatic patients, the total covid index in the BCG cohort (48 ± 11, n = 31) was significantly reduced versus the placebo group (152 ± 70, n = 20; p = 0.04). The average infectious disease index also showed a significant decrease (BCG 13 ± 2, n = 31 versus placebo 23 ± 7, n = 20; p = 0.04). This indicates that BCG vaccination reduced the severity and duration of all infectious disease symptoms compared with placebo. Symptomatic patients in the BCG group also reported significantly fewer numbers of days of missed work compared with the placebo group (Figure 4D; BCG 0.77 ± 0.28; placebo 2.26 ± 0.84, p = 0.02). All individual average symptom scores (12 out of 12) were more severe in the placebo group versus the BCG group (Figure 4E, left panel). For 11 out of 12 symptoms, there also was a higher percentage of patients in the placebo group versus the BCG group (Figure 4E, right panel).
BCG recipients had comparable or milder infectious disease symptoms compared with their household members, whereas most placebo recipients had more severe symptoms compared with their household members
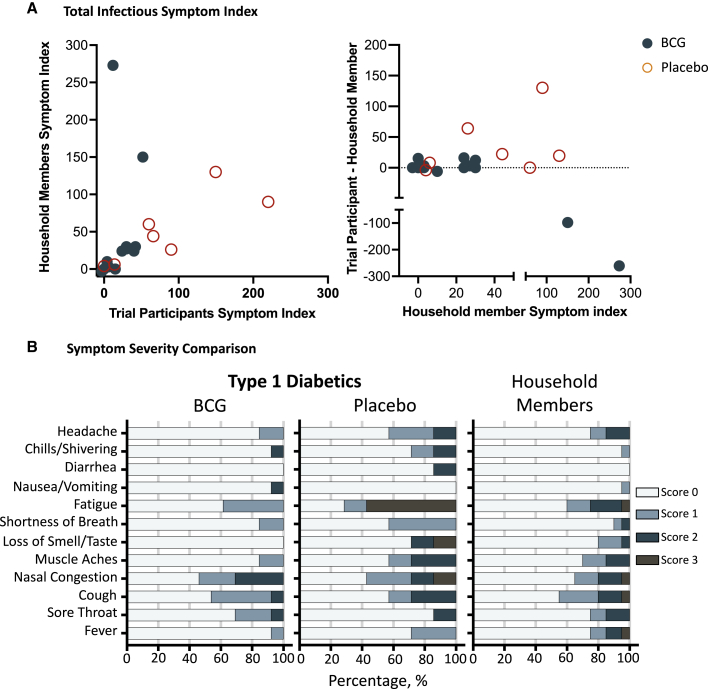
We, on an exploratory basis, studied infectious disease severity of trial participants compared with non-diabetic adults living in the same household. We collected infectious disease symptom information for trial participants and for co-habitating adult partners in 20 households (13 BCG and 7 placebo) and determined the differences in total infectious index for each household (Figures 5A and S3A). The data show that BCG recipients had comparable or milder symptoms compared with their household members, whereas most placebo recipients had more severe disease compared with their household members. The symptom scores between participants and household members were compared between the BCG-treated group and the placebo-treated group using a two-sample Wilcoxon test (p = 0.049, two-tailed). The stacked horizontal bars in Figure 5B show the distributions of the infectious disease symptom scores in each group. BCG recipients overall had milder symptoms compared with placebo recipients and even compared with non-diabetic household member controls. Number at risk data are shown in Figure S4.
Figure 5.
Infection symptoms of trial participants versus adult household members
(A) Infection symptoms in BCG and placebo groups compared with non-diabetic adult partners living in the same households. We collected surveys of symptoms of infectious diseases from all trial participants and household members of 13 BCG families and 7 placebo families. The BCG-treated trial participants had comparable or lower total infectious symptoms indexes compared with their partners living in the same household, whereas most placebo-treated trial participants had more severe symptoms compared with their partners. Statistical analysis of the differences by two-sample Wilcoxon testing was significant (two-tailed; p = 0.049; BCG n = 13 and placebo n = 7). Left panel: xy plot of symptom index of trial participants versus their household members. Right panel: the same data are also shown as an xy plot of household members versus the difference of trial participants and their household members. This plot makes it easier to visualize which groups had milder or more severe disease by introducing positive and negative differences.
(B) Distribution of individual infectious disease symptoms in BCG- and placebo-treated participants and infected household members across the four scoring possibilities (0: no symptoms; 1: mild; 2: moderate; 3: severe symptoms). Number at risk data are shown in Figure S4.
These data support previously published data that type 1 diabetics not only have more severe COVID-19 but also have more severe and more infectious disease events.49 Lastly, the distribution of symptoms in Figure 5B shows once again that for BCG-treated trial participants, individual symptoms were minimized with vaccinations compared with placebo.
Discussion
This randomized, double-blinded, placebo-controlled trial shows that multi-dose BCG vaccination is safe and prevents COVID-19 with an efficacy of 92%–100% (depending on case definition) relative to placebo. This trial was conducted in the United States in previously unvaccinated at-risk type 1 diabetic subjects. BCG-vaccinated adults also had reduced incidence and severity of all infections. This randomized, double-blinded study is now a rigorous manner of formal testing and substantiates a large body of epidemiological, observational, and clinical trial evidence that BCG vaccines might, in some settings, provide a broad platform for infectious disease protection. These data are from a study conducted in the United States as a multi-dose BCG vaccine therapy and administered 2 years prior to the pandemic to allow sufficient time for full effectiveness.2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16
This clinical trial has several strengths. First, this trial is, to our knowledge, the first peer-reviewed randomized double-blinded trial of multi-dose BCG for COVID-19 protection and infection protection in a United States population. A prior trial of single-dose BCG, with immediate COVID-19 disease monitoring, showed neither protection from COVID-19 infections nor protection from disease severity.7 This trial was likely too short in duration to find an effect based on the evidence from other off-target effects (see below) indicating years to show clinical efficacy. Second, our trial uses a very potent strain of BCG, Tokyo-172. BCG strain differences for other off-target indications are important, and this strain of BCG exhibits some of the highest in vitro potency and is highly immunogenic.52, 53, 54, 55, 56, 57 Indeed, it is now appreciated that Japan, as a country with mandatory BCG vaccines and as one of the oldest populations in the world, has remarkable resistance to COVID-19.58 Third, the diabetic study population is high risk for infections, suggesting that the vaccine is efficacious in populations susceptible to infections. Fourth, the study uses rigorous molecular methods to define a case of COVID-19 based on both PCR and/or SARS-CoV-2-directed immunoglobulin production to very specific regions of the virus to confirm current or past infection. Fifth, there was no patient attrition. Sixth, the subjects are from the United States. This is important because all subjects, prior to enrollment, were confirmed by diagnostics and by history to be unexposed to tuberculosis and lacking prior BCG vaccinations. The United States has never had a country policy of neonatal BCG vaccinations. Two properties of the vaccine are especially noteworthy. BCG’s 100-year-strong safety record and its international use as a neonatal vaccine should help to overcome vaccine hesitancy. Seventh, this trial was conducted in subjects who all had negative serology for prior COVID-19 infections and also had not yet received the approved Moderna and Pfizer vaccines that could confound the effect of BCG’s protection alone. Finally, the BCG vaccine is affordable, which is especially important in the developing world, where it offers almost immediate availability.
In conclusion, the BCG vaccine effectively protects against COVID-19 and provides broad infectious disease protection as now tested with a formal double-blinded and randomized clinical trial. Also, the BCG vaccine is safe, effective, affordable, and potentially protective against every changing viral variant of the COVID-19 pandemic, based on its broad-based protection against other infections.
Limitations of the study
The most important limitation of our trial is that off-target effects take time to systemically manifest, but, once they do appear, they may offer broad-based infectious disease protection over the long term. BCG does not work as fast as the antigen-specific COVID-19 vaccines and also likely requires multiple treatments over time to be effective, at least in BCG-naive adults. Antigen-specific vaccines generally take weeks to show an effect, although protection is limited by a specific strain and then, downstream, by short durability. In the case of the BCG vaccine, the off-target platform protection from infections has a slower onset (months to years) but offers perhaps life-long durability. The other off-target effects of BCG are in its reset of autoimmune diseases, benefits that take 2 years to show an effect.18, 19, 20, 21 Nonetheless, the benefits may last for decades thereafter as well, at least 8 years in randomized clinical trials. Many of the epidemiologic studies show off-target protection after neonatal or adult vaccination, including one study showing >60 years of protection from a neonatal BCG vaccine.19,59 This “long immunity” is both long to start and perhaps long lasting. The slow onset of therapeutic effects and perhaps lifelong durability could be due to the gradual migration of the BCG organism from the vaccine injection site to the bone marrow, where it may infect resident stem cells, a process that is hastened by the intravenous (i.v.) administration of BCG.19,60,61 Longitudinal mechanistic data from our lab show that BCG, over time, alters key metabolic and immune signaling pathways via methylation of genes over a 2- to 3-year time period.61, 62, 63, 64 Others have shown that more acute changes are brought about by chemical modifications (methylation, acetylation, etc.) of the histones in the promoters and enhancers of the genes.65,66 The slow reset of key immune genes by BCG-driven methylation and demethylation of select host genes and in almost all lymphoid cell types, such as T cells and monocytes, shows that this long immunity is multi-lineage. This could be due to possible stem cell-driven re-modeling of the human immune system by this very synergistic organism. This randomized, double-blinded clinical trial documents, in humans, the broad infectious benefits of host microbe interactions.
Other potential limitations for this BCG study, as well as other studies that test COVID-19 vaccines in specialized patient populations, such as type 1 diabetic subjects, healthcare workers, or the elderly, is that the translation of vaccine efficacy to the general public, in countries with diverse populations and different neonatal vaccination histories, may not always be applicable.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| BCG vaccine (Bacillus Calmette-Guérin) | Japan BCG Laboratory, Tokyo, Japan | N/A |
| Critical commercial assays | ||
| AntiCoV-ID™ IgG ELISA | Akston BioSciences, Beverly, MA | SKU: 600016 |
| SARS-CoV-2 Protein Microarray: 2-in-1 protein and peptide assay | CDI Labs, Baltimore, MD. (http://cdi.bio) | CDICOV2-001.0 |
| Point of Care SARS-CoV-2 testing (PCR, Rapid Antigen test, Antibody test) | Quest Diagnostics, Secaucus, NJ | N/A |
| Software and algorithms | ||
| StudyTrax Electronic Data Capture System for Clinical Research | StudyTrax, Macon, GA, USA | StudyTrax |
| Other | ||
| BD Vacutainer Serum Blood Collection Tubes (Red Top) | Becton and Dickinson, Franklin Lakes, NJ | Ref. 366430 |
| BD Vacutainer Push Button Blood Collection Set | Becton and Dickinson, Franklin Lakes, NJ | Ref. 367344 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Denise Faustman (dfaustman@mgh.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
We assessed the safety and efficacy of ≥3 BCG vaccinations (Tokyo-172 strain) versus placebo for prevention of COVID-19 and other infectious diseases in a community-based, single-center, Phase ⅔ randomized, double-blind, placebo-controlled clinical trial. The trial duration was 15 months, starting at the beginning of the SARS-CoV-2 pandemic in the US (01/0½020) and ending with the US launch of COVID-19 vaccines in April 2021 (Figure 1A). All participants only received the placebo or BCG vaccine and did not receive COVID-19 antigen specific vaccines. Clinical trial participants were volunteers ages 18–50 years old with a co-morbid condition, type 1 diabetes (Figure 1B). The current trial was a parallel study using already enrolled double-blinded participants in a multi-dose BCG vaccine trial assessing its 5-year impact on other outcomes unrelated to infections (US FDA Investigational New Drug [IND]#16434; Massachusetts General Hospital [MGH] protocol #2013P002633). At the three-year time point of the original study, this parallel study on COVID-19 protection was initiated, while the original trial continued in a double-blinded fashion. The original clinical trial and this parallel clinical trial were pre-registered in the clinicaltrials.gov NCT site as: NCT02081326.
The protocol for the current COVID-19 prevention trial was approved as an amendment to the original FDA IND and MGH protocols as well as a new MGH protocol #2020P001462. A study synopsis is provided (Figure S6). The current trial and the original trial were performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Conference on Harmonization (ICH)/Good Clinical Practice, applicable regulatory requirements, and the MGH policy on Bioethics. Informed consent was written for the intervention; informed consent could be oral for the COVID-19 symptom surveys.
A total of 150 subjects were recruited for the original trial. All subjects were recruited with a requirement to have no previous or current tuberculosis and no history of BCG vaccinations, even during childhood. All subjects were US born and followed from the beginning of the original trial for all infection diseases (viral, bacterial, parasitic, etc) and any sort of antibiotic usage through a direct MedDra documentation system. The US has never offered newborn or childhood BCG vaccinations. The original trial recruited 150 subjects (2:1 BCG:placebo) and at the start of this COVID-19 trial two years later, 144 subjects were enrolled for this parallel trial design. The parallel infectious disease study design studied the off-target effects of repeat BCG vaccinations for COVID-19 and infectious disease protection (96 BCG treated, 48 placebo treated). There was no patient attrition over the 15-month time course of the COVID-19 study. The original exclusion criteria included positive purified protein derivative (PPD) test, positive T-spot test for tuberculosis, or born in a foreign country with mandatory BCG vaccinations. The goal of these exclusion criteria was to prevent prior ongoing and durable protection from Mycobacterium bovis (the origin of the BCG vaccine) or Mycobacterium tuberculosis (TB) exposures causing long-term protection. Exclusion criteria also included no active glucocorticoids treatment, chronic immunosuppressive medications, or currently living with an immunosuppressed individual to prevent an adverse event from the administration of this live vaccine. All subjects lived within the United States (Figure S5). The largest contingent of participants, 36, was from Massachusetts, the second largest, 16, was from New York, and the next largest, 11, was from Texas. During this time in the US for this COVID-19 study (January 2020 - April 2021) the Delta variant was identified in May 2021 so this study addresses BCG protection from all infectious diseases and resistance to predominantly the Alpha variant of the SARS-CoV-2 pandemic. Importantly both the diagnostic PCR testing and serology testing performed to confirm COVID-19 disease, detected all genetic variants of the virus.
This trial had three types of oversight. At 6-month intervals, this trial had audits from either Massachusetts General Brigham (MGB) Division of Quality Management or from outside auditors (Advanced Clinical Trials, Deerfield, IL). All trial data was processed by two unblinded statisticians (D.S., H.Z.), and an independent data and safety monitoring board (DSMB) met at 6-month intervals to monitor subject safety and reporting compliance.
The MGH Research Pharmacy generated the randomization code for the administration to study participants of either the BCG or the saline placebo vaccine. This randomization code was held in the MGH pharmacy. All drug within the syringe had an identical appearing fluid for the BCG and placebo vaccine in the 0.1 cc volume. With this vaccine there are absolutely no systemic symptoms after administration so blinding of study staff and also subjects collecting post-vaccine adverse systemic events is easier than antigen specific vaccines that frequently yield multi-day systemic reactions and sometimes missed workdays. A separate third party study nurse collected systemic reactions to the vaccine (none reported) and also consulted with participants for any skin reactions. Skin reactions were kept in separate locked storage center and not within the Studytrax or EPIC data management center. All investigators and study staff remained blinded when studying both the primary and parallel study outcomes of COIVID-19 and infectious diseases. Subject and staff unblinding will occur at the end of the primary study.
Method details
Procedures
The BCG vaccine or saline placebo (both 0.1 mL volume per dose) was administered intradermally over a period of 2.5–3 years prior to the start of this parallel trial. Two BCG doses were given 4 weeks apart in the beginning of year 1, and an identical booster dose was given in year 2. Site staff were responsible for reporting all drug- and non-drug-related safety information and were unaware of group assignments.
Monitoring for COVID-19 or infectious disease over the 15-month surveillance period included emailed surveys (every 2 months), as well as surveys during every visit to our clinic. The survey asked about COVID-19 and any other infections subjects had over the previous three months. It was completed by the patient via e-mail, over the phone with research staff at the 6 months visit time or online through Studytrax. Studytrax is an FDA compliant electronic data capture system for clinical research. At each 6-month clinic visit 10 mL of blood was drawn. The survey was also completed by the patient for any adult household member with infectious symptoms. Participants also directly reached out to the clinic to report infections. Household members’ blood for confirmation of SARS-CoV-2 infection was locally obtained by a national based chain of blood drawing centers (Quest Diagnostics, Secaucus, NJ). The symptoms questionnaire for study participants and infected household members was in compliance with FDA Guidance for Industry.51 For each symptom, the participants provided a severity score of 0 (none), 1 (mild), 2 (moderate) or 3 (severe). The length of the infectious illness in days was also reported. From the individual symptom scores a Total Symptom Score and an Average Symptom Score were tabulated. Then, multiplying by the length of illness, the Total Infectious Symptom Index or the Average Infectious Symptom Index was assessed (Figure S3A).
For SARS-CoV-2 antibodies detected, the average and SD of antibody levels prior to the onset of COVID-19 pandemic (Pre-2020) was determined. The average of the levels after the start of the pandemic (2020 and 2021 data) was also calculated. Using these averages and the Pre-2020 SD the Z score per patient was then calculated. The Z score thus represents the difference in average pre-COVID and average COVID signal levels, expressed as the number of standard deviations of pre-COVID. A Z score of ≥3 was considered to represent a statistically significant difference.
Outcomes
This trial had the following co-primary efficacy endpoints on potential benefits of multi-dose BCG: prevention of symptomatic and molecularly-confirmed COVID-19 (see case criteria below); and reduction of infectious disease (including COVID-19) symptomatology and severity by the Total or Average Infectious Disease Index. The study synopsis is provided (Figure S6). These outcomes conformed to other primary US based trials for the antigen-specific vaccines (Pfizer-BioNTech and Moderna trials). Secondary outcomes were average workdays missed, and individual infection symptom severity, and household members’ total infectious symptom index.
COVID-19 is defined according to FDA by the presence of at least one of 12 symptoms51 (headache, chills/shivering, diarrhea, nausea/vomiting, fatigue, shortness of breath, loss of smell or taste, muscle aches, nasal congestion, cough, sore throat and fever) and molecular confirmation. These symptoms, other than the loss of smell or taste, are symptoms of most infectious diseases, so the survey was used for both purposes.
Our criteria for symptomatic and molecularly confirmed COVID-19 had to be rigorous while overcoming the problem that PCR testing was not available at the origin of this trial and/or not equally accessible in all parts of the US. PCR testing only became widely available seven months into the trial. In addition, PCR detects SARS-CoV-2 virus in a smaller time window than molecular antibody confirmation through the measurement of host direct SARS-CoV-2 antibodies. Therefore for symptomatic and molecularly confirmed COVID-19 we required subjects to exhibit one or more FDA-defined symptoms of COVID-191 together with positive findings on ≥5 out of 10 assays (9 SARS-CoV-2 IgG antibody binding assays and a 10th assay that included PCR testing for viral RNA) (Figure S1). A subject was deemed positive for a particular SARS-CoV-2 antibody assay if the serology antibody Z score was ≥3, i.e., at least 3 standard deviations higher than during the pre-COVID period (baseline). Serum was obtained in all cases within 3 months of the infection for the detection of IgG antibodies.
Quantification and statistical analysis
The sample size and power analysis of this trial were based on the primary randomized Phase II clinical trial that started 2–3 years prior to this parallel trial focusing on COVID-19. Vaccine efficacy is defined by (p1 – p2)/p1 x 100, where p1 is the % Covid-positive in the placebo group and p2 is the % Covid-positive in the BCG group. For comparison to published Covid vaccine trials such as the mRNA trials, we also calculated the posterior probability that the vaccine efficacy is greater than 30% using a Bayesian Beta binomial model fitted using WinBUGS.67 We used a non-informative beta prior with parameters (1,1) and took 5000 MCMC samples to compute the posterior probability for efficacy to be greater than 30%. The 95% credible interval for the posterior probability for efficacy to be >30% is 98.9–100%. Further details are provided in the Figure legends and these method mirror past studies on COVID-19 vaccine efficacy.68 Average antibody levels were compared using Student’s T-testing in Prism (Graphpad Software, San Diego, CA) or Microsoft Excel. The number of patients positive in the BCG versus the Placebo cohort were compared using Fisher Exact Test (available online at https://www.graphpad.com/quickcalcs/contingency1/). Differences in the symptoms scores between participants and household members were compared between the BCG-treated group and the placebo-treated group using a Two-Sample Wilcoxon test. Statistics were considered significant at p < 0.05.
Additional resources
This trial is listed on clinicaltrials.gov (https://clinicaltrials.gov/ct2/show/NCT02081326).
Acknowledgments
We thank all the participants who volunteered for this study for their dedication to continue to participate through the COVID-19 pandemic. We specifically thank Dr. Deneen Vojta for her insight and advocacy. We thank UnitedHealth Group for their insight to support this clinical trial. We also thank Dr. Miriam Davis for her editing of the manuscript with the highest of standards.
Author contributions
All authors met the criteria for authorship set by the International Committee of Medical Journal Editors. D.L.F. was the principal investigator and W.M.K. was the co-principal investigator of this randomized clinical trial. E.R.H., N.C.N., and G.W. were clinical trial coordinators and interacted with participants, gathered participant data, and worked on formatting figures with processed data. J.B.’s role was as a registered nurse, tracking vaccine side effects and seeing subjects. A.L., A.A., G.F.S., L.T., H.T., and H.F.D. performed various assays related to serum obtained from patients. H.Z. and D.A.S. performed the statistical analysis with the data provided by W.M.K. All authors had access to the blinded data. The data interpretation and data verification were performed at many levels but most by D.L.F., D.A.S., H.Z., and W.M.K. Authors D.A.S., H.Z., and W.M.K. were unblinded subjects that processed the data; as outlined, the original randomized trial is still active and ongoing. Blinded audits of data integrity and FDA compliance were performed by an outside monitoring company (Advanced Clinical, Inc.) as well as blinded compliance audits once per week by E.R.H., D.L.F., G.W., and N.C.N. All primary data will be shared at this time but are still blinded data because the primary outcome trial is still ongoing until approximately August 2023. All unblinded data will be shared after the primary trial is completed with patient identifiers removed for HIPPA compliance.
Declaration of interests
No author or author family member owns patents on this work. No author has any ownership rights to the study drug. No authors receive consulting or research support from Japan Laboratories.
Inclusion and diversity
All subjects were chosen for eligibility based on non-bias selection for sex or select ethnic diversity.
Published: August 15, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100728.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Anonymous BCG vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2018 [Google Scholar]
- 2.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N., et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 3.Biering-Sorensen S., Aaby P., Lund N., Monteiro I., Jensen K.J., Eriksen H.B., Schaltz-Buchholzer F., Jorgensen A.S.P., Rodrigues A., Fisker A.B., Benn C.S. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin. Infect. Dis. 2017;65:1183–1190. doi: 10.1093/cid/cix525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biering-Sorensen S., Aaby P., Napirna B.M., Roth A., Ravn H., Rodrigues A., Whittle H., Benn C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guerin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012;31:306–308. doi: 10.1097/INF.0b013e3182458289. [DOI] [PubMed] [Google Scholar]
- 5.de Castro M.J., Pardo-Seco J., Martinon-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin. Infect. Dis. 2015;60:1611–1619. doi: 10.1093/cid/civ144. [DOI] [PubMed] [Google Scholar]
- 6.Garly M.L., Martins C.L., Bale C., Balde M.A., Hedegaard K.L., Gustafson P., Lisse I.M., Whittle H.C., Aaby P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21:2782–2790. doi: 10.1016/s0264-410x(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 7.Giamarellos-Bourboulis E.J., Tsilika M., Moorlag S., Antonakos N., Kotsaki A., Dominguez-Andres J., Kyriazopoulou E., Gkavogianni T., Adami M.E., Damoraki G., et al. Activate: randomized clinical trial of BCG vaccination against infection in the elderly. Cell. 2020;183:315–323.e9. doi: 10.1016/j.cell.2020.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkridge A., Hatherill M., Little F., Goetz M.A., Barker L., Mahomed H., Sadoff J., Hanekom W., Geiter L., Hussey G., South African B.C.G.t.t. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ. 2008;337:a2052. doi: 10.1136/bmj.a2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kristensen I., Aaby P., Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ. 2000;321:1435–1438. doi: 10.1136/bmj.321.7274.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N., Mabwe S., Makhethe L., Erasmus M., Toefy A., et al. Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N. Engl. J. Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponnighaus J.M., Fine P.E., Sterne J.A., Wilson R.J., Msosa E., Gruer P.J., Jenkins P.A., Lucas S.B., Liomba N.G., Bliss L. Efficacy of BCG vaccine against leprosy and tuberculosis in northern Malawi. Lancet. 1992;339:636–639. doi: 10.1016/0140-6736(92)90794-4. [DOI] [PubMed] [Google Scholar]
- 12.Roth A., Gustafson P., Nhaga A., Djana Q., Poulsen A., Garly M.L., Jensen H., Sodemann M., Rodriques A., Aaby P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005;34:540–547. doi: 10.1093/ije/dyh392. [DOI] [PubMed] [Google Scholar]
- 13.Shann F. The non-specific effects of vaccines. Arch. Dis. Child. 2010;95:662–667. doi: 10.1136/adc.2009.157537. [DOI] [PubMed] [Google Scholar]
- 14.Stensballe L.G., Nante E., Jensen I.P., Kofoed P.E., Poulsen A., Jensen H., Newport M., Marchant A., Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23:1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Walk J., de Bree L.C.J., Graumans W., Stoter R., van Gemert G.J., van de Vegte-Bolmer M., Teelen K., Hermsen C.C., Arts R.J.W., Behet M.C., et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019;10:874. doi: 10.1038/s41467-019-08659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardhana, Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011;43:185–190. [PubMed] [Google Scholar]
- 17.Calmette A. Preventive vaccination against tuberculosis with BCG. Proc. R Soc. Med. 1931;24:1481–1490. [PMC free article] [PubMed] [Google Scholar]
- 18.Faustman D.L., Wang L., Okubo Y., Burger D., Ban L., Man G., Zheng H., Schoenfeld D., Pompei R., Avruch J., Nathan D.M. Proof-of-concept, randomized, controlled clinical trial of Bacillus-Calmette-Guerin for treatment of long-term type 1 diabetes. PLoS One. 2012;7:e41756. doi: 10.1371/journal.pone.0041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhtreiber W.M., Tran L., Kim T., Dybala M., Nguyen B., Plager S., Huang D., Janes S., Defusco A., Baum D., et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines. 2018;3:23. doi: 10.1038/s41541-018-0062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paolillo A., Buzzi M.G., Giugni E., Sabatini U., Bastianello S., Pozzilli C., Salvetti M., Ristori G. The effect of Bacille Calmette-Guerin on the evolution of new enhancing lesions to hypointense T1 lesions in relapsing remitting MS. J. Neurol. 2003;250:247–248. doi: 10.1007/s00415-003-0967-6. [DOI] [PubMed] [Google Scholar]
- 21.Ristori G., Buzzi M.G., Sabatini U., Giugni E., Bastianello S., Viselli F., Buttinelli C., Ruggieri S., Colonnese C., Pozzilli C., Salvetti M. Use of bacille Calmette-Guerin (BCG) in multiple sclerosis. Neurology. 1999;53:1588–1589. doi: 10.1212/wnl.53.7.1588. [DOI] [PubMed] [Google Scholar]
- 22.Bagheri N., Montazeri H. On BCG vaccine protection from COVID-19: a review. SN Compr. Clin. Med. 2021;3:1261–1271. doi: 10.1007/s42399-021-00835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis N., Sparrow A., Ghebreyesus T.A., Netea M.G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395:1545–1546. doi: 10.1016/S0140-6736(20)31025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escobar C.E. Correction for Escobar et al., BCG vaccine protection from severe coronavirus disease 2019 (COVID-19) Proc. Natl. Acad. Sci. USA. 2020;117:27741–27742. doi: 10.1073/pnas.2019438117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klinger D., Blass I., Rappoport N., Linial M. Significantly improved COVID-19 outcomes in countries with higher BCG vaccination coverage: a multivariable analysis. Vaccines (Basel) 2020;8 doi: 10.3390/vaccines8030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moorlag S., van Deuren R.C., van Werkhoven C.H., Jaeger M., Debisarun P., Taks E., Mourits V.P., Koeken V., de Bree L.C.J., Ten Doesschate T., et al. Safety and COVID-19 symptoms in individuals recently vaccinated with BCG: a retrospective cohort study. Cell Rep. Med. 2020;1:100073. doi: 10.1016/j.xcrm.2020.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivas M.N., Ebinger J.E., Wu M., Sun N., Braun J., Sobhani K., Van Eyk J.E., Cheng S., Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Invest. 2021;131 doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickramasinghe D., Wickramasinghe N., Kamburugamuwa S., Arambepola C., Samarasekera D. Correlation between immunity from BCG and the morbidity and mortality of COVID-19. Trop. Dis. Travel Med. Vaccines. 2020;5:17. doi: 10.1186/s40794-020-00117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg M.K., Yu Q., Salvador C.E., Melani I., Kitayama S. Mandated Bacillus Calmette-Guerin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci. Adv. 2020;6:eabc1463. doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joy M., Malavika B., Asirvatham E.S., Sudarsanam T.D., Jeyaseelan L. Is BCG associated with reduced incidence of COVID-19? A meta-regression of global data from 160 countries. Clin. Epidemiol. Glob. Health. 2021;9:202–203. doi: 10.1016/j.cegh.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinoshita M., Tanaka M. Impact of routine infant BCG vaccination on COVID-19. J. Infect. 2020;81:625–633. doi: 10.1016/j.jinf.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauer J., Fischer U., Auer F., Borkhardt A. Regional BCG vaccination policy in former East- and West Germany may impact on both severity of SARS-CoV-2 and incidence of childhood leukemia. Leukemia. 2020;34:2217–2219. doi: 10.1038/s41375-020-0871-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gursel M., Gursel I. Is global BCG vaccination-induced trained immunity relevant to the progression of SARS-CoV-2 pandemic? Allergy. 2020;75:1815–1819. doi: 10.1111/all.14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks N.A., Puri A., Garg S., Nag S., Corbo J., Turabi A.E., Kaka N., Zemmel R.W., Hegarty P.K., Kamat A.M. The association of Coronavirus Disease-19 mortality and prior bacille Calmette-Guerin vaccination: a robust ecological analysis using unsupervised machine learning. Sci. Rep. 2021;11:774. doi: 10.1038/s41598-020-80787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samrah S.M., Al-Mistarehi A.W., Ibnian A.M., Raffee L.A., Momany S.M., Al-Ali M., Hayajneh W.A., Yusef D.H., Awad S.M., Khassawneh B.Y. COVID-19 outbreak in Jordan: epidemiological features, clinical characteristics, and laboratory findings. Ann. Med. Surg. (Lond) 2020;57:103–108. doi: 10.1016/j.amsu.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozdemir C., Kucuksezer U.C., Tamay Z.U. Is BCG vaccination affecting the spread and severity of COVID-19? Allergy. 2020;75:1824–1827. doi: 10.1111/all.14344. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A., Misra S., Verma V., Vishwakarma R.K., Kamal V.K., Nath M., Prakash K., Upadhyay A.D., Sahu J.K. Global impact of environmental temperature and BCG vaccination coverage on the transmissibility and fatality rate of COVID-19. PLoS One. 2020;15:e0240710. doi: 10.1371/journal.pone.0240710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Upton C.M., van Wijk R.C., Mockeliunas L., Simonsson U.S.H., McHarry K., van den Hoogen G., Muller C., von Delft A., van der Westhuizen H.M., van Crevel R., et al. Safety and efficacy of BCG re-vaccination in relation to COVID-19 morbidity in healthcare workers: a double-blind, randomised, controlled, phase 3 trial. EClinicalMedicine. 2022;48:101414. doi: 10.1016/j.eclinm.2022.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moorlag S., Taks E., Ten Doesschate T., van der Vaart T.W., Janssen A.B., Muller L., Ostermann P., Dijkstra H., Lemmers H., Simonetti E., et al. Efficacy of Bacillus Calmette-Guerin vaccination against respiratory tract infections in the elderly during the Covid-19 pandemic. Clin. Infect. Dis. 2022 doi: 10.1093/cid/ciac182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li W.X. Worldwide inverse correlation between Bacille Calmette-Guerin (BCG) immunization and COVID-19 mortality. Infection. 2021;49:463–473. doi: 10.1007/s15010-020-01566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pepin J., Labbe A.C., Carignan A., Parent M.E., Yu J., Grenier C., Beauchemin S., De Wals P., Valiquette L., Rousseau M.C. Does BCG provide long-term protection against SARS-CoV-2 infection? a case-control study in Quebec, Canada. Vaccine. 2021;39:7300–7307. doi: 10.1016/j.vaccine.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Chaisemartin C., de Chaisemartin L. Public Health Emergency Collection. Oxford University Press; 2020. BCG Vaccination in Infancy Does Not Protect against COVID-19. Evidence from a Natural Experiment in Sweden. [Google Scholar]
- 43.Hensel J., McAndrews K.M., McGrail D.J., Dowlatshahi D.P., LeBleu V.S., Kalluri R. Protection against SARS-CoV-2 by BCG vaccination is not supported by epidemiological analyses. Sci. Rep. 2020;10:18377. doi: 10.1038/s41598-020-75491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamiel U., Kozer E., Youngster I. SARS-CoV-2 rates in BCG-vaccinated and unvaccinated young adults. JAMA. 2020;323:2340–2341. doi: 10.1001/jama.2020.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates M.N., Herron T.J., Lwi S.J., Baldo J.V. BCG vaccination at birth and COVID-19: a case-control study among U.S. military Veterans. Hum. Vaccin. Immunother. 2022;18:1981084. doi: 10.1080/21645515.2021.1981084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su W.J., Chang C.H., Wang J.L., Chen S.F., Yang C.H. COVID-19 severity and neonatal BCG vaccination among young population in Taiwan. Int. J. Environ. Res. Public Health. 2021;18 doi: 10.3390/ijerph18084303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amirlak L., Haddad R., Hardy J.D., Khaled N.S., Chung M.H., Amirlak B. Effectiveness of booster BCG vaccination in preventing Covid-19 infection. Hum. Vaccin. Immunother. 2021;17:3913–3915. doi: 10.1080/21645515.2021.1956228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carey I.M., Critchley J.A., DeWilde S., Harris T., Hosking F.J., Cook D.G. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41:513–521. doi: 10.2337/dc17-2131. [DOI] [PubMed] [Google Scholar]
- 49.Barrett C.E., Park J., Kompaniyets L., Baggs J., Cheng Y.J., Zhang P., Imperatore G., Pavkov M.E. Intensive care unit admission, mechanical ventilation, and mortality among patients with type 1 diabetes hospitalized for COVID-19 in the U.S. Diabetes Care. 2021;44:1788–1796. doi: 10.2337/dc21-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moorlag S., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 51.FDA . Center for Biologics Evaluation and Research Center for Drug Evaluation and Research. FDA; 2020. Assessing COVID-19 related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for Industry. [Google Scholar]
- 52.Abdallah M., Mahgoub A., Ahmed H., Chaterji S. Author correction: athena: automated tuning of k-mer based genomic error correction algorithms using language models. Sci. Rep. 2020;10:2390. doi: 10.1038/s41598-020-59141-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Angelidou A., Conti M.G., Diray-Arce J., Benn C.S., Shann F., Netea M.G., Liu M., Potluri L.P., Sanchez-Schmitz G., Husson R., et al. Licensed Bacille Calmette-Guerin (BCG) formulations differ markedly in bacterial viability, RNA content and innate immune activation. Vaccine. 2020;38:2229–2240. doi: 10.1016/j.vaccine.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi D., Takii T., Fujiwara N., Fujita Y., Yano I., Yamamoto S., Kondo M., Yasuda E., Inagaki E., Kanai K., et al. Comparable studies of immunostimulating activities in vitro among Mycobacterium bovis bacillus Calmette-Guerin (BCG) substrains. FEMS Immunol. Med. Microbiol. 2009;56:116–128. doi: 10.1111/j.1574-695X.2009.00559.x. [DOI] [PubMed] [Google Scholar]
- 55.Rentsch C.A., Birkhauser F.D., Biot C., Gsponer J.R., Bisiaux A., Wetterauer C., Lagranderie M., Marchal G., Orgeur M., Bouchier C., et al. Bacillus Calmette-Guerin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur. Urol. 2014;66:677–688. doi: 10.1016/j.eururo.2014.02.061. [DOI] [PubMed] [Google Scholar]
- 56.Ritz N., Dutta B., Donath S., Casalaz D., Connell T.G., Tebruegge M., Robins-Browne R., Hanekom W.A., Britton W.J., Curtis N. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am. J. Respir. Crit. Care Med. 2012;185:213–222. doi: 10.1164/rccm.201104-0714OC. [DOI] [PubMed] [Google Scholar]
- 57.Shann F. Editorial commentary: different strains of Bacillus Calmette-Guerin vaccine have very different effects on tuberculosis and on unrelated infections. Clin. Infect. Dis. 2015;61:960–962. doi: 10.1093/cid/civ454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roser M. Our World of Data. Covid-19 data explorer. 2022. https://ourworldindata.org/explorers/coronavirus-data-explorer
- 59.Aronson N.E., Santosham M., Comstock G.W., Howard R.S., Moulton L.H., Rhoades E.R., Harrison L.H. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: a 60-year follow-up study. JAMA. 2004;291:2086–2091. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 60.Cirovic B., de Bree L.C.J., Groh L., Blok B.A., Chan J., van der Velden W., Bremmers M.E.J., van Crevel R., Handler K., Picelli S., et al. BCG vaccination in humans elicits trained immunity via the hematopoietic progenitor compartment. Cell Host Microbe. 2020;28:322–334.e5. doi: 10.1016/j.chom.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Darrah P.A., Zeppa J.J., Maiello P., Hackney J.A., Wadsworth M.H., 2nd, Hughes T.K., Pokkali S., Swanson P.A., 2nd, Grant N.L., Rodgers M.A., et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature. 2020;577:95–102. doi: 10.1038/s41586-019-1817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keefe R.C., Takahashi H., Tran L., Nelson K., Ng N., Kuhtreiber W.M., Faustman D.L. BCG therapy is associated with long-term, durable induction of Treg signature genes by epigenetic modulation. Sci. Rep. 2021;11:14933. doi: 10.1038/s41598-021-94529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias H.F., Kuhtreiber W.M., Nelson K.J., Ng N.C., Zheng H., Faustman D.L. Epigenetic changes related to glucose metabolism in type 1 diabetes after BCG vaccinations: a vital role for KDM2B. Vaccine. 2021 doi: 10.1016/j.vaccine.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Kuhtreiber W.M., Takahashi H., Keefe R.C., Song Y., Tran L., Luck T.G., Shpilsky G., Moore L., Sinton S.M., Graham J.C., Faustman D.L. BCG vaccinations upregulate Myc, a central switch for improved glucose metabolism in diabetes. iScience. 2020;23:101085. doi: 10.1016/j.isci.2020.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Heijden C., Noz M.P., Joosten L.A.B., Netea M.G., Riksen N.P., Keating S.T. Epigenetics and trained immunity. Antioxid Redox Signal. 2018;29:1023–1040. doi: 10.1089/ars.2017.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fanucchi S., Dominguez-Andres J., Joosten L.A.B., Netea M.G., Mhlanga M.M. The intersection of epigenetics and metabolism in trained immunity. Immunity. 2021;54:32–43. doi: 10.1016/j.immuni.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Lunn D.J., Thomas A., Best N., Spiegelhalter D. WinBugs — a Bayesian modelling framework: concepts, structure, and extensibility. Stat. Comput. 2000;10:325–337. [Google Scholar]
- 68.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.