Abstract
Background
Although several assays are used to measure anti-receptor-binding domain (RBD) antibodies induced after severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) vaccination, the assays are not fully comparable in practice. This study evaluated the immunogenicity of the BNT162b2 mRNA vaccine in healthy adults using two immunoassays.
Methods
This prospective cohort study included SARS-CoV-2-naïve adults, predominantly healthcare workers, aged 20–64 years, who received two BNT162b2 vaccine doses between March and May 2021. Blood samples were collected before the first vaccination (S0), before the second vaccination (S1), 4 weeks after the second vaccination (S2), and 6 months after the second vaccination (S3). anti-RBD antibodies were measured using the Architect SARS-CoV-2 IgG II Quant (Abbott Laboratory) and Elecsys anti-SARS-CoV-2 S (Roche Diagnostics) assays.
Results
Among the 385 participants, the geometric mean antibody titers (GMTs) on the Architect assay (AU/mL) were 7.5, 693, 7007, and 1030 for S0, S1, S2, and S3, respectively. The corresponding GMTs on the Elecsys assay (U/mL) were 0.40, 24, 928, and 659, respectively. The GMT ratio (S3/S2) was 0.15 on the Architect and 0.71 on the Elecsys assay. The correlation between antibody titers measured with the two assays were strong at all time points after vaccination (Spearman's correlation coefficient: 0.74 to 0.86, P < 0.01 for all). GMT was significantly lower in the older age group after vaccination (P < 0.01), with no significant differences according to sex. Seroprotection (≥5458 AU/mL on the Architect assay and ≥ 753 U/mL on the Elecsys) at each time point was 0 %, 1 %, 67 %, and 1 % on the Architect assay and 0 %, 1 %, 62 %, and 43 % on the Elecsys, respectively.
Conclusions
Two BNT162b2 vaccine doses resulted in adequate anti-RBD antibody response, which varied by age. As the two assays showed different kinetics, the results of single immunoassays should be interpreted with caution.
Keywords: SARS-CoV-2, Vaccination, Immunoassay, mRNA vaccine, Japan, BNT162b2, anti-RBD antibody response, Architect SARS-CoV-2 IgG II Quant, Elecsys Anti-SARS-CoV-2 S
1. Introduction
Messenger RNA vaccines use a novel technology [1], and have been shown to have a high level of efficacy against coronavirus disease (COVID-19) in pre-licensure phase 3 clinical trials [2], [3]. Recent reports have shown that vaccine effectiveness wanes over time in real-world settings [4], [5]. Although post-marketing surveillance of effectiveness is desirable, it may not be feasible in populations with a low incidence of COVID-19. As an alternative, vaccine effectiveness can be predicted by measuring antibodies to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) receptor-binding domain spike protein IgG (anti-RBD) [5], [6], [7] which correlates with virus neutralization [8]. As with changes in vaccine effectiveness, anti-RBD titers sharply increase after vaccination and then gradually decrease [9], [10]. The antibody response after vaccination has been reported to be attenuated by older age [11], [12], [13], male sex [11], [12], [13], immunosuppressive and anticancer drugs [11], [13], [14], [15], hemodialysis [16], and underlying diseases such as cancer [17] and diabetes mellitus [11]. However, results are inconsistent.
In Japan, the Pfizer-BioNTech BNT162b2 mRNA vaccine was approved on February 14, 2021, and the Moderna mRNA-1273 vaccine and the AstraZeneca ChAdOx1nCoV-19 adenoviral vector vaccine were approved on May 21, 2021. The primary objectives of this post-marketing observational study were to evaluate the anti-RBD titer up to 6 months after the second dose of the BNT162b2 vaccine, and to determine differences in immunogenicity according to age, sex, and body mass index (BMI). A secondary objective was to assess the differences between anti-RBD titers measured using two different immunoassays, because several different assays are used to measure anti-RBD antibodies [18] and although standardization of measurement is possible [19], different assays are not fully comparable in practice [20], [21].
2. Methods
2.1. Study design
This prospective cohort study was conducted at Osaka Metropolitan University Hospital, Osaka, Japan. Here, we provide a report summarizing the results between March 2021 and November 2021. The eligibility criteria were as follows: 1) 20–64 years old at enrolment; 2) healthcare workers of Osaka Metropolitan University Hospital, employees of the Osaka City Health Bureau, and faculty members and students of the School/Graduate School of Medicine and Graduate School of Nursing of Osaka Metropolitan University; 3) individuals scheduled to receive two doses of the COVID-19 vaccines approved in Japan; and 4) individuals who voluntarily provided written consent to participate in the study. Those with a history of COVID-19 infection or vaccination and contraindications for vaccination were excluded. The study protocol was developed in compliance with the Declaration of Helsinki and was approved by the Osaka Metropolitan University Hospital Certified Review Board (approval number: OCU010E, registration number: jRCT1051200143). Informed consent was given by participants after the nature and possible consequences of the study were fully explained.
2.2. Vaccination, and sample and data collection
At the time of enrolment, information on the basic characteristics of the participants, such as profession, ethnicity, sex, age, height, weight, medical history or underlying disease, smoking habit, and drinking habit, was self-reported by the participants using the research electronic data capture system. All participants were vaccinated according to the package insert. Regarding BNT162b2 vaccine, the standard interval between the first and second doses was 21 days. Participants were vaccinated with 0.3 mL of diluted BNT126b2 by intramuscular injection in the deltoid muscle. Blood samples were collected at four time points: within 1 week before the first vaccination (S0), within 1 week before the second vaccination (S1), 4–5 weeks after the second vaccination (S2), and 6 months (24–28 weeks) after the second vaccination (S3).
2.3. Measurement of antibody titer
Titers of anti-RBD and anti-SARS-CoV-2 nucleocapsid protein (anti-N) were measured in the collected blood samples using two assays: Architect SARS-CoV-2 IgG II Quant (Abbott Laboratories, Illinois, USA) [22] and Elecsys anti-SARS-CoV-2 S (Roche Diagnostics, Basel, Switzerland) [23].
-
1)
Architect
The quantitative range was 6.8–80000 (AU/mL) in this study, and the cut-off value for a positive anti-RBD test was ≥ 50 (AU/mL) [22]. The cut-off value for a positive anti-N test was ≥ 1.4.
-
2)
Elecsys
The quantitative range was 0.04–25000 (U/mL) in this study, and the cut-off value for a positive anti-RBD test was ≥ 0.8 (U/mL) [23]. The cut-off value for a positive anti-N test result was ≥ 1.0.
2.4. Statistical analysis
The geometric mean antibody titer (GMT) and GMT ratio (GMTR) of the anti-RBD antibody titer were calculated. Seropositivity was defined as the proportion of participants with a titer ≥ 50 (AU/mL) on the Architect assay, and ≥ 0.8 (U/mL) on the Elecsys assay. Seroprotection was defined based on the proportion of participants with a titer ≥ 775 BAU/mL, according to the international standard [19] and 90 % vaccine efficacy threshold against symptomatic COVID-19, as shown in a previous clinical trial [5]. The value, 775 BAU/mL, was converted to 5458 AU/mL (BAU/mL × 7.042) for the Architect assay and 753 U/mL (BAU/mL × 0.971) for the Elecsys assay [19], [20]. For the stratified analysis, participant age was divided into five groups (20–29, 30–39, 40–49, 50–59, and 60–64 years). The BMI (kg/m2) was calculated as weight/(height)2 and was divided into three categories (<18.5, 18.5–24.9, ≥25) based on the Japanese criteria for the classification of underweight and obesity [24].
The correlation between anti-RBD antibody titers measured by the Architect assay and those measured by the Elecsys assay was evaluated using Spearman's correlation coefficient. The GMT and GMTR between the categories were compared using the Jonckheere-Terpstra trend test. The Mantel-Haenstzel's χ-square test or Fisher's exact test was used to compare seropositivity and seroprotection. Multiple linear regression analysis was performed with the anti-RBD titer at each time point as the objective variable and age (continuous), sex, and BMI (continuous) as the explanatory variables.
SAS version 9.4 and R version 4.1.0 were used for the analysis. Statistical significance was set at P < 0.05.
3. Results
3.1. Participants
As of November 14, 2021, 388 of the 508 participants had completed the two doses of vaccination and the four antibody titer measurements as scheduled. The three participants with positive anti-N values in both assays were considered to have been naturally infected with SARS-CoV-2. Therefore, a total of 385 participants were included in the analysis. All participants were vaccinated using the BNT162b2 vaccine between March and May 2021, and the median interval between the first and second doses was 21 days (range, 19–28 days).
The characteristics of the participants are presented in Table 1 . Approximately-two-thirds were healthcare workers (72 %) and female (67 %), and approximately-one-third of participants (34 %) were aged 40–49 years. Seventy-two percent of the participants had a BMI within the normal range (18.5–24.9). Two percent of participants were treated with steroids or immunosuppressive drugs within the previous 6 months, 4 % were current smokers, and 62 % were current drinkers. The most common underlying diseases were hypertension (11 %), dyslipidemia (9 %), and bronchial asthma (6 %) (Supplementary Table 1).
Table 1.
Characteristics of the participants (N = 385).
| n (%) | |
|---|---|
| Profession | |
| Healthcare workers | 279 (72) |
| Other | 106 (28) |
| Ethnicity | |
| Japanese | 376 (98) |
| Non-Japanese Asians | 9 (2) |
| Sex | |
| Male | 126 (33) |
| Female | 259 (67) |
| Age (years) | |
| Median (interquartile range) | 43 (34–50) |
| 20–29 | 63 (16) |
| 30–39 | 88 (23) |
| 40–49 | 132 (34) |
| 50–59 | 88 (23) |
| 60–64 | 14 (4) |
| Body mass index(kg/m2) | |
| Median (interquartile range) | 21.7 (19.9–23.7) |
| <18.5 | 40 (10) |
| 18.5–25 | 279 (72) |
| ≥25 | 66 (17) |
| Oral administration of steroids or immunosuppressants (previous 6 months) | |
| Yes | 9 (2) |
| Cigarette smoking habits | |
| Non-smoker | 313 (81) |
| Ex-smoker | 57 (15) |
| Current smoker | 15 (4) |
| Alcohol drinking habits | |
| Non-drinker | 113 (29) |
| Ex-drinker | 33 (9) |
| Current drinker | 239 (62) |
3.2. Longitudinal changes in anti-RBD antibody titers among all participants
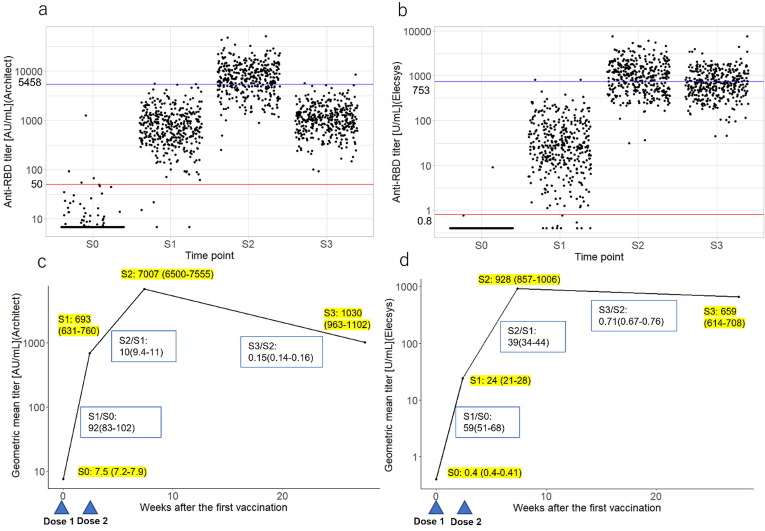
The anti-RBD titer measured by the Architect assay increased rapidly after the first vaccination (S1) and further increased at S2 but decreased substantially at S3 (Fig. 1 -a). The fold rise (GMTR) from S0 to S1 and S1 to S2 was 92-fold and 10-fold, respectively, decreasing to 0.15-fold from S2 to S3 (Fig. 1-b). Conversely, the anti-RBD titer measured by the Elecsys assay showed a slow and variable increase at S1. However, in most of the participants this increased to a high titer at S2 and remained relatively high at S3 (Fig. 1-c). The fold rise (GMTR) from S0 to S1 and S1 to S2 was 59-fold and 39-fold, respectively, and 0.71-fold from S2 to S3 (Fig. 1-d).
Fig. 1.
Longitudinal changes of the anti-RBD antibody titers among all participants. (a) Scatterplot of participant antibody titers at each time point according to the Architect assay; (b) Scatterplot of participant antibody titers at each time point according to the Elecsys assay; (c) GMT (95 % CI) and GMT ratio (95 % CI) of antibody titers over time after the first vaccination according to the Architect assay; (d) GMT (95 % CI) and GMT ratio (95 % CI) of antibody titers over time after the first vaccination according to the Elecsys assay. BMI: body mass index; CI: confidence interval; anti-RBD: anti-SARS-CoV-2 receptor binding domain spike protein IgG; GMT: geometric mean titer; Architect: Architect SARS-CoV-2 IgG Ⅱ Quant (Abbott Laboratories); Elecsys: Elecsys anti-SARS-CoV-2 S (Roche Diagnostics); S0: Within 1 week before the first vaccination, S1: Within 1 week before the second vaccination, S2: 4–5 weeks after the second vaccination, and S3: 6 months after the second vaccination.
Additionally, anti-RBD titers measured by the Elecsys assay were higher at S3 than at S2 in 117 (30 %) of the total subjects. In these 117 participants, anti-RBD titers measured using the Architect assay showed a gradual decrease from S2 to S3 (Supplementary Fig. 1).
3.3. Correlation between anti-RBD antibody titer measured by Architect assay and that measured by Elecsys assay
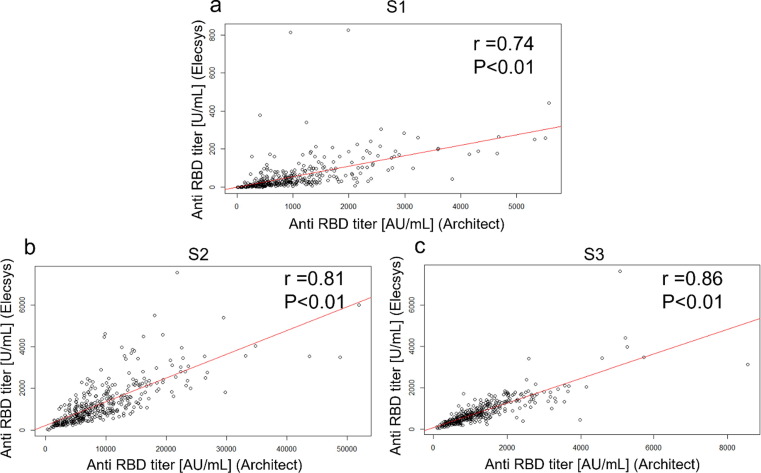
The correlation between the anti-RBD antibody titer measured by the Architect assay and the antibody titer measured by the Elecsys assay at S1, S2, and S3 are shown in scatter plots with the regression lines (Fig. 2 , a-c). Spearman's correlation coefficients were 0.74 (P < 0.01) for S1, 0.81 (P < 0.01) for S2, and 0.86 (P < 0.01) for S3.
Fig. 2.
The correlation between anti-RBD antibody titers measured by the Architect assay and those measured by the Elecsys assay at each time point. (a) Scatterplot showing correlation between anti-RBD antibodies measured by the Architect assay and anti-RBD antibodies measured by the Elecsys assay at S1; (b) Scatterplot showing correlation between anti-RBD antibodies measured by the Architect assay and anti-RBD antibodies measured by the Elecsys assay at S2; (c) Scatterplot showing correlation between anti-RBD antibodies measured by the Architect assay and anti-RBD antibodies measured by the Elecsys assay at S3; anti-RBD: anti-SARS-CoV-2 receptor binding domain spike protein IgG; Architect: Architect SARS-CoV-2 IgG Ⅱ Quant (Abbott Laboratories); Elecsys: Elecsys anti-SARS-CoV-2 S (Roche Diagnostics); S1: Within 1 week before the second vaccination, S2: 4–5 weeks after the second vaccination, and S3: 6 months after the second vaccination. The correlation between anti-RBD antibody titers measured by the Architect assay and those measured by the Elecsys was evaluated using Spearman's correlation coefficient (r). The red line indicates the regression line. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. anti-RBD antibody titer at each time point stratified by age, sex, and BMI category
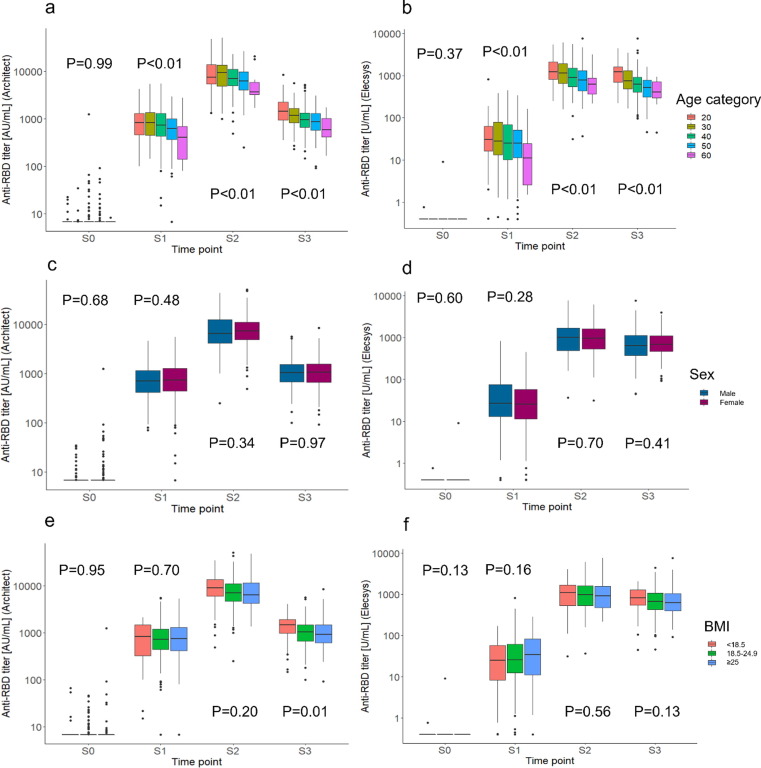
For both assays, the anti-RBD titers in the older age categories were lower in S1, S2, and S3 than in the younger age categories. However, there was no clear difference in the responses between the sexes or the BMI categories (Fig. 3 , a-f).
Fig. 3.
Anti-RBD titers at each time point according to age, sex, and body mass index category. (a) Box-and-whisker plot showing anti-RBD antibody titers by age category according to the Architect assay; (b) Box-and-whisker plot showing anti-RBD antibody titers by age category according to the Elecsys assay; (c) Box-and-whisker plot showing anti-RBD antibody titers by sex according to the Architect assay; (d) Box-and-whisker plot showing anti-RBD antibody titers by sex according to the Elecsys assay; (e) Box-and-whisker plot showing anti-RBD antibody titers by BMI category according to the Architect assay; (f) Box-and-whisker plot showing anti-RBD antibody titers by BMI category according to the Elecsys assay. BMI: Body mass index (kg/m2); anti-RBD: anti-SARS-CoV-2 receptor binding domain spike protein IgG. Architect: Architect SARS-CoV-2 IgG Ⅱ Quant (Abbott Laboratories); Elecsys: Elecsys anti-SARS-CoV-2 S (Roche Diagnostics). S0: Within 1 week before the first vaccination, S1: Within 1 week before the second vaccination, S2: 4–5 weeks after the second vaccination, and S3: 6 months after the second vaccination. P values were calculated using the Jonckheere-Terpstra trend test.
3.5. GMT and GMTR of anti-RBD antibodies compared by age, sex, and BMI category
-
(1)
Architect
The GMTs at S1, S2, and S3 and the GMTRs for S1/S0, S2/S0, and S3/S2 decreased significantly in the older age categories (P < 0.01, P < 0.01, P < 0.01, P < 0.01, P < 0.01, P = 0.03, respectively). The GMT at S3 was significantly lower in the high BMI group (P = 0.01) (Table 2 , Supplementary Fig. 2).
-
(2)
Elecsys
Table 2.
Comparison of the geometric mean titer (GMT) and GMT ratio of the anti-RBD antibodies by age, sex, and BMI.
| N | GMT (95 % CI) |
GMT ratio |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| S0 | S1 | S2 | S3 | S1/S0 | S2/S1 | S2/S0 | S3/S2 | ||
| Architect (Abbott) | |||||||||
| Overall | 385 | 7.5 (7.2–7.9) | 693 (631–760) | 7007 (6500–7555) | 1030 (963–1102) | 92 | 10 | 930 | 0.15 |
| Age | |||||||||
| 20–29 | 63 | 7.2 (6.8–7.7) | 782 (650–941) | 7772 (6421–9406) | 1481(1266–1734) | 108 | 9.9 | 1076 | 0.19 |
| 30–39 | 88 | 7.0 (6.7–7.3) | 853 (727–1001) | 8424 (7134–9947) | 1128 (988–1287) | 122 | 9.9 | 1207 | 0.13 |
| 40–49 | 132 | 7.9 (7.2–8.8) | 711 (603–839) | 6920 (6181–7748) | 979 (875–1095) | 90 | 9.7 | 873 | 0.14 |
| 50–59 | 88 | 7.9 (7.1–8.7) | 549 (439–685) | 5908 (4993–6991) | 854 (742–982) | 70 | 11 | 751 | 0.14 |
| 60–64 | 14 | 6.9 (6.7–7.1) | 364 (203–653) | 4544 (2972–6948) | 596 (393–906) | 53 | 12 | 659 | 0.13 |
| P = 0.99 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P = 0.46 | P < 0.01 | P = 0.03 | ||
| Sex | |||||||||
| Male | 126 | 7.4 (7.0–7.8) | 691 (596–800) | 6573 (5671–7620) | 1030 (910–1167) | 93 | 9.5 | 887 | 0.16 |
| Female | 259 | 7.6 (7.2–8.1) | 694 (616–781) | 7229 (6632–7879) | 1030 (950–1116) | 91 | 10 | 952 | 0.14 |
| P = 0.68 | P = 0.48 | P = 0.34 | P = 0.97 | P = 0.51 | P = 0.22 | P = 0.39 | P = 0.25 | ||
| BMI(kg/m2) | |||||||||
| <18.5 | 40 | 7.9 (6.7–9.3) | 602 (414–875) | 7710 (5746–10346) | 1236 (967–1580) | 76 | 13 | 977 | 0.16 |
| 18.5–24.9 | 279 | 7.3 (7.0–7.5) | 697 (630–771) | 6914 (6347–7533) | 1017 (945–1095) | 96 | 9.9 | 951 | 0.15 |
| ≥25 | 66 | 8.5 (7.0–10) | 735 (569–950) | 6997 (5825–8405) | 971 (797–1182) | 87 | 9.5 | 823 | 0.14 |
| P = 0.95 | P = 0.70 | P = 0.20 | P = 0.01 | P = 0.75 | P = 0.053 | P = 0.18 | P = 0.25 | ||
| Elecsys (Roche) | |||||||||
| Overall | 385 | 0.40 (0.40–0.41) | 24 (21–28) | 928 (857–1006) | 659 (614–708) | 59 | 39 | 2298 | 0.71 |
| Age (years) | |||||||||
| 20–29 | 63 | 0.40 (0.40–0.41) | 32 (23–43) | 1199 (1004–1431) | 1047 (908–1209) | 78 | 38 | 2967 | 0.87 |
| 30–39 | 88 | 0.40 (0.40–0.40) | 30 (23–39) | 1111 (939–1315) | 767 (674–873) | 75 | 37 | 2779 | 0.69 |
| 40–49 | 132 | 0.41 (0.39–0.43) | 23 (17–29) | 871 (763–995) | 607 (538–686) | 55 | 39 | 2128 | 0.70 |
| 50–59 | 88 | 0.40 (0.40–0.40) | 20 (14–28) | 756 (633–903) | 497 (430–575) | 50 | 38 | 1889 | 0.66 |
| 60–64 | 14 | 0.40 (0.40–0.40) | 9.2 (4.1–21) | 626 (395–992) | 399 (251–635) | 23 | 68 | 1564 | 0.64 |
| P = 0.37 | P < 0.01 | P < 0.01 | P < 0.01 | P < 0.01 | P = 0.86 | P < 0.01 | P = 0.03 | ||
| Sex | |||||||||
| Male | 126 | 0.40 (0.40–0.41) | 28 (22–36) | 941 (811–1093) | 627 (543–724) | 69 | 34 | 2341 | 0.67 |
| Female | 259 | 0.40 (0.40–0.41) | 22 (19–27) | 922 (838–1014) | 675 (623–731) | 55 | 41 | 2277 | 0.73 |
| P = 0.60 | P = 0.28 | P = 0.70 | P = 0.41 | P = 0.25 | P = 0.15 | P = 0.69 | P = 0.27 | ||
| BMI(kg/m2) | |||||||||
| <18.5 | 40 | 0.41 (0.39–0.42) | 17 (10–28) | 920 (678–1248) | 733 (565–950) | 41 | 55 | 2263 | 0.80 |
| 18.5–24.9 | 279 | 0.40 (0.40–0.41) | 24 (21–29) | 930 (847–1021) | 651 (601–705) | 60 | 38 | 2299 | 0.70 |
| ≥25 | 66 | 0.40 (0.40–0.40) | 28 (19–40) | 927 (768–1118) | 650 (535–790) | 70 | 33 | 2317 | 0.70 |
| P = 0.13 | P = 0.16 | P = 0.56 | P = 0.13 | P = 0.14 | P = 0.04 | P = 0.63 | P = 0.40 | ||
BMI: Body mass index; CI: confidence interval; anti-RBD: anti-SARS-CoV-2 receptor binding domain spike protein IgG.
S0: Within 1 week before the first vaccination, S1: within 1 week before the second vaccination, S2: 4–5 weeks after the second vaccination, S3: 6 months after the second vaccination.
Architect: Architect SARS-CoV-2 IgG Ⅱ Quant (Abbott Laboratories), Elecsys: Elecsys anti-SARS-CoV-2 S (Roche Diagnostics).
P values were calculated using the Jonckheere-Terpstra trend test.
The GMTs at S1, S2, and S3 and the GMTRs for S1/S0, S2/S0, and S3/S2 were significantly lower in the older age categories (P < 0.01, P < 0.01, P < 0.01, P < 0.01, P < 0.01, P = 0.03, respectively). The GMTR for S2/S1 was significantly lower in the high BMI group (P = 0.04) (Table 2, Supplementary Fig. 2).
3.6. Seropositivity and seroprotection compared by age, sex, and BMI category
The proportion of participants satisfying the criteria for seropositivity and seroprotection was assessed according to age, sex, and BMI category (Table 3 ).
-
(1)
Architect
Table 3.
Seropositivity and seroprotection according to age, sex, and BMI.
| Characteristics | N | Anti-RBD titer (Architect) |
Anti-RBD titer (Elecsys) |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seropositivity ≥ 50 [AU/mL] |
Seroprotection ≥ 5458 [AU/mL] |
Seropositivity ≥ 0.8 [U/mL] |
Seroprotection > 753 [U/mL] |
||||||||||||||||
| S0 | S1 | S2 | S3 | S0 | S1 | S2 | S3 | S0 | S1 | S2 | S3 | S0 | S1 | S2 | S3 | ||||
| Overall | 385 | 4 (1) | 381 (99) | 385(100) | 385(100) | 0(0) | 2 (1) | 258a (67) | 2 (1) | 1 (<1) | 373 (97) | 385(100) | 385(100) | 0(0) | 2 (1) | 240b (62) | 165c (43) | ||
| Age (years) | |||||||||||||||||||
| 20–29 | 63 | 0 (0) | 63 (100) | 0 (0) | 47 (75) | 1 (2) | 0 (0) | 62 (98) | 2 (3) | 50 (79) | 43 (68) | ||||||||
| 30–39 | 88 | 0 (0) | 88 (100) | 1 (1) | 64 (73) | 1 (1) | 0 (0) | 87 (99) | 0 (0) | 64 (73) | 43 (49) | ||||||||
| 40–49 | 132 | 2 (2) | 130 (98) | 1 (1) | 91 (69) | 0 (0) | 1 (1) | 128 (97) | 0 (0) | 78 (59) | 50 (38) | ||||||||
| 50–59 | 88 | 2 (2) | 86 (98) | 0 (0) | 52 (59) | 0 (0) | 0 (0) | 82 (93) | 0 (0) | 44 (50) | 26 (30) | ||||||||
| 60–64 | 14 | 0 (0) | 14 (100) | 0 (0) | 4 (29) | 0 (0) | 0 (0) | 14 (100) | 0 (0) | 4 (29) | 3 (21) | ||||||||
| P = 0.65 | P = 0.65 | P = 1.00 | P < 0.01 | P = 0.35 | P = 1.00 | P = 0.31 | P = 0.10 | P < 0.01 | P < 0.01 | ||||||||||
| Sex | |||||||||||||||||||
| Male | 126 | 0 (0) | 126 (100) | 0 (0) | 76 (60) | 1 (1) | 0 (0) | 124 (98) | 2 (2) | 79 (63) | 56 (44) | ||||||||
| Female | 259 | 4 (2) | 255 (98) | 2 (1) | 182 (70) | 1 (<1) | 1 (<1) | 249 (96) | 0 (0) | 161 (62) | 109 (42) | ||||||||
| P = 0.31 | P = 0.31 | P = 1.00 | P = 0.051 | P = 0.55 | P = 1.00 | P = 0.35 | P = 0.11 | P = 1.00 | P = 0.66 | ||||||||||
| BMI(kg/m2) | |||||||||||||||||||
| <18.5 | 40 | 2 (5) | 38 (95) | 0 (0) | 33 (83) | 0 (0) | 0 (0) | 36 (90) | 0 (0) | 27 (68) | 22 (55) | ||||||||
| 18.5–24.9 | 279 | 0 (0) | 278 (99) | 2 (1) | 187 (67) | 1 (<1) | 1 (<1) | 272 (97) | 2 (1) | 173 (62) | 118 (42) | ||||||||
| 25–40 | 66 | 2 (3) | 65 (98) | 0 (0) | 38 (58) | 1 (2) | 0 (0) | 65 (98) | 0 (0) | 40 (61) | 25 (38) | ||||||||
| P < 0.01 | P = 0.04 | P = 1.00 | P < 0.01 | P = 0.48 | P = 1.00 | P = 0.046 | P = 1.00 | P = 0.52 | P = 0.11 | ||||||||||
Values in the table are n (%). The thresholds for seropositivity and seroprotection were defined as 1.0 AU/mL and 5458 AU/mL, respectively using the Architect assay; and 0.8 U/mL and 753 U/mL, respectively using the Elecsys assay. P values were calculated using the Mantel-Haenszel chi-square test or Fisher's exact test, depending on the number of participants that exceeded the seropositivity or seroprotection threshold.
BMI: body mass index; anti-RBD: anti-SARS-CoV-2 receptor binding domain spike protein IgG; Architect: Architect SARS-CoV-2 IgG Ⅱ Quant (Abbott Laboratories); Elecsys: Elecsys anti-SARS-CoV-2 S (Roche Diagnostics); S0: Within 1 week before the first vaccination, S1: Within 1 week before the second vaccination, S2: 4–5 weeks after the second vaccination, S3: 6 months after the second vaccination.
Overall, seropositivity (≥50 [AU/mL]) was 1 % at S0, 99 % at S1, 100 % at S2, and 100 % at S3. Seroprotection (≥5458 [U/mL]) was 0 % at S0, 1 % at S1, 67 % at S2, and 1 % at S3. Seroprotection at S2 was significantly lower in the older age group (P < 0.01) and high BMI category (P < 0.01). Seropositivity at S1 was significantly lower in the low BMI category (P = 0.04).
-
(2)
Elecsys
Overall, seropositivity (≥0.8 [U/mL]) was < 1 % at S0, 97 % at S1, 100 % at S2, and 100 % at S3. Seroprotection (>753 [U/mL]) was 0 % at S0, 1 % at S1, 62 % at S2, and 43 % at S3. Seroprotection at S2 and S3 was significantly lower in the older category (P < 0.01 each). Seropositivity and seroprotection did not significantly differ by sex. Furthermore, seropositivity at S1 was significantly lower in the low BMI group (P = 0.046).
3.7. Multivariate linear regression analysis to identify predictors of the anti-RBD antibody titer
In the multivariate analysis, which included age, sex, and BMI (Table 4 ), older age as a continuous variable was significantly associated with lower anti-RBD titers before the second vaccination, and at 4 weeks and 6 months after the second vaccination. Sex was not significantly associated with the anti-RBD titer on either assay. A higher BMI was significantly associated with higher anti-RBD titers before the first and second vaccinations on the Architect assay.
Table 4.
Multivariate linear regression of AU/ml (Architect) or U/ml (Elecsys) adjusted by age, sex, and BMI (kg/m2).
| Architect (Abbott) |
Elecsys (Roche) |
||||||
|---|---|---|---|---|---|---|---|
| Beta (95 %CI) | Standardized beta | P value | Beta (95 %CI) | Standardized beta | P value | ||
| S0 | Age (in years, continuous) | −0.04(-0.65; 0.57) | −0.01 | 0.90 | 0.001(-0.004; 0.005) | 0.02 | 0.76 |
| Sex (female vs male) | 12.30(-1.87; 26.47) | 0.09 | 0.09 | 0.03(-0.07; 0.13) | 0.03 | 0.57 | |
| BMI (kg/m2, continuous) | 3.75(1.67; 5.84) | 0.19 | < 0.01 | −0.001(-0.02; 0.01) | −0.01 | 0.90 | |
| S1 | Age (in years) | −11.05(-19.29; −2.81) | −0.14 | 0.01 | −0.89(-1.69; −0.10) | −0.11 | 0.03 |
| Sex (female vs male) | 101.33(-90.79; 293.45) | 0.05 | 0.30 | −14.73(–33.33; 3.86) | −0.08 | 0.12 | |
| BMI (kg/m2, continuous) | 32.69(4.44; 60.94) | 0.12 | 0.02 | 2.47(-0.26; 5.21) | 0.10 | 0.08 | |
| S2 | Age (in years, continuous) | −109.98(-174.20; −45.76) | −0.18 | < 0.01 | −18.60(-28.28; −8.92) | −0.20 | < 0.01 |
| Sex (female vs male) | 295.93(-1201.10; 1792.95) | 0.02 | 0.70 | −51.95(-277.58; 173.68) | −0.02 | 0.65 | |
| BMI (kg/m2) | 16.92(-203.20; 237.05) | 0.01 | 0.88 | 7.08(-26.10; 40.25) | 0.02 | 0.68 | |
| S3 | Age (in years, continuous) | –23.05(-31.73; −14.37) | −0.27 | < 0.01 | −19.69(-25.88; −13.50) | −0.31 | < 0.01 |
| Sex (female vs male) | −15.60(-217.87; 186.66) | −0.01 | 0.88 | −7.56(-151.90; 136.79) | < 0.01 | 0.92 | |
| BMI (kg/m2, continuous) | 0.37(-29.37; 30.11) | < 0.01 | 0.98 | 10.72(-10.51; 31.94) | 0.05 | 0.32 | |
BMI, Body mass index (kg/m2); CI, confidence interval.
Architect: Architect SARS-CoV-2 IgG II Quant (Abbott Laboratories); Elecsys: Elecsys anti-SARS-CoV-2 S (Roche Diagnostics).
S0: Within 1 week before the first vaccination, S1: within 1 week before the second vaccination, S2: 4–5 weeks after the second vaccination, S3: 6 months after the second vaccination.
4. Discussion
In this study, 4 weeks after receiving two doses of the BNT162b2 vaccine, all study participants met the criterion for seropositivity and two-thirds met the criterion for seroprotection according to both assays. However, 6 months after the second vaccination, the antibody titer was greatly attenuated according to the Architect assay and to a lesser extent according to the Elecsys assay. With both assays, the anti-RBD titers were significantly lower at all time points after vaccination in the older age group than in the younger age group, although there were no significant differences according to sex. Findings regarding the effect of BMI differed between the two assays.
The increase in the anti-RBD titer and the durability of the increase, differed significantly by age. Similar results have been reported in other studies that targeted healthcare workers in Israel [11], Greece [12], and Japan [13]. Immunosenescence develops with aging, leading to a lower immune response to vaccines [25]. There was no significant difference in the anti-RBD titer by sex, although some studies have found lower anti-RBD titers in men than in women [11], [12], [13]. This inconsistency may be attributable to differences in factors such as ethnicity or the time-points at which antibodies were assessed. Both underweight and obesity were associated with lower anti-RBD titers at some time-points. BMI as a continuous variable does not predict the magnitude of the antibody response [10], [26]. We analyzed the BMI as a categorical variable; therefore, a possible bimodal effect of BMI on the post-vaccine immune response was identified.
We unexpectedly found that the anti-RBD titers measured using the two assays displayed significantly different kinetics, despite a strong correlation between the assays at all time points (S1, S2, S3) after vaccination. Four weeks after the second vaccination, the pre-defined seroprotection criterion of 90 % vaccine effectiveness was met by 62–67 % of the participants using both assays. By age group, 75–79 % of participants in their 20 s met the criterion, compared with 29 % of those in their 60 s. However, at 6 months, few participants met the criterion for seroprotection according to the Architect assay, while 43 % of participants met the criterion according to the Elecsys assay. A previous study measuring anti-RBD antibody titers after vaccination with a viral-vector vaccine also showed differences in kinetics between titers measured by the Architect assay and those measured by the Elecsys assay [27]. These results suggest that it is not appropriate to determine the timing of booster vaccination based on the results of only one type of immunoassay.
The different kinetics observed between the assays may be due to differences in the antibody detection methods [18]. The Architect assay uses a chemiluminescent microparticle immunoassay (CLIA) [22], which uses secondary antibodies to cause an antigen–antibody reaction against human IgG antibodies, to measure the antibody titer. In contrast, the Elecsys assay uses the double-antigen sandwich method [23] which does not require secondary antibodies. The double-antigen sandwich method has been reported to have better sensitivity and specificity for hepatitis B virus core antibodies than the chemiluminescent microparticle immunoassay [28]. Another possible explanation is that the repertoire and avidity of the antibodies affects the titers and changes over time [29]. In general, the avidity (affinity for the antigen) of IgG remains low after vaccine administration. The time interval and the administration of additional doses of vaccine enhances the maturity of elicited antibodies and increases their avidity toward targeting antigens [30]. The Elecsys platform is more likely to detect antibodies with higher avidity [31]. In SARS-CoV-2-naïve individuals, a single dose of mRNA vaccine generates antibodies of incomplete maturity, and high-affinity antibodies are only elicited after the second dose [32]. Differentiating high- and low-affinity antibodies may have significant clinical implications, since lower serum IgG avidity is likely associated with higher risk of infection [33]. Our study also showed that the correlation of antibodies between two assays became stronger with time following the vaccination, which is consistent with the results from a previous study [27]. Factors other than the affinity/avidity maturation of antibodies that may affect the kinetics of antibody following vaccination require further evaluation.
Although we did not directly measure neutralizing activity in this study, it may be worthwhile to compare our findings with the previously reported kinetics of neutralizing antibody titers. In a study comparing anti-RBD antibody titers (CLIA method) and neutralizing activity every 2 months for 6 months after two doses of BNT126b2 vaccine, anti-RBD antibody titers have shown a constant and dramatic decay (as observed in the Architect titers from the present study) while neutralizing activity remained rather preserved following the initial decline during the first 3 months post-vaccination [9]. Another cohort study has also shown contrasting decline patterns between anti-RBD antibody titers and neutralizing activity [34]. Interestingly, the reported relatively slow decay of neutralizing activity resembled the longitudinal evolution of Elecsys titers shown in the present study (Supplementary Fig. 3, d). The measured kinetics of antibody titers and neutralizing activity are reflective of the biphasic immune responses to SARS-CoV-2 infection and, likewise, vaccination; a rapidly peaking acute response followed by a stage of long-term memory which gradually decays [35], [36]. The excessive fluctuation observed in Architect titers from 4 weeks (S2) to 6 months (S3) after the second vaccination may be closely related to the shift among these two phases; from the acute response rich in low avidity antibodies produced by short-lived plasma cells (S2) to the chronic stage where high-avidity antibodies produced by long-lived plasma cells prevail (S3). Since the affinity maturation of antibodies are known to enhance the SARS-CoV-2 neutralizability of the antibodies [37], the decline in residual circulating IgG is seemingly countered by its continuous affinity maturation, resulting in the relative preservation of neutralizing activity. With high-affinity antibodies more strongly contributing to neutralizing activity, the fluctuation of titers (decline from S2 to S3) reported by the Architect assay, which capture specific-antibodies irrespective of their degree of avidity, may be too exaggerated a representation of the change in the actual neutralizing activity. Whether the Elecsys assay, better tuned for high-avidity antibody detection, more closely correlate to neutralizing activity during the early phase of post-vaccination is an intriguing question, not directly assessed in the present study. Further investigations are warranted, especially following the additional boosters.
A strength of this study is that, by targeting healthcare workers, who tend to be highly compliant with vaccination requirements, we obtained immunogenicity data at regular time intervals after vaccination. Furthermore, we determined the specific kinetics of the antibody titers based on different types of assays.
A limitation of the study is the small number of participants aged over 60 years. Therefore, we were unable to evaluate antibody responses in the older adult population. Furthermore, it would be useful to assess additional measures of immune protection, such as in-vitro neutralizability, cellular immune responses, and the avidity index.
In healthy Japanese adults aged 20–64 years, two doses of BNT162b2 vaccine produced an adequate antibody response, with lower antibody response and response durability in older adults than in younger adults. The effects of other factors, such as underlying disease and lifestyle, on post-vaccination immunogenicity need to be evaluated. Additionally, the kinetics of the anti-RBD titers during the 6 months after the second vaccination differed according to the immunoassay. This suggests that the results of a single immunoassay should be interpreted with caution. SARS-CoV-2 is a novel virus, and the mRNA vaccine is a novel vaccine platform and therefore, the characteristics of each assay need to be explored in depth, including their correlation with functional antibodies and vaccine efficacy/effectiveness.
Funding
This study was supported by a Grant-in-Aid for Investigation of Promotion of Health Labor Administration (Research Project for Promotion of Policies for Emerging and re-emerging Infectious Diseases and Immunization) [Principal Investigator: Yoshio Hirota; Grant Number: 20HA2001], and Japan Agency for Medical Research and Development (AMED) [Grant No: JP20he1122001 (Yasutoshi Kido) and JP20jk0110021 (Yu Nakagama)]. We received support from Osaka Metropolitan University’s “Special Reserves” fund for COVID-19.
CRediT authorship contribution statement
Tomoka Matsuura: Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Wakaba Fukushima: Conceptualization, Methodology, Investigation, Data curation, Project administration, Writing – original draft, Writing – review & editing. Yu Nakagama: Methodology, Investigation, Writing – original draft, Writing – review & editing. Yasutoshi Kido: Conceptualization, Methodology, Investigation, Project administration, Writing – original draft, Writing – review & editing. Tetsuo Kase: Conceptualization, Methodology, Investigation, Data curation, Writing – original draft, Writing – review & editing. Kyoko Kondo: Data curation, Writing – review & editing. Natsuko Kaku: Investigation, Formal analysis, Writing – review & editing. Kazuhiro Matsumoto: Investigation, Writing – review & editing. Asae Suita: Investigation, Writing – review & editing. Eriko Komiya: Investigation, Writing – review & editing. Emiko Mukai: Investigation, Writing – review & editing. Yuko Nitahara: Investigation, Writing – review & editing. Ayako Konishi: Investigation, Writing – review & editing. Ayane Kasamatsu: Investigation, Writing – review & editing. Etsuko Nakagami-Yamaguchi: Supervision, Writing – review & editing. Satoko Ohfuji: Conceptualization, Methodology, Supervision, Writing – review & editing. Yukihiro Kaneko: Conceptualization, Supervision, Writing – review & editing. Akira Kaneko: Conceptualization, Supervision, Writing – review & editing. Hiroshi Kakeya: Conceptualization, Project administration, Writing – review & editing. Yoshio Hirota: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Dr. Hisako Yoshida, Dr. Rie Onodera, Ms. Keiko Ota, Dr. Hisako Fujii, Ms. Yuki Yamada, Ms. Yoshiko Yagi, and Professor Ayumi Shintani (Department of Medical Statistics, Graduate School of Medicine, Osaka Metropolitan University, and Center for Clinical Research Innovation, Osaka Metropolitan University Hospital) for protocol review, data center and data management, monitoring, and clinical research coordinator. We would like to thank Ms. Michiyo Hattori for chief assistance in the registration process, Dr. Akitoshi Hakoda (Center for Clinical Research Innovation, Osaka Metropolitan University Hospital) for his help in registration and blood collection, and Dr. Hiroshi Kubota (Central Clinical Laboratory, Osaka Metropolitan University Hospital) for management of blood collection for antibody titer measurement. We would like to thank Mrs. Chihiro Watanabe, Mrs. Sachie Nakagama, and Mrs. Takako Kobayashi from the Department of Parasitology, Graduate School of Medicine, Osaka Metropolitan University, for technical assistance in the processing of specimens and the measurement of antibodies. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.08.018.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Accorsi E.K., Britton A., Fleming-Dutra K.E., Smith Z.R., Shang N., Derado G., et al. Association between 3 doses of mRNA COVID-19 Vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. 2022;327(7):639. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.Peterhoff D., Glück V., Vogel M., Schuster P., Schütz A., Neubert P., et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 2021;49(1):75–82. doi: 10.1007/s15010-020-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campo F., Venuti A., Pimpinelli F., Abril E., Blandino G., Conti L., et al. Antibody Persistence 6 Months Post-Vaccination with BNT162b2 among Health Care Workers. Vaccines (Basel) 2021;9(10):1125. doi: 10.3390/vaccines9101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lustig Y., Sapir E., Regev-Yochay G., Cohen C., Fluss R., Olmer L., et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Psichogiou M., Karabinis A., Poulakou G., Antoniadou A., Kotanidou A., Degiannis D., et al. Comparative immunogenicity of BNT162b2 mRNA vaccine with natural SARS-CoV-2 infection. Vaccines (Basel) 2021;9(9):1017. doi: 10.3390/vaccines9091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kageyama T, Ikeda K, Tanaka S, Taniguchi T, Igari H, Onouchi Y, et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin Microbiol Infect. 2021; 27: 1861.e1-e5. https://doi.org/10.1016/j.cmi.2021.07.042. [DOI] [PMC free article] [PubMed]
- 14.Marinaki S., Adamopoulos S., Degiannis D., Roussos S., Pavlopoulou I.D., Hatzakis A., et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goshen-Lago T., Waldhorn I., Holland R., Szwarcwort-Cohen M., Reiner-Benaim A., Shachor-Meyouhas Y., et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507. doi: 10.1001/jamaoncol.2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau K., Abe K.T., Naimark D., Oliver M.J., Perl J., Leis J.A., et al. Evaluation of the SARS-CoV-2 antibody response to the BNT162b2 vaccine in patients undergoing hemodialysis. JAMA Netw Open. 2021;4(9):e2123622. doi: 10.1001/jamanetworkopen.2021.23622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., del Molino del Barrio I., Alaguthurai T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safiabadi Tali S.H., LeBlanc J.J., Sadiq Z., Oyewunmi O.D., Camargo C., Nikpour B., et al. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin Microbiol Rev. 2021;34(3) doi: 10.1128/cmr.00228-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knezevic I., Mattiuzzo G., Page M., Minor P., Griffiths E., Nuebling M., et al. WHO International Standard for evaluation of the antibody response to COVID-19 vaccines: call for urgent action by the scientific community. Lancet Microbe. 2022;3(3):e235–e240. doi: 10.1016/S2666-5247(21)00266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lukaszuk K., Kiewisz J., Rozanska K., Dabrowska M., Podolak A., Jakiel G., et al. Usefulness of IVD kits for the assessment of SARS-CoV-2 Antibodies to evaluate the humoral response to vaccination. Vaccines (Basel) 2021;9(8):840. doi: 10.3390/vaccines9080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez-Saez J, Zaballa ME, Yerly S, Andrey DO, Meyer B, Eckerle I, et al. Persistence of anti-SARS-CoV-2 antibodies: immunoassay heterogeneity and implications for serosurveillance. Clin Microbiol Infect. 2021; 27: 1695.e7-e12. https://doi.org/10.1016/j.cmi.2021.06.040. [DOI] [PMC free article] [PubMed]
- 22.Abbott ARCHITECT SARS-CoV-2 IgG II Quant Reagent -Instructions for Use- US Food and Drug administration. https://www.fda.gov/media/146371/download [accessed on 8 February 2022].
- 23.Elecsys Anti-CoV-2 S -Instructions for use- US Food and Drug administration. https://www.fda.gov/media/137605/download [accessed on 8 February 2022].
- 24.Japan Society for the Study of Obesity. Guidelines for the management of obesity diseases 2016 (in Japanese). http://www.jasso.or.jp/contents/magazine/journal.html [accessed on 7 April 2022].
- 25.Poland G.A., Ovsyannikova I.G., Kennedy R.B. Personalized vaccinology: a review. Vaccine. 2018;36:5350–5357. doi: 10.1016/j.vaccine.2017.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36 doi: 10.3390/vaccines9070685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkmann T., Mucher P., Perkmann-Nagele N., Radakovics A., Repl M., Koller T., et al. The comparability of anti-spike SARS-CoV-2 antibody tests is time-dependent: a prospective observational study. Microbiol Spectr. 2022;10(1):e0140221. doi: 10.1128/spectrum.01402-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li A.n., Yuan Q., Huang Z., Fan J., Guo R., Lou B., et al. Novel double-antigen sandwich immunoassay for human hepatitis B core antibody. Clin Vaccine Immunol. 2010;17(3):464–469. doi: 10.1128/CVI.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nitahara Y., Nakagama Y.u., Kaku N., Candray K., Michimuko Y.u., Tshibangu-Kabamba E., et al. High-resolution linear epitope mapping of the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 mRNA vaccine recipients. Microbiol Spectr. 2021;9(3):e0096521. doi: 10.1128/Spectrum.00965-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Saez J, Zaballa ME, Yerly S, Andrey DO, Meyer B, Eckerle I, et al. Persistence of anti-SARS-CoV-2 antibodies: immunoassay heterogeneity and implications for serosurveillance. Clin Microbiol Infect. 2021; 27: 1695.e7-e12. https://doi.org/10.1016/j.cmi.2021.06.040. [DOI] [PMC free article] [PubMed]
- 31.Nakagama Y.u., Nitahara Y., Kaku N., Tshibangu-Kabamba E., Kido Y., McAdam A.J. A dual-antigen SARS-CoV-2 serological assay reflects antibody avidity. J Clin Microbiol. 2022;60(2) doi: 10.1128/jcm.02262-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Struck F., Schreiner P., Staschik E., Wochinz‐Richter K., Schulz S., Soutschek E., et al. Vaccination versus infection with SARS-CoV-2: Establishment of a high avidity IgG response versus incomplete avidity maturation. J Med Virol. 2021;93(12):6765–6777. doi: 10.1002/jmv.27270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int J Infect Dis. 2021;106:61–64. doi: 10.1016/j.ijid.2021.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malipiero G., D’Agaro P., Segat L., Moratto A., Villalta D. Long-term decay of anti-RBD IgG titers after BNT162b2 vaccination is not mirrored by loss of neutralizing bioactivity against SARS-CoV-2. Clin Chim Acta. 2022;524:11–17. doi: 10.1016/j.cca.2021.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turner J.S., Kim W., Kalaidina E., Goss C.W., Rauseo A.M., Schmitz A.J., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595(7867):421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 36.Radbruch A., Chang H.-D. A long-term perspective on immunity to COVID. Nature. 2021;595(7867):359–360. doi: 10.1038/d41586-021-01557-z. [DOI] [PubMed] [Google Scholar]
- 37.Muecksch F, Weisblum Y, Barnes CO, Schmidt F, Schaefer-Babajew D, Wang Z, et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity 2021; 54: 1853-1868.e7. https://doi.org/10.1016/j.immuni.2021.07.008. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.