Abstract
We assessed antibiotic prescribing in practical dentistry in the Czech Republic, as antibiotics are widely prescribed by dental practitioners and warning signals of their overuse can be observed. The individual antibiotic prescriptions were extracted from the database of the General Health Insurance Company and further analysed. The proportion of dentists’ prescription within the whole primary health-care sector and the rate of prescriptions of particular antibiotics were both in defined daily doses per 1,000 insurees and day (DID) and in number of prescriptions calculated. The proportion of antibiotic use in dentistry increased from 0.63 DID in 2006 to 0.75 DID. We found a decline in use of narrow-spectrum penicillins by 4.8%, tetracyclines by 3.5% and macrolides by 3.6%, accompanied by increasing rate of prescription of aminopenicillins combined with beta-lactamase inhibitor by 8.9% and lincosamides by 8.5%. The consumption of clindamycin and amoxicillin combined with clavulanate in DID has increased by approximately 60% since 2006 thanks to the exclusive prescribing of two commercial oral products only. Factors contributing to this unfavourable trend are commercial influence or defensive medicine practice.
Key words: General dental practitioners, prescribing, antibiotics, database study
INTRODUCTION
Antibiotics are a category of drugs widely prescribed by dental practitioners. Even in this specialisation antibiotics should be used rationally, by attempting to ensure their maximum effectiveness with minimal risk of side effects or development of resistance, as general practitioners and practitioners for children and adolescents should do.
The use of antibiotics in dentistry can be characterised as predominantly empirical, when the antibiotic/antibacterial therapy is carried out on the basis of well-known clinical and epidemiological factors. As with other practitioners in the Czech Republic bacteriological culture is not usually performed before antibiotic is administered, which is quite often seen as a waste of time and money, perhaps somewhat justifiably, if we know that the spectrum of bacterial pathogens causing odontogenic infections is relatively narrow. Moreover, as found elsewhere, the therapeutic and prophylactic use of antibiotics by dental practitioners is often incorrect1., 2., 3., 4., 5., 6., 7., 8., 9., 10.. Administration of prophylactic antibiotics to individuals at risk of developing infectious endocarditis of odontogenic origin constitutes a negligible percentage of indications. Moreover, in recent years, antibiotic prophylaxis has been increasingly discussed. It also is difficult to justify the relatively widespread prophylactic antibiotic use in oral surgery. A typical feature is overuse of broad-spectrum antibiotics, particularly boosted aminopenicillins and macrolide antibiotics, and redundant and questionable use of lincosamides, especially oral clindamycin, because of their good penetration in bone tissue6., 7., 8.. We are not aware of a study that has evaluated this issue in the Czech Republic.
METHODS
All systemic antibiotics, ATC (Anatomical Therapeutic Chemical classification) group J01 (Antibacterials for Systemic Use) in the Czech Republic are subject to medical prescription. The General Health Insurance Company (NGA), which insures 62% of the population, have a database summarising data of its insured individuals based on the supply of medicinal products in pharmacies. One row in the database corresponds to one individual prescription. Physician specialty is identified. The patients’ personal identification numbers were automatically encoded, to ensure the patients’ anonymity. There is therefore no possibility of disclosing any personal information other than sex and year of birth. Nevertheless, the Ethical board independently reviewed the study although informed consent was not obtained. The diagnosis is not mandatory in the instructions, and therefore this information is not included in the database. For the purposes of this study we extracted the expenditures relating ATC group J01, prescribed by primary care physicians, GPs (general practitioner, practitioner for children and adolescents, dentist) in the period 2006–2012.We evaluated the proportion of dentists’ antibiotics prescriptions within primary health care and the trend in consumption in defined daily doses (DDDs) or in number of prescriptions of clindamycin and aminopenicillins combined with beta-lactamase inhibitors. We discovered preferences for some brand name products. In men older than 18 years we also have identified the proportion of individuals who received amoxicillin with clavulanic acid in the lowest distributed dose of 375 mg.
RESULTS
Dental practitioners prescribed 6.5% and 8.5% of antibiotic prescriptions by all practitioners providing primary health care in 2006 and 2012, respectively; this increase is also observable in DDDs (6.2% and 7.7%, 0.63 DID and 0.75 DID, respectively). Dental practitioners prescribed over 27 antibiotics for every 1,000 insured individuals. That means that roughly one in 36 insurees gets one prescription for antibiotics per year for dental indications (Table 1). In the older middle age patients group the dentists prescriptions comprised up to 12% of antibiotic prescriptions, whereas to children and elderly they prescribed <1% of antibiotic prescriptions.
Table 1.
The percentage rate of dentists’ antibiotic prescriptions and number of defined daily doses (DDDs) within the whole primary health-care sector
| Year | % of prescriptions | % of DDDs (DID) | Prescriptions/1000 insurees/year |
|---|---|---|---|
| 2006 | 6.5 | 6.2 (0.63) | 23.9 |
| 2007 | 6.7 | 6.3 (0.68) | 25.8 |
| 2008 | 6.9 | 6.3 (0.67) | 27.3 |
| 2009 | 7.3 | 6.5 (0.70) | 28.5 |
| 2010 | 7.9 | 7.3 (0.74) | 29.8 |
| 2011 | 7.7 | 7.0 (0.75) | 29.9 |
| 2012 | 8.5 | 7.7 (0.79) | 30.1 |
Defined daily dose set by the World Health Organisation Collaborating Centre for Drug Statistics Methodology, Oslo,2012 (http://www.whocc.no/atc_ddd_index/). DID, Defined daily doses per 1,000 insurees and day.
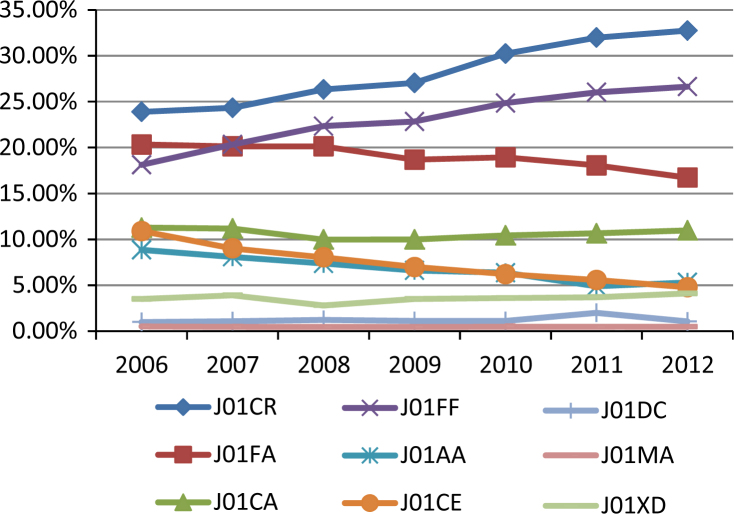
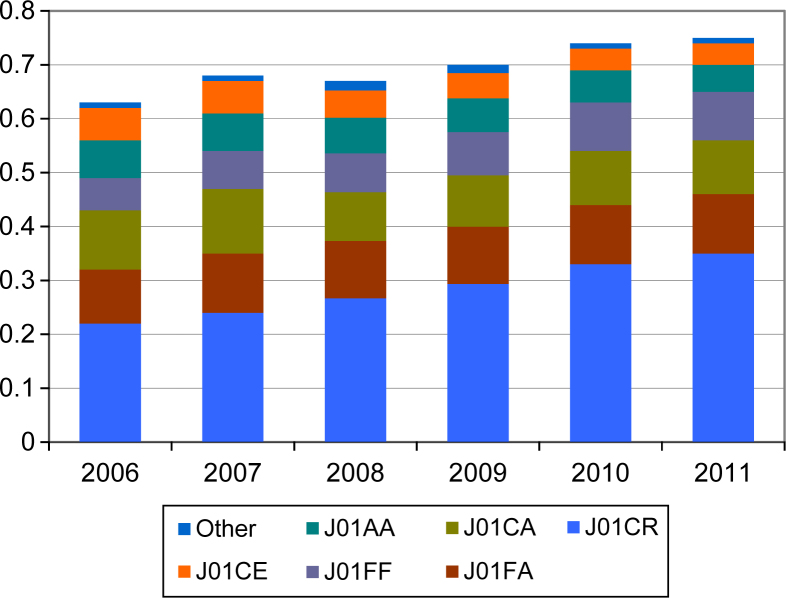
We also found a decline in the use of narrow-spectrum penicillins (4.8%), tetracyclines (3.5%) and macrolides (3.6%), accompanied by increasing rate in prescriptions of aminopenicillins combined with beta-lactamase inhibitor (8.9%) and lincosamides (8.5%) (Figure 1). In DDDs per 1,000 insurees and day, the differences were more pronounced (Figure 2), mainly with regard to amoxicillin combined with clavulanic acid, for which consumption increased from 0.217 DID in 2006 to 0.350 DID in 2011. The increase in clindamycin consumption is also prominent, although the proportion of prescribing is lower (0.054 DID in 2006 and 0.089 DID in 2012). The consumption of each of these two antibiotics increased by approximately 60%. Dentists prescribed approximately 80% of lincosamides used in the primary health care overall (Table 2). Similarly, dentists prescribe metronidazole to a greater extent than other GPs (57.5% and 62.2% of all metronidazole prescriptions in primary health care in 2006 and 2012, respectively).
Figure 1.
The proportion of prescriptions of particular ATC (Anatomical Therapeutic Chemical Classification) groups of antibiotics (100% represents all antibiotic prescriptions by dental practitioners). Legend: J01CR, aminopenicillins with beta-lactamase inhibitor; J01FA, macrolides; J01CA, aminopenicillins; J01FF, lincosamides; J01AA, tetracyclines; J01CE narrow spectrum penicillines; J01DC, second generation cephalosporines; J01MA, fluoroquinolones; J01XD, nitroimidazoles.
Figure 2.
The consumption of particular ATC (Anatomical Therapeutic Chemical Classification) groups in DID (defined daily doses per 1,000 insurees and day) in dentistry. Legend: J01CR, aminopenicillins with beta-lactamase inhibitor; J01FA, macrolides; J01CA, aminopenicillins; J01FF, lincosamides; J01AA, tetracyclines; J01CE, narrow spectrum penicillines.
Table 2.
The rate of clindamycin and amoxicillin combined with clavulanic acid use in dentistry as a proportion of its prescriptions issued in primary health care and their use in dentistry as a proportion of all antibiotics administered by dentists
| Year | Proportion of clindamycin prescriptions within the primary care in DDDs, % (prescriptions, %) | The proportion of clindamycin use in dentistry in number of prescriptions, % (consumption in DID) | The proportion of amoxicillin with clavulanic acid use within primary care in DDDs, % (prescriptions %) | The proportion of amoxicillin with clavulanic acid use in number of prescriptions in dentistry (consumption in DID) |
|---|---|---|---|---|
| 2006 | 81.2 (86.0) | 17.9 (0.054) | 13.8 (13.5) | 23.1% (0.217) |
| 2007 | 82.0 (87.2) | 20.3 (0.065) | 13.3 (13.0) | 23.7% (0.241) |
| 2008 | 80.5 (85.2) | 22.2 (0.071) | 13.7 (13.1) | 25.8% (0.264) |
| 2009 | 80.6 (85.3) | 23.0 (0.078) | 14.4 (13.7) | 27.0% (0.291) |
| 2010 | 80.5 (84.9) | 23.5 (0.085) | 17.1 (16.1) | 29.1% (0.329) |
| 2011 | 78.4 (82.3) | 24.6 (0.089) | 17.4 (16.6) | 30.8% (0.350) |
| 2012 | 78.4 (83.0) | 25.1 (0.094) | 19.0 (18.7) | 31.4% (0.373) |
DDD, Defined daily doses; DID, defined daily doses per 1,000 insurees and day.
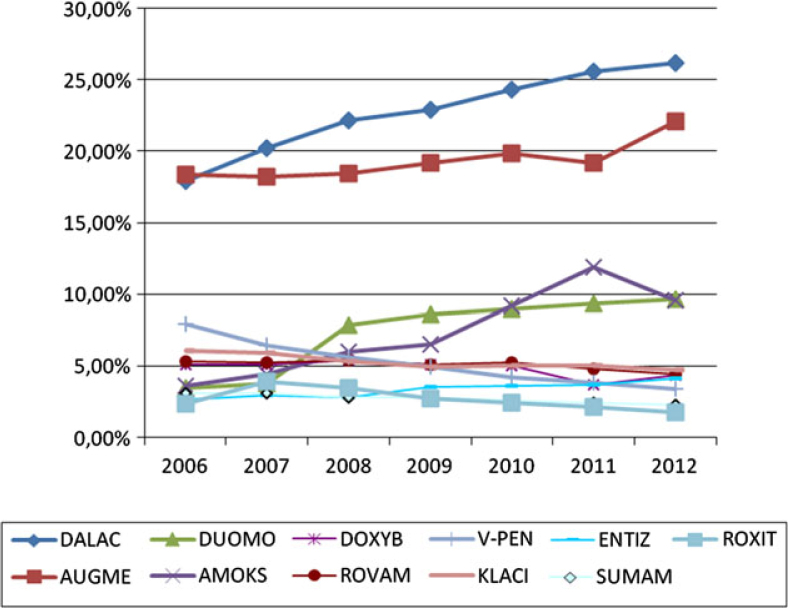
Commercial oral products Dalacin C and Augmentin were the major preparations prescribed (around 20%). Other brand names, even though of the same composition were prescribed two to three times less frequently (Figure 3).
Figure 3.
Stomatologist’ preference of various antibiotics brand names. Legend: DALAC, dalacin; DUOMO, duomox; DOXYB, doxybene; V-PEN, V-penicilin; ENTIZ, Entizol; ROXIT, Roxithromycin; AUGME, augmentin; AMOKS, amoksiklav; ROVAM, rovamycin, KLACI, klacid; SUMAM, Sumamed.
While in 2006 probably 5.9% of men were treated with an insufficient dose, in 2012 it was only 2.1% of those taking 375 mg amoxicillin + clavulanic acid. Similar positive trends were noted in clindamycin prescribing. One package of Dalacin C 150 mg was prescribed in 17.5% and 8.4% in 2006 and 2012, respectively. Clindamycin was mostly used in 4-day treatment regimens, as determined from the average number of DDDs per one patient.
DISCUSSION
We found an absolute increase in the use of antibiotics by the dentists and a growing trend in the number of antibiotic prescriptions per insured person per year. There was also a high proportion of broad-spectrum antibiotics prescribed, namely amoxicillin combined with clavulanic acid, for which consumption was pronouncedly higher than for other antibiotics in the study period and, additionally, had been increasing. In this aspect, the behaviour of dentists was not significantly different from the trends recorded in outpatient settings in the Czech Republic and some other European countries12 or in surveys of paediatricians elswhere13. The specific phenomenon of contemporary dental care is very often prescribed and misused clindamycin. We have discussed the reasons and consequences of increased consumption of clindamycin and amoxicillin with clavulanate, which were caused almost exclusively by prescribing two brand names only (Dalacin C and Augmentin). Clindamycin came to the fore in dental practice in the 1990s14. For a long time it was used in the prophylaxis of infectious endocarditis (IE), mainly in patients with verified penicillin allergy, and there was huge clinical evidence in 2005 about its efficacy and safety in the management of dental infections15. In contrast, there are reports about the association with the occurence of methicillin-resistant Staphylococcus aureus (MRSA) and in some countries it is used as a reserve antibiotic for the treatment of serious infections of inpatients16 Clindamycin is favoured for its ability to penetrate bones and joints, where it is used to treat infections caused by S. aureus and anaerobes17. We consider the routine use of clindamycin in dentistry, as found in this study, quite unfounded. Both lincosamides and combinations of amoxicillin + clavulanate, relatively more often cause severe pseudomembranous colitis associated with Clostridium difficile overgrowth18. A high proportion of the prescribing of these suggests that the reason for their use is often ‘buck-passing’ in terms of so-called ‘defensive medicine’. The predominance of prescribing amoxicillin among all antibiotics has been also reported in other studies4., 6., 19., 20.. Perhaps higher average age of Czech dentists (in 2009 stood at 50.3 years21) contributes to this phenomenon. In contrast, Lithuanian research revealed that dentists with longer practice experience, although prescribing antibiotics more often, are more likely to prescribe narrow-spectrum penicillin and less often aminopenicillins compared with younger physicians1. In most cases, oral streptococci are causal pathogens of odontogenic bacterial infections, and are reported to be sensitive to beta-lactam antibiotics in general22. Acid-stable penicillin V (phenoxymethylpenicillin) remains the most appropriate antimicrobial agent of choice for initial empirical antibiotic therapy: it is safe, highly effective and inexpensive. The investigation carried out in the Czech Republic, at the University Hospital Hradec Kralove, found that 94% of the strains of obligate anaerobes and 100% of the strains of oral streptococci and beta-haemolytic streptococci isolated from patients with orofacial bacterial infections were sufficiently sensitive to penicillin V23. Clindamycin is probably used in all patients self-reporting a history of penicillin allergy as it is described in obstetrics24, although it is known that penicillin allergy is over-reported25. Amoxicillin reveals few indications in the routine treatment of odontogenic infections, although it is the drug of choice for prophylaxis of IE and better than penicillin V for higher serum levels. However, the number of resistant strains of oral streptococci producing beta-lactamase is increasing. This happens especially in patients previously treated with beta-lactamase antibiotic26 and this factor should be taken into account in the choice of treatment not only in dentistry. We observed an increase in the proportion of use of aminopenicillins combinations over merely aminopenicillins and narrow-spectrum penicillins, which unfortunately corresponds with the findings that the oral cavity is more often inhabited by bacterial beta-lactamase-producing strains27. Other factors that can contribute to this trend are undoubtedly considerable pressure from commercial companies – producers and distributors of these drugs in dentistry. Another reason is that the medical issues as the rational pharmacotherapy of infectious deseases and pharmacotherapy at all, are only of marginal interest in dentistry, although without their deeper knowledge the dentists cannot work successfully.
Synthetic nitroimidazole drugs, mostly metronidazole, are currently irreplaceable in the treatment of stages of advanced periodontitis of aggressive or chronic type as the antimicrobial adjuvant chemotherapy in which we try to eliminate selectively the overgrowth of so called periopatogens, especially Gram-negative rods and oral spirochetes.
Some recent studies have also revealed underdosing and inappropriate treatment periods2., 6.. We are aware of this problem, but to date, it has not been evaluated. The actual dosing regimens are difficult to identify from the database. Therefore, rating of incorrect antibiotic dosing (especially the frequent underdosing) was based on the evaluation of a prescribing of packages with different contents of amoxicillin + clavulanic acid. The prerequisite was that the dose of 375 mg of amoxicillin and clavulanic acid in men older than 18 years provides an insufficient amount of antibiotic for therapeutic use, and carries the risk of developing resistance of sensitive microorganisms. Over time, fortunately, we observed a reversal of the trend towards using such inappropriate ‘weak’ dosages.
Because the NGA data do not allow one to determine the indication for antibiotic administration, we cannot assess whether the type of antibiotic, dosage and duration of treatment was chosen correctly for each individual patient. However suboptimal, unfit to improper use of antibiotics by dentists has been found elsewhere in the world, such as in France2, Lithuania1 and Iran4. Dental emergencies very similar to problems in our country are described in the UK4. The patient is sent home with antibiotics instead of receiving a thorough treatment of the tooth because the dental surgery traditionally lacks the space for proper care. The question in this discussion is also the legitimacy of prophylactic administration of antibiotics for the prevention of infectious endocarditis (IE). It should be noted that in 2008 the National Institute for Health and Clinical Excellence (NICE) recommended terminating antibiotic prophylaxis before dental procedures in the UK and there has not been an increased incidence in IE upto 201228. There is a lack of evidence for the efficacy of penicillin in the prophylaxis of IE and it is also unclear whether the potential harms and costs of antibiotic administration outweigh the benefit11. The implications of this are concerns over patients with an increased risk of developing IE in dental treatment associated with impaired integrity of vascularised tissue, such as tooth extraction29. Therefore, the ethically minded dentists should discuss potential harms and benefits with their patients and make decisions about antibiotic administration on an individual basis11.
Still unsolved and undebated is the subject of so-called surgical prophylaxis, performed in difficult extraction of teeth, especially the lower third molars, in particular in the introduction of dental implants, regenerative surgical procedures (e.g. bone augmentations and guided tissue regeneration and guided bone regeneration) in the Czech Republic. This trend is increasing in direct proportion with the financial demands of therapy paid directly by the patient. The shift from the prescribing of narrow-spectrum penicillins or oral aminopenicillin to lincosamides is perhaps also indirect evidence. However, the main precaution to eliminate any early postoperative complications remains a carefully executed surgery without tissue damage and effective decontamination of the oral cavity carried out immediately before surgery using appropriate and sufficiently effective mucosal antiseptic (chlorhexidine, octenidine). This discussion does not apply to indications for antibiotic use in maxillofacial surgery and oncological surgery, which does occur within primary dental care.
In future dentists should receive the maximum information about antibiotics (and all other dosage groups) during their undergraduate study in the various subjects taught (e.g. oral microbiology, pharmacology, periodontics, minor oral surgery, dental implantology, etc.). Expertise in this period is chiefly theoretical, unsupported practical experience obtained in addition to competing other equally important medical knowledge. After graduating and leaving the medical faculty, the dentist’s knowledge is largely overwhelmed by the relatively greater information needed, while they resort to therapeutic use of antibiotics de facto only in problematic situations. Dental practitioners undergo refreshing of these findings only by virtue of voluntarily and sporadically offered training events of varying quality.
We could not determine the percentage of correctly and incorrectly indicated antibiotic prescribed by the dental practitioners from the NGA database. However, we noted some trends in their prescription, including a growing share of the total consumption of antibiotics in the primary health-care area, the rising consumption of broad-spectrum antibiotics, and can thus estimate the proportion of patients with poorly chosen doses of antibiotics. None of these outstanding issues have yet been described in public or discussed in the Czech dentist community.
ACKNOWLEDGEMENTS
We thank the General Health Insurance Company for providing the data. This study was supported by the Charles University in Prague (Project SVV 267 005).
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Skučaitė N, Pečiulienė V, Manelienė R, et al. Antibiotic prescription for the treatment of endodontic pathology: a survey among Lithuanian dentists. Medicina (Kaunas) 2010;46:806–813. [PubMed] [Google Scholar]
- 2.Dupon M, Cohere-Moleres MF, Dupon C, et al. Evaluation of antibiotherapy in dental surgery. Presse Med. 1994;23:1803–1808. [PubMed] [Google Scholar]
- 3.Roy KM, Bagg J. Antibiotic prescribing by general dental practitioners in the Greater Glasgow Health Board, Scotland. Br Dent J. 2000;188:674–676. doi: 10.1038/sj.bdj.4800574. [DOI] [PubMed] [Google Scholar]
- 4.Vessal G, Khabiri A, Mirkhani H, et al. Study of antibiotic prescribing among dental practitioners in Shiraz, Islamic Republic of Iran. East Mediterr Health J. 2011;17:763–769. [PubMed] [Google Scholar]
- 5.Dailey YM, Martin MV. Are antibiotics being used appropriately for emergency dental treatment? Br Dent J. 2001;191:391–393. doi: 10.1038/sj.bdj.4801190. [DOI] [PubMed] [Google Scholar]
- 6.Murti A, Morse Z. Dental antibiotic prescription in Fijian adults. Int Dent J. 2007;57:65–70. doi: 10.1111/j.1875-595x.2007.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 7.Mainjot A, D’Hoore W, Vanheusden A, et al. Antibiotic prescribing in dental practice in Belgium. Int Endod J. 2009;42:1112–1117. doi: 10.1111/j.1365-2591.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- 8.Anderson R, Calder L, Thomas DW. Antibiotic prescribing for dental conditions: general medical practitioners and dentists compared. Br Dent J. 2000;188:398–400. doi: 10.1038/sj.bdj.4800493. [DOI] [PubMed] [Google Scholar]
- 9.Tanwir F, Marrone G, Lundborg CS. Knowledge and reported practice of antibiotic prescription by dentists for common oral problems. J Coll Physicians Surg Pak. 2013;23:276–281. [PubMed] [Google Scholar]
- 10.Dar-Odeh NS, Abu-Hammad OA, Shehabi AA. Antibiotic prescribing practices by dentists: a review. Ther Clin Risk Manag. 2010;6:301–306. doi: 10.2147/tcrm.s9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver R, Roberts GJ, Hooper L, et al. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst Rev. 2008;8:CD003813. doi: 10.1002/14651858.CD003813.pub3. [DOI] [PubMed] [Google Scholar]
- 12.European Centre for Disease Prevention and Control . ECDC; Stockholm: 2013. Surveillance of Antimicrobial Consumption in Europe, 2010. [Google Scholar]
- 13.Rossignoli A, Clavenna A, Bonati M. Antibiotic prescription and prevalence rate in the outpatient paediatric population: analysis of surveys published during 2000-962005. Eur J Clin Pharmacol. 2007;63:1099–1106. doi: 10.1007/s00228-007-0376-3. [DOI] [PubMed] [Google Scholar]
- 14.van der Bilj P. Clindamycin in dentistry. J Dent Assoc. 1994;49:563–566. [PubMed] [Google Scholar]
- 15.Brook I, Lewis MA, Sándor GK, et al. Clindamycin in dentistry: more than just effective prophylaxis for endocarditis? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:550–558. doi: 10.1016/j.tripleo.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 16.Prabhu K, Rao S, Rao V. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Lab Physicians. 2011;3:25–27. doi: 10.4103/0974-2727.78558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baird P, Hughes S, Sullivan M. Penetration into bone and tissues of clindamycin phosphate. Postgrad Med J. 1978;54:65–67. doi: 10.1136/pgmj.54.628.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conly JM. Clostridium difficile-associated diarrhea – the new scourge of the health care facility. Can J Infect Dis. 2000;11:25–27. doi: 10.1155/2000/608154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dayer MJ, Chambers JB, Prendergast B, et al. NICE guidance on antibiotic prophylaxis to prevent infective endocarditis: a survey of clinicians’ attitudes. QJM. 2013;106:237–243. doi: 10.1093/qjmed/hcs235. [DOI] [PubMed] [Google Scholar]
- 20.Kudiyirickal MG, Hollinshead F. Antimicrobial prescribing practice by dentists: a study from two primary care centres in UK. Minerva Stomatol. 2011;60:495–500. [PubMed] [Google Scholar]
- 21.Ústav zdravotnických informací a statistiky ČR. Lékaři, zubní lékaři a farmaceuti 2009, ISSN: 1214-9888 (1211-8230): p 17.
- 22.Warnke PH, Becker ST, Springer IN, et al. Penicillin compared with other advanced broad spectrum antibiotics regarding antibacterial activity against oral pathogens isolated from odontogenic abscesses. J Craniomaxillofac Surg. 2008;36:462–467. doi: 10.1016/j.jcms.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Kudiyirickal MG. Charles University in Prague, Faculty of Medicine in Hradec Kralove; 2012. Effect of Antimicrobial Agents on Oral Microorganisms, Dissertation Thesis. [Google Scholar]
- 24.Paccione KA, Wiesenfeld HC. Guideline adherence for intrapartum Group B Streptococci prophylaxis in penicillin-allergic patients. Infect Dis Obstet Gynecol. 2013;2013:917304. doi: 10.1155/2013/917304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopac P, Zidarn M, Kosnik M. Epidemiology of hypersensitivity reactions to penicillin in Slovenia. Acta Dermatovenerol Alp Panonica Adriat. 2012;21:65–67. [PubMed] [Google Scholar]
- 26.Kuriyama T, Nakagawa K, Karasawa T, et al. Past administration of beta-lactam antibiotics and increase in the emergence of beta-lactamase-producing bacteria in patients with orofacial odontogenic infections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:186–192. doi: 10.1067/moe.2000.102040. [DOI] [PubMed] [Google Scholar]
- 27.Rams TE, Degener JE, van Winkelhoff AJ. Prevalence of β-lactamase-producing bacteria in human periodontitis. J Periodontal Res. 2013;48:493–499. doi: 10.1111/jre.12031. [DOI] [PubMed] [Google Scholar]
- 28.Thornhill MH. Infective endocarditis: the impact of the NICE guidelines for antibiotic prophylaxis. Dent Update. 2012;39:6–10. doi: 10.12968/denu.2012.39.1.6. 12. [DOI] [PubMed] [Google Scholar]
- 29.Thomas DW, Hill CM. An audit of antibiotic prescribing in third molar surgery. Br J Oral Maxillofac Surg. 1997;35:126–128. doi: 10.1016/s0266-4356(97)90688-4. [DOI] [PubMed] [Google Scholar]