Abstract
Many publications are available on the topic of compliance with infection prevention and control in oral health-care facilities all over the world. The approaches of developing and developed countries show wide variation, but the principles of infection prevention and control are the same globally. This study is a systematic review and global perspective of the available literature on infection prevention and control in oral health-care facilities. Nine focus areas on compliance with infection-control measures were investigated: knowledge of infectious occupational hazards; personal hygiene and care of hands; correct application of personal protective equipment; use of environmental barriers and disposable items; sterilisation (recirculation) of instruments and handpieces; disinfection (surfaces) and housekeeping; management of waste disposal; quality control of dental unit waterlines, biofilms and water; and some special considerations. Various international studies from developed countries have reported highly scientific evidence-based information. In developed countries, the resources for infection prevention and control are freely available, which is not the case in developing countries. The studies in developing countries also indicate serious shortcomings with regard to infection prevention and control knowledge and education in oral health-care facilities. This review highlights the fact that availability of resources will always be a challenge, but more so in developing countries. This presents unique challenges and the opportunity for innovative thinking to promote infection prevention and control.
Key words: Dentistry, dental, oral health care, infection control, compliance with guidelines
INTRODUCTION
During the early 1980s, most oral health-care workers (OHCWs) practiced oral health care without wearing gloves, masks or eye protection1. The identification of infection with the human immunodeficiency virus (HIV) in 1981, which resulted in acquired immune-deficiency syndrome (AIDS), possibly had one of the most significant impacts on the oral health-care profession2., 3.. At that time, the routes of transmission and biology of HIV were poorly understood. As a direct result of the growing HIV/AIDS epidemic, infection control, especially in the clinical oral health-care environment, changed almost overnight. More than three decades later, patient profiles have changed considerably, and treatment regimens have thus adapted towards early diagnosis and preventive approaches2. Today, there is generally a better understanding of disease transmission and prevention in oral health care, which has led to a greater focus on practising infection prevention and control4.
Oral health-care facilities have led the way in implementing infection-control practices by routinely incorporating hand hygiene and sterilisation procedures3. This has contributed positively to the reduction of various disease-transmission challenges. Additionally, since the mid-1980s, before any of the other health professions, oral health-care facilities have rapidly incorporated hepatitis B virus (HBV) vaccinations for personnel members3.
A systematic review of studies published from January 2008 to September 2013 that address compliance with infection-control guidelines and recommendations in developed as well as in developing countries, was undertaken, and will be reflected in this article.
RESEARCH MATERIALS AND METHODS
As just noted, a systematic review of global literature addressing infection-control compliance in oral health care was undertaken. A similar review has previously been published, covering the same literature and applicable to South Africa, but only up to 20075. The present review covers global studies published from January 2008 to September 2013. It focuses particularly on adherence to infection-control practices and includes all the categories of OHCWs, namely dental practitioners, dental therapists, dental assistants, oral hygienists and students.
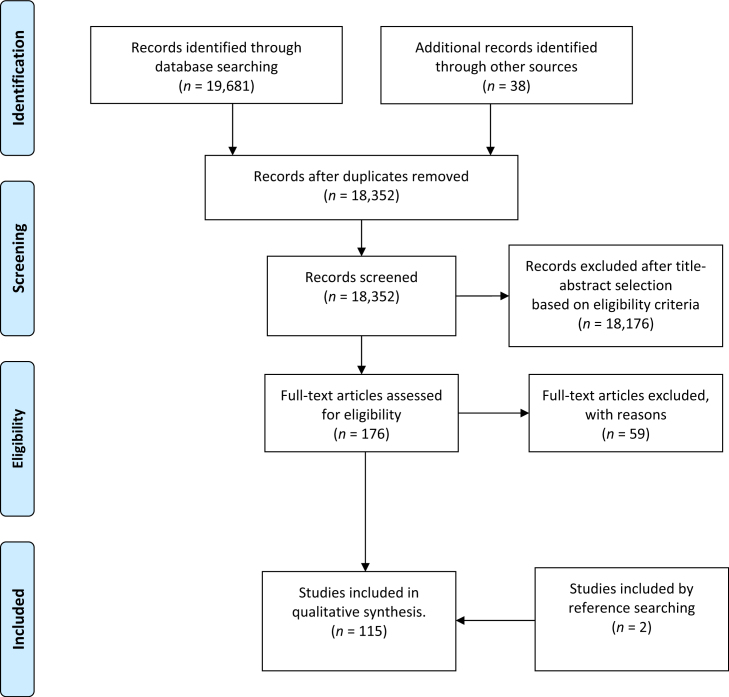
International electronic databases were searched, including Medline (EBSCOhost), Academic Search Premier (EBSCOhost), Science Direct, SA ePublications, SACAT and ISAP (by the National Library of South Africa), as well as the theses and dissertations from universities for the period January 2008 to September 2013. The search terms included, ‘infection control’, ‘dentistry’, ‘dental’, ‘oral health’ and ‘compliance’. Responses to these search terms were then searched again, in more depth. The search produced 19,681 publications, of which 176 were selected containing quantitative data, while those containing mere recommendations were excluded (Figure 1). All selected publications were further scrutinised for adherence to the following questions:
Figure 1.
PRISMA 2009 flow diagram (Moher et al., 2009)117.
-
•
Does the literature provide details on infection control in oral health care?
-
•
Can the contents of the selected literature be applied for compliance with infection control in oral health-care facilities?
The outcome measures used as the baseline for infection-control practices are similar to those used in the earlier publication5. These outcome measures were selected according to international recommendations of the British Dental Association, the United States Centers for Disease Control and Prevention (CDC) and the Australian Dental Association6., 7., 8., 9., 10.. The outcomes focused on: knowledge of infectious hazards, personal hygiene and care of hands; wearing of personal protective equipment; environmental barriers; sterilisation (recirculation) of instruments and handpieces; disinfection (surfaces) and housekeeping; waste disposal; quality control and maintenance of dental unit waterlines, biofilms and water supply; and other special considerations.
COMPLIANCE WITH INFECTION CONTROL IN DENTISTRY: A 5-YEAR REVIEW OF STUDIES IN DEVELOPED AND DEVELOPING COUNTRIES
Focus area one: Knowledge of infectious hazards
Current epidemiological data outline the risk of exposure and possibility of transmitting diseases when providing oral health-care treatment11. The World Dental Federation (FDI) thus recommends that all oral health-care professionals keep their knowledge and skills current. With the application of up-to-date knowledge and skills, transmission of infectious diseases could be managed in oral health-care facilities11. As stated, three decades ago it was the fear of the HIV/AIDS epidemic that motivated infection-control preventive measures2., 12.. Today, recommendations, guidelines and policy statements assist oral health-care professionals to prevent and control infectious risks by routinely following standard infection-control precautions6., 7., 8., 11., 13., 14., 15., 16., 17..
Recent media reports on breaches of infection control in USA oral health-care facilities have increased public concern18., 19., 20.. Compliance with infection control and factors associated with the implementation of CDC infection-control guidelines were investigated by Cleveland et al. from the USA21. The authors linked compliance with infection control to continuous professional education through various modes/events of education21. Examples of the modes of learning and education included workshops, journal articles and Internet-based learning. Furthermore, Cleveland et al.21 reported that younger dental practitioners, who had been in their current practice for less than 30 years, were more likely to implement infection-control guidelines. Exposure to more intensive and varying types of infection-control education were highlighted as possible reasons for better compliance among younger oral health-care practitioners21. Apart from the age of practitioners, it was also reported that the size of facilities played a role in compliance with infection control. The results indicated that larger facilities, employing nine or more oral health-care practitioners and other personnel, were more likely to have implemented guidelines and also to have more knowledge to comply with infection control when compared with solo or smaller facilities21.
Educational methods in infection-control procedures were questioned in a study from the UK, in which 77% of personnel confirmed that they had received specific training in this field. However, it should be noted that training for instrument-decontamination procedures was provided for mainly by demonstration (97%) or observed practice (88%)22. In addition to these results, the majority of dental assistant and dental practitioner responders from the same study were unfamiliar with the international indicator symbol for a single-use item22. This has highlighted the need for theoretical and practical education and training in infection control.
Cleveland et al.21 investigated the knowledge about surgical irrigation methods in the USA. They found that dental practitioners were aware that they should use sterile water or saline during surgical procedures. However, only about half of the dental practitioners ever used sterile water or sterile saline during surgical procedures, such as gingivectomy, extraction of an impacted third molar, soft-tissue biopsy or bone recontouring.
Basic knowledge of infection prevention and control varies among countries. In a study investigating the education and knowledge of Turkish dental practitioners, only 43% of participants were able to define ‘cross-infection’ correctly23. In Brazil, education and knowledge was agreed to contribute to improved infection-control attitudes and behaviour24. However, upon further investigation of the compliance with infection control, the results in practice were worrying24. Similarly, findings in India indicated that oral health-care professionals have good knowledge of infection control25. However, the authors admitted that the compliance levels with infection control were low. Singh et al.26 concluded that infection-control guideline training among oral health-care personnel and cooperation with local hazardous waste-disposal authorities were identified as priorities.
An association was made between the knowledge of infection control and the injuries that occurred among Taiwanese dental practitioners27. The results from this study indicated that the overall knowledge of infection-control procedures among dental practitioners was insufficient. Cheng et al.27 reported that, although younger dental practitioners had fewer needlestick and sharps injuries, those oral health-care providers routinely exposed to injuries tended to be more concerned about knowledge of infectious hazards and compliance with infection prevention and safety measures. Studies among oral health-care providers and dental students in the USA reported a lack of understanding of the basics of infection prevention and control28
Focus area two: personal hygiene and care of hands
Personal hygiene and care of hands have been identified as the most important infection-control precautions to prevent transmission of diseases29., 30.. Transfer of health care-associated cross-infections has been linked to the hands of health-care workers in an estimated 20–40% of cases31. To enable OHCWs to execute routine hand hygiene before and after each patient contact session, the minimum requirements include the availability of clean water, adequate hand-washing facilities, patient-placement facilities, correct storage of sterile supplies and other conditions relevant to the physical working environment32. Fixed hand-hygiene facilities, including separate basins for instrument cleaning, hand hygiene and patient rinsing, are some of the routine challenges for providing patient care33. These challenges are doubly experienced when community oral health-care procedures are executed in mobile dental units or community centres, such as schools or other venues34. These facilities are usually not specifically designed or equipped for oral health-care procedures.
In 2002, new evidence-based practices for hand hygiene in health care were published by the CDC35. This guideline promotes the use of alcohol-based hand-sanitisers or hand rubs to be utilised as replacement for routine washing with soap and water, particularly when hand-wash basins are not available. The use of these products is contraindicated when hands are visibly contaminated35. During 2009, the World Health Organisation (WHO) endorsed these guidelines to improve hand-hygiene practices throughout all health-care facilities32.
In addition, findings from Europe indicate that oral health-care professionals there do not wash their hands according to the CDC recommendations for oral health-care facilities30., 36.. However, as a result of the implementation of the Protection Against Infection Act in Germany, a decrease of errors in hand hygiene, and an increase in the use of skin antiseptics and surface disinfection, were reported37.
Adequate hand-hygiene practices, such as frequent use of soap and water and sometimes alcohol-based hand sanitisers, were maintained by more than 75% of oral health-care practitioners investigated in the USA38. In the UK it was reported that compliance with hand hygiene was not high enough, and, when applied, the methods used were outdated22. Results of hand-hygiene practices in this UK study point out that bar soaps were still used and nail brushes were present in 22% of facilities22. In actual fact, the use of bar soaps and nail brushes is discouraged in current UK guidelines/recommendations15.
Studies on personal hygiene and the care of hands in oral health-care facilities in developing countries are limited. In one study, Nigerian respondents strongly agreed that the transmission of diseases to patients can be prevented through application of appropriate hand hygiene33. In Brazil, the use of soap and paper towels in public oral health-care facilities was found to be significantly less than in private practices (P < 0.001)39. Bar soaps used in oral health-care facilities in India were found to be contaminated with organisms such as Pseudomonas aeruginosa, Acinetobacter, Enterobacter spp, Staphylococcus aureus and Staphylococcus epidermidis in more than 90% of the samples taken40. This supports the use of automated soap dispensers and liquid hand hygiene products, as recommended in the 2003 CDC guidelines for oral health care, which actively discourages the use of bar soaps.
The use of mobile devices in oral health-care facilities, especially while busy with patient-care procedures, is also a concern. The results of a study in India revealed that mobile phones may act as an infection risk in oral health-care facilities, as frequent touching heavily contaminates these devices with pathogens41. Therefore, it is important for educators to instruct OHCWs to limit the touching of personal mobile devices, and to avoid interruptions during contaminating treatment procedures. Higher compliance with hand-hygiene practices and routine surface disinfection of mobile devices should further be advocated.
Focus area three: personal protective equipment
The areas most vulnerable and at risk for transmission of diseases include the eyes, face and hands of OHCWs42. Personal protective equipment (PPE), including protective clothing, masks, protective eyewear and disposable gloves, should be worn during any clinical contact. PPE acts as an important safety barrier to prevent exposure of the skin and mucous membranes of the OHCWs43. This theory embraces the broader concept of ‘standard precautions’, as incorporated in current infection-control recommendations36.
At the foundation of any infection-control programme is the use of standard precautions, which includes wearing PPE, which should be applied at all times in oral health-care procedures, regardless of a patient’s suspected or confirmed medical history of infection44. When used appropriately and in combination with other protective measures, PPE forms an effective barrier against transmission of any infection15., 36..
Studies found that the use of PPE among Lithuanian dentists, particularly the use of gloves and changing of gloves after each patient, was relatively high (85%)45. In contrast, although the general level of use of masks was high, changing of those masks was low (28%). In this study, the use of protective eyewear/face shields was less than 50%45. Furthermore, in a Russian study, the results indicated that many dental practitioners used double gloving after being informed of patients’ infectious conditions46. Similarly, in India, most dental practitioners included additional precautions when patients indicated a medical history of infection. However, in some cases, treatment was refused (21%)47.
In Brazil, the wearing of PPE was evaluated over a 10-year time period. In 1995, more than 95% of students wore protective clothing, face masks and rubber gloves during all patient procedures24. However, the wearing of protective eyewear was considerably less (66.1%). After reassessing the use of PPE in 2005, similar results were obtained for the wearing of protective clothing, face masks and rubber gloves. However, a decline of 11% in the use of protective eyewear is of particular concern24.
In a US study, a large percentage of respondents, including students and professional OHCWs, incorrectly indicated that gloves provided full protection28. Furthermore, some students and professional OHCWs also mistakenly believed that gloves provide adequate protection as long as they are not visibly torn. Some respondents also stated that they never changed gloves in lengthy procedures, some of which lasted for up to 3 hours28.
Research has shown that the unpredictable perforation rate of gloves presents specific challenges, particularly during high-exposure procedures such as oral and maxillofacial surgery48. The results of a Japanese study suggested that double gloving may offer a protection rate of up to 95%48. In a study in Iran, improved compliance was reported for the use of double gloving while performing intravenous procedures and working in emergency areas49.
Constant use of gloves also has health implications. An increase in allergic reactions, as a result of continuous contact with the latex content of gloves and other protective products, has been noted among many OHCWs and patients28. As a result, products manufactured from new materials, such as vinyl and nitrile, have been introduced to avoid these allergic reactions50.
In contrast to the case in developed countries, in developing countries affordability, unavailability, limited resources and shortage of equipment have been put forward as reasons for low compliance with PPE guidelines45., 47., 51..
Focus area four: environmental barriers
The production of aerosols and spatters during oral health-care procedures, such as while operating high-speed dental handpieces and ultrasonic scalers, has been well documented52., 53., 54., 55., 56., 57.. These aerosols, as well as spatters, have been identified as potentially hazardous, as they may contain infectious agents originating from the patient’s oral cavity or the dental unit waterlines52., 57., 58.. As a preventive measure against infectious material from the oral health-care environment, and to minimise contamination of surfaces and equipment by the hands of oral health-care workers, protective environmental barriers should be applied on frequently touched areas.
Changing environmental barriers for every patient can be costly and impractical in some clinical environments, such as during screening or orthodontic follow-up appointments. Costs are determined by the number and amount of clinical contact surfaces to be covered, as well as the number of patients treated during a working day59. The relative risk of exposure, effectiveness of the barrier, time and cost will ultimately determine the choice of protection applied. For example, it was determined that inexpensive food-wrap material is an equally effective environmental barrier as some expensive, commercially available, environmental barrier products60.
However, the effects of environmental barriers on the power output results from dental light-curing units after application, is one area that presents challenges. The physical changes to the output of light-curing tips should be monitored. The thickness and translucency of the barrier may have a negative effect on the curing depth in light-activated resin-composite procedures60.
The National Dental Practice-Based Research Network Collaborative Group in the USA reported on the use of a rubber dam during root canal treatment and suggested that improved infection control, patient protection and treatment efficacy were some of the advantages offered by the rubber dam61. A significant reduction of spatter during treatments with the application of a combination rubber dam and high-volume evacuation was reported62.
Focus area five: sterilisation
Sterilisation includes the safe and effective recycling of instruments as a key element of any infection prevention and control programme63. The Spaulding Classification Scheme is a rational approach to disinfection and sterilisation that is used by all health-care professionals as a guide for the decontamination and reprocessing of items63. The gold standard recommended for sterilisation of heat-tolerant instruments or devices is vacuum autoclaving63., 64.. It is also recommended that dental handpieces be steam autoclaved15., 17., 36.. Most instruments used in oral health-care facilities today are heat tolerant and can thus be heat sterilised65. Application of liquid chemical sterilants is only intended for the processing of heat-sensitive instruments and for instruments with acute cutting edges6., 36., 43., 63., 64..
Effective instrument processing depends on systematic processes, involving a sequence of specific steps. These processes should ideally be executed in a specific, separate area, designed to promote routine workflow from ‘dirty’ towards ‘clean’ areas14. During these processes the following should be considered as equally important aspects: occupational health and safety issues; the processing of different instrument types, equipment and supplies; sterilisation verification; and stock control17., 36., 63..
Current global recommendations suggest that automated cleaning devices and ultrasonic baths should be utilised to facilitate a thorough cleaning process before sterilisation15., 17., 36., 63.. In Germany, however, contradictory results indicated that some dental materials, such as cement, can only be removed manually or with an ultrasonic bath66. These results thus contrast current regulations as enforced in the UK, where the use of a washer-disinfector is compulsory15.
Various studies have reported on the effectiveness of cleaning, disinfection and sterilisation of instruments. In a study among 30 oral health-care facilities in south-west England, processed instruments, such as matrix bands with retainers, diamond and stainless-steel burs, extraction forceps and hand scalers, were investigated67. The best dental instrument cleaning result was obtained after automated washer-disinfector cleaning. A study in Poland investigated cleaning methods in 43 oral health-care facilities. The results indicated that manual cleaning and ultrasonic baths were applied in more than 50% of the facilities, whilst only 23% used washer-disinfectors68.
Studies on the sterilisation methods used for critical instruments have revealed varying results. A Russian study revealed that dental practitioners had a poor understanding of Spaulding’s classification46. In spite of that, most Russian practitioners indicated that they always pre-packed instruments and applied sterilisation for critical instruments. This study also revealed that many practitioners used autoclaves (72%) and dry heat sterilisers (64%), while glass-bead sterilisers were still in use in more than a third of the investigated practices. Alcohol is still widely used for disinfection (83%).
Findings from India indicated that many practitioners used autoclaves47. However, the results from this study revealed that the majority used locally manufactured pressure cookers for sterilisation and thus never packed instruments for sterilisation and storage47. Further reports from India also indicated that many dentists (71%) used boiling water as the sterilising medium26. In Turkey, the majority of dental practitioners used dry heat sterilisation, although autoclave (47%) and other sterilisation methods, such as chemical solutions (35%) and boiling water (2%), were also applied23. A study from Brazil revealed that autoclaves were used by more than 60% of the dental practitioners69. However, many practitioners (83%) did not use chemical and biological indicators to verify effective sterilisation69. Similarly, Indian practitioners never used biological indicators to verify steriliser efficiency47. Results from Poland indicated that all sterilisation processes were performed in steam autoclaves, and a third verified sterilisation using chemical indicators. Biological verification was rarely carried out68. These reports confirm earlier reports from Poland, identifying the need to improve monitoring and documentation of sterilisation processes70.
In Africa and Asia, procedures by traditional healers, including tooth extractions, have been performed for centuries, often without any western technologies, such as radiographs, pharmaceuticals or surgical instruments71. WHO reports state that more than 80% of some Asian and African countries rely on traditional healers and indigenous knowledge for their primary health care72. It has been reported that patients prefer treatment by traditional healers because it is inexpensive, and there is a 93% satisfaction rate with the treatment provided73. In Cameroon, however, cases of extraction of teeth by traditional healers, using crude and dirty instruments without any sterilisation, has been reported73. It is of concern that many traditional medicine practices have often been adopted in different cultures and regions without international standards or guidelines. Tooth extractions without infection prevention and control could be potentially life-threatening for both oral care workers and patients.
Focus area six: disinfection (surfaces) and housekeeping
Disinfection is defined as the physical or chemical destruction of microorganisms, including pathogens43. Disinfection is a less lethal process than sterilisation because it destroys most, but not necessarily all, pathogens; for example, it does not destroy bacterial spores43. Effective use of disinfectants first requires effective dilution of the chemical product and second that the product is applied for an adequate period of contact time, as indicated by the manufacturer6., 36.. These instructions need to be followed meticulously to prevent incorrect use or ineffective application.
In different oral health-care facilities, different intra-oral and extra-oral surfaces present different challenges to decontaminate or clean effectively8. The most difficult surface to clean is textured vinyl, followed by smooth vinyl, enamelled metal, service line rubber hosing and brushed aluminium74. In a study in Italy it was demonstrated that, when applying disinfection and cleaning with a sodium-lauryl-sulphate-based detergent (the wipe–rinse method), the application was cost effective and practical59. This study also illustrated equivalence with placement of disposable barriers to reduce methicillin-resistant S. aureus (MRSA) contamination on dental chairs59. Patel et al.75 identified computers, keyboards and other components positioned near the patient treatment areas as a potential risk for cross-infection from and to patients and operators. However, findings from the study indicated that routine cleaning, followed by disinfection with 70% isopropanol wipes, reduced the microbial load on computer keys by at least 96%75. Another challenge for cleaning has been identified in orthodontic facilities, where decontamination of photographic retractors, often manufactured from heat-sensitive material, has been reported as being technique sensitive76. The findings indicate that the application of a washer-disinfector for the retractors is most effective76.
In a Brazilian study, surface contamination with S. aureus was investigated around patients, dental students and in the oral health-care environment77. By far, the majority of microbial colonies (74%) were obtained from the nose, tongue and hands of patients. The results also clearly indicated that dental students were already contaminated before commencement of the clinical appointment, with the highest colony counts found on gloved hands, followed by the tongue and ungloved hands77. Upon investigation of the clinical oral health care environment during this study, the count of S. aureus colonies significantly increased to 10.3% contamination of the surfaces, where 575 colonies were identified after the appointment (P < 0.05), of which the store room and auxiliary table were the most contaminated77. These results could be a result of the intense circulation of people in the clinical dental area, as well as the use of high-speed dental handpieces during dental appointments. It is speculated that much of the S. aureus contamination detected in the clinical environment came from direct contact, skin exfoliation or improper handling of equipment77.
A record of evidence of work relating to decontamination or general housekeeping should be maintained for audit purposes15. Results from Poland revealed incorrect documentation of instrument and surface decontamination in oral health-care facilities68. Further findings from Poland also indicated three common failures during disinfection, namely multiple re-use of disinfectant by topping up disinfectant instead of using freshly prepared mixture, continuously adding additional instruments to the disinfectant and not following the manufacturers’ instructions70.
No specific data are available on housekeeping in oral health-care facilities, which offers an opportunity for further investigation.
Focus area seven: waste management
Waste generated in oral health-care facilities, including sharps and other infectious waste, is classified as hazardous and poses a serious risk to human health and the general environment78. Most countries have their own classification of hazardous or health-care risk waste, which often includes infectious waste, pathological waste, sharps, chemical waste and radioactive waste. To reduce the risk of hazardous waste to human health and the general environment, the WHO has defined eight steps to manage health-care waste, including waste minimisation, waste generation, waste segregation, intermediate storage, centralised storage, external transport, treatment and disposal79. By segregating waste, oral health-care facilities can reduce the hazardous waste that requires special treatment and safe disposal.
In the UK it was reported that the segregation and disposal of health-care risk waste in oral health-care facilities was carried out according to waste-management guidelines22. However, one exception was noted in this study, namely that anaesthetic cartridges were disposed of in plastic bags rather than in rigid puncture-proof sharps containers22. Furthermore, studies from the UK also reported that all orthodontic facilities used ‘yellow bags’ to dispose of clinical waste and had puncture-proof sharps containers, which were in accordance with waste-management recommendations80.
Waste from oral health-care facilities poses an infectious risk. In Malaysia, various types of bacterial agents, including Enterobacter spp., Salmonella spp., Klebsiella spp., Pseudomonas spp., Serratia spp., Proteus mirabilis, Escherichia spp., Staphylococcus spp., Enterococcus spp. and Streptococcus spp., were detected in waste collected from oral health-care facilities81., 82.. The results of a specific Indian study are particularly worrying, as the majority of general dentists included (67%) had disposed of hazardous waste, such as syringes, blades and ampoules, in normal dustbins, which were emptied in domestic municipal waste26.
Focus area eight: dental unit waterlines, biofilms and water quality
The water in dental unit waterlines is often contaminated with high concentrations of bacterial agents. Bacteria multiply and cling to the inner walls of the waterline plastic tubing, which continues to accumulate into biofilms83., 84.. Biofilm formation in waterlines can be removed by breaking the biofilm into individual bacteria through a cleaning and decontamination process, such as flushing or purging the air- and water lines routinely84. Results from Germany indicated that when the water quality is also tested, this may be helpful, as mould contamination can provide a sign of biofilm formation before a high total colony count is obtained85.
Contaminated dental unit water, used during oral health-care treatment, could be potentially life-threatening to vulnerable people such as the immunocompromised, the elderly and people with chronic conditions, such as diabetes, cancer, AIDS or tuberculosis (TB)86. In February 2011, an 82-year-old woman, with no underlying disease, was admitted to an intensive care unit in Italy with fever and respiratory distress87. Two days later she died as a result of Legionnaires’ disease. Her death was attributed to the presence of Legionella pneumophila in dental unit waterlines, a high-speed handpiece and the oral health-care facility’s taps87. Pathogenic bacterial agents, such as Legionella and Pseudomonas spp., have been the reason for increasing concern and a topic of discussion over the past four decades15., 43., 88., 89., 90..
Special considerations
Special considerations include aspects directed at dental handpieces and other devices attached to air and waterlines; single-use or disposable devices, including saliva ejectors; pre-procedural mouth rinses; dental radiology; the dental laboratory; Mycobacterium tuberculosis; the risk of contracting Creutzfeldt–Jakob and other prion diseases; sharps injuries and postexposure management; and the vaccination of OHCWs.
Dental handpieces and other devices attached to air- and waterlines
OHCWs involved in clinical procedures are exposed to the sprays and spatters generated during oral health-care procedures. The sprays and spatters produced by dental unit handpieces have the potential to transmit pathogenic agents through airborne or waterborne modes91., 92.. The CDC states that ‘handpieces that cannot be heat sterilised should not be used’ during oral health-care procedures6. According to the UK Department of Health17 and Rutala et al.63, vacuum autoclaving is recommended to achieve sterility of instruments, such as dental handpieces with lumens, cavities or indentations. A Polish study revealed that one-third of the dental practitioners questioned used non-vacuum autoclaving (type B) for dental handpieces68. In Scotland, decontamination and autoclaving of handpieces between patients were investigated in a study involving 179 oral health-care facilities93. The results indicated that most of the practitioners (97%) autoclaved their handpieces between patient treatments. However, the majority of respondents manually decontaminated their dental handpieces externally with a disinfectant wipe rather than washing them, and then processed them in type N bench top steam sterilisers93. In a study among dentists in Beijing, autoclaving of dental handpieces between patients increased from 41% to 96% in a 10-year survey94.
The use of non-water-soluble lubricants in handpieces may be problematic. Such lubricants may block the narrow lumen and also prevent effective cleaning of the inner parts of the handpieces before sterilisation. Furthermore, processed handpieces are recontaminated if lubricated after sterilisation. A Scottish study showed that most handpieces were lubricated with non-water-soluble lubricants after cleaning and before sterilisation (91%), and a number (24%) of participants also lubricated handpieces after sterilisation93.
Single-use or disposable devices
Application of single-use or disposable devices has become common in oral health care42. Examples of single-use or disposable devices include prophylaxis cups and brushes, saliva ejectors, high-volume evacuator tips, hypodermic syringes, needles, blades, endodontic irrigation tips/needles, plastic impression trays, air/water syringe tips, gloves and masks, among others. These items are not designed by their manufacturers to be cleaned and reused, and are thus classified as single-use or disposable items.
A study in the UK revealed that 23% of oral health-care facilities gave guidance on when to choose single-use as opposed to reusable instruments when both were commercially available. For 47% of facilities there was an internal policy on the re-use of devices labelled as single use, of which only 37% specified that re-use was never allowed22.
Pre-procedural mouth rinses
The major source of pathogens in oral health-care facilities is the oral cavities of patients, each laden with high concentrations of oral microbial flora95. Having patients rinse with a pre-procedural mouth rinse has been proven as an effective precautionary infection-control measure to reduce the microbial counts in the oral cavity43. It has been shown, in an Indian study, that bacterial cross-infection from dental aerosols can be reduced when chlorhexidine is used as a simple, non-expensive and effective pre-procedural rinse, before procedures with ultrasonic scalers and high-speed handpieces96. A Brazilian study confirmed this and showed that rinses containing 0.05% cetylpyridinium chloride (CPC), 0.12% chlorhexidine and water were equally effective in lowering the bacterial counts97. Because CPC has fewer side effects than chlorhexidine, it could be considered as a good choice for pre-procedural rinsing97.
Dental radiology
Sensors used during digital intra-oral radiography are heat sensitive and cannot be autoclaved. Thus, to prevent cross-contamination, protective barrier envelopes that cover the sensors are used while capturing the radiographs98. The sensors, contained inside the plastic barrier envelopes, always remain a potential source of contamination with saliva. Recommendations suggest disinfection of the digital intra-oral radiography sensors and equipment upon removal of the contaminated outer envelope, and the aseptic re-placement of a new protective envelope36. A Canadian study revealed that contamination of digital sensors can still occur owing to the compromised integrity of the protective envelopes and the techniques applied during placement and removal of the envelopes, despite various precautions to prevent cross-infection98. An Iranian study indicated significant differences between the bacterial counts on radiographic equipment and surrounding surfaces before and after disinfection99. In a comparison of four disinfectant products, Deconex demonstrated the highest disinfectant efficacy on radiographic equipment and the surrounding surfaces99.
Dental laboratory
Materials or instruments/equipment transferred to and received from the dental laboratory, such as impression materials, impression trays and dispensers, have the potential to transmit disease100. It has been reported that impression material cartridges and handgun dispensers are easily and heavily contaminated with pathogenic agents, such as MRSA, during clinical prosthetic procedures100. This places oral health-care workers, as well as dental laboratory personnel, at risk for acquiring infections that are difficult to treat or possibly life-limiting. All standard precautions, such as careful handling of sharp instruments, hand washing, use of protective barriers and wearing of PPE (such as gloves, masks, protective eyewear and protective clothing) for infection prevention and control should therefore also be extended to the dental laboratory36.
For optimal consumer protection, clear communication between oral health-care facilities and dental laboratories is crucial. It is generally recommended that the responsibility of the cleaning and disinfection of impressions, before despatching to dental laboratories, should lie with the dental practitioner8. The same applies to dental technicians when sending completed products and dispensers, such as prosthetic or orthodontic appliances and impression trays, among others, back to the oral health-care facility8.
Impression decontamination and disinfection practices among oral health-care practitioners and dental technicians have been investigated in a number of studies. Results from the UK indicated that 37% of participants rinsed impressions with water and 3% brushed debris away before disinfection101. Although 75% of the participating practitioners had claimed that they informed dental laboratories of impression disinfection, the large majority (95%) of participating dental technicians still received blood-contaminated impressions101.
Approximately 61% of dental-practitioner participants in studies in Russia indicated that they disinfected impressions46. A study in Saudi Arabia to evaluate the efficacy of sodium hypochlorite (1:10) and iodophor disinfectants, found sodium hypochlorite to be highly effective when applied to alginate impressions102. Furthermore, the results indicated that gypsum does not have any inherent antibacterial properties. The presence of opportunistic pathogenic organisms, such as streptococci (100%), staphylococci (65.4%), Candida (46.2%), MRSA (15.4%) and P. aeruginosa (7.7%), which could be life-threatening to immune-compromised persons, was demonstrated on selective agar cultures from impressions and gypsum casts in a study among Japanese dentists103. Upon investigating different Japanese disinfecting methods on alginate impressions, the findings suggested that application of a solution of 0.5% sodium hypochlorite for 15 minutes was a feasible disinfection method104.
Proper disinfection of impressions thus provides adequate cross-contamination protection between the oral health-care facility and the dental laboratory102. A study of Iranian dental laboratories revealed that the most popular chemical materials used by dental technicians for disinfection included household bleach, glutaraldehyde and alcohol105. Alarming results from this study also indicated that dental technicians rarely wore gloves (14%) and protective eyewear (8%) while handling used equipment. Only half of the technicians in this study had been vaccinated against HBV.
Mycobacterium tuberculosis
Transmission of M. tuberculosis occurs through aerosols generated by coughing, sneezing and speaking106. M. tuberculosis can remain airborne within small droplets for several hours, and susceptible individuals can still become infected43. Some countries have policies or recommendations that oral health-care workers should avoid treating patients with suspicious symptoms of TB until it is confirmed the patient does not have TB, or is not infectious107. If emergency oral health-care treatment needs to be executed on suspected TB patients, respiratory protection such as N95, N99 or N100 respirators should be worn107.
The incidence of M. tuberculosis infection among oral health-care patients was assessed at a large tertiary hospital in Nigeria. Ten out of 78 sputum samples tested positive for M. tuberculosis108. These findings emphasise the risk of active TB cases among patients and the need to implement specific infection-prevention precautions and policies for TB in oral health-care facilities. Particular challenges identified in training institutions, hospitals and public health-care facilities include a lack of TB-specific infection-control training and a need for infrastructure improvement and better ventilation systems in existing and new facilities109.
Creutzfeldt–Jakob and other prion diseases
Creutzfeldt–Jakob disease (CJD) is caused by a proteinaceous infectious agent, or prion, which has an unusual resistance to standard methods of decontamination110. A variant form of CJD (vCJD), acquired from cattle, has recently been identified as a hazard for all health-care professions, especially those exposed to blood and nerve tissue. The potential risk of further human-to-human transmission of the disease through contaminated instruments is a further concern64. Recently, a study in the UK indicated that the risk of vCJD transmission during oral health-care procedures was higher than previously expected111. This study also revealed transmission of vCJD after exposure of a patient’s gingival tissues to a contaminated endodontic file, and not just from nerve tissue exposure, as previously suggested.
A study in south England compared the different cleaning methods applied in oral health-care facilities67. The study measured the amounts of protein left on different types of instruments after manual cleaning, manual cleaning plus ultrasonic bath cleaning and use of the automated washer-disinfector. Several shortcomings were observed in all three methods, which could be indicative of the potential risk of transmitting CJD between patients67. Although current evidence suggests that the possibility of prion contamination from dental instruments may be low, this may not be the case should endodontic instruments and reamers be applied15. When a strict and reliable cleaning regime cannot be executed for endodontic instruments and reamers, the application of a single-use, disposable policy may be the safer alternative15.
Sharps injuries and post-exposure management
The highest risk of infection is associated with accidental punctures with used and/or contaminated needles, or injuries with sharp instruments91. The most common occupational risks to which oral health-care workers and dental patients are exposed include exposure to blood-borne pathogens, in particular HBV, hepatitis C virus (HCV) and HIV43., 112., 113..
The nature of oral health care easily results in exposure incidents. In 2012, Cleveland et al. from the CDC reported that 6% of dental practitioners and 14% of other oral health-care personnel had experienced at least one or more percutaneous injuries in the 12 months before the study. In a second study among dental students in the USA, percutaneous injuries had occurred in 88% of the respondents114. In a nationwide survey among dental practitioners in Taiwan, the results indicated that the risk of occupational needlestick and sharps injuries increased in correlation to practitioner age27. In a study conducted with dental students in Shiraz, Iran, 73% of the participants experienced needlestick and sharps injuries in the 12 months before the study49. More than half of the injuries occurred during patient treatment procedures, of which needle re-capping was the most frequent problem. More alarming, however, is the fact that 85% of the respondents did not report their injury after it happened49. The reasons indicated for non-reporting included not knowing the mechanism of reporting, not realising that all needlestick injuries required reporting and evaluation, as well as not knowing who to report to49. In a study of oral health-care facilities in Brazil, occupational accidents caused by cutting and piercing objects were reported by half of the participating facilities69. Of all the respondents, only 26% had had specialised follow-up medical appointments after the accidents69.
Vaccination of OHCWs
HBV transmission is the greatest infectious risk to which dental patients or members of the oral health-care team can be exposed91. In Brazil, vaccination against HBV and post-vaccination tests among oral health-care practitioners raised concerns115. The results of the study revealed that, although 74% of the respondents had received all three doses of the vaccine as required, only 15% had undergone the follow-up post-vaccination test115. In Nigeria, compliance with the recommended HBV immunisations was poor116. Of all respondents, 20% had received three doses of the hepatitis B vaccine, 49% had received either two doses or a single dose and 31.4% were not vaccinated. The reasons reported by the respondents who were not vaccinated as recommended, included lack of opportunity for vaccination and the fear of side effects of the vaccines116.
CONCLUSION
Many publications are available on the topic of compliance with infection prevention and control practices in oral health-care facilities all over the world. The approaches in developing and developed countries vary widely, although the principles of infection prevention and control are the same globally. The availability of resources is, and will always be, a challenge, perhaps more so in developing countries. This review has indicated serious deviations in the compliance with infection-control guidelines and recommendations internationally. Although there was often good knowledge and high compliance with infection-control guidelines in developed countries, the lack of knowledge and compliance with infection-control guidelines in developing countries is low and particularly disturbing.
In both developed and developing countries, hand hygiene and the care of hands were not consistently carried out according to international recommendations. The fact of frequent touching in oral health care has been identified as an area of specific concern, and should, similarly to all other health-care professions, be addressed as one of the most important infection-control areas. The use and common application of modern technology, including digital devices, mobile devices and cell phones in oral health-care facilities, and the potential for cross-contamination between the patient’s oral cavity and these appliances, presents a further challenge, as frequent touching may heavily contaminate these devices with pathogens. The younger generation of oral health-care professionals seem to comply better with the wearing of personal protective equipment, but areas of some concern are not replacing these between every patient. Affordability, unavailability, limited resources and shortage of equipment/supplies have been indicated as reasons for non-compliance with the routine use of PPE in developing countries.
The application of protective environmental barriers is widely promoted and applied in developed countries, but the lack of relevant studies in developing countries could conceal serious shortcomings. In spite of guidelines promoting the safety of workers and many studies indicating that the best instrument-cleaning results are obtained in an automated washer-disinfector, manual cleaning is still widely used. Most participant practitioners used autoclaves, but the majority in developing countries had never used biological indicators, and many still use chemical solutions for reprocessing of critical instruments. In many developing countries, boiling water is widely used to ‘sterilise’ appliances and alcohol is still utilised for disinfection, while used handpieces are not sterilised between all patients and single-use items are reused.
Additionally, wide-ranging research has indicated that waste segregation and disposal is undertaken incorrectly. Whilst immunisation against hepatitis B has improved among oral health-care personnel, many do not maintain immunity with boosters or carry out postvaccine testing. The hygiene and maintenance of waterlines and the use of sterile water or saline during surgical procedures are areas of notable concern. No data are available with regard to the quality of the water from dental units that are used in developing countries. In dental laboratories, poor compliance with clinical infection-control and -prevention practices, and inadequate knowledge about the topic among technicians, are serious problems.
Finally, although developed countries obviously have more resources, there are some areas in which specific reports on compliance with their infection prevention and control precautions show functional shortcomings. The more significant areas identified as such include the application of environmental barriers, quality control and maintenance of dental unit waterlines and water supply, the development of biofilms and some special considerations. The special considerations that require further investigation include pre-procedural rinses, radiology, the treatment of, or protection from, patients with TB, the risk of Creutzfeldt–Jakob and other prion diseases, as well as the timely vaccination of OHCWs. At present, in most developing countries the application of infection prevention and control measures, or the lack thereof in oral health-care facilities, is nothing short of a nightmare and in many instances is an outright health and safety hazard to both patients and OHCWs.
Acknowledgements
We thank Sr Laura Ziady, Nurse Educator/IPC Assessor from Mediclinic Southern Africa, who assisted with language editing.
Conflict of interest
None declared.
Funding sources
This investigation was partially financed by the National Research Foundation, South Africa, the Central University of Technology, Free State and the Dentistry Development Foundation of the South African Dental Association.
REFERENCES
- 1.De Paola LG. Infectious diseases in the oral healthcare environment. Inside Dental Assisting [serial on the Internet]. 2012; November/December: Available from: http://ida.cdeworld.com/courses/4636
- 2.Kaste LM, Bednarsh H. The third decade of HIV/AIDS: a brief epidemiologic update for dentistry. J Can Dent Assoc. 2007/2008;73:941–944. [PubMed] [Google Scholar]
- 3.Molinari JA, Harte JA. 3rd ed. Lippincott, Williams and Wilkens, a Wolters Kluwer business; Baltimore, Philadelphia: 2010. Cottone’s Practical Infection Control in Dentistry. [Google Scholar]
- 4.Kuhar DT, Henderson DK, Struble KA, et al. Updated US public health service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol [serial on the Internet] 2013;34:875–892. doi: 10.1086/672271. US Public Health Service Working Group, Available from: http://www.jstor.org/stable/10.1086/672271. [DOI] [PubMed] [Google Scholar]
- 5.Oosthuysen J, Potgieter E, Blignaut E. Compliance with infection control recommendations in South African dental practices: a review of studies published between 1990 and 2007. Int Dent J. 2010;60:181–189. [PubMed] [Google Scholar]
- 6.Centers for Disease Control. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. 2008 [cited 2013 3 April]; Available from: http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Disinfection_Nov_2008.pdf
- 7.Australian Dental Association . Australian Dental Association Inc; St Leonards, Australia: 2012. Australian Dental Association Guidelines for Infection Control, Second ed, Vol. II (Fryer FS, editor) pp. 1–50. Available from: http://www.ada.org.au/app_cmslib/media/lib/1203/m356702_v1_infection%20control%20guidelines%202012.pdf. [Google Scholar]
- 8.British Dental Association . British Dental Association; London: 2011. Advice Sheet A12 Infection Control in Dentistry (England) [Google Scholar]
- 9.Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care [database on the Internet]. Centers for Disease Control and Prevention. 2011 [cited 2013-04-03]. Available from: http://www.cdc.gov/HAI/pdfs/guidelines/standatds-of-ambulatory-care-7-2011.pdf
- 10.Infection Prevention Checklist for Outpatient Settings: Minimum Expectations for Safe Care [database on the Internet]. Centers for Disease Control and Prevention. 2011 [cited 2013-04-03]. Available from: http://www.cdc.gov/HAI/pdfs/guidelines/ambulatory-care-checklist-07-2011.pdf
- 11.Fédération Dentaire Internationale . FDI World Dental Federation; Singapore: 2009. FDI Policy Statement on Infection Control in Dental Practice: Merging of ‘Human Immunodeficiency Virus Infection and Other Blood Borne Infections (2000)’, ‘Infection Control in Dentistry’ (2007), and ‘Sterilization and Cross Infection Control in the Dental Practice’ (2005). Revised version adopted by the General Assembly: 4th September 2009. [cited 2013 9 February]; Available from: http://www.fdiworldental.org/media/11259/Infection-control-in-dental-practice-2009.pdf. [Google Scholar]
- 12.Amritraj A, Rajan PS, Shenoy N, et al. Awareness among young dentists about transmission of HIV and preventive measures. Int J Bioassays [serial on the Internet] 2013;2:701–704. [cited 2013 April], Available from: http://ebioscholar.com/ojs/index.php/ijb/article/view/283. [Google Scholar]
- 13.Australian/New Zealand Standard. Office-based health care facilities – Reprocessing of reusable medical and surgical instruments and equipment, and maintenance of the associated environment. In: New Zealand, SAS, editor.: Jointly published by Standards Australia, GPO Box 476, Sydney, NSW 2001 and Standards New Zealand, Private Bag 2439, Wellington 6020; 2006
- 14.British Dental Association . British Dental Association; London: 2003. Infection Control in Dentistry. Advice Sheet A12 of the British Dental Association. [Google Scholar]
- 15.Department of Health United Kingdom. Health Technical Memorandum 01-05: Decontamination in primary care dental practices. Health Technical Memoranda [serial on the Internet]. 2013 [cited 2013 26 March]: Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/170689/HTM_01-05_2013.pdf
- 16.Department of Health United Kingdom. Health Technical Memorandum 07-01: Safe management of healthcare waste. Health Technical Memoranda [serial on the Internet]. 2013 [cited 2013 20 March]; 2013: Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/167976/HTM_07-01_Final.pdf
- 17.Department of Health United Kingdom. Health Technical Memorandum 01-05 Decontamination in primary care dental practices. Health Technical Memorandum 01-05. 2008 (Oct 2008):1-60
- 18.Michmershuizen F. Dental associations respond to infection control breach in Oklahoma. Dental Tribune – The World’s Dental Newspaper. 2013 4 April
- 19.Bradley K, Fox J, Wilson J et al. Investigation of Healthcare-Associated Hepatitis C Virus Transmission in a Dental Surgical Clinic – Oklahoma, 2013. 2013 Annual CSTE Conference - Epi on the Edge; June 2013; Pasadena California. http://www.csteconference.org/2013
- 20.Eaton K. Associated Press; Oklahoma City (AP): 2013. Tulsa Oral Surgeon Accused in Health Scare Sued. [cited 2013 7 September]; Available from: http://www.sfgate.com/news/article/Tulsa-oral-surgeon-accused-in-health-scare-sued-4786971.php. [Google Scholar]
- 21.Cleveland J, Foster M, Barker L, et al. Advancing infection control in dental care settings: factors associated with dentists’ implementation of guidelines from the Centers for Disease Control and Prevention. J Am Dent Assoc. 2012;143:1127–1138. doi: 10.14219/jada.archive.2012.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith A, Creanor S, Hurrell D, et al. Management of infection control in dental practice. J Hosp Infect. 2009;71:353–358. doi: 10.1016/j.jhin.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 23.Yüzbasioglu E, Saraç D, Canbaz SY, et al. A survey of cross-infection control procedures: knowledge and attitudes of Turkish dentists. J Appl Oral Sci. 2009;17:565–569. doi: 10.1590/S1678-77572009000600005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Abreu MHNG, Lopes-Terre MC, Braz LF, et al. Attitudes and behavior of dental students concerning infection control rules: a study with a 10-year interval. Braz Dent J. 2009;20:221–225. doi: 10.1590/s0103-64402009000300009. [DOI] [PubMed] [Google Scholar]
- 25.Jain M, Sawla L, Mathur A, et al. Knowledge, attitude and practice towards droplet and airborne isolation precautions amongs dental health care professionals in India. Med Oral Patol Oral Cir Bucal. 2010;15:e957–e961. doi: 10.4317/medoral.15.e957. [DOI] [PubMed] [Google Scholar]
- 26.Singh BP, Khan SA, Agrawal N, et al. Current biomedical waste management practices and cross-infection control procedures of dentists in India. Int Dent J. 2012;62:111–116. doi: 10.1111/j.1875-595X.2011.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng HC, Su CY, Yen AM, et al. Factors affecting occupational exposure to needlestick and sharps injuries among dentists in Taiwan: A nationwide survey. Public Library of Science. 2012;7:e34911. doi: 10.1371/journal.pone.0034911. Available from http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0034911#pone-0034911-g001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanjirath PP, Coplen AE, Chapman JC, et al. Effectiveness of gloves and infection control in dentistry: student and provider perspectives. J Dent Educ. 2009;73:571–580. [PubMed] [Google Scholar]
- 29.World Health Organization . WHO Press; Geneva, Switzerland: 2009. Guide to Implementation: A Guide to the Implementation of the WHO Multimodal Hand Hygiene Improvement Strategy. Save Lives Clean Your Hands. [cited 2012 3 August]; Available from: WHO_IER_PSP_2009.02_eng.pdf. [Google Scholar]
- 30.De Amorim-Finzi MB, Cury MVC, Costa CRR, et al. Rate of compliance with hand hygiene by dental healthcare personnel (DHCP) within a dentistry healthcare first aid facility. Eur J Dent. 2010;4:233–237. [PMC free article] [PubMed] [Google Scholar]
- 31.Weber DJ, Rutala WA, Miller MB, et al. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;35(5 supplement 1):S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization . WHO Press; Geneva, Switzerland: 2009. The WHO Guidelines on Hand Hygiene in Health Care 2009. [cited 2012 3 August]; Available from: http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf. [PubMed] [Google Scholar]
- 33.Omogbai JJ, Azodo CC, Ehizele AO, et al. Hand hygiene amongst dental professionals in a tertiary dental clinic. Afr J Clin Experiment Microbiol. 2011;12:9–14. [Google Scholar]
- 34.Radcliffe RA, Bixler D, Moorman A, et al. Hepatitis B virus transmissions associated with a portable dental clinic, West Virginia, 2009. J Am Dent Assoc. 2013;144:1110–1118. doi: 10.14219/jada.archive.2013.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention Guideline for hand hygiene in health-care settings. Morb Mortal Wkly Rep. 2002;51:1–85. (RR-16) [Google Scholar]
- 36.Centers for Disease Control and Prevention Guidelines for infection control in dental health-care settings – 2003. Morb Mortal Wkly Rep. 2003;52:1–68. (Number RR-17) [Google Scholar]
- 37.Heudorf U, Eikmann T, Exner M. Ten years’ German Protection against Infection Act: evaluation of the implementation of infection control visits in the ambulatory medical setting. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:455–465. doi: 10.1007/s00103-012-1627-8. [DOI] [PubMed] [Google Scholar]
- 38.Myers R, Larson E, Cheng B, et al. Hand hygiene among general practice dentists: a survey of knowledge, attitudes and practices. J Am Dent Assoc [serial on the Internet] 2008;139:948–957. doi: 10.14219/jada.archive.2008.0282. [DOI] [PubMed] [Google Scholar]
- 39.Bellissimo-Rodrigues WT, Bellissimo-Rodrigues F, Machado AA. Infection control practices among a cohort of Brazilian dentists. Int Dent J. 2009;59:53–58. [PubMed] [Google Scholar]
- 40.Pradeep S, Aravindhan TR, Chinnaiah R, et al. Bar soaps in dental clinic might be a bacterial reservoir. Int J Contemp Dent. 2011;2:115–120. [Google Scholar]
- 41.Singh S, Acharya S, Bhat M, et al. Mobile phone hygiene: potential risks posed by use in the clinics of an Indian dental school. J Dent Educ. 2010;74:1153–1158. [PubMed] [Google Scholar]
- 42.Martin MV, Fulford MR, Preston AJ. Quintessence Publishing Co. Ltd.; London: 2009. Quintessentials of Dental Practice, Clinical Practice Number 3 Infection Control for the Dental Team. Nairn HFW, editor. [Google Scholar]
- 43.Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings - 2003. Morb Mortal Wkly Rep. 2003;52(RR-17):1–61. Centers for Disease Control and Prevention (CDC) [PubMed] [Google Scholar]
- 44.Harte JA. Standard and transmission-based precautions: an update for dentistry. J Am Dent Assoc. 2010;141:572–581. doi: 10.14219/jada.archive.2010.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rimkuvienë J, Pûrienë A, Peèiulienë V, et al. Use of personal protective equipment among Lithuanian dentists. Sveikatos Mokslai. 2011;21:29–36. [Google Scholar]
- 46.Budnyak MA, Gurevich KG, Fabrikant KG, et al. Dental infection control and occupational safety in the Russian Federation. J Contemp Dent Pract. 2012;13:703–712. doi: 10.5005/jp-journals-10024-1213. [DOI] [PubMed] [Google Scholar]
- 47.Puttaiah R, Shetty S, Bedi R, et al. Dental infection control in India at the turn of the century. World J Dent. 2010;1:1–6. [Google Scholar]
- 48.Kuroyanagi N, Nagao T, Sakuma H, et al. Risk of surgical glove perforation in oral and maxillofacial surgery. Int J Oral Maxillofac Surg. 2012;41:1014–1019. doi: 10.1016/j.ijom.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Askarian M, Malekmakan L, Memish ZA, et al. Prevalence of needle stick injuries among dental, nursing and midwifery students in Shiraz, Iran. GMS Krankenhaushygiene Interdisziplinär [serial on the Internet] 2012;7:Doc05. doi: 10.3205/dgkh000189. [cited 2012 4 April], Available from: http://www.egms.de/pdf/journals/dgkh/2012-7/dgkh000189.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coplen A, Chapman J, Kanjirath P et al., editors. Nitrile, latex, and vinyl gloves permeabiliy testing using serratia marcescens. Presentation at the Clinical Research Symposium on Clinical Research to Clinical Practice: September 11–13, 2008. Managing Challenges at the Cutting Edge; 2008; University of Michigan
- 51.Uti OG, Agbelusi GA, Jeboda SO, et al. Infection control knowledge and practices related to HIV among Nigerian dentists. J Infect Dev Countries. 2009;3:604–610. doi: 10.3855/jidc.552. [DOI] [PubMed] [Google Scholar]
- 52.Gross KB, Overman PR, Cobb C, et al. Aerosol generation by two ultrasonic scalers and one sonic scaler: a comparative study. J Dent Hyg. 1992;66:314–318. [PubMed] [Google Scholar]
- 53.Micik RE, Miller RL, Mazzarella MA, et al. Studies on dental aerobiology, part I: bacterial aerosols generated during dental procedures. J Dent Res. 1969;48:49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 54.Miller RL. Generation of airborne infection by high speed dental equipment. J Am Soc Prev Dent. 1976;6:14–17. [PubMed] [Google Scholar]
- 55.Miller RL, Micik RE, Abel C, et al. Studies on dental aerobiology, part II: microbial splatter discharged from the oral cavity of dental patients. J Dent Res. 1971;50:621–625. doi: 10.1177/00220345710500031701. [DOI] [PubMed] [Google Scholar]
- 56.Holbrook WP, Muir KF, Macphee IT, et al. Bacteriological investigation of the aerosol from ultrasonic scalers. Br Dent J. 1978;144:245–247. doi: 10.1038/sj.bdj.4804072. [DOI] [PubMed] [Google Scholar]
- 57.Larato DC, Ruskin PF, Martin A, et al. Effect of a dental air turbine drill on the bacterial counts in air. J Prosthet Dent. 1966;16:758–765. [Google Scholar]
- 58.Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petti S, Polimeni A, Dancer SJ. Effect of disposable barriers, disinfection, and cleaning on controlling methicillin-resistant Staphylococcus aureus environmental contamination. Am J Infect Control. 2012;21:1–5. doi: 10.1016/j.ajic.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 60.Dunne S. Summary of: the effect of disposable infection control barriers and physical damage on the power output of light curing units and light curing tips. Br Dent J. 2011;210:358–359. doi: 10.1038/sj.bdj.2011.290. [DOI] [PubMed] [Google Scholar]
- 61.Anabtawi MF, Gilbert GH, Bauer MR, et al. Rubber dam use during root canal treatment. Findings from the Dental Practice-Based Research Network. J Am Dent Assoc. 2013;144:179–186. doi: 10.14219/jada.archive.2013.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dahlke WO, Cottam MR, Herring MC, et al. Evaluation of the spatter-reduction effectiveness of two dry-field isolation techniques. J Am Dent Assoc. 2012;143:1199–1204. doi: 10.14219/jada.archive.2012.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rutala WA, Weber DJ, HICPAC . Centers for Disease Control and Prevention, Department of Health and Human Services; Atlanta, Georgia: 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. [Google Scholar]
- 64.Rutala WA, Weber DJ. Guideline for disinfection and sterilization of prion-contaminated medical instruments. Infect Control Hosp Epidemiol. 2010;31:107–117. doi: 10.1086/650197. [DOI] [PubMed] [Google Scholar]
- 65.Scarlett MI. Disinfection and sterilization: a primer. Dentaltown. 2007;2007:52–60. July. [Google Scholar]
- 66.Franz A, Bristela M, Stauffer F. Reprocessing of dental instruments in washer-disinfectors: does a representative test soil exist in dentistry? GMS Krankenhaushygiene Interdisziplinär [serial on the Internet] 2012;7:1–6. doi: 10.3205/dgkh000197. [cited 2012 4 April], Available from: http://www.egms.de/pdf/journals/dgkh/2012-7/dgkh000197.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bagg J. Summary of: a quantitative assessment of residual protein levels on dental instruments reprocessed by manual, ultrasonic and automated cleaning methods. Br Dent J. 2011;210:418–419. doi: 10.1038/sj.bdj.2011.341. [DOI] [PubMed] [Google Scholar]
- 68.Röhm-Rodowald E, Jakimiak B, Chojecka A, et al. Assessment of decontamination processes: cleaning, disinfection and sterilization in dental practice in Poland in the years 2011-2012. Przegląd Epidemiologiczny. 2012;66:635–641. [PubMed] [Google Scholar]
- 69.Matsuda JK, Grinbaum RS, Davidowicz H. The assessment of infection control in dental practices in the municipality of São Paulo. Braz J Infect Dis. 2011;15:45–51. [PubMed] [Google Scholar]
- 70.Podgórska M, Jakimiak B, Röhm-Rodowald E, et al. Assessment of disinfection and sterilization processes in dental practice as an important factors in prevention of infections. Przegląd Epidemiologiczny. 2009;63:545–550. [PubMed] [Google Scholar]
- 71.Willis MS, Harris LE, Hergenrader PJ. On traditional dental extraction: case reports from Dinka and Nuer en route to restoration. Br Dent J. 2008;204:121–124. doi: 10.1038/bdj.2008.46. [DOI] [PubMed] [Google Scholar]
- 72.World Health Organization . World Health Organization Media Centre; Geneva: 2008. Traditional Medicine: Fact Sheet N°134. [cited 2013 28 April]; Available from: http://www.who.int/mediacentre/factsheets/fs134/en/index.html. [Google Scholar]
- 73.Agbor AM, Singh S, Mbia AM. The role of traditional healers in tooth extractions in Lekie Division, Cameroon. J Ethnobiol Ethnomed [serial on the Internet] 2011;7:1–8. doi: 10.1186/1746-4269-7-15. Available from: http://www.ethnobiomed.com/content/7/1/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palenik CJ. The effect of long-term disinfection on clinical contact surfaces. J Am Dent Assoc. 2012;143:472–477. doi: 10.14219/jada.archive.2012.0207. [DOI] [PubMed] [Google Scholar]
- 75.Patel S, Porter K, Sammons RL. Are computer keyboards a cross-infection risk in a dental clinic? J Infect Prev [serial on the Internet] 2010;11:206–211. [cited 2010 8 October], Available from: http://bji.sagepub.com/content/11/6/206.full.pdf+html. [Google Scholar]
- 76.Walker J. Summary of: the cleaning of photographic retractors; a survey, clinical and laboratory study. Br Dent J. 2010;208:306–307. doi: 10.1038/sj.bdj.2010.310. [DOI] [PubMed] [Google Scholar]
- 77.Negrini T, Duque C, De Oliveira ACM, et al. Staphylococcus Aureus contamination in a pediatric dental clinic. J Clin Pediatric Dentistry. 2009;34:13–18. doi: 10.17796/jcpd.34.1.n435k10291222035. [DOI] [PubMed] [Google Scholar]
- 78.Eberle J, Allain L, Nersesian P. USAID Deliver Project, Task Order 1 from the US Agency for International Development; Arlington, VA: 2009. Logistics of Health Care Waste Management: Information and Approaches for Developing Country Settings. [Google Scholar]
- 79.World Health Organization . WHO Press; Geneva, Switzerland: 2014. Safe management of wastes from health-care activities, Second ed., Vol. II (Chartier Y, Emmanual J, Pieper U, Prüss A., Rushbrook P, Stringer R, Townend W, Wilbum S and Zghondi R, editors). pp. 1–329. Available from http://apps.who.int/iris/bitstream/10665/85349/1/9789241548564_eng.pdf?ua=1. [Google Scholar]
- 80.Shah R, Collins JM, Hodge TM, et al. A national study of cross infection control: ‘are we clean enough?’. Br Dent J. 2009;207:267–274. doi: 10.1038/sj.bdj.2009.824. [DOI] [PubMed] [Google Scholar]
- 81.Hossain S, Rahman NNNA, Balakrishnan V, et al. Infectious risk assessment of unsafe handling practices and management of clinical solid waste. Int J Environ Res Public Health [serial on the Internet] 2013;10:556–567. doi: 10.3390/ijerph10020556. [cited 2013 31 January], Available from: www.mdpi.com/journal/ijerph. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vieira CD, De Carvalho MAR, De Menezes Cussiol NA, et al. Count, identification and antimicrobial susceptibility of bacteria recovered from dental solid waste in Brazil. Waste Manag (Oxford) 2011;31:1327–1332. doi: 10.1016/j.wasman.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 83.Miller CH, Palenik CJ, editors. Infection Control and Management of Hazardous Materials for the Dental Team. 4th ed. Elsevier Mosby; St. Louis, Missouri: 2010. [Google Scholar]
- 84.Schmidtke DW. The University of Alabama at Birmingham; Birmingham, Alabama: 2011. Efficacy of Sterisil in the treatment of dental unit waterlines. Thesis: Master of Science. [Google Scholar]
- 85.Kramer A, Assadian O, Bachfeld D, et al. Purge- and intensive-purge decontamination of dental units contaminated with biofilm. GMS Krankenhaushygiene Interdisziplinär [serial on the Internet] 2012;7:1–4. doi: 10.3205/dgkh000195. [cited 2012 4 April], Available from: http://www.egms.de/pdf/journals/dgkh/2012-7/dgkh000195.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samaranayake L. 4th ed. Churchill Livingstone Elsevier; Hong Kong: 2012. Essential Microbiology for Dentistry. Taylor, A, editor. [Google Scholar]
- 87.Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. Lancet. 2012;379:684. doi: 10.1016/S0140-6736(12)60074-9. [DOI] [PubMed] [Google Scholar]
- 88.Garg SK, Mittal S, Kaur P. Dental unit waterline management: historical perspectives and current trends. J Investig Clin Dent [serial on the Internet] 2012;3:247–252. doi: 10.1111/j.2041-1626.2012.00135.x. [cited 2012 27 August], Available from:/doi/10.1111/j.2041-1626.2012.00135.x/pdf. [DOI] [PubMed] [Google Scholar]
- 89.Coleman DC, O’Donnell MJ, Shore AC, et al. Biofilm problems in dental unit water systems and its practical control. J Appl Microbiol. 2009;106:1424–1437. doi: 10.1111/j.1365-2672.2008.04100.x. [DOI] [PubMed] [Google Scholar]
- 90.Singh TS, Mabe OD. Review: occupational exposure to endotoxin from contaminated dental unit waterlines. S Afr Dent J. 2009;64:8–14. [PubMed] [Google Scholar]
- 91.Laheij AMGA, Kistler JO, Belibasakis GN et al. Healthcare-associated viral and bacterial infections in dentistry. European Oral Microbiology Workshop (EOMW) 2011: CoAction; 2012 [cited 2013 20 April]; Available from: 10.3402/jom.v4i0.17659 [DOI] [PMC free article] [PubMed]
- 92.Cristina ML, Spagnolo AM, Sartini M, et al. Evaluation of the risk of infection through exposure to aerosols and spatters in dentistry. Am J Infect Control [serial on the Internet] 2008;4:304–307. doi: 10.1016/j.ajic.2007.07.019. [cited 2008 4 April], Available from: http://www.ajicjournal.org/article/S0196-6553%2807%2900807-3/pdf. [DOI] [PubMed] [Google Scholar]
- 93.Smith GW, Smith AJ, Creanor S, et al. Survey of the decontamination and maintenance of dental handpieces in general dental practice. Br Dent J. 2009;207:E7. doi: 10.1038/sj.bdj.2009.761. discussion 160-161. [DOI] [PubMed] [Google Scholar]
- 94.Su J, Deng X-H, Sun Z. A 10-year survey of compliance with recommended procedures for infection control by dentists in Beijing. Int Dent J. 2012;62:148–153. doi: 10.1111/j.1875-595X.2011.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aravind NKS, Reddy S, Manjunath C, et al. Complete care to cure: an update of the current knowledge of infection control in dental practice. Ann Essen Dent. 2012;4:43–49. [Google Scholar]
- 96.Purohit B, Priya H, Acharya S, et al. Efficacy of pre-procedural rinsing in reducing aerosol contamination during dental procedures. J Infect Prev. 2009;10:90. [Google Scholar]
- 97.Feres M, Figueiredo LC, Faveri M, et al. The effectiveness of a preprocedural mouthrinse containing cetylpyridinium chloride in reducing bacteria in the dental office. J Am Dent Assoc. 2010;141:415–422. doi: 10.14219/jada.archive.2010.0193. [DOI] [PubMed] [Google Scholar]
- 98.MacDonald DS, Waterfield JD. Infection control in digital intraoral radiography: evaluation of microbiological contamination of photostimulable phosphor plates in barrier envelopes. J Can Dent Assoc. 2011;77:1–5. [PubMed] [Google Scholar]
- 99.Ardakani FE, Zandi H, Mohammadi Z, et al. Comparing the disinfecting efficacies of Micro 10, Deconex, Alprocid and Microzid AF on the microorganisms on radiographic equipments. J Dent Res Dent Clin Dent Prospects. 2008;2:48–52. doi: 10.5681/joddd.2008.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Westergard EJ, Romito LM, Kowolik MJ, et al. Controlling bacterial contamination of dental impression guns. J Am Dent Assoc. 2011;142:1269–1274. doi: 10.14219/jada.archive.2011.0112. [DOI] [PubMed] [Google Scholar]
- 101.Almortadi N, Chadwick RG. Disinfection of dental impressions - compliance to accepted standards. Br Dent J. 2010;209:607–611. doi: 10.1038/sj.bdj.2010.1134. [DOI] [PubMed] [Google Scholar]
- 102.Haralur SB, Al-Dowah OS, Gana NS, et al. Effect of alginate chemical disinfection on bacterial count over gypsum cast. J Adv Prosthetics. 2012;2012:84–88. doi: 10.4047/jap.2012.4.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Egusa H, Watamoto T, Abe K, et al. An analysis of the persistent presence of opportunistic pathogens on patient-derived dental impressions and gypsum casts. Int J Prosthetics. 2010;21:62–68. [PubMed] [Google Scholar]
- 104.Hiraguchi H, Kaketani M, Hirose H, et al. Effect of immersion disinfection of alginate impressions in sodium hypochlorite solution on the dimensional changes of stone models. Dent Mater J. 2012;31:280–286. doi: 10.4012/dmj.2010-201. [DOI] [PubMed] [Google Scholar]
- 105.Hashemipour M, Mosharafian S, Mohammadalizadeh S, et al. Evaluation of infection control situation among dental laboratories in Kerman. J Isfahan Dent School. 2008;4:18–24. [Google Scholar]
- 106.Jensen PA, Lambert LA, Iademarco MF, et al. Centers for Disease Control and Prevention Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. Morb Mortal Wkly Rep. 2005;54(RR-17):1–142. [PubMed] [Google Scholar]
- 107.Cleveland JL, Robison VA, Panlilio AL. Tuberculosis epidemiology, diagnosis and infection control recommendations for dental settings: an update on the centers for disease control and prevention guidelines. J Am Dent Assoc. 2009;140:1092–1099. doi: 10.14219/jada.archive.2009.0335. [DOI] [PubMed] [Google Scholar]
- 108.Cadmus SI, Okoje VN, Taiwo BO, et al. Exposure of dentists to Mycobacterium tuberculosis, Ibadan, Nigeria. Emerg Infect Dis. 2010;2010:1479–1481. doi: 10.3201/eid1609.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mphahlele MT, Tudor C, Van der Walt M, et al. An infection control audit in 10 primary health-care facilities in the Western Cape Province of South Africa. Int J Infect Control. 2012;8:1–5. [Google Scholar]
- 110.Azarpazhooh A, Leake JL. Prions in dentistry–what are they, should we be concerned, and what can we do? J Can Dent Assoc. 2006;72:53–60. Feb. [PubMed] [Google Scholar]
- 111.Kirby E, Dickinson J, Vassey M, et al. Bioassay studies support the potential for iatrogenic transmission of variant Creutzfeldt Jakob Disease through dental procedures. PLoS One [serial on the Internet] 2012;7:e49850. doi: 10.1371/journal.pone.0049850. [cited 2012 30 November], Available from: http://www.plosone.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kohli A, Puttaiah R. Thompson Printers; New Delhi, India: 2008. Dental Infection Control and Occupational Safety for Oral Health Professionals. [Google Scholar]
- 113.Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention (CDC) in 2008–2011. [database on the Internet]. CDC. 2013 [cited 10 February 2013]. Available from: http://www.cdc.gov/hepatitis/Outbreaks/HealthcareHepOutbreakTable.htm
- 114.Myers JE, Myers R, Wheat ME, et al. Dental students and bloodborne pathogens: occupational exposures, knowledge, and attitudes. J Dent Educ. 2012;76:479–486. [PubMed] [Google Scholar]
- 115.Resende VLS, Abreu MHG, Paiva SM, et al. Research concerns regarding hepatitis B vaccination and post-vaccination test among Brazilian dentists. Virol J [serial on the Internet] 2010;7:1–9. doi: 10.1186/1743-422X-7-154. Available from: http://www.virologyj.com/content/7/1/154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Azodo CC, Ehizele AO, Uche I, et al. Hepatitis-B vaccination status among dental surgeons in Benin City, Nigeria. Ann Med Health Sci Res. 2012;2:24–28. doi: 10.4103/2141-9248.96932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med 6: e1000097. British Medical Journal. 2009;2009:332–336. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]