Abstract
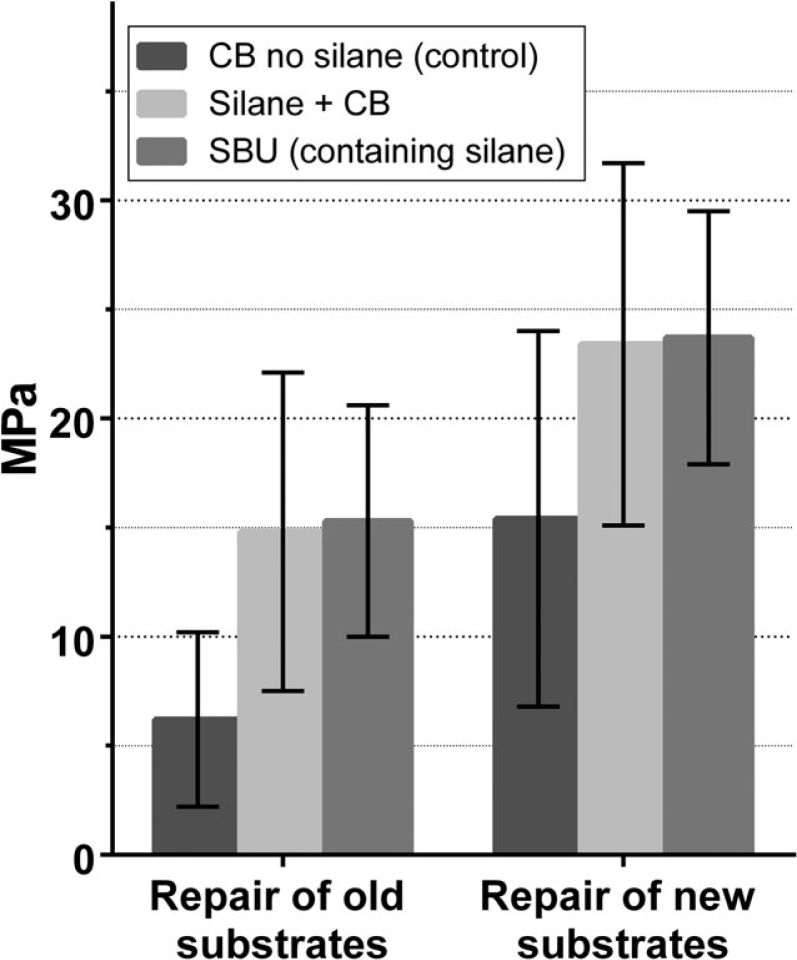
Objective: The aim of this study was to investigate the effect of silane in the repair of old and new resin-composite restorations. Method: Part 1: repair of old composite was performed on 60 resin-composite substrates that were 6 years old and were made of six different brands of composite. Three experiments were performed. In the first experiment, the substrates were ground flat and composite was fixed to the surface with bonding agent without silane (i.e. Clearfil Bond SE only, the control). Shear bond strength (SBS) was tested according to ISO/TS 11405 after thermocycling. In the second experiment, the same 60 substrates were ground again and treated with bis-silane a 2-part silane mixed shortly before application before applying bonding agent (Clearfil Bond SE plus silane) and repair composite before SBS testing. In the third experiment, the same substrates were ground again and a one-step bonding product containing silane (Scotchbond Universal bond containing silane) was used for the repair procedure before SBS testing. Part 2: to evaluate the repair of newly made composite restorations, 66 composite substrates were made and stored in water for 2 months. The specimens were divided into three groups and were tested using the same protocols as used to evaluate repair of old composite. Results: Mean SBS (±standard deviation), in MPa, for repair of old composite was 6.2 ± 4.0 (Clearfil Bond SE only, control), 14.8 ± 7.8 (Clearfil Bond SE plus silane) and 15.3 ± 5.6 (Scotchbond Universal bond with silane), whereas for new composite mean SBS was 15.4 ± 8.6 (Clearfil Bond SE only, control), 23.4 ± 8.3 (Clearfil Bond SE with silane) and 23.7 ± 5.8 (Scotchbond Universal containing silane). A significant difference was observed between the control and the test groups with silanising agents, both in Part 1 (P < 0.001) and in Part 2 (P < 0.005). Conclusion: Silanising agents increase the bond strength of the resin composite repair.
Key words: Composite repair, silanising, resin-based fillings, bonding agents, ageing, shear bond strength
INTRODUCTION
One of the reasons for using resin-based composite restorations is that they are repairable1 and they represent the modern philosophy of ‘Minimal Intervention Dentistry’2., 3.. Composite restorative materials have been through tremendous development over the last decades concerning material strength, handling properties, aesthetic features and longevity4., 5.. Still, the main reasons for restoration failure are caries and fractures6., 7., 8., 9. posing the question to the clinician: repair or replace. The remaining composite restoration may have been in the oral environment for a long, and often unknown, period of time, and the dental clinician is often unaware of the product used to create the initial restoration. Despite these facts, the dentist may find it desirable, or even favourable, to keep all or part of the old restoration in place10. The reasons may be financial, in that a small filling represents a lower cost, or practical, in order to make the procedure more conservative by sacrificing less of the adjacent sound tooth tissue and/or to simplify the replacement restoration. Simpler restorations may be time saving and enhance moisture control. The treatment goals are long-lasting restorations of good quality, and repair of resin-based composites is reported to have a favourable outcome for the longevity and quality of the restoration without compromising sound tooth tissue more than necessary11., 12., 13..
Regarding bonding to dentin and enamel, the use of bonding agents in the repair of composite restorations is an established procedure. There is strong evidence for increased bond strength in vitro between new and old composite when using dentin-bonding agents in the repair procedure12., 14., 15., 16., 17.. In addition, other pretreatments have been suggested to improve composite-to-composite bonding, but it has not been possible to identify an optimal procedure18. In this study we are specifically investigating the effects of using silane in composite repair because old composite consists of inorganic filler particles that should be resilanised to improve bonds to organic monomers in the repair material.
Silanising agents are important in all composite technology as coupling agents19. They link inorganic filler particles to organic resin polymers. The use of silanising agents in combination with bonding agents has been discussed and investigated lately14, and at least one company (3M ESPE, St Paul, MN, USA) has recently incorporated a silanising agent in their newest bonding agent. This prompted an evaluation of the importance of silanising agents in composite repair.
The following null hypothesis was proposed: the use of additional silaniser has no effect upon bond strength when repairing a composite restoration.
MATERIALS AND METHODS
Part 1: Test of old composite repair
The materials used are given in Table 1. The methacrylate-based composites selected were the most commonly used products in Norway in 2006. Test substrate bases were made by packing composite in copper rings (8 mm diameter, 10 mm height), placed on a mylar strip on a table. The composite cylinders were light-cured in increments of approximately 2 mm, from the top of the cylinder, according to the manufacturer’s specifications, using a Demetron VCL 400 halogen curing lamp (Kerr Hawe, Orange, CA, USA) with an irradiance of 859 mW/cm², as measured by the Norwegian Radiation Protection Authorities (Österaas, Norway). After curing, the copper rings were carefully split with a carbide disk and removed. Ten test substrates were produced of each composite resin product, in total 60 substrates. These were made in July 2008, and have been stored in distilled water at room temperature since production and after use in a composite repair study in 2008/200917.
Table 1.
Materials used in the study
| Material | Lot | Repair composite |
|---|---|---|
| Old substrates | ||
| Filtek Z 250 (3M ESPE, St Paul, MN, USA) | 2007 11 03 7LU | Filtek Supreme XTE (lot nos N510285 and N486561; 3M ESPE) |
| Filtek Silorane (3M ESPE) | 2007 11 12 7AT | |
| Charisma (Heraeus Kulzer, Hanau, Germany) | 010307 2011-10 | |
| CeramX Mono (Dentsply, York, PA, USA) | 0803002878 | |
| Tetric Evo Ceram (Vivadent-Ivoclar, Schaan, Liechtenstein) | L08982 | |
| Filtek Supreme XTE (3M ESPE) | 2008 03 30/2008 02 29 | |
| New substrates | ||
| Filtek Supreme XTE (3M ESPE) | Lots N510285 and N486561 | Filtek Supreme XTE (lot nos N510285 and N486561; 3M ESPE) |
| Bonding agent | ||
| Clearfil SE Bond (Kuraray Noritake Dental Inc., Okayama, Japan) | 000035 | |
| Scotchbond Universal (3M ESPE) | 518662 | |
| Silanising agent | ||
| Bis-Silane (Bisco Inc., Schaumburg, IL, USA) | 1300000623 | |
The old test substrates were used three times for testing the following three different bonding procedures before repair: (1) Clearfil Bond SE without sialising agent (CB) (control); (2) Clearfil Bond SE with bis-silane pretreatment (CB+SA); and (3) Scotchbond Universal, containing silanising agent (SBU).
The aged composite cylinders were wet-ground manually with grinding paper (Fepa P #500, silicon carbide, waterproof; Struers, Copenhagen, Denmark) to imitate preparation with a superfine diamond bur and to minimise the potential of micro-mechanical interlocking. The ground surface was dried by air blowing for 10 seconds and was masked with plastic tape with a circular aperture of 3 mm in diameter.
The bonding agent was applied according to the manufacturer’s specifications. After bonding application the repair composite was fixed to the old substrate using the equipment described in ISO/TS 11405:200320 and light-cured (Elipar S10 LED curing lamp, irradiance: 1150 mW/cm²; 3M ESPE). All test specimens were thermocycled (5000 cycles/5–55 °C) before measuring shear bond strength (SBS), according to ISO/TS 11405:200320 as previously described17. All the substrates were again ground flat, as described above, before procedures 2 and 3, respectively.
Part 2: Test of new composite repair
Sixty-six new test substrates were made, as described above, from the material of choice (Filtek Supreme XTE) at the student training clinic, University of Oslo. After 60 days in water storage, the new substrates were divided into three groups of 22. This number was chosen for statistical strength as there should be at least 15 specimens in the sample. They were tested using exactly the same protocol as used for repair of old composite.
Statistics
The Student’s t-test for two-tailed samples was performed to compare the results for each treatment procedure.
RESULTS
The results of the repair experiments are given in Figure 1., Figure 2., Figure 3.. The bond strength when repairing old composite was significantly lower than that when repairing new composite (P < 0.01). The use of silanising agents significantly improved the bond strength in composite repair (P < 0.01) and the increase in bond strength was greater when repairing old composite substrates. The increase in repair strength of old substrates was approximately 139%, compared with that of newly made substrates, which had an increase, in our study, of approximately 51% (Table 2). Binding between Silorane and methacrylate (MA)-based composites gave poorer results than binding between MA-based composites (data not shown), probably because of differences in composition.
Figure 1.
Shear bond strength (SBS) after repairing old and new substrates. Bars represent the mean test value and error bars represent the standard deviation. CB no silane, Clearfil Bond SE without silane (control); Silane + CB, silanising of composite substrate + Clearfil Bond SE; SBU, Scotchbond Universal.
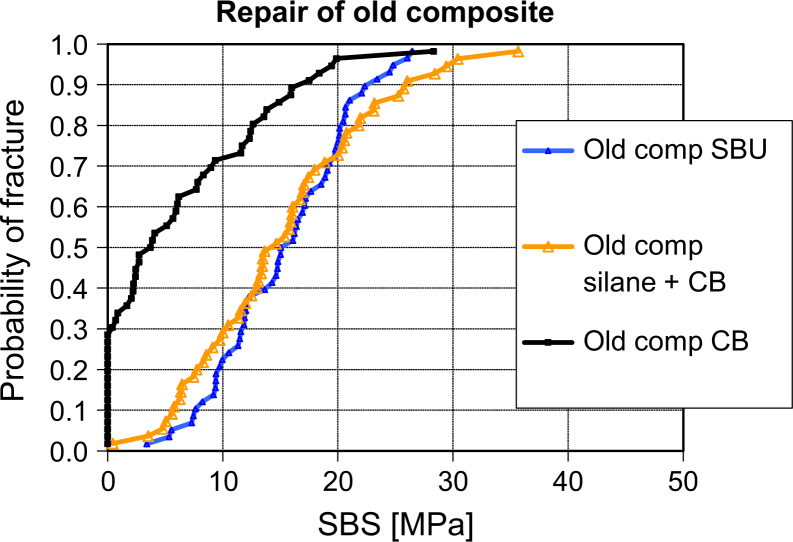
Figure 2.
Weibull distribution of shear bond strength (SBS) for repair of old composite, showing a marked increase for SBS when using silane. n = 60 for each group. CB, Clearfil Bond SE without silane (control); silane + CB, silanising of composite substrate + Clearfil Bond SE; SBU, Scotchbond Universal.
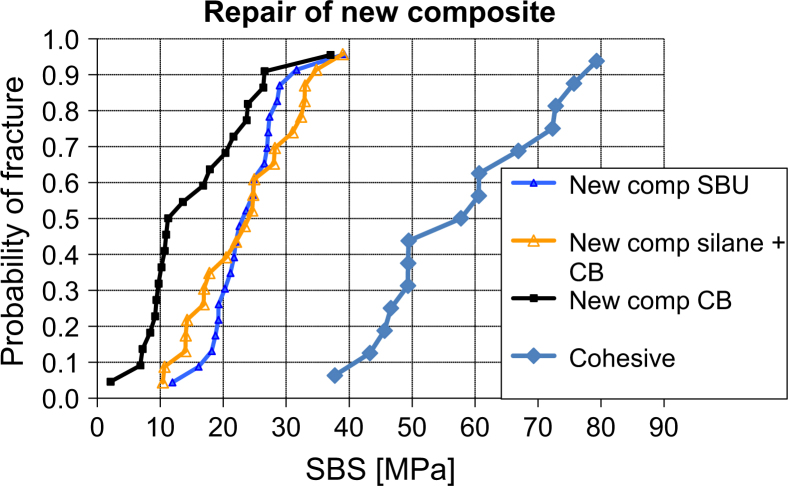
Figure 3.
Weibull distribution of shear bond strength (SBS) for repair of new composite, showing a marked increase for SBS when using silane. Cohesive, SBS values of inherent strength of the new composite; CB no silane, Clearfil Bond SE without silane (control); Silane + CB, silanising of composite substrate + Clearfil Bond SE; SBU, Scotchbond Universal.
Table 2.
Shear bond strength after repairing old and new composite substrates
| Procedure | Test results (MPa) | |
|---|---|---|
| Part 1: Repair of old substrates | ||
| 1 | CB no silane (control) | 6.2 ± 4.0 |
| 2 | Silane + CB | 14.8 ± 7.3* |
| 3 | SBU (containing silane) | 15.3 ± 5.3* |
| Part 2: Repair of new substrates | ||
| 1 | CB no silane (control) | 15.4 ± 8.6 |
| 2 | Silane + CB | 23.4 ± 8.3† |
| 3 | SBU (containing silane) | 23.7 ± 5.8† |
Values are given as mean ± standard deviation.
Statistically significant difference from the controls (P < 0.001).
Statistically significant difference from the controls (P < 0.005).
CB no silane, Clearfil Bond SE without silane; Silane + CB, silanising of composite substrate + Clearfil Bond SE; SBU, Scotchbond Universal.
DISCUSSION
The results of this in vitro investigation (Figure 1., Figure 2., Figure 3.) show a clear difference in SBS between repair with and repair without silanising agents (P < 0.01), for both old and new composite, confirming the results of previous studies21., 22.. However, the SBS in the repair interface is significantly weaker than the inherent strength of the composite itself. A preliminary test was performed on 15 new substrates (Figure 3). There was no significant difference between repair strength using a separate silanising step (CB+SA) and using a bonding agent containing silane (SBU) (P > 0.7). The latter even had a smaller standard deviation, indicating a more predictable outcome. Both methods of silanising are easy to handle chair side, and require no extra equipment14., 23.. The conclusion of these findings is that the null hypothesis has to be rejected.
It should be noted that these tests, repair of both old and new composite, were performed 14 days after the bonding procedure followed by thermocycling (5000 cycles/5–55 °C), and they cannot predict the long-term stability of the repair24.
It is unclear if the favourable effects of silane are long lasting. The main problem with silanes is their long-term hydrolytic instability, which causes hydrolysis, splitting the Si–O cation bridges over time. When repairing a restoration, all surfaces are covered with at least one single sheet of water molecules, which may not be the ideal condition. The bonding strength will partly become dependent on hydrogen bonds and molecular attraction forces, such as Van der Waals forces, and not the stronger covalent or ionic types. For this reason, the bonding between the silaniser and old composite is always vulnerable to hydrolysis of the relatively weak bonds25., 26., 27..
Hydrolysis leads to degradation of the interface bonding26., 28., 29.. The content of hydrophilic monomers, such as 2-hydroxyethyl methacrylate (HEMA), in the bonding agent is of importance for the water content in the repair area. HEMA attracts water and keeps it there, thereby increasing hydrolytic reaction. The stoichiometric configuration of the molecules, especially the silanes, may, on the other hand, prevent water movement and sorption in the area, making an impact on the long-term stability. Much research remains to be completed in this field26.
SBU was tested in this study. It contains prehydrolysed silanes, which are reported by the manufacturer to be stable for at least 1 year of storage, and both priming and bonding chemicals are contained in this one mixture. In this short-term test, the silanes performed well. The success of SBU lies in its ability to split off hydroxyl groups and form oxygen bridges to surface cations. The complex chemistry is not publicly available (propriety information), but the product showed remarkably good results in this short-term in vitro study. The performance over a longer period of time remains to be seen as it has only been commercially available for a few years. Scepticism of simplified systems is still connected to the problems concerning evaporation of solvents and hydrophilic monomers, causing water sorption and hydrolysis, when all the ingredients are mixed in one bottle24., 30..
The good results for SBU may be a result of the presence of another recognized ingredient, 10-methacryloyloxydecyl dihydrogenphosphate (10-MDP), a 10-carbon phosphorylated acidic monomer that shows very good ability to form stable bonds to cations31. However, CB also contains MDP. When CB was used with additional silane, the test results were significantly better than those obtained for CB without extra silane, indicating a positive effect of the silaniser. We chose two bonding agents containing MDP to avoid confounders. Likewise, we chose #500 grinding paper to minimise the micro-mechanical interlocking effects at the bonding interface.
One might speculate that the positive effect on SBS when using silanes was a result of resilanising of the filler particles at the prepared restoration surface, improving the bond between the filler particles in the old composite and the resin matrix in the repair composite. The possibility of obtaining a chemical bond between the resins in the old substrate and the bonding agent decreases with time. The resin no longer has free radicals available for bonding and polymerisation, owing to a half-life of approximately 48 hours32 and a slow chemical aftercure combined with hydrolysis of the available double bonds, leaving the resin without the possibility to form new bonds. The much higher percentage increase of the repair strength for old composite (140%), compared with the increase of repair strength for new composite (50%), and that all the failures for repair of the old composite were of the adhesive type, strengthen the idea that silane has a strong ability to form siloxane bonds to filler particles in composite also when the composite is old. The improved effect of silane in old composite substrates may reflect the fact that hydrolytic degradation is higher in older specimens, which was also observed as reduced strength in the material over time14.
Another property of silanes, which may be beneficial to bond strength, is their ability to change surface energy in order to enhance wetting of the surface of inorganic materials, which is essential for the intimate contact needed between different materials to obtain good bonding19., 27..
To put the silane test into perspective, it is of interest to compare the bond strength to composite with bond strength to dentine. We have no proof that silanes form bonds to cations in organic substrates such as collagen/dentine26. To investigate the role of silanes when bonding composites to dentine, preliminary tests demonstrated no difference in bond strength between bonding with and without silanising agents (data not shown).
CONCLUSION
Composite repair with the use of a silanising agent performed significantly better than repair without a silanising agent. Bonding to older composites gave poorer results, but repair of old restorations is still recommended.
Acknowledgements
The work was financed by NIOM and the University of Oslo. All materials were purchased through local dental suppliers. The authors have no conflict of interest.
REFERENCES
- 1.Demarco FF, Correa MB, Cenci MS, et al. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Ericson D. The concept of minimally invasive dentistry. Dent Update. 2007;34:9–10. doi: 10.12968/denu.2007.34.1.9. 12-4, 17-8. [DOI] [PubMed] [Google Scholar]
- 3.Tyas MJ, Anusavice KJ, Frencken JE, et al. Minimal intervention dentistry–a review. FDI Commission Project 1-97. Int Dent J. 2000;50:1–12. doi: 10.1111/j.1875-595x.2000.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferracane JL. Resin composite–state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.van Meerbeek B, Yoshihara K, Yoshida Y, et al. State of the art of self-etch adhesives. Dent Mater. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Heintze SD, Rousson V. Clinical effectiveness of direct class II restorations – a meta-analysis. J Adhes Dent. 2012;14:407–431. doi: 10.3290/j.jad.a28390. [DOI] [PubMed] [Google Scholar]
- 7.Hickel R, Manhart J. Longevity of restorations in posterior teeth and reasons for failure. J Adhes Dent. 2001;3:45–64. [PubMed] [Google Scholar]
- 8.Kopperud SE, Tveit AB, Gaarden T, et al. Longevity of posterior dental restorations and reasons for failure. Eur J Oral Sci. 2012;120:539–548. doi: 10.1111/eos.12004. [DOI] [PubMed] [Google Scholar]
- 9.Opdam NJ, van de Sande FH, Bronkhorst E, et al. Longevity of posterior composite restorations: a systematic review and meta-analysis. J Dent Res. 2014;93:943–949. doi: 10.1177/0022034514544217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum IR, Lynch CD, Wilson NH. Factors influencing repair of dental restorations with resin composite. Clin Cosmet Investig Dent. 2014;6:81–87. doi: 10.2147/CCIDE.S53461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordan VV, Garvan CW, Blaser PK, et al. A long-term evaluation of alternative treatments to replacement of resin-based composite restorations: results of a seven-year study. J Am Dent Assoc. 2009;140:1476–1484. doi: 10.14219/jada.archive.2009.0098. [DOI] [PubMed] [Google Scholar]
- 12.Maneenut C, Sakoolnamarka R, Tyas MJ. The repair potential of resin composite materials. Dent Mater. 2011;27:e20–e27. doi: 10.1016/j.dental.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Mjor IA, Gordan VV. Failure, repair, refurbishing and longevity of restorations. Oper Dent. 2002;27:528–534. [PubMed] [Google Scholar]
- 14.Eliasson S, Tibballs J, Dahl J. Effect of different surface treatments and adhesives on repair bond strength of resin composites after one and 12 months of storage using an improved microtensile test method. Oper Dent. 2014;39:E206–E216. doi: 10.2341/12-429-L. [DOI] [PubMed] [Google Scholar]
- 15.Papacchini F, Dall’oca S, Chieffi N, et al. Composite-to-composite microtensile bond strength in the repair of a microfilled hybrid resin: effect of surface treatment and oxygen inhibition. J Adhes Dent. 2007;9:25–31. [PubMed] [Google Scholar]
- 16.Rathke A, Tymina Y, Haller B. Effect of different surface treatments on the composite-composite repair bond strength. Clin Oral Investig. 2009;13:317–323. doi: 10.1007/s00784-008-0228-2. [DOI] [PubMed] [Google Scholar]
- 17.Staxrud F, Dahl JE. Role of bonding agents in the repair of composite resin restorations. Eur J Oral Sci. 2011;119:316–322. doi: 10.1111/j.1600-0722.2011.00833.x. [DOI] [PubMed] [Google Scholar]
- 18.Loomans BA, Cardoso MV, Roeters FJ, et al. Is there one optimal repair technique for all composites? Dent Mater. 2011;27:701–709. doi: 10.1016/j.dental.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Anusavice KJ, Shen C, Rawls HR. Saunders; St. Louis, MO: 2013. Phillips Science of Dental Materials. [Google Scholar]
- 20.ISO . ISO; Geneva: 2003. International Organisation of Standardization,Testing of dental materials to tooth structure. ISO/TS 11405:2003. Dental Materials. [Google Scholar]
- 21.Ozcan M, Barbosa SH, Melo RM, et al. Effect of surface conditioning methods on the microtensile bond strength of resin composite to composite after aging conditions. Dent Mater. 2007;23:1276–1282. doi: 10.1016/j.dental.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan M, Corazza PH, Marocho SM, et al. Repair bond strength of microhybrid, nanohybrid and nanofilled resin composites: effect of substrate resin type, surface conditioning and ageing. Clin Oral Investig. 2013;17:1751–1758. doi: 10.1007/s00784-012-0863-5. [DOI] [PubMed] [Google Scholar]
- 23.Brendeke J, Ozcan M. Effect of physicochemical aging conditions on the composite-composite repair bond strength. J Adhes Dent. 2007;9:399–406. [PubMed] [Google Scholar]
- 24.van Meerbeek B, Peumans M, Poitevin A, et al. Relationship between bond-strength tests and clinical outcomes. Dent Mater. 2010;26:e100–e121. doi: 10.1016/j.dental.2009.11.148. [DOI] [PubMed] [Google Scholar]
- 25.Breschi L, Mazzoni A, Ruggeri A, et al. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Lung CY, Matinlinna JP. Aspects of silane coupling agents and surface conditioning in dentistry: an overview. Dent Mater. 2012;28:467–477. doi: 10.1016/j.dental.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Ruyter I. Chemistry of current adhesive agents. Today’s Dent 1987. Special edition, 11–18.
- 28.Lagouvardos PE, Pissis P, Kyritsis A, et al. Water sorption and water-induced molecular mobility in dental composite resins. J Mater Sci Mater Med. 2003;14:753–759. doi: 10.1023/a:1025080103857. [DOI] [PubMed] [Google Scholar]
- 29.Malacarne J, Carvalho RM, de Goes MF, et al. Water sorption/solubility of dental adhesive resins. Dent Mater. 2006;22:973–980. doi: 10.1016/j.dental.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Peumans M, Kanumilli P, de Munck J, et al. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dent Mater. 2005;21:864–881. doi: 10.1016/j.dental.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida Y, Yoshihara K, Nagaoka N, et al. Self-assembled Nano-layering at the Adhesive interface. J Dent Res. 2012;91:376–381. doi: 10.1177/0022034512437375. [DOI] [PubMed] [Google Scholar]
- 32.Burtscher P. Stability of radicals in cured composite materials. Dent Mater. 1993;9:218–221. doi: 10.1016/0109-5641(93)90064-w. [DOI] [PubMed] [Google Scholar]