Abstract
Carbazole 1,9a-dioxygenase (CARDO) from Pseudomonas sp. strain CA10 is a multicomponent enzyme that catalyzes the angular dioxygenation of carbazole, dibenzofuran, and dibenzo-p-dioxin. It was revealed by gas chromatography-mass spectrometry and 1H and 13C nuclear magnetic resonance analyses that xanthene and phenoxathiin were converted to 2,2′,3-trihydroxydiphenylmethane and 2,2′,3-trihydroxydiphenyl sulfide, respectively. Thus, for xanthene and phenoxathiin, angular dioxygenation by CARDO occurred at the angular position adjacent to the oxygen atom to yield hetero ring-cleaved compounds. In addition to the angular dioxygenation, CARDO catalyzed the cis dihydroxylation of polycyclic aromatic hydrocarbons and biphenyl. Naphthalene and biphenyl were converted by CARDO to cis-1,2-dihydroxy-1,2-dihydronaphthalene and cis-2,3-dihydroxy-2,3-dihydrobiphenyl, respectively. On the other hand, CARDO also catalyzed the monooxygenation of sulfur heteroatoms in dibenzothiophene and of the benzylic methylenic group in fluorene to yield dibenzothiophene-5-oxide and 9-hydroxyfluorene, respectively. These results indicate that CARDO has a broad substrate range and can catalyze diverse oxygenation: angular dioxygenation, cis dihydroxylation, and monooxygenation. The diverse oxygenation catalyzed by CARDO for several aromatic compounds might reflect the differences in the binding of the substrates to the reaction center of CARDO.
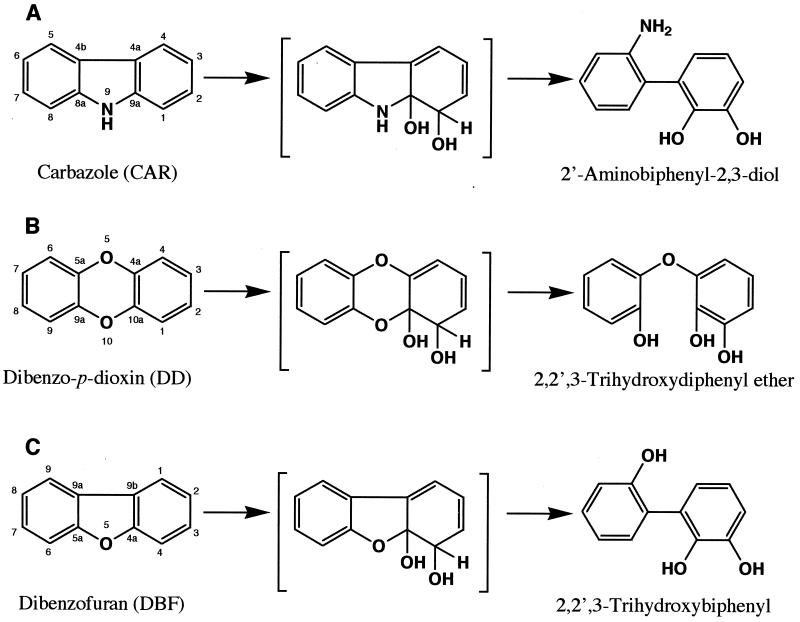
Carbazole 1,9a-dioxygenase (CARDO) from Pseudomonas sp. strain CA10 is a multicomponent enzyme that catalyzes the angular dioxygenation of carbazole (CAR) to yield an unstable dihydroxylated intermediate which is considered to be converted to 2′-aminobiphenyl-2,3-diol spontaneously (Fig. 1A). All of the structural genes encoding CARDO have been cloned, and their nucleotide sequences have been determined (26). Functional analysis revealed that CARDO consists of terminal oxygenase (CarAa), ferredoxin (CarAc), and ferredoxin reductase (CarAd). Although terminal oxygenase components of well-known multicomponent dioxygenase systems consist of large (α) and small (β) subunits, that of CARDO consists of a single protein, CarAa. The CarAa protein showed about 27 to 30% homology with the large subunits of terminal oxygenase components in other multicomponent dioxygenase systems, and phylogenetic analysis of the large subunits of terminal oxygenases revealed that CarAa was an evolutionarily novel oxygenase (26).
FIG. 1.
Conversion of CAR (A), DD (B), and DBF (C) catalyzed by CARDO from Pseudomonas sp. strain CA10. The structures shown in brackets are unstable intermediates that have not been characterized. Absolute stereochemistry is not intended.
Some of the multicomponent dioxygenases oxidizing aromatic compounds are reported to have broad substrate specificities and to perform several types of oxidation reactions. For example, dibenzofuran 4,4a-dioxygenase (dioxin dioxygenase) from Sphingomonas sp. strain RW1 catalyzes the angular dioxygenation of dibenzofuran (DBF) and dibenzo-p-dioxin (DD) (3). This well-investigated angular dioxygenase was also presumed to catalyze the angular dioxygenation of 9-fluorenone and the sulfoxidation of dibenzothiophene (3). Naphthalene 1,2-dioxygenase from Pseudomonas sp. strain NCIB 9816-4 has a broad substrate range for catalyzing the monooxygenation of benzylic methylenic groups (7, 21, 24, 30) and sulfur heteroatoms (1, 17, 22), the O dealkylation of anisole and phenetole (20), and the desaturation of phenetole and 1,2-dihydronaphthalene (20, 29).
In an earlier study, we demonstrated that DD and DBF were converted by CARDO to 2,2′,3-trihydroxydiphenyl ether and 2,2′,3-trihydroxybiphenyl, respectively, suggesting that CARDO attacks at the angular positions adjacent to the heteroatoms of these substrates, as in the case of CAR (Fig. 1B and C) (26). It was also suggested that CARDO has the ability to oxidize a wide variety of polyaromatic compounds, including dibenzothiophene, biphenyl, and polycyclic aromatic hydrocarbons (PAHs) (26). In this study, we identified the products generated by CARDO from several aromatic compounds, such as heterocyclic aromatic compounds, PAHs, and biphenyl, to clarify the oxygenation reaction catalyzed by CARDO for various aromatic compounds and to obtain information on substrate recognition by CARDO.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Escherichia coli JM109 harboring pUCARA (26), which directs the CARDO genes to be expressed under the control of the isopropylthiogalactopyranoside (IPTG)-inducible lac promotor, was used for the expression of CARDO. Because pUCARA is derived from pUC119, E. coli JM109 harboring pUC119 was used in control experiments. Cells were grown on 2× YT medium (25), which was supplemented with ampicillin at a final concentration of 50 μg/ml, at 37°C. For plate cultures, 2× YT medium solidified with 1.6% (wt/vol) agar was used.
DNA manipulation.
Plasmid DNA was prepared from E. coli cells by the alkaline lysis method (2). DNA fragments were extracted from the agarose gel by the glass powder method (GENECLEAN II kit; Bio 101, Inc., La Jolla, Calif.) according to the manufacturer’s instructions. Transformation of E. coli cells was performed as described by Hanahan (11).
Biotransformation of CAR analogues, PAHs, and biphenyl.
E. coli JM109(pUCARA) cells were precultured in 10 ml of 2× YT medium supplemented with ampicillin at 37°C for 16 h and then transferred to 1 liter of the same medium to which IPTG had been added to a final concentration of 1 mM. After incubation for another 8 h, the cells were harvested by centrifugation, washed twice with minimal medium (19), and resuspended in 100 ml of minimal medium. The optical density at 600 nm of the resultant cell suspension solution was 20 to 25.
As substrates, we used heterocyclic aromatic compounds (xanthene, phenoxathiin, and dibenzothiophene), PAHs (naphthalene, anthracene, phenanthrene, fluorene, and fluoranthene), and biphenyl. Each substrate was dissolved in dimethyl sulfoxide or ethanol (10 mg/ml), and then a 50-μl aliquot of the solution was added to 5 ml of cell suspension solution. The reaction mixture was incubated on a reciprocal shaker (300 strokes/min) at 30°C for 16 h. The biotransformation of CAR was used as a positive control to monitor CARDO activity. The products were extracted with ethyl acetate and concentrated as described previously (27), except for extraction under neutral conditions (pH 6.8 to 7.0). The extraction recovery of each substrate was about 80 to 100%. After analytical thin-layer chromatography (TLC), a portion of each extract was directly analyzed by gas chromatography-mass spectrometry (GC-MS) after trimethylsilylation with N-methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) at 70°C for 20 min. To quantify the substrate remaining and the compounds formed, we compared the peak area for the total ion current of the compounds extracted from the reaction mixture containing E. coli cells harboring pUCARA with that of the substrate extracted from the reaction mixture containing E. coli cells harboring pUC119. We repeated the above experiments two or three times and determined the amount of substrate remaining and the yields of the compounds formed during the biotransformation of aromatic compounds.
To identify the compounds formed by 1H and 13C nuclear magnetic resonance (NMR) analyses, we conducted a modified biotransformation experiment with several aromatic compounds. To obtain larger amounts of products, 100 mg of each substrate dissolved in 1 ml of dimethyl sulfoxide or ethanol was added to 100 ml of a cell suspension solution of E. coli JM109(pUCARA) prepared as described above. Incubation was carried out for 16 h, and the resultant reaction mixture was extracted with ethyl acetate at pH 6.8 to 7.0 as described above. After purification by preparative TLC or silica gel column chromatography, 0.5 to 10 mg of each compound was obtained. The purified compounds were dissolved in CDCl3 and used for NMR analysis and GC-MS analysis.
Analytical and purification methods.
Analytical TLC was performed on 0.25-mm-thick, precoated silica gel plates containing a fluorescence indicator (E. Merck AG, Darmstadt, Germany) and developed with a solvent system of toluene-dioxane (18:5, by volume).
For GC-MS, a model JMS-Automass 150 GC-MS system (JEOL, Ltd., Tokyo, Japan) fitted with a fused-silica chemically bonded capillary column (DB-5; 0.25 mm [inside diameter] by 15 m, 0.25-μm film thickness; J & W Scientific Inc., Folsom, Calif.) was used. After trimethylsilylation with MSTFA, each sample was injected into the column at 80°C in the splitless mode. After 2 min at 80°C, the column temperature was increased at 16°C/min to 280°C. The head pressure of the helium carrier gas was 65 kPa. The amount of each purified compound used for GC-MS analysis was approximately 1 to 10 ng for each injection.
Preparative TLC was carried out as follows. The sample was developed on a precoated silica gel plate (1 mm thick; Merck) with a solvent system of n-hexane–ethyl acetate (1:1, by volume). The desired band was scraped off and eluted with H2O-saturated ethyl acetate. The resultant eluate was dried over Na2SO4 before evaporation and used for NMR analysis.
Silica gel column chromatography was carried out as follows. The sample was loaded on a column of silica gel (15 mm [inside diameter] by 8 cm) and eluted with increasing amounts of ethyl acetate in n-hexane. After analytical TLC, the eluates containing the products were combined and used for NMR analysis.
The 1H and 13C NMR spectra of the compounds were recorded with a JNM-A500 spectrometer (JEOL) operated at 500 and 125 MHz, respectively, with tetramethylsilane as an internal standard. NAORAC H5X/FG and JEOL TUNABLE/H (5)500 probes were used for 1H and 13C NMR analyses, respectively. The number of spectra accumulated was 16 to 64. Two-dimensional NMR experiments for the determination of direct 1H-13C connectivity (HMQC), long-range (two- and three-bond) 1H-13C connectivity (HMBC), nuclear Overhauser enhancement measurements (NOESY), and 1H-1H shift-correlated spectroscopy (1H-1H COSY) were performed under the above conditions. About 0.5 to 10 mg of each purified compound was used in the NMR analyses.
Chemicals.
CAR, DBF, fluorene, and 1-naphthol were purchased from Tokyo Kasei Kogyo Co., Ltd. (Tokyo, Japan). Phenoxathiin, naphthalene, 2-naphthol, anthracene, and biphenyl were purchased from Kanto Chemical Co., Inc. (Tokyo, Japan). Xanthene, dibenzothiophene, fluoranthene, and MSTFA were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). DD and phenanthrene were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Dibenzothiophene-5-oxide, 2-hydroxyfluorene, 9-fluorenone, 9-hydroxyfluorene, and 2- and 3-hydroxybiphenyl were purchased from Sigma Aldrich Japan Co. (Tokyo, Japan).
RESULTS
Xanthene.
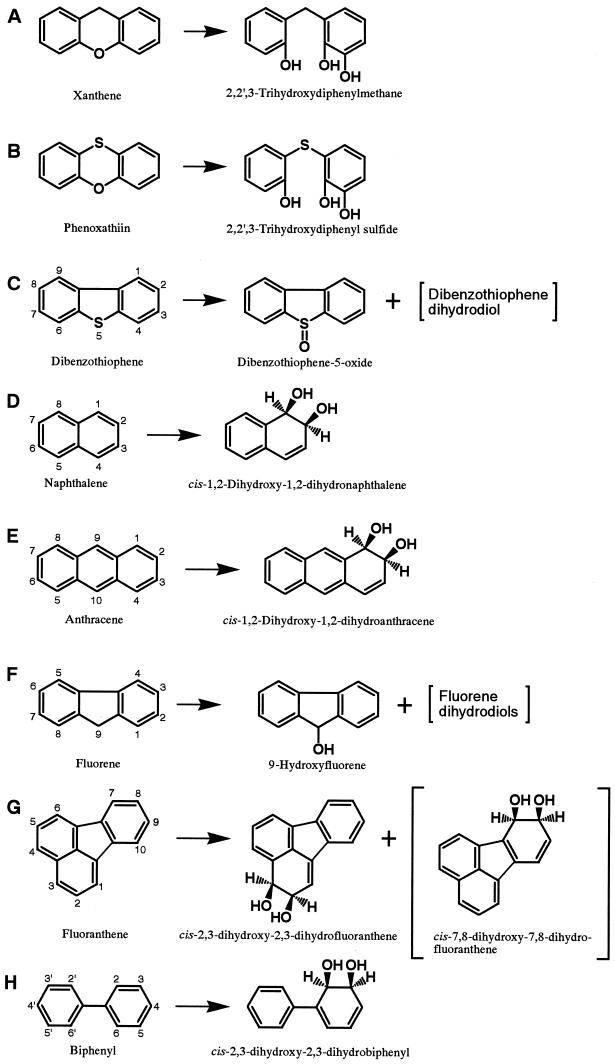
In xanthene biotransformation experiments with E. coli JM109(pUCARA), we detected a compound having an Rf of 0.34 on analytical TLC (Table 1). The mass spectrum of the trimethylsilyl (TMS) derivative of this compound (Table 1) indicated a molecular ion peak at m/z 432 and the presence of three hydroxy groups, suggesting that this compound was produced via cleavage of a heterocyclic ring. The 1H NMR data for this compound are shown in Table 2. Based on the correlations with the 1H-1H COSY spectrum (data not shown), this compound was identified as 2,2′,3-trihydroxydiphenylmethane (Fig. 2A). This result indicates that the oxygenation of xanthene by CARDO occurs at the angular position adjacent not to the methylenic carbon atom but to the oxygen atom.
TABLE 1.
Physical properties of the compounds formed during the biotransformation of heterocyclic aromatic compounds, PAHs, and biphenyl by CARDO
| Substratea (% remainingb) | Rf on TLCc | GC-MS datad
|
Yield (%)b | Identification or possible structure | |
|---|---|---|---|---|---|
| Rf (min) | Principal ions and relative abundance (% base peak) | ||||
| Xanthene (35–40) | 0.34 | 11.6 | 432 (M+, 33), 417 (3), 329 (47), 179 (45), 73 (100) | 60–65 | 2,2′,3-Trihydroxydiphenylmethanee |
| Phenoxathiin (35–45) | 0.34 | 12.2 | 450 (M+, 61), 435 (43), 347 (94), 331 (26), 73 (100) | 55–65 | 2,2′,3-Trihydroxydiphenyl sulfidee |
| Dibenzothiophene (35–55) | 0.40 | 10.9 | 200 (M+, 48), 184 (100), 171 (50), 152 (35), 139 (57) | 40–60 | Dibenzothiophene-5-oxideef |
| 11.1 | 362 (M+, 16), 272 (12), 184 (36), 147 (17), 73 (100) | 2–3 | Dihydrodiol | ||
| 10.7 | 272 (M+, 100), 257 (74), 241 (96), 226 (19), 197 (16) | 2–3 | Monohydroxydibenzothiophene | ||
| Naphthalene (tr) | 0.33 | 8.1 | 306 (M+, 10), 275 (6), 203 (38), 191 (100), 147 (70) | 90–95 | cis-1,2-Dihydroxy-1,2-dihydronaphthalenee |
| 0.64 | 7.1 | 216 (M+, 85), 201 (100), 185 (73), 141 (14), 73 (44) | 5–10 | 1-Naphtholf | |
| Anthracene (20–35) | 0.30 | 11.4 | 356 (M+, 3), 253 (20), 191 (65), 147 (19), 73 (100) | 60–80 | cis-1,2-Dihydroxy-1,2-dihydroanthracenee |
| 0.60 | 11.0 | 266 (M+, 100), 251 (39), 235 (43), 191 (20), 165 (25) | 0–5 | Monohydroxyanthracene | |
| Phenanthrene (10–25) | 10.5 | 356 (M+, 6), 253 (2), 191 (2), 147 (61), 73 (100) | Tr | Dihydrodiol | |
| 11.2 | 356 (M+, 4), 253 (4), 191 (52), 147 (9), 73 (100) | 20–25 | Dihydrodiol | ||
| 11.5 | 356 (M+, 2), 253 (8), 191 (80), 147 (20), 73 (25) | 50–55 | Dihydrodiol | ||
| 11.0 | 266 (M+, 79), 251 (59), 235 (13), 176 (18), 73 (100) | Tr | Monohydroxyphenanthrene | ||
| 11.1 | 266 (M+, 100), 251 (77), 235 (51), 176 (17), 73 (46) | 5–10 | Monohydroxyphenanthrene | ||
| 11.3 | 266 (M+, 100), 251 (93), 235 (13), 176 (22), 73 (21) | Tr | Monohydroxyphenanthrene | ||
| Fluorene (65–80) | 0.65 | 8.9 | 254 (M+, 100), 239 (89), 211 (6), 178 (16), 165 (42) | 5–10 | 9-Hydroxyfluorenef |
| 9.7 | 254 (M+, 100), 239 (89), 211 (6), 178 (16), 165 (42) | 15–20 | Monohydroxyfluorene | ||
| 9.8 | 254 (M+, 100), 239 (89), 211 (6), 178 (16), 165 (42) | Tr | Monohydroxyfluorene | ||
| 10.0 | 254 (M+, 100), 239 (89), 211 (6), 178 (16), 165 (42) | Tr | Monohydroxyfluorene | ||
| 0.74 | 10.1 | 254 (M+, 100), 239 (89), 211 (6), 178 (16), 165 (42) | 1–5 | 2-Hydroxyfluorenef | |
| Fluoranthene (90–95) | 0.36 | 12.8 | 380 (M+, 8), 290 (22), 218 (23), 190 (31), 73 (100) | 3–7 | cis-2,3-Dihydroxy-2,3-dihydrofluoranthenee |
| 0.65 | 12.6 | 290 (M+, 97), 275 (60), 259 (100), 244 (12), 215 (41) | 2–3 | 7-Hydroxyfluoranthenee | |
| Biphenyl (tr) | 0.32 | 9.2 | 332 (M+, 22), 243 (8), 227 (12), 211 (8), 73 (100) | 85 | cis-2,3-Dihydroxy-2,3-dihydrobiphenyle |
| 0.59 | 8.5 | 242 (M+, 65), 227 (100), 211 (90), 165 (9), 152 (25) | 7–8 | 3-Hydroxybiphenylf | |
| 0.65 | 7.5 | 242 (M+, 36), 227 (67), 211 (100), 165 (6), 152 (12) | 7–8 | 2-Hydroxybiphenylf | |
The starting concentration of substrates in the biotransformations used to estimate the yields of substrates remaining and the compounds formed was 100 μg/ml.
To quantify the yields of substrates and products after the biotransformation experiment with CARDO, we compared the peak area for the total ion current of each compound extracted from the reaction mixture prepared with E. coli cells harboring pUCARA with that of each substrate extracted from the reaction mixture prepared with E. coli cells harboring pUC119. We repeated the experiments two or three times and estimated the yields of the compounds.
Analytical TLC was done with the solvent system toluene-dioxane (18:5, by volume).
GC-MS data for all TMS derivatives but dibenzothiophene-5-oxide are shown.
Identification was made by 1H and 13C NMR analyses.
Identification was made by Rf and spectral comparisons with authentic samples in GC-MS analyses.
TABLE 2.
NMR analysis of the compounds formed in biotransformation experiments
| Substrate | Chemical shifts (δ)a
|
Identification | |
|---|---|---|---|
| 1H NMR | 13C NMR | ||
| Xanthene | 7.27 (H-6′, d, J = 7.5 Hz), 7.09 (H-4′, t, J = 7.5 Hz), 6.90 (H-5′, t, J = 7.5 Hz), 6.79 (H-3′, d, J = 7.5 Hz), 6.73–6.82 (H-4,5,6), 3.90 (CH2, s) | ND | 2,2′,3-Trihydroxydiphenylmethane |
| Phenoxathiin | 7.35 (H-6′, d, J = 7.5 Hz), 7.24 (H-4′, t, J = 7.5 Hz), 6.95 (H-3′, d, J = 7.5 Hz), 6.87 (H-5′, t, J = 7.5 Hz), 6.85 (H-4,6, d, J = 8.0 Hz), 6.74 (H-5, t, J = 8.0 Hz) | 155.8, 144.0, 143.4, 134.6, 130.9, 125.0, 121.6, 121.3, 119.4, 118.7, 116.0, 115.8 | 2,2′,3-Trihydroxydiphenyl sulfide |
| Dibenzothiophene | 7.98 (2H, d, J = 7.5 Hz), 7.80 (2H, d, J = 7.5 Hz), 7.59 (2H, t, J = 7.5 Hz), 7.49 (2H, t, J = 7.5 Hz) | ND | Dibenzothiophene-5-oxide |
| Naphthalene | 7.12–7.54 (H-5,6,7,8), 6.54 (H-4, d, J = 10 Hz), 6.07 (H-3, dd, J = 10 Hz, 4.5 Hz), 4.70 (H-1, d, J = 4.5 Hz), 4.39 (H-2, t, J = 4.5 Hz) | ND | cis-1,2-Dihydroxy-1,2-dihydronaphthalene |
| Anthracene | 7.45–7.98 (H-5,6,7,8,9,10), 6.73 (H-4, d, J = 9.8 Hz), 6.15 (H-3, dd, J = 9.8 Hz, 4.0 Hz), 4.87 (H-1, d, J = 4.0 Hz), 4.46 (H-2, t, J = 4.0 Hz) | ND | cis-1,2-Dihydroxy-1,2-dihydroanthracene |
| Fluoranthene | 7.70–7.73 (H-7,10), 7.60 (H-6, d, J = 7.1 Hz), 7.40–7.42 (H-4,5,8), 7.32 (H-9, t, J = 7.0 Hz), 6.65 (H-1, d, J = 4.8 Hz), 4.98 (H-3, d, J = 4.8 Hz), 4.79 (H-2, t, J = 4.8 Hz) | 141.9 (C), 138.6 (C), 136.9 (C), 135.9 (C), 134.3 (C), 132.8 (C), 129.7 (C-5), 129.3 (C-8), 127.3 (C-9), 125.1 (C-4), 122.4 (C-10), 122.1 (C-1), 120.9 (C-7), 70.1 (C-2), 69.5 (C-3) | cis-2,3-Dihydroxy-2,3-dihydrofluoranthene |
| 8.11 (H-4 or 6, d, J = 6.5 Hz), 7.95 (H-1, d, J = 7.0 Hz), 7.86 (H-3, d, J = 8.5 Hz), 7.82 (H-4 or 6, d, J = 8.5 Hz), 7.62–7.66 (H-2,5), 7.56 (H-10, d, J = 8.0 Hz), 7.26 (H-9, t, J = 8.0 Hz), 6.81 (H-8, d, J = 8.0 Hz) | ND | 7-Hydroxyfluoranthene | |
| Biphenyl | 7.55 (H-2′,6′, d, J = 4.0 Hz), 7.37 (H-3′,5′, t, J = 4.0 Hz), 7.30 (H-4′, t, J = 4.0 Hz), 6.37 (H-6, d, J = 5.5 Hz), 6.11 (H-5, m), 5.90 (H-4, d, J = 9.5 Hz), 4.61 (H-3, m), 4.51 (H-2, d, J = 6.0 Hz) | ND | cis-2,3-Dihydroxy-2,3-dihydrobiphenyl |
Determined at 500 MHz (1H NMR) or 125 MHz (13C NMR) in CDCl3. Chemical shift multiplicities are abbreviated as follows: s, singlet; d, doublet; t, triplet; m, multiplet; dd, doublet of doublets. (C), 13C chemical shifts of quaternary carbons; ND, not determined.
FIG. 2.
Oxidation reaction catalyzed by CARDO from Pseudomonas sp. strain CA10 and identified by GC-MS or NMR analysis for xanthene (A), phenoxathiin (B), dibenzothiophene (C), naphthalene (D), anthracene (E), fluorene (F), fluoranthene (G), and biphenyl (H). The compounds shown in brackets are the hypothetical products generated by CARDO. Monohydroxylated compounds were also identified in the biotransformation experiments with dibenzothiophene, naphthalene, anthracene, fluorene, fluoranthene, and biphenyl. The presence of cis-7,8-dihydroxy-7,8-dihydrofluoranthene and fluorene dihydrodiols was presumed because of the identification of 7-hydroxyfluoranthene and monohydroxyfluorenes. Absolute stereochemistry is not intended.
Phenoxathiin.
Phenoxathiin was converted to one major product (Table 1). In the GC-MS analysis of its TMS derivative, the molecular ion peak was observed at m/z 450 (Table 1). The molecular weight was in accordance with that of a heterocyclic ring-cleaved derivative of phenoxathiin with trimethylsilylation at three positions. Therefore, oxygenation probably occurred at an angular position relative to the oxygen or the sulfur atom to give 2,2′,3-trihydroxydiphenyl sulfide or 2,3-dihydroxy-2′-mercaptodiphenyl ether. Eventually, the structure of the compound was determined to be 2,2′,3-trihydroxydiphenyl sulfide based on an analysis of the 1H and 13C NMR spectra (Table 2) and the 1H-1H COSY spectrum (data not shown). This result indicates that CARDO attacks at an angular position adjacent to the oxygen atom and that the resultant dihydroxylated intermediate is spontaneously converted to 2,2′,3-trihydroxydiphenyl sulfide (Fig. 2B).
Dibenzothiophene.
Dibenzothiophene was mainly converted to a compound having an Rf of 0.40 on analytical TLC (Table 1). In the GC-MS analysis, the retention time (Rt) and the full-scan mass spectrum of this compound were identical to those of authentic dibenzothiophene-5-oxide (Table 1). In addition, 1H NMR data for this compound were also identical to those of authentic dibenzothiophene-5-oxide and those reported by Resnick and Gibson (22) (Table 2). Thus, the major product of the oxidation of dibenzothiophene by CARDO was identified as dibenzothiophene-5-oxide (Fig. 2C).
The GC-MS analytical data implied the presence of dibenzothiophene dihydrodiol and monohydroxydibenzothiophene (Table 1). Although the amounts of these compounds were too small for further structural analysis, these results suggest that CARDO can also catalyze cis dihydroxylation at benzylic carbon atoms of dibenzothiophene, because it is well-known that dihydrodiols of aromatic compounds easily undergo the specific loss of water to yield monohydroxylated aromatic compounds.
Naphthalene.
Naphthalene was mainly converted to a product having an Rf of 0.33 on analytical TLC (Table 1). The GC-MS data for its TMS derivative indicated that this product was a naphthalene dihydrodiol (Table 1), and its 1H NMR data (Table 2) were identical to those of cis-1,2-dihydroxy-1,2-dihydronaphthalene reported by Jerina et al. (12). Thus, the results indicated that naphthalene was converted to cis-1,2-dihydroxy-1,2-dihydronaphthalene by CARDO (Fig. 2D). This oxygenation reaction was similarly catalyzed by many naphthalene dioxygenases (4).
On the other hand, a small amount of 1-naphthol was also identified by GC-MS analysis of the reaction mixture (Table 1). Because the yield of 1-naphthol increased when extraction with ethyl acetate was conducted under acidic conditions (pH 2 to 3) (data not shown), 1-naphthol was considered to be derived from cis-1,2-dihydroxy-1,2-dihydronaphthalene.
Anthracene.
Anthracene was mainly converted by CARDO to a compound having an Rf of 0.30 on analytical TLC (Table 1). This compound was identified as cis-1,2-dihydroxy-1,2-dihydroanthracene (Fig. 2E) by comparison of its 1H NMR spectrum (Table 2) with that reported by Jerina et al. (13). A compound whose molecular ion peak was observed at m/z 266 in the GC-MS analysis was suggested to be monohydroxyanthracene (Table 1). Considering that 1-naphthol was detected as shown above, this compound was considered to be 1- or 2-hydroxyanthracene derived from cis-1,2-dihydroxy-1,2-dihydroanthracene.
Phenanthrene.
Based on the GC-MS analysis, phenanthrene was considered to be converted to three phenanthrene dihydrodiols by CARDO (Table 1), suggesting that CARDO attacks at distinct positions of phenanthrene. Small amounts of three monohydroxyphenanthrenes which were considered to be formed by the dehydration of the dihydrodiols were also detected in the GC-MS analysis (Table 1).
Fluorene.
In the GC-MS analysis, 9-hydroxyfluorene was identified as an oxidation product of fluorene (Table 1), suggesting that CARDO can catalyze the monooxygenation of the benzylic methylenic group of fluorene. In addition to 9-hydroxyfluorene, 2-hydroxyfluorene was identified as a compound formed in the biotransformation experiment by comparison of the mass spectrum and Rt of the TMS derivative with those of authentic 2-hydroxyfluorene (Table 1). As shown in Table 1, we also detected three compounds whose mass spectra were similar to that of the TMS derivative of authentic 2-hydroxyfluorene. Therefore, these three compounds were suggested to be 1-, 3-, and 4-hydroxyfluorene. These monohydroxyfluorenes were considered to be derived from the dihydrodiols formed by CARDO, as in the case of naphthalene, although we could not detect the dihydrodiols formed by CARDO.
Fluoranthene.
The conversion rate for fluoranthene was lower than those for the other aromatic compounds. In the TLC analysis, we detected two compounds (Table 1). The compound having an Rf of 0.36 was identified as cis-2,3-dihydroxy-2,3-dihydrofluoranthene (Fig. 2G) based on the results of 1H-1H COSY, HMQC, HMBC, and NOESY experiments (data not shown), allowing the assignment of the 1H and 13C NMR chemical shifts shown in Table 2.
On the other hand, the compound having an Rf of 0.65 was identified as monohydroxyfluoranthene by the GC-MS analysis (Table 1). Based on the results of 1H-1H COSY and NOESY experiments (data not shown), this compound was identified as 7-hydroxyfluoranthene (Fig. 2G), allowing the assignment of the 1H NMR chemical shifts shown in Table 2. Although this compound was considered to be formed by the dehydration of cis-7,8-dihydroxy-7,8-dihydrofluoranthene, this cis-dihydrodiol was not detected in the GC-MS analysis (data not shown). cis Dihydroxylation at the 7 and 8 positions of fluoranthene was also reported as the initial oxygenation reaction for fluoranthene with Mycobacterium sp. strain PYR-1 (15, 16).
Biphenyl.
Based on the GC-MS data (Table 1) and 1H NMR chemical shifts (Table 2), cis-2,3-dihydroxy-2,3-dihydrobiphenyl was identified as a product in the biotransformation of biphenyl by CARDO (Fig. 2H). This cis dihydroxylation was the same initial oxygenation reaction catalyzed by the well-investigated biphenyl dioxygenase cloned from biphenyl- and polychlorinated biphenyl-utilizing bacteria (6, 10). Small amounts of 2- and 3-hydroxybiphenyl were also identified in the reaction mixture by the GC-MS analysis (Table 1). These monohydroxybiphenyls were considered to be derived by the dehydration of cis-2,3-dihydroxy-2,3-dihydrobiphenyl.
DISCUSSION
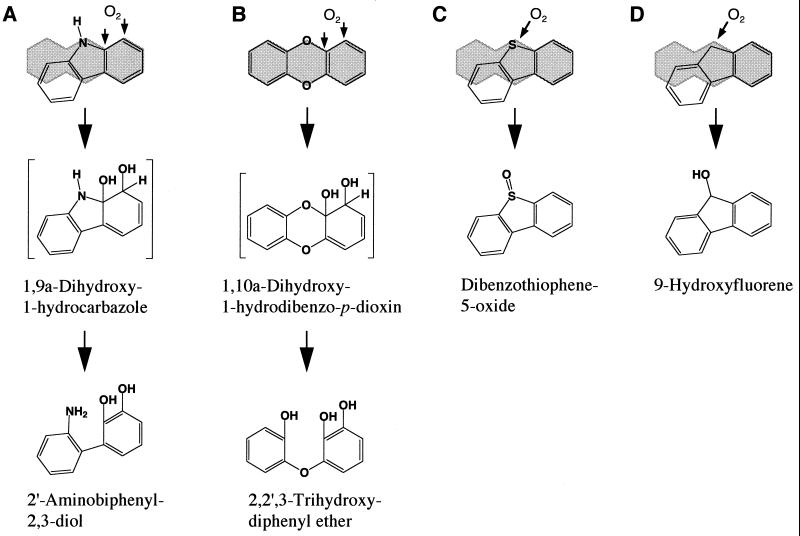
The angular dioxygenation catalyzed by CARDO occurred at the angular position adjacent to an oxygen or nitrogen atom but not to a sulfur or carbon atom (Fig. 1 and 2A, B, C, and F). Dibenzofuran 4,4a-dioxygenase, which was purified from Sphingomonas sp. strain RW1, was reported to catalyze the angular dioxygenation of DBF and DD but not of CAR (3). This angular dioxygenase was also presumed to catalyze the angular dioxygenation of 9-fluorenone to yield 1,9a-dihydroxy-1-hydrofluoren-9-one (3). In addition, no dibenzofuran 4,4a-dioxygenase oxygenation product of PAHs, such as naphthalene, has been detected (3). Up to now, 9-fluorenone angular dioxygenases have been reported for Terrabacter sp. strain DBF63 (14, 18) (strain DBF63 was formerly identified as Staphylococcus auriculans), Pseudomonas sp. strain F274 (8, 28) and Brevibacterium sp. strain DPO1361 (5). It was also reported that Pseudomonas sp. strain F274 can catalyze the angular dioxygenation of DBF but not of CAR, although this transformation was seen with whole cells (8, 9). When washed cells of E. coli JM109(pUCARA) were incubated with 9-fluorenone, no oxidation product was detected (data not shown). Therefore, we concluded that CARDO cannot oxidize the angular and its adjacent positions (9a and 1 positions) of 9-fluorenone, although the possibility that 9-fluorenone was transported into E. coli cells cannot be excluded. The above results indicate that the substrate specificity of CARDO is different from those of dibenzofuran 4,4a-dioxygenase from strain RW1 and the angular dioxygenase from strain F247 and that the amino acid residues involved in substrate recognition are probably different among these angular dioxygenases.
Monohydroxylated compounds were also detected as minor oxidized compounds formed during the biotransformation of dibenzothiophene, PAHs, and biphenyl. Considering the fact that the amounts of monohydroxylated compounds were increased when extraction from the reaction mixture by use of ethyl acetate was conducted under acidic conditions (pH 2 to 3) (data not shown), these monohydroxylated compounds were considered to be formed by nonenzymatic dehydration of the corresponding cis-dihydrodiols produced by CARDO.
In addition to dioxygenation, CARDO also catalyzed the sulfoxidation of dibenzothiophene to dibenzothiophene-5-oxide as the dominant reaction (Table 1 and Fig. 2C). A similar sulfoxidation of dibenzothiophene by naphthalene 1,2-dioxygenase from Pseudomonas sp. strain NCIB 9816-4 (22) was reported, although the dominant reaction was cis dihydroxylation of dibenzothiophene at 1,2 positions (the yield of sulfoxidation was 9 to 14%). On the other hand, dibenzothiophene was presumed to be converted to dibenzothiophene-5-oxide and dibenzothiophene-5,5-dioxide by dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1 (3). Similarly, Pseudomonas sp. strain F247 was reported to catalyze the sulfoxidation of dibenzothiophene to dibenzothiophene-5-oxide and dibenzothiophene-5,5-dioxide, although this transformation was seen with whole cells (9). It was also revealed that CARDO can convert fluorene to 9-hydroxyfluorene, although the yield of this product was low (5 to 10%). This result suggests that CARDO is also able to catalyze the monooxygenation of the benzylic methylenic group of fluorene. In a study by Bünz and Cook (3), no dibenzofuran 4,4a-dioxygenase oxygenation product of fluorene was detected. While the monooxygenation of fluorene to 9-hydroxyfluorene by Pseudomonas sp. strain F247 was reported (9), there has been no evidence that the angular dioxygenase from strain F247 catalyzes monooxygenation. Thus, CARDO is the first angular dioxygenase which can catalyze the monooxygenation of the benzylic methylenic group of fluorene. Considering that the benzylic methylenic group of xanthene was not hydroxylated by E. coli JM109(pUCARA) and that sulfoxidation did not occur for phenoxathiin, CARDO catalyzes angular dioxygenation rather than monooxygenation for xanthene and phenoxathiin.
It was reported that naphthalene 1,2-dioxygenase from Pseudomonas sp. strain NCIB 9816-4 catalyzed the cis dihydroxylation of fluorene (3 and 4 positions), DBF (1 and 2 positions), and dibenzothiophene (1 and 2 positions) as the dominant reactions (22) and that this broad-substrate-range dioxygenase was not able to catalyze the angular dioxygenation of CAR and DBF (22, 23). These results indicate that molecular dioxygen mainly attacks at the opposite side of the methylenic carbon atom of fluorene or the heteroatoms of DBF and dibenzothiophene when naphthalene 1,2-dioxygenase oxidizes these compounds. In contrast, CARDO attacks at the same side of the heteroatoms of CAR and DBF when it oxidizes these compounds (Fig. 1 and 3A). When CARDO oxygenates dibenzothiophene and fluorene, it also mainly attacks at the same side of the sulfur atom of dibenzothiophene and the methylenic carbon atom of fluorene (Fig. 3C and D). These results indicate that CARDO recognizes the common structures of CAR analogues and that the type of oxygenation catalyzed by CARDO is affected by heteroatoms (CAR, DBF, and dibenzothiophene) or the methylenic carbon atom (fluorene). It is quite possible that CAR analogues fit the substrate binding site of CARDO differently and that the diverse reactions catalyzed by CARDO for CAR analogues were dependent upon the relative reactivities of heteroatoms, the methylenic carbon atom, and the vicinal aromatic carbon atoms after the binding of substrates to CARDO.
FIG. 3.
Proposed initial attack on CAR (A) and the CAR analogues DD (B), dibenzothiophene (C), and fluorene (D) by CARDO from Pseudomonas sp. strain CA10. The small arrows indicate the positions of the enzymatic incorporation of oxygen. The structures shown in brackets are unstable intermediates that have not been characterized. Absolute stereochemistry is not intended. The shaded areas represent the structure of anthracene or DD.
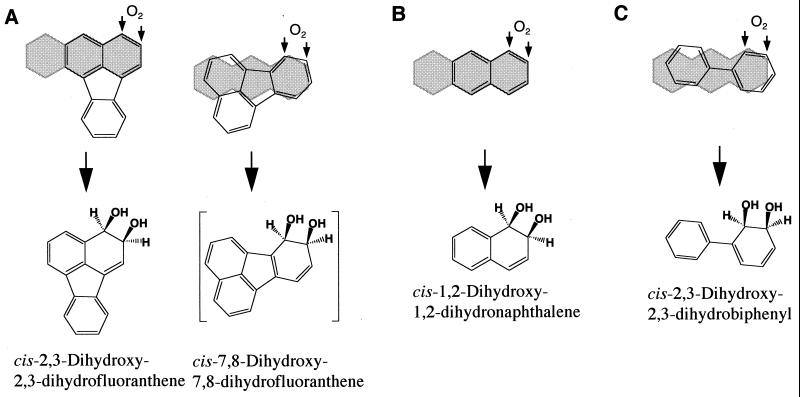
When CARDO catalyzes the cis dihydroxylation of PAHs and biphenyl, the substrates are considered to bind loosely to the reaction center of CARDO. In fact, fluoranthene was dihydroxylated at two distinct positions (Fig. 4A), because 7-hydroxyfluoranthene was produced via the dehydration of cis-7,8-dihydrodiol. This result suggests that fluoranthene can approach the reaction center of CARDO in at least two different ways. The diverse cis dihydroxylation of PAHs and biphenyl catalyzed by CARDO might reflect differences in the binding of the substrates to the active site. The hypothetical positions of cis dihydroxylation for PAHs and biphenyl (Fig. 4) do not overlap with the angular positions of CAR and DD (Fig. 3A and B), suggesting that the binding of the PAHs or biphenyl to the active site of CARDO in cis dihydroxylation is different from that of CAR or DD.
FIG. 4.
Proposed initial attack on fluoranthene (A), naphthalene (B), and biphenyl (C) by CARDO from Pseudomonas sp. strain CA10. The small arrows indicate the positions of the enzymatic incorporation of oxygen. The structures shown in brackets are the hypothetical products of cis hydroxylation by CARDO that have not been characterized. Absolute stereochemistry is not intended. The shaded areas represent the structure of anthracene or DD.
Although, to date, the occurrence of many aromatic compound dioxygenases has been reported, CARDO is the only dioxygenase which can catalyze cis dihydroxylation, monooxygenation, and angular dioxygenation, as described above. Therefore, it will be quite interesting to clarify the similarities and differences between the three-dimensional structures of substrate binding sites and reaction centers of CARDO and those of other dioxygenases. We are currently carrying out the purification, crystallization, and determination of the three-dimensional structure of CarAa (the catalytic component of CARDO). The three-dimensional structure of CarAa will give us valuable information on the amino acid residues involved in substrate recognition by CARDO and on the mechanisms of angular dioxygenation, cis dihydroxylation, and monooxygenation.
ACKNOWLEDGMENT
This work was partly supported by the New Energy and Industrial Technology Development Organization, Tokyo, Japan (Project ID 8B-090-1).
REFERENCES
- 1.Allen C C R, Boyd D R, Dalton H, Sharma N D, Haughey S A, McMordie R A S, McMurray B T, Sheldrake G N, Sproule K. Sulfoxides of high enantiopurity from bacterial oxygenase-catalyzed oxidation. J Chem Soc Chem Commun. 1995;1995:119–120. [Google Scholar]
- 2.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bünz P V, Cook A M. Dibenzofuran 4,4a-dioxygenase from Sphingomonas sp. strain RW1: angular dioxygenation by a three-component enzyme system. J Bacteriol. 1993;175:6467–6475. doi: 10.1128/jb.175.20.6467-6475.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerniglia C E. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation. 1992;3:351–368. [Google Scholar]
- 5.Engesser K H, Strubel V, Christoglou K, Fischer P, Rast H G. Dioxygenolytic cleavage of aryl ether bonds: 1,10-dihydro-1,10-dihydroxyfluorene-9-one, a novel arene dihydrodiol as evidence for angular dioxygenation of dibenzofuran. FEMS Microbiol Lett. 1989;65:205–210. doi: 10.1016/0378-1097(89)90392-3. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa K, Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986;166:392–398. doi: 10.1128/jb.166.2.392-398.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson D T, Resnick S M, Lee K, Brand J M, Torok D S, Wackett L P, Schocken M J, Haigler B E. Desaturation, dioxygenation, and monooxygenation reactions catalyzed by naphthalene dioxygenase from Pseudomonas sp. strain 9816-4. J Bacteriol. 1995;177:2615–2621. doi: 10.1128/jb.177.10.2615-2621.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoll M, Selifonov S A, Chapman P J. Evidence for a novel pathway in the degradation of fluorene by Pseudomonas sp. strain F274. Appl Environ Microbiol. 1994;60:2437–2449. doi: 10.1128/aem.60.7.2438-2449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grifoll M, Selifonov S A, Chapman P J. Transformation of substituted fluorenes and fluorene analogs by Pseudomonas sp. strain F274. Appl Environ Microbiol. 1995;61:3490–3493. doi: 10.1128/aem.61.9.3490-3493.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haddock J D, Nadim L M, Gibson D T. Oxidation of biphenyl by a multicomponent enzyme system from Pseudomonas sp. strain LB400. J Bacteriol. 1993;175:395–400. doi: 10.1128/jb.175.2.395-400.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 12.Jerina D M, Daly J W, Jeffrey A M, Gibson D T. cis-1,2-Dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971;142:394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- 13.Jerina D M, Selander H, Yagi H, Wells M C, Davey J F, Mahadevan V, Gibson D T. Dihydrodiols from anthracene and phenanthrene. J Am Chem Soc. 1976;98:5988–5996. doi: 10.1021/ja00435a035. [DOI] [PubMed] [Google Scholar]
- 14.Kasuga K, Nojiri H, Yamane H, Kodama T, Omori T. Cloning and characterization of the genes involved in the degradation of dibenzofuran by Terrabacter sp. strain DBF63. J Ferment Bioeng. 1997;84:387–399. [Google Scholar]
- 15.Kelley I, Cerniglia C E. The metabolism of fluoranthene by a species of Mycobacterium. J Ind Microbiol. 1991;7:19–26. [Google Scholar]
- 16.Kelley I, Freeman J P, Evans F E, Cerniglia C E. Identification of a carboxylic acid metabolite from the catabolism of fluoranthene by a Mycobacterium sp. Appl Environ Microbiol. 1991;57:636–641. doi: 10.1128/aem.57.3.636-641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K, Brand J M, Gibson D T. Stereospecific sulfoxidation by toluene and naphthalene dioxygenase. Biochem Biophys Res Commun. 1995;212:9–15. doi: 10.1006/bbrc.1995.1928. [DOI] [PubMed] [Google Scholar]
- 18.Monna L, Omori T, Kodama T. Microbial degradation of dibenzofuran, fluorene, and dibenzo-p-dioxin by Staphylococcus auriculans DBF63. Appl Environ Microbiol. 1993;59:285–289. doi: 10.1128/aem.59.1.285-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouchiyama N, Zhang Y, Omori T, Kodama T. Biodegradation of carbazole by Pseudomonas spp. CA06 and CA10. Biosci Biotechnol Biochem. 1993;57:455–460. [Google Scholar]
- 20.Resnick S M, Gibson D T. Biotransformation of anisole and phenetole by aerobic hydrocarbon-oxidizing bacteria. Biodegradation. 1993;4:195–203. [Google Scholar]
- 21.Resnick S M, Gibson D T. Regio- and stereospecific oxidation of 9,10-dihydroanthracene and 9,10-dihydrophenanthrene by naphthalene dioxygenase: structure and absolute stereochemistry of metabolites. Appl Environ Microbiol. 1996;62:3355–3359. doi: 10.1128/aem.62.9.3355-3359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Resnick S M, Gibson D T. Regio- and stereospecific oxidation of fluorene, dibenzofuran, and dibenzothiophene by naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816-4. Appl Environ Microbiol. 1996;62:4073–4080. doi: 10.1128/aem.62.11.4073-4080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Resnick S M, Torok D S, Gibson D T. Oxidation of carbazole to 3-hydroxycarbazole by naphthalene 1,2-dioxygenase and biphenyl 2,3-dioxygenase. FEMS Microbiol Lett. 1993;113:297–302. doi: 10.1111/j.1574-6968.1993.tb06530.x. [DOI] [PubMed] [Google Scholar]
- 24.Resnick S M, Torok D S, Lee K, Brand J M, Gibson D T. Regiospecific and stereoselective hydrogenation of 1-indanone and 2-indanone by naphthalene dioxygenase and toluene dioxygenase. Appl Environ Microbiol. 1994;60:3323–3328. doi: 10.1128/aem.60.9.3323-3328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Sato S, Nam J-W, Kasuga K, Nojiri H, Yamane H, Omori T. Identification and characterization of genes encoding carbazole 1,9a-dioxygenase in Pseudomonas sp. strain CA10. J Bacteriol. 1997;179:4850–4858. doi: 10.1128/jb.179.15.4850-4858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato S, Ouchiyama N, Kimura T, Nojiri H, Yamane H, Omori T. Cloning of genes involved in carbazole degradation of Pseudomonas sp. strain CA10: nucleotide sequences of genes and characterization of meta-cleavage enzymes and hydrolase. J Bacteriol. 1997;179:4841–4849. doi: 10.1128/jb.179.15.4841-4849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selifonov S A, Grifoll M, Gurst J E, Chapman P J. Isolation and characterization of (+)-1,1a-dihydroxy 1-hydrofluorene-9-one formed by angular dioxygenation in the bacterial catabolism of fluorene. Biochem Biophys Res Commun. 1993;193:67–76. doi: 10.1006/bbrc.1993.1591. [DOI] [PubMed] [Google Scholar]
- 29.Torok D S, Resnick S M, Brand J M, Cruden D L, Gibson D T. Desaturation and oxygenation reactions of 1,2-dihydronaphthalene by toluene and naphthalene dioxygenase. J Bacteriol. 1995;177:5799–5805. doi: 10.1128/jb.177.20.5799-5805.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wackett L P, Kwart L D, Gibson D T. Benzylic monooxygenation catalyzed by toluene dioxygenase from Pseudomonas putida. Biochemistry. 1988;27:1360–1367. doi: 10.1021/bi00404a041. [DOI] [PubMed] [Google Scholar]