Abstract
Objectives: To compare the erosion protection efficacy of a stabilised, stannous fluoride (SnF2) dentifrice versus a sodium fluoride (NaF) dentifrice using a modified in situ clinical model. Methods: This study, a randomised parallel group in situ design with in vivo product use and ex vivo acid challenge, compared: A, a dentifrice containing 1,450 ppm F as NaF; B, a dentifrice containing 1,450 ppm F (1,100 ppm F as SnF2 + 350 ppm F as NaF); and T, tap water. Sample size was n = 4 per group (total of 12 subjects) and within each subject appliances were placed on each side of the mouth (left and right). Enamel specimens were placed in different positions of the mouth (front, mid-front, mid-rear, rear) in each appliance (total = 8 specimens per subject). Product treatment was twice per day (lingual brushing for 30 seconds followed by swishing for 90 seconds with the resultant product/saliva slurry) in vivo for 15 days, and ex vivo acid treatment (0.02 m citric acid 5 minutes four times per day; total exposure time = 300 minutes). Data were analysed using a general linear repeated measures model with treatment, side and position as fixed effects. Within subjects, correlations were modelled assuming a different correlation and variance for treatment B relative to the other groups. Pairwise treatment differences were performed using a 5% two-sided significance level. Results: Enamel loss (in μm) was significantly lower (P < 0.005) for treatment B versus treatments A and T. Treatment B reduced enamel surface loss by 86.9% relative to treatment A. There was no statistical difference in mean enamel loss (P = 0.51) between treatments A and T. Enamel loss was not statistically different for side (left vs. right; P = 0.44) or position (front, mid-front, mid-rear, rear; P = 0.36). Conclusion: This modified in situ erosion model confirmed the enhanced erosion protection benefits of a stabilised SnF2 dentifrice versus a conventional NaF dentifrice, validating the ability of the model to safely and effectively demonstrate differences in the erosion protection potential of oral care products.
Key words: Erosion, dentifrice, stannous, in situ clinical
INTRODUCTION
It is clear that dentifrices play an important role in the maintenance of oral health. In addition to aiding in the prevention of caries1, dentifrices have been demonstrated to provide additional benefits such as plaque/gingivitis control2, hypersensitivity reduction3, calculus prevention4, bad breath reduction5 and whitening enhancement6. A more recent benefit provided by some dentifrices is protection against the initiation and progression of dental erosion7.
Dental erosion results from aggressive acid challenges to exposed tooth surfaces that overwhelm the natural protective pellicle barrier, softening the surface enamel and making it highly susceptible to abrasive forces present in the mouth. Significant increases in the consumption of acid-containing food and drink over the past several decades are often cited as the primary cause for the reported increases in dental erosion globally8.
Similar to caries, one of the initial steps in the dental erosion process is a softening of the enamel. In the caries process, however, this softening generally occurs under plaque-covered surfaces, which provide a certain level of protection to the tooth surface. Acids penetrate through the natural tooth surface and damage generally occurs underneath the surface, with the outer layer of the tooth surface remaining structurally intact9. In the case of dental erosion, softening of the enamel occurs on exposed tooth surfaces that are not plaque covered. Unfortunately, this softened mineral can be easily lost as a result of abrasion, with abrasion being the result of a number of factors, such as brushing, eating, abfraction, contact with the tongue, etc10., 11., 12..
Unlike caries, which is a reversible process (at least during the early stages of initiation), dental erosion is generally considered to be irreversible from its earliest stages in vivo13. Dental erosion occurs as a result of a higher level of acid challenge compared with cariogenic acid attack. Whereas caries acids attack tooth enamel with a low concentration of acids over a prolonged period of time, dental erosion is the result of a higher level of acid attack for shorter periods of time, overwhelming the natural protective pellicle and aggressively challenging exposed tooth surfaces. Tooth surfaces that have softened as a result of the erosive acid challenge have a low likelihood of rehardening in vivo12. Once softened, these surfaces become more susceptible to abrasive tooth surface loss. This is the primary reason that dental erosion is generally considered to be an irreversible process.
It is possible to develop models that simulate the process of dental erosion, enabling the evaluation of products designed to protect against this growing problem. Many of these studies have focused on the ability of fluoride to help protect against erosive tooth surface loss, as fluoride is well known for its ability to help strengthen enamel14., 15.. Despite fluoride’s proven ability to strengthen enamel against caries, there is considerable evidence to support that there is a significant difference among the various fluoride sources most commonly used in over-the-counter dentifrices with respect to their ability to protect tooth surfaces against erosive acid damage. There is growing acceptance in the literature that the most effective agent used in over-the-counter dentifrices to aid in the prevention of dental erosion is stannous fluoride (SnF2) 16.
The aim of the present study was to assess the relative erosion protection potential of two marketed dentifrices, both of which contain a total of 1,450 ppm F, using a modification of a previously published (2007) human in situ erosion prevention clinical model7. In addition, this study, a pilot study, was intended to provide outcomes that could be used to help size future studies using this model design. In the present study, one product was formulated with 1,450 ppm F as NaF, and the other contained a combination of 1,100 ppm F as SnF2 + 350 ppm F as NaF. The 2007 study7, which demonstrated enhanced protective benefits for a stabilised SnF2 dentifrice, was conducted with dentifrice containing 1,100 ppm F as SnF2. Of interest in the present study was how the stabilised SnF2 dentifrice would perform using a modified version of the 2007 model, enabling an assessment of both the dentifrice formulation and the robustness of the modified model. This modified version of the model incorporates a 30-second lingual brushing of teeth to effect in vivo dilution of the test products with naturally stimulated saliva, followed by swishing of the products in the mouth for an additional 90 seconds. In the original model, subjects swished with a pre-prepared, 1:3 dilution of dentifrice:water for treatment. In addition, this modified model also incorporated the use of ex-vivo acid treatments, ensuring not only a consistent level of challenge to all study samples but also eliminating the potential for excessive acid exposure of the subject’s natural teeth.
MATERIALS AND METHODS
A blinded, two-treatment/one water control parallel group study using in situ devices was conducted. Subjects were asked to read and sign an informed consent before their inclusion in the study. Ethical approval for the study was granted from the Freiburger Ethik-Kommission International, Freiberg, Germany and all participants gave oral and written consent to participate. The study was conducted according to standards of good clinical practice.
Generalised study protocol
Subjects, who were randomly assigned to one of three treatment groups, wore two in situ devices that retained eight human enamel samples (four per side) during the study periods (Figure 1). Tooth samples were obtained following ethical approval; all samples were unerupted third molars, which minimised the potential for any fluoride exposure before the study, from individuals over 18 years of age. Teeth were sterilised, sectioned and polished to expose a smooth enamel surface, masked to expose a 2–3 mm zone of enamel and placed in the intraoral appliances (Figure 2). Subjects wore the appliances for approximately 6 hours/day, excluding 1 hour for lunch. No food or drink could be consumed while the appliances were in the mouth, with the exception of water. Overnight, during the 1-hour lunch period and at weekends, the appliances retaining the samples were kept moistened in a closed container to prevent dehydration of the enamel.
Figure 1.
Colour-coded in situ devices containing four enamel specimens each.
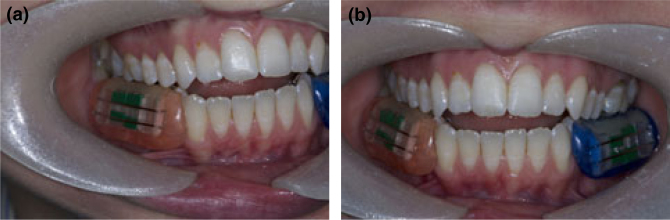
Figure 2.
Colour-coded in situ devices are fitted onto buccal positions in each subject.
Immediately before the start of the treatment phase of the study, there was a 7-day period of acclimatisation using standard dentifrice products (manual toothbrush and fluoride dentifrice: Table 1). Subjects continued to use the standard products at home, both morning and evening, for the duration of the study to maintain their own personal hygiene. The in situ devices were not present in the mouth when these products were used.
Table 1.
Treatment codes, with product and usage procedures followed
| Treatment | Product descriptions and usage procedures |
|---|---|
| B | Oral-B® Pro-Expert All Around Protection dentifrice (UK version, 0.454% SnF2 and 350 ppm F from NaF) and Oral-B® Indicator 35 medium manual toothbrush. Subjects brushed lingually with a full brushhead of toothpaste for 30 seconds and then gently swished the generated slurry around the in situ devices for a further 90 seconds. Subjects rinsed twice daily (09:00 hours and 13:00 hours) with 10 ml of tap water for 10 seconds |
| A | Crest® Decay Prevention dentifrice (UK version, 1,450 ppm F as NaF) and Oral-B® Indicator 35 medium manual toothbrush. Subjects brushed lingually with full brushhead of toothpaste for 30 seconds, and then gently swished the generated slurry around the in situ devices for a further 90 seconds. Subjects rinsed twice daily (09:00 hours and 13:00 hours) with 10 ml of tap water for 10 seconds |
| T | Subjects brushed lingually with tap water only for 30 seconds, and then gently swished 10 ml of tap water around the in situ devices for a further 90 seconds. This was followed by a final rinse with twice daily (09:00 hours and 13:00 hours) rinsing with 10 ml of tap water for 10 seconds |
| Standard products | Crest® Decay Prevention dentifrice (UK version, 1,450 ppm F as NaF) and Oral-B Indicator 35 medium manual toothbrush, used morning and evening ad libitum for personal oral hygiene. The in situ devices were not worn during periods when personal oral hygiene was conducted |
The treatment phase of the study lasted 15 days (three working weeks). Subjects wore the in situ devices for approximately 6 hours (from 07.30 hours ±30 minutes to 15.30 hours ±30 minutes) Monday to Friday, removing the devices for 1 hour at lunchtime while they ate. No eating was allowed while the devices were in place, and only a restricted list of beverages (tea, coffee) could be consumed when the devices were temporarily allowed to be removed during the morning and afternoon to allow consumption of the beverages.
The in situ devices were sterilised in 0.2% chlorhexidine gluconate (Corsodyl; GlaxoSmithKline Consumer Healthcare, Brentford, UK) by the study coordinator on the day before first use, each evening at least 30 minutes after the final erosive acid challenge of the day and each Friday evening. After sterilisation, the devices were immediately placed in a sealed, moist container for the subject to take home. In situ devices were reinserted in the mouth (at home) after breakfast the next morning (or on Monday after the weekend break). Each morning, subjects reported to the laboratory upon arrival and the treatment paste (or water) was then applied, in vivo, following the specified protocol (Table 1). A second treatment occurred each day during the lunch break. All product treatments were conducted in vivo, while acid treatments were done ex vivo to protect the subject’s own teeth from potential acid damage.
At least 60 minutes after treatment, subjects removed their in situ devices before exposure to 0.02 m citric acid for 5 minutes in a disposable cup, without agitation at room temperature (approximately 21°C). After a water rinse, the devices were reinserted. A further three citric acid challenges occurred throughout each treatment day. Treatment with product and citric acid challenges occurred each weekday for 15 days. There were no treatments, no wearing of the devices and no citric acid challenges overnight or at the weekends.
Surface topography and efficacy measurements
Enamel surface topography measurements were only conducted after the treatment. Baseline measurements were not taken. Previous clinical work has shown that baseline measurements are not useful as a covariate in the final statistical model17. Blinded measurements were made using a Mitutoyo Surftest SV-2000, contact profilometer (Mitutoyo Instruments, Andover, UK); this is a contact profilometer with diamond stylus. For each sample, two readings across the eroded gap were averaged.
Inclusion criteria
Key inclusion criteria in this study, which was limited to company employees age 18 years or older, consisted of a signed, informed consent document, the ability to understand and comply with directions, good general oral health and the ability to accommodate the lower, bilateral buccal intraoral in situ devices.
Exclusion criteria
Key exclusion criteria for this study included subjects who were pregnant or lactating, had any disease that may affect assessments, susceptibility to acid regurgitation, orthodontic devices, restorations, bridgework or dentures that would interfere with study evaluations, excessive gingival inflammation or periodontal disease, or significant signs of dental erosion.
Other key study requirements
Subjects were requested to refrain from taking any acidic medication (pH < 5.3), antacids or vitamin C preparations during study treatment days.
Recruitment
Employees who expressed an interest in participating in this particular study were seen by study dentists to have their medical history recorded, an oral soft tissue examination and inclusion/exclusion criteria checked. Once deemed suitable, potential volunteers were asked to read and sign the informed consent document. Maxillary and mandibular impressions were taken to enable manufacture of the in situ devices by a qualified dental hospital (Bristol Dental Hospital, Bristol, UK).
Preparation of the lower buccal in situ devices
All devices and enamel samples were supplied by the Bristol Dental Hospital under the supervision of Professor N. X. West, managed in compliance with the UK Human Tissue Legislation18. Lower left and right buccal in situ devices were prepared by the Bristol Dental Hospital in the Dental Laboratories. A lower alginate impression was recorded in a perforated stock tray. The impressions were then poured in dental stone and two lower buccal in situ devices (lower right and lower left) were constructed from self-curing acrylic. The in situ devices were constructed to fit suitable posterior teeth to aid retention. A channel with retaining wires was made along the buccal aspect of each in situ device to accommodate the enamel samples. The acrylic used to make the in situ devices was colour coded to distinguish the right side from the left side (Figures 1, 2a,b).
Preparation of the enamel samples
Enamel samples were prepared at the study site according to the appropriate standard operating procedure (SOP) held by the research laboratories within the Clinical Trials Unit, Bristol Dental School and Hospital. Caries-free human third molars that had been recently extracted and donated from patients aged over 18 years, of either gender, were used for the enamel samples. Before donation, each patient signed an ethically approved informed consent form, allowing their teeth to be used for research purposes. To comply with UK law, human molars were sourced through appropriately licensed Tissue Banks and were tracked and disposed of in compliance with Human Tissue Legislation. Teeth for this study were issued from the ethically approved Bristol Dental School and Hospital Tooth Tissue Bank, REC ref: 07/NIR01/20, renewal ref: 11/NI/0145.
Upon donation to the Tissue Bank, the teeth were soaked for 24 hours in a 20 g/l available chlorine solution (Haz tabs; Guest Medical, Aylesford, UK) for at least 24 hours. The teeth were scraped clean of any remaining tissue with a scalpel and the root sectioned from the crown to enable dental pulp removal and disposal, then soaked for a subsequent 24 hours in the 20 g/l available chlorine solution. The teeth were then washed in distilled water and stored in the tooth tissue bank in the chlorine solution until use.
Teeth obtained from the tissue bank were rinsed thoroughly in deionised water and sectioned into slices using a water-cooled diamond precision annular saw (Microslice; Ultratec, Santa Ana, CA, USA). The slices were then cut into samples with a high-speed hand piece and diamond bur. Each enamel sample was then placed with the test surface face-down in a polyurethane vacuum packed mould 6 × 8 × 2 mm (width, length and depth, respectively) and filled with epoxy resin. After 24 hours, when the epoxy resin had cured, the samples were removed.
Once set, the back of the sample was flattened using a stainless steel jig on a lapping and polishing unit fitted with P600 silicon carbide paper. Any resin flash remaining on the surface was then removed by the lapping and polishing machine with P1200 grit silicon carbide paper. Samples were then polished by hand using a slurry of P1200 grit silica powder on a glass slab. Hand polishing was carried out using small rotational figure-of-eight movements until the samples were deemed smooth by visual observation. Samples were then ultrasonicated in deionised water to remove any powder debris. The final stage of polishing was carried out by hand using a glass slab and slurry of 0.3 μm alpha alumina powder on a felt cloth. The samples were again polished using figure-of-eight movements until the sample surface was shiny and smooth, and then given a final ultrasonication in deionised water to remove any powder debris. Each sample was indented in the top left corner as reference points in the control area, and then masked with polyvinyl chloride (PVC) tape on either side of a 2-mm wide window of enamel. Each enamel sample was identified with a unique number on the reverse of the sample using a permanent marker.
Sterilisation of specimens
Before and after surfometry readings, enamel specimens were soaked in 0.5% chlorhexidine gluconate and 70% aqueous ethanol (Bristol Royal Infirmary Pharmacy Supplies, Bristol, UK) for a period of at least 20 minutes then stored in a closed, moist container.
Statistical analysis
The erosion measurements of each of the four enamel specimens on each side of the in situ device were analysed using a general linear repeated measures model with treatment group, side (left or right) and position (front, mid-front, mid-rear, rear) as fixed effects, and within-subject correlations were modelled assuming a different correlation and variance for treatment B relative to the other groups. Statistically modelling a different variance structure for treatment B was necessary because that group demonstrated lower variability relative to the other two treatments. Summary statistics of the enamel loss data were displayed graphically, such as averages by treatment and side for each subject (Figure 3) as well as boxplots versus subject-by-treatment of the individual sample measurements (Figure 4). Pairwise treatment differences were performed using a 5% two-sided significance level. The P-values were not adjusted for multiple comparisons, although in this study the observed conclusions described below with respect to the statistical significance level would remain the same, even using the most conservative Bonferroni approach (i.e. simply multiplying each P-value by the number of comparisons or 3 in this case).
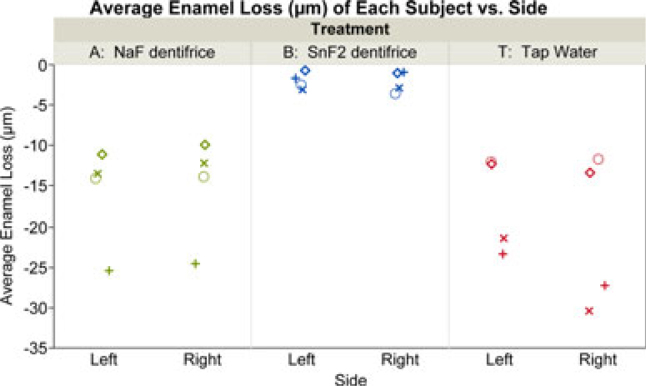
Figure 3.
Average enamel loss (μm) versus side of the subjects by treatment. Within treatment groups, the same symbols were used for subjects on the left and right sides.
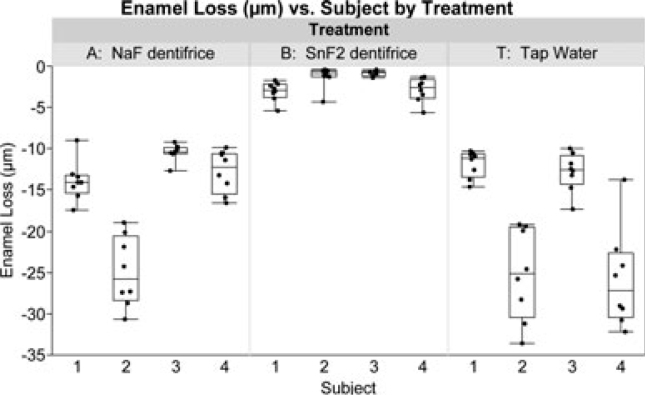
Figure 4.
Boxplots of enamel loss (μm) versus subject of the sample measurements by treatment showing both the between- and within-subject variability. In the graph, the order of the four subjects per group (n = 12 total) was arbitrary.
RESULTS
Twelve subjects were enrolled in the study, and all of them completed it. Averaging by subject and side, enamel loss for the two treatment dentifrices and the tap water control are shown in Figure 3 (for both right and left appliances). Figure 4 displays boxplots of the individual enamel loss sample measurements taken from eight locations in the mouth versus subject number by treatment, which shows both the between- and within-subject variability associated with these data. After 15 days of treatment, the marketed SnF2 dentifrice demonstrated 86.9% lower enamel loss than the NaF dentifrice (Table 2), with means of 2.03 μm (SE 0.57) and 15.53 μm (SE 3.53), respectively. The mean enamel loss for the marketed SnF2 dentifrice group was statistically significantly (P < 0.005) less than both the NaF dentifrice group and the tap water group. Likely because of the smaller sample size in this pilot research, there was no statistically significant mean difference in enamel loss observed between the NaF dentifrice group and the tap water control group (P = 0.51), where the tap water mean was 18.94 (SE 3.53).
Table 2.
Treatment comparisons from the statistical modelling analysis
| Treatment group n = 4 per group (eight observations/subject) | Enamel loss in μm* mean (SE) | % Mean reduction vs. A | Treatment comparison P-value vs. B | Treatment comparison P-value vs. T |
|---|---|---|---|---|
| A: NaF dentifrice | 15.53 (3.53) | 0.0044 | 0.5117 | |
| B: SnF2 + NaF dentifrice | 2.03 (0.57) | 86.9% | 0.0011 | |
| T: Tap water | 18.94 (3.53) |
SE, standard error of the mean.
Enamel loss values were multiplied by −1 for display purposes.
It was notable that the variability in each group tended to increase with greater enamel loss. From the statistical model, the NaF dentifrice and tap water groups had a between-subject pooled standard deviation of 3.45 and a within-subject correlation among sample measurements of 0.78, while the SnF2 dentifrice group showed less variability with a between-subject standard deviation of 1.16 and a within-subject correlation of 0.47. Enamel loss was not statistically significantly different for side of the mouth (left versus right appliance; P = 0.44) or position of the samples within the appliances (front, mid-front, mid-rear, rear; P = 0.36). For enamel loss, side showed a marginal interaction (P = 0.062) with treatment; however, the effect was entirely driven by one subject, and so this interaction was removed from the statistical model. Both test products were well-tolerated, and no adverse events were reported during the study.
DISCUSSION
Evaluation of products designed to treat clinically important oral care problems, such as caries or sensitivity, are often accomplished using models that are designed to simulate, as closely as possible, the condition of interest. In many cases, in vitro models designed to mimic various aspects of each of these conditions are the initial step in the evaluation of products intended to help prevent, or even reverse, each of these conditions. Once demonstrated effective using well-designed in vitro models, products can then progress to more realistic modelling, including, when necessary, in vivo clinical evaluation. In the case of dental erosion, in vivo clinical studies that expose subjects’ teeth to long-term acid challenge would be destructive, irreversible and, logistically, teeth may not be extracted for the purpose of measuring the level of erosion with available profilometry measurement devices. As an alternative to long-term in vivo clinical studies for dental erosion, short-term in situ models are used with minimal controlled exposure of teeth to common dietary acids, or ex vivo acid exposure in the case of this study, for predicting potential effectiveness of oral care products against dental erosion as well as vehicles to demonstrate the relative benefits provided by different formulations7., 19..
The model presented here measures the ability of products to help prevent the effects of erosive acid attack. By beginning the study with sound, intact enamel surfaces and monitoring the ability of each test product to help protect against tooth surface loss, the model is designed to focus not on reversal of damage, but on the ability of each product to maintain strong, healthy tooth surfaces under a high level of erosive acid challenge. This is particularly important when considering how dental erosion both initiates and progresses in vivo. Dental erosion is a progressive condition that cannot be reversed, although the progression of dental erosion can be minimised, halted and kept under control by using the appropriate intervention therapies20. The current pilot study was capable of confirming the relative ability of a stabilised stannous fluoride-containing dentifrice to inhibit dental erosion and protect against its progression relative to a sodium fluoride dentifrice control.
The model was an adaptation of the model of Hooper7. In the Hooper version of the model, dentifrice products were prepared in vitro using a 1:3 dilution of dentifrice:water (w/w), and the subjects swished with this pre-diluted product for 60 seconds. In our modified version of the model, subjects performed a 30-second lingual brushing of teeth to effect in vivo dilution of the test products with naturally stimulated saliva, followed by swishing of the products in the mouth for an additional 90 seconds. The inclusion of natural saliva to dilute the test products is a major step in the evolution of this model, as this adaptation enables the assessment of product performance under realistic conditions of actual product use. Salivary dilution is known to vary between individuals. For that reason, many models incorporate standardisation of dilution, such as a product:diluent ratio of 1:3, to help reduce model variations7., 17.. In the present model, the inclusion of natural, salivary dilution of product under actual brushing conditions enables an assessment of each product in a more realistic environment, with a key assumption being that the performance of a highly effective product will be obvious, regardless of how much dilution occurs.
The present model was capable of demonstrating statistically significant differences between the stabilised SnF2 and the NaF dentifrices, which is consistent with other studies, both in vitro and in situ, that have demonstrated significant enhancement in protection of enamel against erosive acid damage for stannous-containing dentifrices7., 17., 21., 22., 23., 24.. Statistically significant differences were demonstrated using a small number of subjects and a parallel group study design. The design of this study did not expose the subjects’ natural teeth to any erosive acid challenges.
Previous studies have demonstrated that a significant factor with respect to model variation is sample to sample variability17. In the present study, the use of eight specimens per subject, while statistically modelling both between- and within-subject variability, was intended to reduce subject-level measurement error by providing more samples per subject than is usually included in such studies. In addition, the use of a specific concentration, volume and duration of exposure to the erosive citric acid was intended to further minimise variability, ensuring a consistent level of challenge to all specimens for all subjects and all products tested. The use of a static rather than agitated acid challenge was also included to reduce variability. While studies have shown that increased agitation results in increased erosive wear, agitation must be carefully controlled if included in the study design25. In this model, agitation would have been performed by each subject, likely resulting in significant differences in the amount of agitation used. Therefore, a static challenge was incorporated to ensure similarity in acid challenge conditions across the entire study. The results demonstrated no significant differences in response of the enamel to treatment based on position in the appliances, suggesting that all specimens were treated in a similar manner. This effect was likely facilitated by use of the ex vivo acid challenge. One of the original intentions of this study was for it to serve as a pilot to provide a statistical basis for sizing future studies. These results suggest that the effectiveness of a known enamel protection positive control dentifrice can be demonstrated relative to a conventional dentifrice using a modified in situ model with in vivo product use and ex vivo acid challenge. More research would be needed to confirm these findings with additional known products and a potentially larger sample size, depending on the comparisons of interest, to better understand this model.
This study confirmed the ability of the stannous fluoride dentifrice to protect the exposed and acid challenged enamel at a level that was significantly greater than the NaF control, while there was no significant difference in performance comparing the NaF and water control groups. This result is consistent with the model’s intent to demonstrate the relative effectiveness of products with regard to their ability to provide protection against erosive acid damage. The NaF dentifrice included in this study – a conventional fluoride dentifrice – was not formulated to provide enhanced levels of protection against dental erosion. Thus, the level of performance found with this particular product was anticipated, based on the result of in vitro erosion-prevention studies used to predict in vivo performance21., 22., 23..
CONCLUSION
Results from this modified in situ erosion model confirmed the enhanced erosion protection benefits of the stabilised SnF2 dentifrice versus a conventional NaF dentifrice. By more careful control of key study variables (the acid itself, the acid immersion phase, etc.) and increasing the number of specimens analysed per subject, it was possible to reduce study variability and provide a significant result with a relatively small subject sample size and increased number of samples per subject. Importantly, this was done with no risk to the subject’s natural teeth. Thus, results from this study confirm the model’s ability to safely and effectively demonstrate differences in the erosion protection potential of oral care products.
Acknowledgements
This study was funded by The Procter & Gamble Company, UK. P. G. Bellamy (Senior Scientist) is a full-time employee of The Procter & Gamble Company, Reading, UK. R. Harris (Study Coordinator), R. F. Date (Study Manager), A. J. Mussett (Principal Investigator/Medical Monitor) and A. Manly (Study Coordinator) are all contracted to The Procter & Gamble Company, Greater London Innovation Centre, Egham, UK. M. L. Barker (Principal Statistician) is a full-time employee of The Procter & Gamble Company, Mason, OH, USA. N. Hellin (Profilometry Operation Expert) and Professor N. X. West (Professor of Restorative Dentistry) are full-time employees of the University of Bristol Dental School and Hospital, Bristol, UK.
Conflicts of interest
Mr. Bellamy, Mr. Harris, Dr. Date, Dr. Mussett, Mr. Manly and Dr. Barker were employees of, or contracted by, Procter & Gamble at the time the research was conducted. Ms. Hellin and Professor West have no conflicts to dislcose.
REFERENCES
- 1.Featherstone JD. The science and practice of caries prevention. JADA. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 2.Perlich MA, Bacca LA, Bollmer BW, et al. The clinical effect of a stabilized stannous fluoride dentifrice on plaque formation, gingivitis, and gingival bleeding: a six-month study. J Clin Dent. 1995;7:54–58. [PubMed] [Google Scholar]
- 3.He T, Cheng R, Biesbrock AR, et al. Rapid desensitizing efficacy of a stannous-containing sodium fluoride dentifrice. J Clin Dent. 2011;22:40–45. [PubMed] [Google Scholar]
- 4.Winston JL, Fielder SK, Schiff T, et al. An anticalculus dentifrice with sodium hexametaphosphate and stannous fluoride: a six month study of efficacy. J Contemp Dent Pract. 2007;8:1–8. [PubMed] [Google Scholar]
- 5.Gerlach RW, Hyde JD, Poore CL, et al. Breath effects of three marketed dentifrices: a comparative study evaluating single and cumulative use. J Clin Dent. 1998;9:83–88. [PubMed] [Google Scholar]
- 6.Terézhalmy GT, Biesbrock AR, Farrell S, et al. Tooth whitening through the removal of extrinsic stain with two sodium hexametaphosphate-containing whitening dentifrices. Am J Dent. 2007;20:309–314. [PubMed] [Google Scholar]
- 7.Hooper SM, Newcombe RG, Faller R, et al. The protective effects of toothpaste against erosion by orange juice: studies in situ and in vitro. J Dent. 2007;35:476–481. doi: 10.1016/j.jdent.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Lussi A. In: Dental Erosion. From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Erosive tooth wear – a multifactorial condition of growing concern and increasing knowledge; pp. 1–8. [Google Scholar]
- 9.Featherstone JDB. Dental caries: a dynamic disease process. Aust Dent J. 2008;53:286–291. doi: 10.1111/j.1834-7819.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 10.Vieira A, Overweg E, Ruben JL, et al. Toothbrush abrasion, simulated tongue friction and attrition of eroded bovine enamel in vitro. J Dent. 2006;34:336–342. doi: 10.1016/j.jdent.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Ganss C. In: Dental Erosion. From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Definition of erosion and links to tooth wear; pp. 9–16. [Google Scholar]
- 12.Addy M, Shellis RP. In: Dental Erosion. From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Interaction between attrition, abrasion and erosion in tooth wear; pp. 17–31. [DOI] [PubMed] [Google Scholar]
- 13.Pickles MJ. In: The Teeth and Their Environment. Duckworth RM, editor. Karger; Basel: 2006. Tooth wear; pp. 86–104. [Google Scholar]
- 14.ten Cate JM. Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand. 1999;57:325–329. doi: 10.1080/000163599428562. [DOI] [PubMed] [Google Scholar]
- 15.White DJ, Nelson DG, Faller RV. Mode of action of fluoride: application of new techniques and test methods to the examination of the mechanism of action of topical fluoride. Adv Dent Res. 1994;8:166–174. doi: 10.1177/08959374940080020601. [DOI] [PubMed] [Google Scholar]
- 16.Magalhães AC, Wiegand A, Rios D, et al. In: Fluoride and the Oral Environment. Buzalaf M, editor. Karger; Basel: 2011. Fluoride in dental erosion; pp. 158–170. [Google Scholar]
- 17.Hooper S, Seong J, Macdonald E, et al. A randomised in situ trial, measuring the anti-erosive properties of a stannous-containing sodium fluoride dentifrice compared with a sodium fluoride/potassium nitrate dentifrice. Int Dent J. 2014;64(Suppl. 1):35–42. doi: 10.1111/idj.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitman B, Ginham R, Perkins M, et al. The Human Tissue Act 2004: reflections on recent changes in regulatory affairs in the United Kingdom. J Res Admin. 2008;39:91–98. [Google Scholar]
- 19.West NX, Maxwell A, Hughes JA, et al. A method to measure clinical erosion: the effect of orange juice consumption on erosion of enamel. J Dent. 1998;26:329–335. doi: 10.1016/s0300-5712(97)00025-0. [DOI] [PubMed] [Google Scholar]
- 20.Lussi A, Hellwig E. In: Dental Erosion. From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Risk assessment and preventive measures; pp. 190–199. [Google Scholar]
- 21.Faller RV, Eversole SL, Tzeghai GE. Enamel protection: a comparison of marketed dentifrice performance against dental erosion. Am J Dent. 2011;24:205–210. [PubMed] [Google Scholar]
- 22.Eversole SL, Saunders-Burkhardt K, Faller RV. Erosion protection comparison of stabilised SnF2, mixed fluoride active and SMFP/arginine-containing dentifrices. Int Dent J. 2014;64(Suppl. 1):22–28. doi: 10.1111/idj.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faller RV, Eversole SL. Enamel protection from acid challenge – benefits of marketed fluoride dentifrices. J Clin Dent. 2013;24:25–30. [PubMed] [Google Scholar]
- 24.Huysmans MC, Jager DH, Ruben JL, et al. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011;45:518–523. doi: 10.1159/000331391. [DOI] [PubMed] [Google Scholar]
- 25.Paepegaey A-M, Barker ML, Bartlett DW, et al. Measuring enamel erosion: a comparative study of contact profilometry, non-contact profilometry and confocal laser scanning microscopy. Dent Mat. 2013;29:1265–1272. doi: 10.1016/j.dental.2013.09.015. [DOI] [PubMed] [Google Scholar]