Abstract
Objectives: The aim of the present study was to assess the levels of awareness, knowledge about signs and risk factors of mouth (oral) cancer, and attitudes towards early diagnosis and treatment among dental outpatients. Material and methods: A total of 1,200 adult outpatients attending dental clinics at the University of Jordan Hospital for dental examination and treatment were randomly selected to participate in the study. An 18-item pretested close-ended questionnaire was used for the study. Descriptive statistics were generated and chi-square tests, t-tests, one-way analysis of variance (ANOVA), and Spearman’s rho test were used to examine differences between groups. Results: Only 45.6% of the subjects had heard about oral cancer. Some 66.9% and 33.8%, respectively, were able to correctly identify tobacco and alcohol as risk factors. Some 24.1% had no knowledge about any signs of oral cancer. Male subjects, smokers, alcohol drinkers, older participants (>40 years), and participants with less than a university education were significantly less aware, and had much less knowledge, of the signs and risk factors of oral cancer (P < 0.05). Conclusion: Awareness about oral cancer among Jordanian dental outpatients is low. These dental patients, especially those in high-risk groups for mouth cancer and those of lower socio-economic status (SES), are less well informed about the signs and risk factors of oral cancer. Interventions to improve public knowledge about oral cancer and attitudes towards early diagnosis and treatment are urgently indicated.
Key words: Oral, cancer, public, awareness, Jordan
INTRODUCTION
Oral cancer is a significant world health problem: it is the sixth most common cause of cancer-related death worldwide1., 2.. The aetiology of oral cancer is primarily linked to tobacco use, alcohol consumption3., 4., 5., 6., 7., 8. and betel use9., 10., and combinations of these habits11. Human papillomavirus (HPV) infection is implicated in oropharyngeal cancer, and ultraviolet light is implicated in lip cancer12., 13.. Socio-economic status (SES) is influential14., 15., 16.. Other factors possibly implicated in mouth and oropharyngeal cancers include immunosuppression17., 18., familial and genetic factors19., 20., 21., 22., 23., diet24., 25., 26., marijuana27, and exercise28.
There is a 5-year survival rate of approximately 50% overall29., 30., 31., the poor prognosis being largely attributed to delays in diagnosis and treatment32., 33.. Previous research has indicated that lack of public awareness about signs and risk factors of oral cancer can contribute to the late diagnosis and poor prognosis34.
Jordan is a Middle Eastern country with a relatively small population of about 7 million. Almost half of this population comprises adolescents and youths (<40 years)35. About 30% of the Jordanian population smoke tobacco in the form of cigarettes or narghile – a tobacco pipe that draws the smoke through water; a hookah, arghila, qalyān, or shisha35.
There are limited data on the prevalence and risk factors of oral cancer in Jordan; however, the data that are available indicate that the impact of oral cancer in Jordan is probably underestimated36. In addition, little is known about public awareness and beliefs regarding oral cancer.
Therefore, the purpose of the present study was to determine the levels of public awareness, knowledge about the signs and risk factors of oral cancer, and attitudes towards treatment and early diagnosis. It was planned to use the results obtained from this study to assist in the implementation of a health-education programme to enhance public knowledge about oral cancer and attitudes towards early diagnosis and treatment.
MATERIALS AND METHODS
The study was conducted in the Department of Dentistry at The University of Jordan Hospital during the period August 2013 to January 2014. The Faculty of Dentistry Research Ethics Committee reviewed and approved the study. The research was conducted in full accordance with the World Medical Association Declaration of Helsinki.
All study patients were dental patients older than 16 years of age attending dental clinics for examination and dental treatment. Patients who were diagnosed with oral cancer or referred for assessment of a suspicious oral mucosal lesion were excluded from the study.
An 18-item pretested close-ended questionnaire that requires 3–6 minutes to complete was employed. The purpose of the study was explained and verbal consent was obtained from all participants. The questionnaire elicited information about patients‘ demographics and tobacco and alcohol habits (six items), knowledge about oral cancer (eight items), and attitude towards early diagnosis and treatment (four items). Illiterate individuals were directly interviewed and their responses were recorded by the authors. Responses to questions about risk factors and signs of oral cancer were assessed as correct or incorrect and knowledge scores were calculated for each respondent.
Statistical analysis was performed using SPSS for Windows release 16.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were generated. The chi-square test, Student’s t-test, one-way analysis of variance (ANOVA), and Spearman’s rho test were used to examine differences between groups. Results were considered significant at P <0.05.
RESULTS
Demographic characteristics and tobacco and alcohol habits
The sample of 1,200 Jordanian subjects comprised 685 (57.1%) female subjects and 515 (42.9%) male subjects. The mean age of study patients was 35.7 ± 16 (range: 16–86) years. In terms of education, 61.3% had a university education, 30.3% had high school education only, 5.6% had elementary school education, and 2.8% were illiterate. Out of the total group investigated, 33.2% were smokers (14.9% cigarettes, 12% narghile, 6.3% both) and 8.1% reported alcohol drinking.
Oral cancer awareness
Only 45.6% of participants had heard about oral cancer. In patients who were aware of oral cancer, the public media (TV, newspapers, and the Internet) were the main sources of information (59.8%). Only 15% had heard about oral cancer from their dentists, 12.1% had heard about it from their physicians, and 12.7% from friends and relatives.
Older patients (>40 years of age) and alcohol drinkers had the lowest level of awareness (P < 0.05). No significant association was found between oral cancer awareness and the level of education, smoking habits, gender, and regular dental visits (P > 0.05) (Table 1).
Table 1.
Factors associated with awareness about oral cancer
| Variable | Awareness |
P value | |
|---|---|---|---|
| Yes (%) | No (%) | ||
| Age | |||
| >40 years | 37.3 | 63.7 | 0.000* |
| <40 years | 50.1 | 49.9 | |
| Gender | |||
| Male | 45.6 | 54.4 | 0.98 |
| Female | 45.5 | 54.5 | |
| Education | |||
| Less than university | 49.3 | 50.7 | 0.13 |
| University | 43.2 | 56.8 | |
| Smoking | |||
| Yes | 42.6 | 57.4 | 0.15 |
| No | 47.1 | 52.9 | |
| Alcohol use | |||
| Yes | 35.1 | 64.9 | 0.03* |
| No | 46.5 | 53.5 | |
Indicates statistical significance at P <0.05.
Knowledge about oral cancer
When asked about the cause of oral cancer, 66.9% and 33.8%, respectively, identified tobacco and alcohol as risk factors. About one-third (27.8%) believed that oral cancer can be inherited, and 20.2% believed that the occurrence of oral cancer is just a matter of luck. About one-quarter (25.4%) attributed oral cancer to inadequate toothbrushing, and 24.3% thought that infectious agents are behind the development of oral cancer.
Knowledge scores for the main risk factors (tobacco and alcohol) ranged from 0 to 2, with 28.1% obtaining a score of 0, 43.8% a score of 1, and 28.1% a score of 2 (Figure 1). Male subjects, smokers, and alcohol drinkers had significantly less knowledge of the risk factors for oral cancer (P < 0.05), whilst participants with a university education had significantly more knowledge (P < 0.05). There was no significant association between participants’ age and regular dental visits and their knowledge of the risk factors for oral cancer (P > 0.05) (Table 2).
Figure 1.
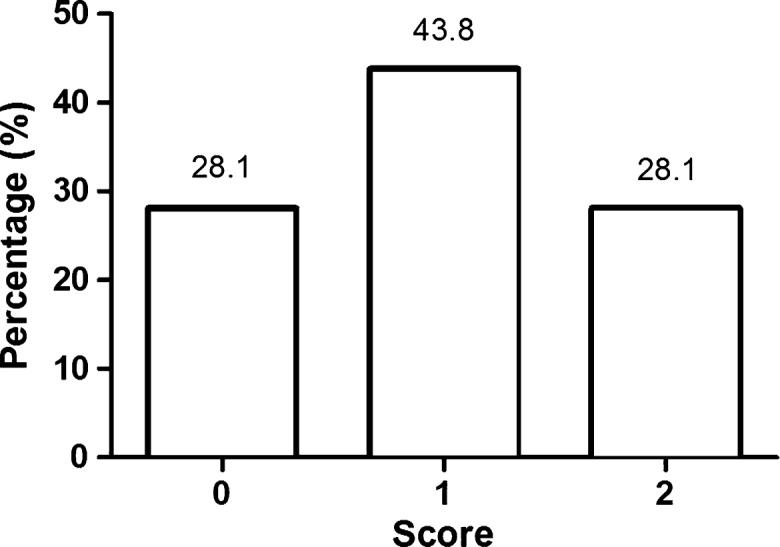
Knowledge about the main risk factors for oral cancer. Participants’ responses regarding knowledge about oral cancer were assessed as correct or incorrect. Score 0 indicates that the participant does not know the two main risk factors for oral cancer (tobacco and alcohol), score 1 indicates that the participant knows only one risk factor, and score 2 indicates that the participants know the two main risk factors.
Table 2.
Factors associated with knowledge about risk factors for oral cancer
| Variable | Knowledge about risk factors |
P value | ||
|---|---|---|---|---|
| Don’t know the two main risk factors (%) | Know one of the two main risk factors (%) | Know the two main risk factors (%) | ||
| Age | ||||
| >40 years | 29.2 | 39.1 | 31.5 | 0.85 |
| <40 years | 28.7 | 40.3 | 31.0 | |
| Gender | ||||
| Male | 33.2 | 43.9 | 22.9 | 0.000* |
| Female | 24.2 | 43.8 | 32.0 | |
| Education | ||||
| Less than university | 31.4 | 44.4 | 24.2 | 0.002* |
| University | 26.0 | 43.4 | 30.6 | |
| Smoking | ||||
| Yes | 34.4 | 43.6 | 21.9 | 0.000* |
| No | 24.9 | 43.9 | 31.2 | |
| Alcohol use | ||||
| Yes | 27.8 | 61.9 | 10.3 | 0.000* |
| No | 28.1 | 42.2 | 29.6 | |
Indicates statistical significance at P <0.05.
When asked about signs of oral cancer, 24.1% knew no signs of oral cancer. However, 43.8%, 41.4%, and 32.9% were able to correctly identify white/red patches, a long-standing swelling, and a non-healing ulcer, respectively, as possible signs of oral cancer. Pain and difficulty in swallowing were identified by 20.3% and 23.7%, respectively, as possible signs. Knowledge scores for signs of oral cancer ranged from 0 to 5 with 24.3% obtaining a score of 0, 21.4% a score of 2, and only 4.6% scoring a maximum of 5 (Figure 2).
Figure 2.
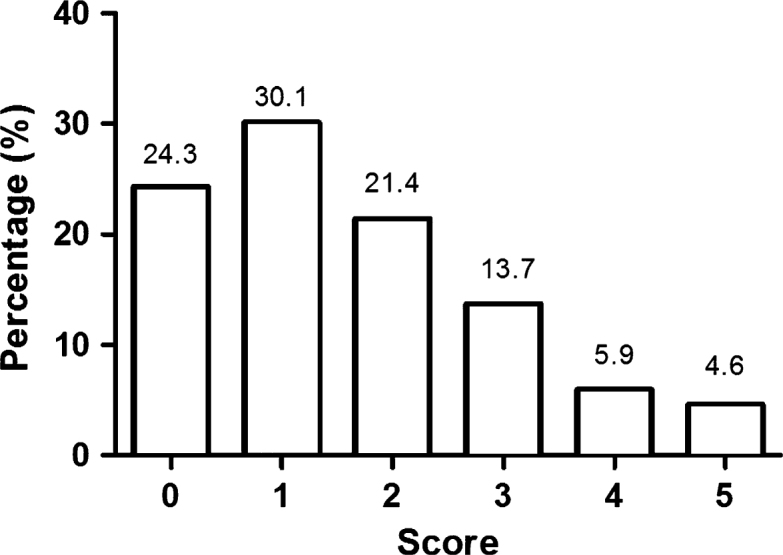
Knowledge about the signs of oral cancer. Responses regarding knowledge about the signs of oral cancer were assessed as correct or incorrect. Score 0 indicates that the participant does not know any sign of oral cancer, score 1 indicates that the participant knows only one sign, score 2 indicates that the participant knows two signs, score 3 indicates that the participant knows three signs, score 4 indicates that the participant knows four signs, and score 5 indicates that the participant knows all five possible signs of oral cancer (swelling, red/white patch, ulcer, pain, difficulty in tongue movement/swallowing).
Male subjects, smokers, and participants with less than a university education had significantly less knowledge about signs of oral cancer (P < 0.05). No significant association was found between knowledge about signs of oral cancer and participants’ gender, age, alcohol drinking habits, and regular dental visits (Table 3).
Table 3.
Factors associated with knowledge about signs of oral cancer
| Variable | Mean score (SD) | P value |
|---|---|---|
| Age | ||
| >40 years | 1.8 (1.3) | 0.71 |
| <40 years | 1.9 (1.2) | |
| Gender | ||
| Male | 1.44 (1.3) | 0.000* |
| Female | 1.72 (1.4) | |
| Education | ||
| Less than university | 1.43(1.4) | 0.000* |
| University | 1.71 (1.35) | |
| Smoking | ||
| Yes | 1.4 (1.26) | 0.000* |
| No | 1.71 (1.42) | |
| Alcohol use | ||
| Yes | 1.53 (1.34) | 0.56 |
| No | 1.61 (1.38) | |
Indicates statistical significance at P <0.05.
Only 22.2% and 15.3%, respectively, were able to correctly identify the tongue and lips as the most common sites of oral cancer. A minority (5.6%) believed that erupted teeth can develop oral cancer, and 36.4% believed that people younger than 40 years of age are most likely to be affected by oral cancer. About one-third (26.2%) believed that oral cancer is untreatable and 15.9% thought that oral cancer is contagious and therefore that they should avoid contact with affected patients. Some 6.8% believed that the use of certain herbs can cure oral cancer.
Attitude towards early diagnosis and treatment
Only 35.5% of the participants reported that they visited a dentist regularly for oral examination. However, 86.1% believed that regular dental visits can help in the early detection of oral cancer and 67.5% thought that dentists are qualified to diagnose oral cancer. No significant association was found between regular dental visits and participants’ age, gender, level of education, smoking, and alcohol consumption habits.
When asked about actions that they would take if they noticed an oral lesion, 39.9% stated that they would consult a dentist, 26.8% that they would consult a physician, 17.9% that they would apply home remedies (olive oil, sesame paste, water and salt, mouth wash, or iodine), and 7.3% would take no action (‘wait and see’). Participants with a university education were more likely to consult a doctor (dentist/physician) regarding a noticeable oral lesion (P < 0.05). Male subjects were more likely to take no action (P < 0.05), whilst female subjects were more likely to apply home remedies (P < 0.05). No significant association was found between the chosen action and participants’ attitude towards regular dental visits, age, smoking, and alcohol consumption habits (P > 0.05).
DISCUSSION
The overall prognosis of oral cancer is not good, with a 5-year survival rate generally of approximately 50%29., 30., 31., the prognosis being influenced by several factors – especially the stage at the time of diagnosis and treatment37., 38.. Unfortunately, most oral cancers, even in developed countries, are detected in late stages39, and delay in diagnosis and treatment has been attributed, at least in part, to the lack of public knowledge and awareness about signs and risk factors of oral cancer34., 40., 41., 42., 43., 44..
The present study examined the level of awareness and knowledge about oral cancer in Jordanian dental patients. The level of participants’ awareness about oral cancer was alarmingly low, with only 45.6% knowing that cancer can occur in the mouth. Similar studies conducted in other countries have revealed variable results, with values ranging from 32.3% to 95% (Table 4)34., 45., 46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59., 60., 61., 62., 63., 64., 65.. Interestingly, studies conducted in countries with a particularly high prevalence of oral cancer, such as India, Sri Lanka, and Malaysia, reported higher levels of public awareness45., 46., 47.. The low level of awareness observed in our study could be attributed to the relatively uncommon nature of oral cancer in Jordan66 and to the current lack of public health education programmes focusing on this type of cancer.
Table 4.
Public and dental patients’ awareness about oral cancer in different countries
| Reference | Country | Level of public awareness (%) |
|---|---|---|
| 57 | Australia | 79 |
| 59 | Germany | 66 |
| 58 | India | 86 |
| 45 | India (Gorakhpur) | 91.2 |
| 49 | India (South) | 60.2 |
| 52 | Iran (Babol) | 24 |
| 46 | Malaysia | 89.9 |
| 53 | Malaysia | 92 |
| 54 | Malaysia | 84.2 |
| 56 | Nigeria | 72 |
| 65 | Portugal | 68.6 |
| 55 | Sri Lanka | 84 |
| 47 | Sri Lanka | 95 |
| 51 | Turkey | 39.3 |
| 63 | UK | 95.6 |
| 34 | UK | 56 |
| 48 | UK | 80 |
| 60 | USA (Florida) | 84.5 |
| 62 | USA (Maryland) | 85 |
| 61 | USA (North Carolina) | 86 |
Tobacco smoking and alcohol consumption are the two main risk factors for oral cancer67. The results of the present study showed that around one-third of Jordanian subjects smoke tobacco in the form of cigarettes or narghile and 8.1% drink alcohol. Although more than two-thirds were aware of the association between tobacco use and oral cancer, only 33.8% were aware that alcohol is a risk factor. Similarly to our findings, most studies have reported greater public awareness of tobacco as a risk factor (47–95%) compared with alcohol consumption (6.6–64%)34., 58., 59., 60., 61., 62., 63., 68.. The greater awareness of tobacco as a risk factor could be attributed to exposure to anti-tobacco messages in various aspects of daily life. Targeting information about the association between alcohol consumption and oral cancer is desirable as the synergistic effect of tobacco and alcohol seems to be little known to the public.
Some 55.4% of the participants obtained scores of 0 and 1 (of a maximal score of 5) when asked about signs of oral cancer, indicating a significant lack of knowledge in this aspect. Similarly to other studies, ulcer, mass, and white/red patches were the most commonly identified signs of oral cancer59., 60., 64.. We have suggested a ‘RULE’ for cancer diagnosis – an acronym based on Red or white lesion, Ulcer, Lump, Exceeding 3 weeks’ duration69.
The results of the present study showed that general awareness about oral cancer was higher in younger subjects (those <40 years of age). This can be attributed to the greater exposure of young individuals to public media, including the Internet. Although level of education was not associated with general awareness about oral cancer, participants with a university education had significantly more knowledge about signs and risk factors of oral cancer, a finding consistent with the results of other studies58., 61.. Interestingly, high-risk groups, such as male subjects, smokers, alcohol drinkers, and participants >40 years of age, had much less knowledge about the risk factors and signs of oral cancer. Improving knowledge of these high-risk groups about oral cancer is therefore particularly important.
More than half (59.8%) of the participants knew about oral cancer through public media (TV, newspaper, and the Internet), which emphasises the importance of using public media to inform society about important health issues, including oral cancer. Only 15% had heard about oral cancer from their dentist. Dentists are therefore encouraged to practice their pivotal role in informing the public about risk factors and signs of oral cancer. In addition, continuous education programmes should focus on updating dentists’ knowledge about oral cancer and improving their clinical skills in oral cancer screening.
Attitudes towards early diagnosis and treatment were generally positive. Most of the participants (86.1%) believed that regular dental visits can help in early detection of oral cancer, and 67.5% believed that dentists are qualified in the diagnosis of oral cancer. However, only 35.5% regularly visit dentists for oral examination. The importance of regular dental visits for oral examination and for consultation regarding an abnormal oral lesion should therefore be emphasised to the public. Practices such as applying home remedies for persistent oral lesions were relatively common among participants (17.9%). This could be attributed to cultural factors and to inherited beliefs about the curative effect of some indigenous herbs and plants; this is particularly common among less-educated and older people in Jordan70.
The present study was primarily a hospital-based survey; however, it revealed some important aspects about public knowledge and awareness regarding oral cancer. Well-designed population-based studies are therefore needed to assess, in greater detail, public knowledge about oral cancer. Nevertheless, the findings of our study suggest that suitable health education materials depicting risk factors and clinical features of oral cancer should be provided to the public, especially high-risk groups71.
Acknowledgement
The authors would like to thank Mrs Najwa for her kind help with administrative work.
Conflict of interest
None declared.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008. GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.La Vecchia C, Tavani A, Franceschi S, et al. Epidemiology and prevention of oral cancer. Oral Oncol. 1997;33:302–312. doi: 10.1016/s1368-8375(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 4.Turati F, Garavello W, Tramacere I, et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers: results from subgroup analyses. Alcohol Alcohol. 2013;48:107–118. doi: 10.1093/alcalc/ags100. [DOI] [PubMed] [Google Scholar]
- 5.Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J Natl Cancer Inst. 2007;99:777–789. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 6.Znaor A, Brennan P, Gajalakshmi V, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105:681–686. doi: 10.1002/ijc.11114. [DOI] [PubMed] [Google Scholar]
- 7.Petti S, Scully C. Oral cancer: the association between nation-based alcohol-drinking profiles and oral cancer mortality. Oral Oncol. 2005;41:828–834. doi: 10.1016/j.oraloncology.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Petti S, Mohd M, Scully C. Revisiting the association between alcohol drinking and oral cancer in nonsmoking and betel quid non-chewing individuals. Cancer Epidemiol. 2012;36:e1–e6. doi: 10.1016/j.canep.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Thomas SJ, Bain CJ, Battistutta D, et al. Betel quid not containing tobacco and oral cancer: a report on a case-control study in Papua New Guinea and a meta-analysis of current evidence. Int J Cancer. 2007;120:1318–1323. doi: 10.1002/ijc.22304. [DOI] [PubMed] [Google Scholar]
- 10.Song H, Wan Y, Xu YY. Betel quid chewing without tobacco: a meta-analysis of carcinogenic and precarcinogenic effects. Asia Pac J Public Health. 2013;10:1177. doi: 10.1177/1010539513486921. [DOI] [PubMed] [Google Scholar]
- 11.Petti S, Masood M, Scully C. The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in south-east Asia: a meta-analysis of observational studies. PLoS One. 2013;8:e78999. doi: 10.1371/journal.pone.0078999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hobbs CG, Sterne JA, Bailey M, et al. Human papillomavirus and head and neck cancer: a systematic review and meta-analysis. Clin Otolaryngol. 2006;31:259–266. doi: 10.1111/j.1749-4486.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 13.Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J Natl Cancer Inst. 2007;99:1801–1810. doi: 10.1093/jnci/djm233. [DOI] [PubMed] [Google Scholar]
- 14.Warnakulasuriya KA, Johnson NW, Linklater KM, et al. Cancer of mouth, pharynx and nasopharynx in Asian and Chinese immigrants resident in Thames regions. Oral Oncol. 1999;35:471–475. doi: 10.1016/s1368-8375(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane GJ, Sharp L, Porter S, et al. Trends in survival from cancers of the oral cavity and pharynx in Scotland: a clue as to why the disease is becoming more common? Br J Cancer. 1996;73:805–808. doi: 10.1038/bjc.1996.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moles DR, Fedele S, Speight PM, et al. Oral and pharyngeal cancer in South Asians and non-South Asians in relation to socioeconomic deprivation in South East England. Br J Cancer. 2008;98:633–635. doi: 10.1038/sj.bjc.6604191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Leeuwen MT, Grulich AE, McDonald SP, et al. Immunosuppression and other risk factors for lip cancer after kidney transplantation. Cancer Epidemiol Biomarkers Prev. 2009;18:561–569. doi: 10.1158/1055-9965.EPI-08-0919. [DOI] [PubMed] [Google Scholar]
- 18.Vajdic CM, van Leeuwen MT. Cancer incidence and risk factors after solid organ transplantation. Int J Cancer. 2009;125:1747–1754. doi: 10.1002/ijc.24439. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi T, Pagon RA, Bird TC, et al. University of Washington, Seattle; Seattle (WA): 2002. Fanconi Anemia. GeneReviews [Internet] [updated 2008]. Available from: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=fa. Accessed 12 January 2014. [Google Scholar]
- 20.Savage SA, Pagon RA, Bird TC, et al. University of Washington, Seattle; Seattle (WA): 2009. Dyskeratosis Congenita. GeneReviews [Internet] Available from: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=dkc. Accessed 12 January 2014. [Google Scholar]
- 21.Kraemer KH, Pagon RA, Bird TC, et al. University of Washington, Seattle; Seattle (WA): 2003. Xeroderma Pigmentosum. GeneReviews [Internet] [updated 2008]. Available from: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=gene&part=xp. Accessed 12 January 2014. [PubMed] [Google Scholar]
- 22.Garavello W, Foschi R, Talamini R, et al. Family history and the risk of oral and pharyngeal cancer. Int J Cancer. 2008;122:1827–1831. doi: 10.1002/ijc.23199. [DOI] [PubMed] [Google Scholar]
- 23.Turati F, Edefonti V, Bosetti C, et al. Family history of cancer and the risk of cancer: a network of case-control studies. Ann Oncol. 2013;24:2651–2656. doi: 10.1093/annonc/mdt280. [DOI] [PubMed] [Google Scholar]
- 24.Tovosia S, Chen PH, Ko AM, et al. Prevalence and associated factors of betel quid use in the Solomon Islands: ahyperendemic area for oral and pharyngeal cancer. Am J Trop Med Hyg. 2007;77:586–590. [PubMed] [Google Scholar]
- 25.Garavello W, Lucenteforte E, Bosetti C, et al. The role of foods and nutrients on oral and pharyngeal cancer risk. Minerva Stomatol. 2009;58:25–34. [PubMed] [Google Scholar]
- 26.Galeone C, Tavani A, Pelucchi C, et al. Coffee and tea intake and risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2010;19:1723–1736. doi: 10.1158/1055-9965.EPI-10-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks MA, Chaturvedi AK, Kelsey K, et al. Association of marijuana smoking with oropharyngeal and oral tongue cancers: pooled analysis from the INHANCE consortium. Cancer Epidemiol Biomarkers Prev. 2014;23:160–171. doi: 10.1158/1055-9965.EPI-13-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolotti N, Chuang SC, Cadoni G, et al. Recreational physical activity and risk of head and neck cancer: a pooled analysis within the international head and neck cancer epidemiology (INHANCE) Consortium. Eur J Epidemiol. 2011;26:619–628. doi: 10.1007/s10654-011-9612-3. [DOI] [PubMed] [Google Scholar]
- 29.Forastiere A, Koch W, Trotti A, et al. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 30.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 31.van der Schroeff MP, Baatenburg de Jong RJ. Staging and prognosis in head and neck cancer. Oral Oncol. 2009;45:356–360. doi: 10.1016/j.oraloncology.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 32.Groome PA, Rohland SL, Hall SF, et al. A population-based study of factors associated with early versus late stage oral cavity cancer diagnoses. Oral Oncol. 2011;47:642–647. doi: 10.1016/j.oraloncology.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Elango KJ, Anandkrishnan N, Suresh A, et al. Mouth self-examination to improve oral cancer awareness and early detection in a high-risk population. Oral Oncol. 2011;47:620–624. doi: 10.1016/j.oraloncology.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Warnakulasuriya KA, Harris CK, Scarrott DM, et al. An alarming lack of public awareness towards oral cancer. Br Dent J. 1999;187:319–322. doi: 10.1038/sj.bdj.4800269. [DOI] [PubMed] [Google Scholar]
- 35.Dar-Odeh NS, Bakri FG, Al-Omiri MK, et al. Narghile (water pipe) smoking among university students in Jordan: prevalence, pattern and beliefs. Harm Reduct J. 2010;7:10. doi: 10.1186/1477-7517-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawashdeh MA, Matalka I. Malignant oral tumors in Jordanians, 1991-2001. A descriptive epidemiological study. Int J Oral Maxillofac Surg. 2004;33:183–188. doi: 10.1054/ijom.2003.0494. [DOI] [PubMed] [Google Scholar]
- 37.Shah JP, Gil Z. Current concepts in management of oral cancer–surgery. Oral Oncol. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massano J, Regateiro FS, Januário G, et al. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 39.La Vecchia C, Lucchini F, Negri E, et al. Trends in oral cancer mortality in Europe. Oral Oncol. 2004;40:433–439. doi: 10.1016/j.oraloncology.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Khoo SP, Shanmuhasuntharam P, Mahadzir WM, et al. Factors involved in the diagnosis of oral squamous cell carcinoma in Malaysia. Asia Pac J Public Health. 1998;10:49–51. doi: 10.1177/101053959801000111. [DOI] [PubMed] [Google Scholar]
- 41.Hollows P, McAndrew PG, Perini MG. Delays in the referral and treatment of oral squamous cell carcinoma. Br Dent J. 2000;188:262–265. doi: 10.1038/sj.bdj.4800449. [DOI] [PubMed] [Google Scholar]
- 42.Scott SE, Grunfeld EA, McGurk M. Patient’s delay in oral cancer: a systematic review. Community Dent Oral Epidemiol. 2006;34:337–343. doi: 10.1111/j.1600-0528.2006.00290.x. [DOI] [PubMed] [Google Scholar]
- 43.Peacock ZS, Pogrel MA, Schmidt BL. Exploring the reasons for delay in treatment of oral cancer. J Am Dent Assoc. 2008;139:1346–1352. doi: 10.14219/jada.archive.2008.0046. [DOI] [PubMed] [Google Scholar]
- 44.Gao W, Guo CB. Factors related to delay in diagnosis of oral squamous cell carcinoma. J Oral Maxillofac Surg. 2009;67:1015–1020. doi: 10.1016/j.joms.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal M, Pandey S, Jain S, et al. Oral cancer awareness of the general public in Gorakhpur city, India. Asian Pac J Cancer Prev. 2012;13:5195–5199. doi: 10.7314/apjcp.2012.13.10.5195. [DOI] [PubMed] [Google Scholar]
- 46.Saini R, Ghani ZI, Rahman NA. The awareness of oral cancer in adult patients attending School of Dental Sciences, Universiti Sains Malaysia: a preliminary study. Singapore Dent J. 2006;28:34–39. [PubMed] [Google Scholar]
- 47.Ariyawardana A, Vithanaarachchi N. Awareness of oral cancer and precancer among patients attending a hospital in Sri Lanka. Asian Pac J Cancer Prev. 2005;6:58–61. [PubMed] [Google Scholar]
- 48.Awojobi O, Scott SE, Newton T. Patients’ perceptions of oral cancer screening in dental practice: a cross-sectional study. BMC Oral Health. 2012;12:55. doi: 10.1186/1472-6831-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srikanth Reddy B, Doshi D, Padma Reddy M, et al. Oral cancer awareness and knowledge among dental patients in South India. J Craniomaxillofac Surg. 2012;40:521–524. doi: 10.1016/j.jcms.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Tadbir AA, Ebrahimi H, Pourshahidi S, et al. Evaluation of levels of knowledge about etiology and symptoms of oral cancer in southern Iran. Asian Pac J Cancer Prev. 2013;14:2217–2220. doi: 10.7314/apjcp.2013.14.4.2217. [DOI] [PubMed] [Google Scholar]
- 51.Peker I, Alkurt MT. Public awareness level of oral cancer in a group of dental patients. J Contemp Dent Pract. 2010;11:049–056. [PubMed] [Google Scholar]
- 52.Motallebnejad MM, Khanian M, Alizadeh R, et al. Community survey of knowledge about oral cancer in Babol: effect of an education intervention. East Mediterr Health J. 2009;15:1489–1495. [PubMed] [Google Scholar]
- 53.Al Dubai SA, Ganasegeran K, Alabsi AM, et al. Awareness and knowledge of oral cancer among university students in Malaysia. Asian Pac J Cancer Prev. 2012;13:165–168. doi: 10.7314/apjcp.2012.13.1.165. [DOI] [PubMed] [Google Scholar]
- 54.Ghani WM, Doss JG, Jamaluddin M, et al. Oral cancer awareness and its determinants among a selected Malaysian population. Asian Pac J Cancer Prev. 2013;14:1957–1963. doi: 10.7314/apjcp.2013.14.3.1957. [DOI] [PubMed] [Google Scholar]
- 55.Amarasinghe HK, Usgodaarachchi US, Johnson NW, et al. Public awareness of oral cancer, of oral potentially malignant disorders and of their risk factors in some rural populations in Sri Lanka. Community Dent Oral Epidemiol. 2010;38:540–548. doi: 10.1111/j.1600-0528.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 56.Lawoyin JO, Aderinokun GA, Kolude B, et al. Oral cancer awareness and prevalence of risk behaviours among dental patients in South-western Nigeria. Afr J Med Med Sci. 2003;32:203–207. [PubMed] [Google Scholar]
- 57.Park JH, Slack-Smith L, Smith A, et al. Knowledge and perceptions regarding oral and pharyngeal carcinoma among adult dental patients. Aust Dent J. 2011;56:284–289. doi: 10.1111/j.1834-7819.2011.01342.x. [DOI] [PubMed] [Google Scholar]
- 58.Elango JK, Sundaram KR, Gangadharan P, et al. Factors affecting oral cancer awareness in a high-risk population in India. Asian Pac J Cancer Prev. 2009;10:627–630. [PubMed] [Google Scholar]
- 59.Hertrampf K, Wenz HJ, Koller M, et al. Public awareness about prevention and early detection of oral cancer: a population-based study in Northern Germany. J Craniomaxillofac Surg. 2012;40:e82–e86. doi: 10.1016/j.jcms.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Tomar SL, Logan HL. Florida adults’ oral cancer knowledge and examination experiences. J Public Health Dent. 2005;65:221–230. doi: 10.1111/j.1752-7325.2005.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 61.Patton LL, Agans R, Elter JR, et al. Oral cancer knowledge and examination experiences among North Carolina adults. J Public Health Dent. 2004;64:173–180. doi: 10.1111/j.1752-7325.2004.tb02748.x. [DOI] [PubMed] [Google Scholar]
- 62.Horowitz AM, Moon HS, Goodman HS, et al. Maryland adults’ knowledge of oral cancer and having oral cancer examinations. J Public Health Dent. 1998;58:281–287. doi: 10.1111/j.1752-7325.1998.tb03010.x. [DOI] [PubMed] [Google Scholar]
- 63.West R, Alkhatib MN, McNeill A, et al. Awareness of mouth cancer in Great Britain. Br Dent J. 2006;200:167–169. doi: 10.1038/sj.bdj.4813197. [DOI] [PubMed] [Google Scholar]
- 64.Devadiga A, Prasad KV. Knowledge about oral cancer in adults attending a Dental hospital in India. Asian Pac J Cancer Prev. 2010;11:1609–1613. [PubMed] [Google Scholar]
- 65.Monteiro LS, Salazar F, Pacheco J, et al. Oral cancer awareness and knowledge in the city of Valongo, Portugal. Int J Dent. 2012;2012:376838. doi: 10.1155/2012/376838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ismail SI, Soubani M, Nimri JM, et al. Cancer incidence in Jordan from 1996 to 2009: a comprehensive study. Asian Pac J Cancer Prev. 2013;14:3527–3534. doi: 10.7314/apjcp.2013.14.6.3527. [DOI] [PubMed] [Google Scholar]
- 67.Muwonge R, Ramadas K, Sankila R, et al. Role of tobacco smoking, chewing and alcohol drinking in the risk of oral cancer in Trivandrum, India: a nested case-control design using incident cancer cases. Oral Oncol. 2008;44:446–454. doi: 10.1016/j.oraloncology.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Rogers SN, Hunter R, Lowe D. Awareness of oral cancer in the Mersey region. Br J Oral Maxillofac Surg. 2011;49:176–181. doi: 10.1016/j.bjoms.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Scully C. RULE for cancer diagnosis. Br Dent J. 2013;215:265–266. doi: 10.1038/sj.bdj.2013.884. [DOI] [PubMed] [Google Scholar]
- 70.Sawair FA. Recurrent aphthous stomatitis: do we know what patients are using to treat the ulcers? J Altern Complement Med. 2010;16:651–655. doi: 10.1089/acm.2009.0555. [DOI] [PubMed] [Google Scholar]
- 71.Petti S, Scully C. Oral cancer knowledge and awareness: primary and secondary effects of an information leaflet. Oral Oncol. 2007;43:408–415. doi: 10.1016/j.oraloncology.2006.04.010. [DOI] [PubMed] [Google Scholar]


