Abstract
Deposition of an acid-resistant barrier onto enamel represents a potentially superior means for delivering protection against dietary, erosive acid challenges. Purpose: The purpose of this study was to demonstrate the ability of a stabilised stannous fluoride (SnF2) dentifrice to: (1) deposit a SnF2 barrier layer onto pellicle-coated enamel surfaces; (2) increase the intensity of the barrier layer over time; and (3) be retained on the enamel surface for hours after product use. Methods: Squares of human enamel were exposed to pooled saliva for 1 hour (pellicle formation) and separated into six sets. Set 1 was treated with the supernatant of a 1:3 slurry of the test dentifrice (Crest® Pro-Health®: water for 2 minutes), then rinsed. Set 2 was treated in the same manner and then placed into saliva (6 hours). Set 3 was cycled through seven repeated treatments. Set 4 was treated for seven cycles and then placed into saliva (6 hours). Set 5 was a water control, and set 6 was a water control that remained in saliva for 6 hours. Surface analysis of specimens was done using laser ablation Inductively Coupled Plasma Mass Spectroscopy (ICP-MS). Results: Deposition of a barrier layer was demonstrated, beginning with the initial treatment, with Sn (using isotopes 117Sn + 120Sn) measured on the enamel surface as the reference marker. Deposition of the barrier layer was greater after seven cycles, and the retention of this layer was highly significant (P = 0.05, anova: 6 hours). Conclusions: This study confirms that: (1) the stabilised SnF2 dentifrice deposits a barrier layer onto the enamel surface, beginning with the first use of the product; (2) this barrier is enhanced following multiple treatments; and (3) the barrier layer is retained on the enamel surface for hours after product use.
Key words: Erosion, toothpaste, fluoride, stannous fluoride, laser ablation
INTRODUCTION
Rather than being an isolated problem in specific populations, tooth surface loss, or dental erosion, is an issue with global implications1. Erosive tooth surface loss can lead to a range of oral care issues, such as pain, dentine hypersensitivity and increased risk of caries, particularly if preventive measures are not taken to avoid the loss of oral hard tissues. As dental erosion becomes more prevalent, the condition and these associated problems are becoming recognised as a serious public health issue2. An awareness of the key factors responsible for initiating and enabling the progression of dental erosion, as well as identification of the most effective preventive strategies, are important in the prevention or management of this condition.
There are many factors with the potential to encourage the initiation and progression of dental erosion, including diets high in acid-containing products3, certain consumer behaviours such as holding acidic beverages in the mouth or swishing them around between the teeth4., 5., brushing teeth soon after ingesting acid-containing foods and beverages6, eating disorders such as bulimia4., 7., where teeth are aggressively challenged with gastric acids, and the widespread use of medications with the potential to reduce salivary levels or flow8; all have the potential to lead to increased tooth surface loss. While all of these factors have the potential to either initiate or aggravate dental erosion, most researchers agree that excessive consumption of acid-containing foods and beverages is a primary factor of this emerging issue9., 10., 11.. Although there is little controversy that dental erosion is both present and increasing, there is not a general agreement among professionals as to the best ways to help prevent the problem from becoming even more prevalent. This is particularly true with respect to the recommended use of oral care products that might provide protective benefits against dental erosion.
As a result of its proven ability to strengthen tooth enamel against bacterial acids that cause caries, fluoride has been studied for its potential to protect dental tissues against erosive acid damage12. Despite the fact that fluoride has been demonstrated to provide some acid protection benefit compared with placebo controls, it is clear that many fluoride-containing products do not provide as great a level of protection as one might desire13., 14.. As opposed to trying to restore erosively softened mineral15, which is highly susceptible to abrasion, a product that is capable of depositing an acid resistant, yet invisible barrier layer onto tooth surfaces during brushing that is then retained on that tooth surface for hours after product use would theoretically provide a more effective approach for preventing dental erosion. Data presented here confirm both the deposition and retention of such a layer onto pellicle-coated enamel specimens, delivered from a stabilised SnF2 dentifrice.
METHODS
This study was conducted following standards for good laboratory practice. Human enamel specimens were prepared using the following procedures.
Specimen collection and preparation
Enamel samples were prepared from human teeth for all studies. Human upper incisors were obtained from local oral surgeons who collected the teeth after removing them, typically for orthodontic reasons. All required precautions were in place to ensure proper handling of tooth samples from the point of collection to the ultimate use in these laboratory studies. Available teeth were individually cleaned and checked for any visible surface cracks or other imperfections. Those with any visible imperfections were discarded. Teeth were stored before use under refrigeration (approximately 5 °C) in a 5% thymol solution.
Enamel specimens were prepared by cutting 4 × 4 × 1 mm blocks from the teeth collected. These blocks were placed individually in pre-numbered wells of tissue culture plates for treatment and specimens were randomly assigned to their individual treatment groups.
Collection of human saliva
Eight to ten healthy volunteers were recruited to provide human saliva for the study. Saliva samples were collected from the volunteers each day of the study, pooled and stored under refrigeration until use. All required precautions were in place to ensure proper handling of saliva from the point of collection to its use in the study. Volunteers chewed paraffin wax, expectorating any stimulated saliva generated into a plastic collection vessel over a period that averaged 30–40 minutes per volunteer per collection period. Saliva was collected early in the morning from each volunteer on the day of the study. Once completed, collection vessels were pooled together, mixed and stored under refrigeration at approximately 5 °C until use.
Specimen handling for treatment
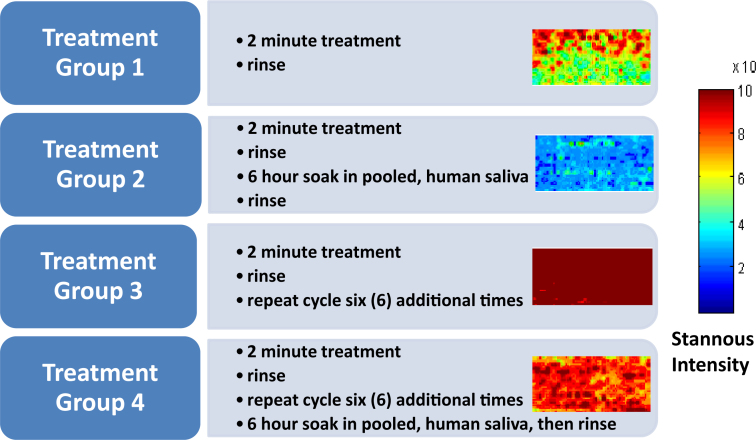
The enamel blocks were exposed for 1 hour to pooled, human saliva to form a pellicle layer. One set of specimens was treated with the supernatant of a 1:3 slurry of the test dentifrice (Crest® Pro-Health, containing 1,100 ppm F as SnF2; The Procter & Gamble Company, Cincinnati, OH, USA): water or the water control for 2 minutes, then rinsed with deionised, distilled water. A second set of samples was treated in the same manner and then placed into saliva for 6 hours. A third set was cycled through repeated treatments (total = seven cycles), then set aside for analyses, and a fourth set of samples was taken through this series of seven treatment cycles and then placed into saliva for 6 hours. A fifth set consisted of pellicle-coated specimens rinsed with water and the final set consisted of specimens left to soak in the pooled, human saliva for an additional 6 hours after development of the initial 1-hour pellicle.
Post-treatment specimen handling
After treatment, specimens were rinsed well in deionised, distilled water (ddiH2O) and stored refrigerated in a humid environment until analysis.
Specimen handling for analyses
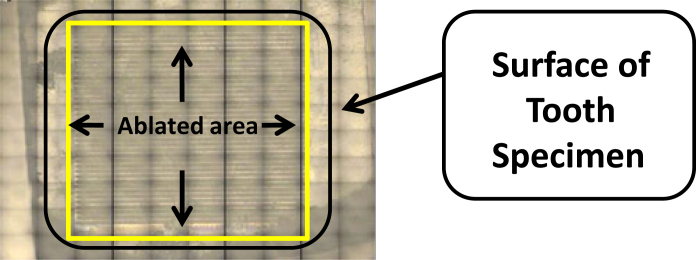
Upon completion of the appropriate treatment(s), each specimen was mounted (treated side face up) for analysis (see Figure 1) using the following laser ablation techniques:
Figure 1.
Image of laser ablation sampling area on treated tooth surface.
-
•
Stage assembly was purged for 10 minutes with a 1:1 UHP/Ar mixture to eliminate background carbon
-
•
Surface analysis of specimens was done using laser ablation ICP-MS. Tiled images in Figure 1 were pieced together automatically with the New Wave software; each tile represents a 790 × 590 μm field of view, forming a 4 × 4 mm field of ablation (or laser sampling)
-
•
Ablation was carried out using New Wave (Merchantek) UP-213 Nd:YAG LASER (New Wave, Fremont, CA, USA)
-
•
All measurements were performed on an Elan 9000DRCII ICP-MS (Perkin-Elmer, Waltham, MA, USA) in normal, peak-hopping mode
-
•
Masses monitored were 117Sn, 120Sn, 88Sr and13C, with the average carbon intensity for each line serving as an internal standard to compensate for any instrument drift
-
•
Sr was monitored to confirm that the laser ablated completely through the pellicle layer and into the enamel surface
-
•
The 4 × 4 mm area sampled was carried out on the flattest portion of each tooth surface
-
•
Data transformation and image generation was done using Matlab R2007a (Mathworks, Nantick, MA, USA)
-
•
Mass intensities were corrected using the average 13C intensity for each row as an internal standard for all corresponding Sn data points in that row; internal standard corrected intensities were then mapped together for an image of the tooth surface.
Statistical methods
Statistical analyses were performed using anova. All comparisons were performed at the 0.05 significance level.
RESULTS
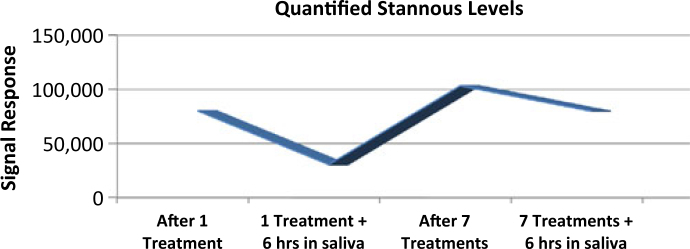
Deposition of a barrier layer onto pellicle-coated human enamel was demonstrated, with Sn (using isotopes 117Sn + 120Sn) measured on the pellicle coated enamel surface as the reference marker. Results for the SnF2 dentifrice treatment groups are presented visually in Figure 2, where the level of deposition for each of these groups can be assessed relative to the Sn intensity scale that accompanies the figure. The metal-rich barrier layer was detectable beginning with the initial 2-minute treatment. Small but insignificant levels of Sn were detected on enamel surfaces treated with the water control and the water control followed by a 6-hour saliva soak (treatment groups 5 and 6; Table 1). These levels are within the error measurement of the detector. Deposition of the Sn-containing barrier layer (treatment groups 1 and 2) visually increased with additional treatment cycles (treatment groups 3 and 4), and each data point was confirmed by the quantified Sn measurements (Table 1, Figure 3) which were determined by relative signal response. Retention of this deposited layer was significant (P = 0.05, anova), even after 6 hours soaking in pooled, human saliva following both the single treatment with the SnF2 dentifrice slurry as well as after a total of seven treatment cycles. At the 6-hour time-point following the initial treatment, approximately 38% of the deposited material was found to remain on the treated surface. After seven treatment cycles, followed by the 6-hour saliva exposure, over 77% of the deposited barrier layer was still detectable on the pellicle-coated enamel surface. These data suggest that increasing the number of treatments results in deposition of a more resilient barrier layer.
Figure 2.
Dentifrice treatment results – Sn intensity levels after treatment and retention with the stabilised SnF2 dentifrice.
Table 1.
Average Sn intensity levels measured on pellicle-coated enamel surfaces
| Treatment | Average stannous intensity level |
|
|---|---|---|
| After treatment | After 6 hours in saliva | |
| Water control | 167 | 185 |
| One treatment | 80,318 | 30,635 |
| Seven treatments | 103,330 | 79,845 |
Figure 3.
Quantified levels of Sn deposition measured on pellicle-coated surfaces
DISCUSSION
Saliva and pellicle both play key roles in the prevention of dental erosion processes16., 17.. The natural flow of saliva is increased in response to different stimuli, such as visual or olfactory stimuli or to chewing. Increased saliva flow not only enhances the buffering capacity within the localised environment, it also assists in clearing excess acids from exposed tooth surfaces during an erosive challenge18. Deposition of the protein-based pellicle layer forms on the surface of a tooth within minutes after being either reduced in thickness by toothbrushing or via removal during a professional cleaning. Pellicle contains salivary mucins that provide protection of tooth surfaces from acid demineralisation19. This natural, protective barrier is essential, as it prevents direct contact between an acid and the tooth’s natural surface. Abrasives used in dentifrice are designed to reduce, though not completely remove, the protective pellicle layer20. Thus, after brushing, an immature pellicle layer is retained on the tooth that continues to mature over time; a result of the constant presence of saliva.
While a separate paper from our group focuses on the mechanism of SnF2 deposition onto powdered hydroxyapatite (HAP) surfaces from both aqueous and dentifrice slurry treatments21, the present study demonstrates mechanistically how the stabilised stannous fluoride dentifrice provides protective benefits on pellicle-coated, human enamel. This study measures the ability of the SnF2-based barrier layer to incorporate into the outer protective pellicle matrix. The method simulates conditions in the mouth during actual product use, which makes it a valuable tool for assessing mechanisms involved in product performance. The 1-hour pellicle coating on the human enamel specimens at the time of product treatment is designed to simulate the pellicle layer that remains on tooth surfaces just after brushing20. Constant bathing in pooled, human saliva enables the maturation of the pellicle over time. As used in this study, laser ablation provides a direct measurement of the metal-rich barrier layer that has been deposited onto the pellicle-coated enamel surfaces from treatment with the stabilised stannous fluoride dentifrice slurry (Figures 1 and 2). This dentifrice slurry was prepared at a dilution that is commonly used in laboratory studies to simulate product use in vivo22. The ability to detect increasing levels of this barrier layer over time, coupled with the retention of the barrier layer at levels well in excess of baseline demonstrates that the protective barrier incorporates into the protective pellicle and remains on the treated tooth surface for many hours after use of the product (Table 1, Figure 3). While the current study was not, in itself, designed to measure efficacy of the stannous fluoride dentifrice, this benefit has been clearly demonstrated for the same stabilised dentifrice in in vitro erosion cycling models23., 24., enamel solubility reduction modelling25 and in situ erosion clinical studies26., 27.. Unlike analytical methods that focus on measuring damage to enamel surfaces after erosive challenges28, laser ablation provides a mechanistic approach for understanding conditions on the pellicle-coated, human enamel surface after treatment with the stabilised SnF2 dentifrice. This enables us to speculate that the enhanced erosive acid protection provided by this type of formulation in both laboratory and human in situ clinical trials23., 24., 25., 26., 27. is the result of both deposition and retention of the active agent on treated tooth surfaces.
CONCLUSION
This study confirmed that: (1) the stabilised SnF2 dentifrice tested deposits a barrier layer onto the enamel surface beginning with the first use of the product; (2) this barrier is enhanced following multiple treatments; and (3) the barrier layer is retained on the enamel surface for hours after use of the product. Coupled with in vitro and in situ demonstrations of enhanced protection against dietary acid challenges, these results strongly suggest that SnF2 represents a unique mechanistic approach for delivering protection against dietary, erosive acid challenges that can lead to irreversible tooth surface loss.
Acknowledgements
This study was funded by The Procter & Gamble Company, Mason, Ohio, USA.
Conflicts of interest
D. Khambe is a Principal Scientist, S. L. Eversole is a Principal Researcher and T. Mills is a Senior Scientist at The Procter & Gamble Company. R. V. Faller is a retired Principal Scientist at The Procter & Gamble Company and is now an Associate Professor at the Kornberg School of Dentistry, Temple University, Philadelphia, PA, USA.
REFERENCES
- 1.Ganss C, Lussi A. Current erosion indices – flawed or valid? Clin Oral Invest. 2008;12(Suppl. 1):1–3. doi: 10.1007/s00784-007-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lussi A. In: Dental Erosion from Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Erosive tooth wear – a multifactorial condition of growing concern and increasing knowledge; pp. 1–8. [Google Scholar]
- 3.Bartlett DW, Fares J, Shirodaria S, et al. The association of tooth wear, diet and dietary habits in adults aged 18–30 years old. J Dent. 2011;39:811–816. doi: 10.1016/j.jdent.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Jarvinen V, Meurman JH, Hyvärinen H, et al. Dental erosion and upper gastrointestinal disorders. Oral Surg Oral Med Oral Pathol. 1988;65:298–303. doi: 10.1016/0030-4220(88)90113-2. [DOI] [PubMed] [Google Scholar]
- 5.O’Sullivan EA, Curzon ME. A comparison of acidic dietary factors in children with and without dental erosion. ASDC J Dent Child. 2000;67:186–192. [PubMed] [Google Scholar]
- 6.Wiegand A, Egert S, Attin T. Toothbrushing before or after an acidic challenge to minimize tooth wear? An in situ/ex vivo study. Am J Dent. 2008;21:13–16. [PubMed] [Google Scholar]
- 7.Schlueter N, Ganss C, Pötschke S, et al. Enzyme activities in the oral fluids of patients suffering from bulimia: a controlled clinical trial. Caries Res. 2012;46:130–139. doi: 10.1159/000337105. [DOI] [PubMed] [Google Scholar]
- 8.Johansson AK. On dental erosion and associated factors. Swed Dent J Suppl. 2002;156:1–77. [PubMed] [Google Scholar]
- 9.Dugmore CR, Rock WP. A multifactorial analysis of factors associated with dental erosion. Br Dent J. 2004;196:283–286. doi: 10.1038/sj.bdj.4811041. [DOI] [PubMed] [Google Scholar]
- 10.Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Res. 2004;38(Suppl. 1):34–44. doi: 10.1159/000074360. [DOI] [PubMed] [Google Scholar]
- 11.Gambon DL, Brand HS, Veerman EC. Dental erosion in the 21st century: what is happening to nutritional habits and lifestyle in our society? Br Dent J. 2012;213:55–57. doi: 10.1038/sj.bdj.2012.613. [DOI] [PubMed] [Google Scholar]
- 12.Ganss C, Lussi A, Grunau O, et al. Conventional and anti-erosion fluoride toothpastes: effect on enamel erosion and erosion-abrasion. Caries Res. 2011;45:581–589. doi: 10.1159/000334318. [DOI] [PubMed] [Google Scholar]
- 13.Larsen MJ. Prevention by means of fluoride of enamel erosion as caused by soft drinks and orange juice. Caries Res. 2001;35:229–234. doi: 10.1159/000047461. [DOI] [PubMed] [Google Scholar]
- 14.Austin RS, Stenhagen KS, Hove LH, et al. A qualitative and quantitative investigation into the effect of fluoride formulations on enamel erosion and erosion-abrasion in vitro. J Dent. 2011;39:648–655. doi: 10.1016/j.jdent.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Barlow AP, Sufi F, Mason SC. Evaluation of different fluoride dentifrice formulations using an in situ erosion remineralization model. J Clin Dent. 2009;20:192–198. Spec Iss. [PubMed] [Google Scholar]
- 16.Hannig M, Balz M. Influence of in vivo formed salivary pellicle on enamel erosion. Caries Res. 1999;33:372–379. doi: 10.1159/000016536. [DOI] [PubMed] [Google Scholar]
- 17.Jager DH, Vieira AM, Ligtenberg AJ, et al. Effect of salivary factors on the susceptibility of hydroxyapatite to early erosion. Caries Res. 2011;45:532–537. doi: 10.1159/000331938. [DOI] [PubMed] [Google Scholar]
- 18.Piangprach T, Hengtrakool C, Kukiattrakoon B, et al. The effect of salivary factors on dental erosion in various age groups and tooth surfaces. J Am Dent Assoc. 2009;140:1137–1143. doi: 10.14219/jada.archive.2009.0341. [DOI] [PubMed] [Google Scholar]
- 19.Cheaib Z, Lussi A. Impact of acquired enamel pellicle modification on initial dental erosion. Caries Res. 2011;45:107–112. doi: 10.1159/000324803. [DOI] [PubMed] [Google Scholar]
- 20.Joiner A, Schwarz A, Philpotts CJ, et al. The protective nature of pellicle towards toothpaste abrasion on enamel and dentine. J Dent. 2008;36:360–368. doi: 10.1016/j.jdent.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Baig AA, Faller RV, Yan J, et al. Protective effects of SnF2 – Part I. Mineral solubilisation studies on powdered apatite. Int Dent J. 2014;64(Suppl. 1):4–10. doi: 10.1111/idj.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stookey GK, Featherstone JDB, Rapozo-Hilo M, et al. The Featherstone laboratory pH cycling model: a prospective, multi-site validation exercise. Am J Dent. 2011;24:322–328. [PubMed] [Google Scholar]
- 23.Faller RV, Eversole SL, Tzeghai GE. Enamel protection: a comparison of marketed dentifrice performance against dental erosion. Am J Dent. 2011;24:205–210. [PubMed] [Google Scholar]
- 24.Eversole SL, Saunders-Burkhardt K, Faller RV. Erosion protection comparison of stabilised SnF2, mixed fluoride active and SMFP/arginine-containing dentifrices. Int Dent J. 2014;64(Suppl. 1):22–28. doi: 10.1111/idj.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faller RV, Eversole SL. Enamel protection from acid challenge – benefits of marketed fluoride dentifrices. J Clin Dent. 2013;24:25–30. [PubMed] [Google Scholar]
- 26.Hooper SM, Newcombe RG, Faller R, et al. The protective effects of toothpaste against erosion by orange juice: studies in situ and in vitro. J Dent. 2007;35:476–481. doi: 10.1016/j.jdent.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy PG, Harris R, Date RF, et al. In situ clinical evaluation of a stabilised stannous fluoride dentifrice. Int Dent J. 2014;64(Suppl. 1):43–50. doi: 10.1111/idj.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attin T. In: Dental Erosion: From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Methods for assessment of dental erosion; pp. 152–172. [Google Scholar]