Abstract
Purpose: This study aimed to investigate the use of 38% silver diamine fluoride (SDF) as a treatment for preventing secondary caries in glass ionomer cement (GIC) and composite resin (CR) restorations. Methods: Six extracted human sound premolars were collected. Four cavities (4 × 2 × 2 mm3) were prepared on each premolar and then allocated to the following restoration groups: group 1, SDF conditioning and GIC restoration; group 2, GIC restoration; group 3, SDF conditioning and CR restoration; and group 4, CR restoration. After thermal cycling and sterilisation, the teeth were soaked in a 5% sucrose solution containing Streptococcus mutans and Lactobacillus acidophilus for 28 days. Micro-computed tomography was used to study demineralisation. The outer lesion depth (OLD) and wall lesion depth (WLD) of the tooth–restoration interface were measured. The OLD and WLD were directly related to the extent of secondary caries. Two-way analysis of variance was used to analyse the effects of SDF conditioning and restorative materials on OLD. Results: The mean ± standard deviation OLD values were 156 ± 45 μm, 235 ± 33 μm, 153 ± 20 μm and 232 ± 24 μm for groups 1–4, respectively. The OLD was less in restorations with SDF conditioning (P < 0.001) than in those without SDF conditioning. No interaction effect on OLD was found between the restorative materials and SDF conditioning (P = 0.062). The WLD was detected only in groups 3 and 4. Clinical significance: Conditioning with 38% SDF can increase resistance of GIC and CR restorations to secondary caries.
Key words: Silver diamine fluoride, secondary caries, glass ionomer, resin composite
INTRODUCTION
Secondary caries has been considered a major reason for failure of direct restorations1., 2.. A study of Dental Practice-Based Research Network practices in the USA reported that secondary caries was the most common reason for repairing or replacing existing restorations3. Another study reported that approximately half of all restorative dentistry is in the form of restoration replacements, with 40% of replacements attributed to secondary caries4. This fact has prompted the development of restorative materials that promise anti-cariogenic properties, such as glass ionomer cement. Glass ionomer cement releases fluoride to promote remineralisation. However, studies found that the antibacterial effect of fluoride released is limited5 and is inadequate to prevent secondary caries development6.
Streptococcus mutans is important for the initiation and progression of caries, and Lactobacillus acidophilus is frequently found in high numbers in both superficial and deep carious lesions. Streptococcus mutans and L. acidophilus are often considered as the two most important cariogenic bacteria associated with dentine caries7. Studies have demonstrated that silver diamine fluoride (SDF) can inhibit the growth of these two cariogenic bacteria7., 8.. SDF is a topical fluoride solution used for arresting caries, although it has been cleared by the US Food and Drug Administration as an anti-hypersensitivity agent. A review concluded that SDF is a safe, effective, efficient and equitable caries-preventive agent that appears to meet the World Health Organization’s Millennium Goals and to fulfil the US Institute of Medicine’s criteria for 21st century medical care9. Studies reported clinical success with SDF in arresting dental caries10., 11., and laboratory studies also found that SDF has an intense antibacterial effect on cariogenic biofilm and hinders caries progression12., 13.. It was reported that SDF did not adversely affect the bond strength of resin composite to non-carious dentine14. SDF-treated carious dentine also represented a biologically acceptable pulpal response15. Therefore, SDF may be useful in preventing secondary caries of dental restorations. However, a search in PubMed and China National Knowledge Infrastructure (CNKI) found that no study in English or Chinese had reported the effect of SDF in the prevention of secondary caries of direct restorations. Therefore, the purpose of this laboratory study was to investigate the effects of SDF conditioning on the prevention of secondary caries formation around direct composite resin and glass ionomer cement restorations. The null hypothesis is that SDF conditioning has no effect on secondary caries prevention in glass ionomer cement and composite resin restorations.
METHODS
Materials selection and specimen preparation
This study received approval from the Institutional Review Board (the University of Hong Kong) under process number IRB UW13-555 and was conducted in full accordance with the Declaration of Helsinki of the World Medical Association. All participants received dental treatment at the Faculty of Dentistry of the University of Hong Kong and provided written informed consent. Parents/guardians provided written consent for teenagers under 18 years of age. The consent procedure was approved by the Institutional Review Board.
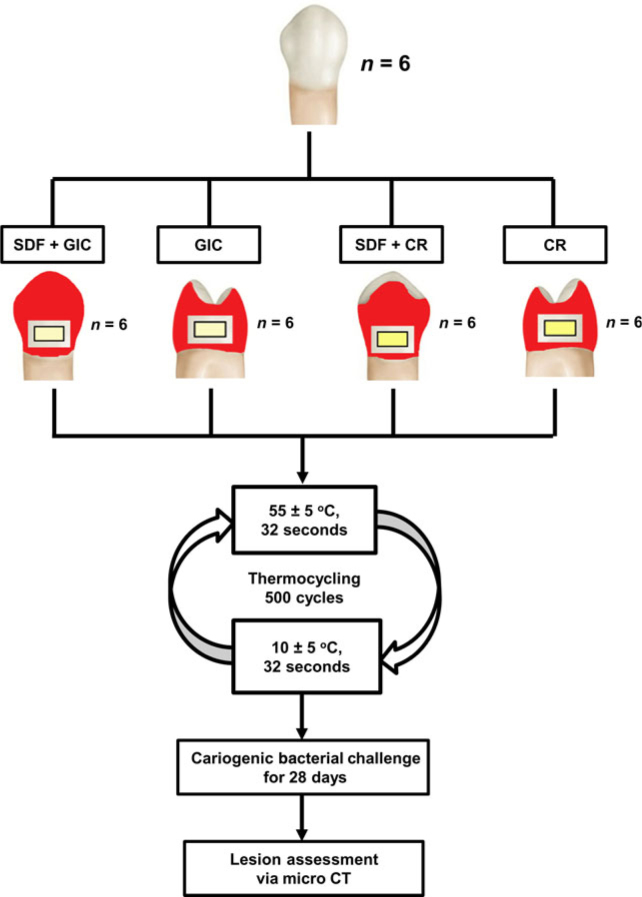
From our previous and pilot studies we expected the mean lesion depth of the test group to be 150 μm. We wanted to detect a difference of at least 100 μm. Assuming a common standard deviation of 60 μm and with power at 0.80 and α = 0.05, the sample size was at least six in each group. Six extracted human premolars, intact and without visible defects, were collected, with the patient’s consent, from teenagers who required orthodontic treatment. After removal of calculus (if any) and soft tissue, and following thorough cleaning, four round cavities of a similar size (4 × 2 × 2 mm3) were prepared on each tooth. The cavities were prepared with a carbide bur (FG 330; SS White, Lakewood, NJ, USA) under copious water-cooling. The four cavities of each tooth were cleaned using 10% polyacrylic acid and allocated to the following four restoration groups:
Group 1: the cavity was conditioned with SDF for 3 minutes, followed by restoration with glass ionomer cement
Group 2: the cavity was bulk filled with glass ionomer cement
Group 3: the cavity was conditioned with SDF for 3 minutes then the exposed surface was treated with a single-step bonding agent. In brief, the bonding agent was applied to the prepared tooth and rubbed for 20 seconds. It was then gently air dried for 5 seconds before being light cured for 10 seconds. Subsequently, the prepared tooth was filled by composite resin using a layering technique.
Group 4: the exposed surface was treated with single-step bonding agent (as described for group 3) and then the cavity was filled with composite resin.
The flow chart of the study is shown in Figure 1. The glass ionomer cement used in this study was Ketac-Molar (3M ESPE, St Paul, MN, USA). The composite resin was Filtek Z250 (3M ESPE). The bonding agent was Scotchbond Universal Adhesive (3M ESPE), and the SDF was Saforide 38% (Toyo Seiyaku Kasei, Osaka, Japan). SDF was topically applied to the specimens using a micro-brush (Micro applicator – regular; Premium Plus International Ltd., Hong Kong, China). The cavities were gently blown dry with a 3-in-1 syringe before restoration.
Figure 1.
Flow chart of the study. CR, composite resin; CT, computed tomography; GIC, glass ionomer cement; SDF, silver diamine fluoride.
Thermocycling
All restored teeth were covered with acid-resistant nail varnish (Clarins, Paris, France), except for a zone approximately 1 mm wide around the restoration. To mimic an aged restoration, the restored teeth were thermocycled for 500 cycles in distilled water baths at 55 ± 5 °C and 10 ± 5 °C, with a 32-second dwell time in each bath and a 14-second interval between baths1. The teeth were then sterilised by autoclaving before challenge with cariogenic bacteria16.
Cariogenic bacterial challenge
The microorganisms used for the cariogenic challenge were S. mutans American Type Culture Collection 35668 and L. acidophilus American Type Culture Collection 92247. The bacteria were grown in blood agar plates until isolated colonies were visible (37 °C for 24 hours, anaerobically). Then, the grown colonies were transferred to tubes containing brain–heart infusion broth + 5% sucrose and incubated for a further 24 hours, anaerobically at 37 °C. At the end of this culture period, the bacterial cell pellets were harvested by centrifugation (1500 g, 37 °C, 10 min) and resuspended in brain–heart infusion broth to a cell density of McFarland 2 (6 × 108 colony-forming units/mL). Each tooth was soaked in a tube containing 10.0 mL brain–heart infusion broth + 5% sucrose and 5.0 mL of the inoculum broth of each bacterium. The teeth were maintained in this bacterial solution at 37 °C for 28 days anaerobically; the medium was refreshed every 48 hours. During the incubation period, Gram stain test of the used medium was performed to check for contaminants8.
Lesion assessment and data collection
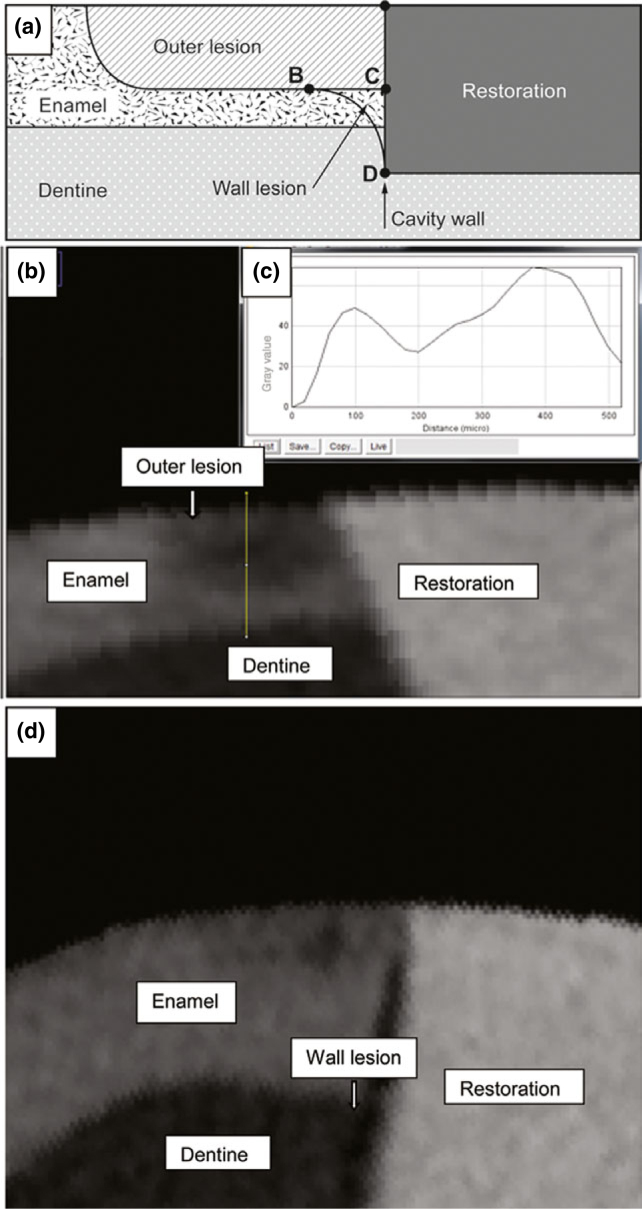
The teeth were scanned using X-ray micro-computed tomography (SkyScan 1172; SkyScan, Antwerp, Belgium) to assess lesion depth. The X-ray source was operated at a voltage of 100 kV and a current of 80 μA. The highest spatial resolution of nine local maxima was used for scanning. The signal-to-noise ratio was 5, and a 1-mm aluminium filter was used to cut off the softest X-rays. SkyScan 1172 has a self-calibrating computed tomography imaging system. Briefly, calibration with aluminium (Al) foils of 20 and 250 μm thickness [an embedded aluminium foil thickness phantom, comprising an embedded set of four aluminium foils of 20, 50, 125 and 250 μm nominal thickness (±10% tolerance); item no. SP 4001; CIRS Computerised Imaging Reference Systems Inc, Norfolk, VA, USA] showed that both thicknesses could be measured accurately, simultaneously. The thickness calibration with 20-μm-thick Al foil was found to be stable over the range of magnifications of ×40 and higher, or pixel sizes 6.8 μm and smaller. The scanning results of each tooth were reconstructed using the reconstruction software NRecon (SkyScan Company). The reconstructed three-dimensional images were viewed and processed using the data-analysis software CTAn (SkyScan Company). From the reconstructed three-dimensional image of each specimen, cross-sectional images of each tooth were identified17. Approximately 100 images were obtained for each restoration; from these lesion images, five were selected by random sampling. Grayscale values of the sound enamel in the image were estimated from the image profile. An image area with a grayscale value of more than 95% of the sound enamel was defined as sound enamel17. Special image-analysis software (Image J; National Institutes of Health, MD, USA) with plot profile was used to determine demineralised enamel in terms of different grayscale values. The method of lesion assessment on the restoration–tooth interface was adapted from Hsu et al.1 by assessing the outer lesion depth (the deepest point of the lesion from the tooth surface) and wall lesion (from the inner border of the outer lesion adjacent to the restoration to the tooth (Figure 2a). The start and end points of the outer lesion were determined according to the corresponding gray value (Figure 2b and c). For each group, the outer lesion depth and wall lesion (to a depth of 500 μm) were assessed using special image-analysis software (Image J; National Institutes of Health).
Figure 2.
Assessment of demineralisation along the restoration margin. (a) Diagrammatic illustration of the lesion assessment (modified from Hsu et al.1) Outer lesion depth, line A-C; wall lesion, area BCD (indicated with an arrow). (b) Micro-computed tomography image of the restoration margin after challenge with cariogenic bacteria. (c) Grayscale value profile along the path (yellow line in b). The start and end points of the demineralised lesion were determined according to the grayscale value. (d) Wall lesion was demonstrated in two restorations, between composite resin and the tooth.
Statistical analysis
The experiment was a randomised complete block with factorial treatment structure (2 × 2 factorial combination with six tooth blocks). The primary outcome measured was outer lesion depth. Therefore, randomised block analysis of variance (ANOVA) with two fixed factors and random block was performed to compare the effects of restorative materials and SDF (as two predicting variables) on secondary caries formation. The computer software SPSS Statistics - V20.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analysis, and the level of statistical significance for all tests was set at 0.05.
RESULTS
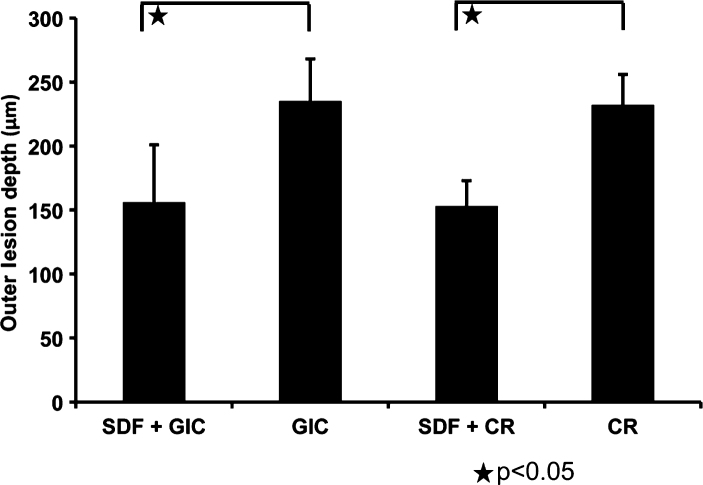
The outer lesion depths (mean ± standard deviation) in groups 1–4 were 156 ± 45 μm, 235 ± 33 μm, 153 ± 20 μm and 232 ± 24 μm, respectively (Figure 3). A statistically significant difference was detected between groups 1 and 2 and groups 3 and 4. Different restorative materials (glass ionomer cement or composite resin) had no significant effect on outer lesion depth (P = 0.797). However, outer lesion depth was reduced in restorations with SDF conditioning (P < 0.001). Randomised block ANOVA with two fixed factors showed that there was no interaction effect on outer lesion depth SDF conditioning and the restorative material (glass ionomer cement or composite resin) (P = 0.963). Different samples did not have a significant impact on outer lesion depths (P = 0.811). Wall lesions were observed in two restorations in both groups 3 and 4 (composite resin groups) (Figure 2d), but not in groups 1 and 2 (glass ionomer cement groups).
Figure 3.
Outer lesion depth of different restoration groups. Randomised block analysis of variance (ANOVA) with two fixed factors and random block was performed to compare the effects of silver diamine fluoride (SDF) and restorative materials (as two predicting variables) on outer lesion depth. A statistically significant difference was detected between group 1 [SDF + glass ionomer cement (GIC)] and group 2 (GIC), and between group 3 [SDF + composite resin (CR)] and group 4 (CR). Different restorative materials (GIC or CR) have no significant effect on outer lesion depth (P = 0.797). However, outer lesion depth was reduced in restorations with SDF conditioning (P < 0.001). Data are presented as mean ± SD, with n = 6 in each group.
DISCUSSION
The study sought first to examine if conditioning with 38% SDF could prevent secondary caries of glass ionomer cement and composite resin restorations. Based on the results of this study, the null hypothesis was rejected. The clinical implication is that SDF can be recommended and incorporated into restorative therapy for the prevention of secondary caries.
A randomised block ANOVA with two fixed factors and random block was performed due to concern for a correlation between restorations in the same tooth. The method of assessment of secondary caries was adapted from a previous study1. Four cavities were prepared on the same premolar. They were allocated to the four experimental groups. This minimised variation of the mineral content of the teeth used13. We used thermocycling treatment to mimic the aging process of the restoration1. The cariogenic bacterial challenge was carried out using two major species of cariogenic bacteria. The experimental duration of this study was 28 days2. This period of time mimicked the clinical situation of cariogenic challenge and allowed development of caries on smooth surface coronal restorations. These in vitro conditions are different from the conditions in which caries develop in vivo and therefore the results should be interpreted with caution.
Conditioning with polyacrylic acid has been recommended before application of glass ionomer cement18. Phosphoric acid conditioning has been reported to have no adverse influence on the micro-shear bond strength of etch-and-rinse bonding systems19. In this laboratory study, we aimed to standardise the sample cavities and used polyacrylic acid to remove the smear layer before application of SDF. This might prevent any unknown effect of SDF with the smear layer on dentine. However, dentists in their clinical practice do not use polyacrylic acid before placing resin composite restorations.
Wall lesion and outer lesion depth were used to evaluate the inhibitory effect on secondary caries. Wall lesion refers to the inner border of the outer carious lesion adjacent to the restoration of the tooth. Ozer and Thylstrup reported that no carious lesions were present along the cavity wall unless large voids or gaps existed20. They also found that wall lesions were associated with the size of the gap between the tooth and the restoration. In our study, we detected wall lesions in the composite resin groups but not in the glass ionomer cement groups. This indicated that the interface between the tooth and the composite resin was less resistant than the interface between the tooth and the glass ionomer cement. This concurs with the finding of a previous study1. Composite resins shrink when they polymerise. The shrinkage tends to cause contraction away from the walls and floor of the prepared tooth, towards the more rigid surface layer, thus jeopardising fit21. Outer lesion depth is the length from the deepest point of the lesion to the tooth surface. It is a parameter commonly used to evaluate the integrity of the tooth restoration interface1. We found that the restorative material was a significant factor for development of the wall lesion. Not all specimens had wall lesion development. Therefore, assessment using outer lesion depth was more predictable than using the wall lesion.
Glass ionomer cement containing calcium and fluoride reacts with poly-acid to produce a gel of hydrated silica. This is an acid–base reaction. Two mechanisms have been proposed by which fluoride may be released from a glass ionomer into an aqueous environment22. The first mechanism is a short-term reaction that involves rapid dissolution from the outer surface into a solution. The second is more gradual and results in the sustained diffusion of ions through bulk cement. However, a study reported that the release of an initial high amount of fluoride from glass ionomer cement rapidly decreased after 1–3 days and subsequently plateaued to a nearly constant level23. Another study found that the concentration of fluoride released decreased significantly to a very low level, about 1–4 parts per million (ppm) (mass/mass), after 60 days24. This could be one of the main reasons for the lack of significant difference in outer lesion depths of glass ionomer cement and composite resin restorations.
Clinical studies demonstrated that 38% SDF prevented and arrested coronal (enamel) caries in preschool children10 and root (dentine) caries in elders with mean age of 78.8 ± 6.2 years25. Laboratory studies have found that SDF has an intense antibacterial effect on cariogenic biofilm7., 8.. It also possesses a potent inhibitory effect on the activity of matrix metalloproteinases26 and cysteine cathepsins27. Treatment with SDF can increase the mineral density of enamel carious lesions17 and the micro-hardness of dentine carious lesions28. The mechanism can be explained from two perspectives9. First, silver has been demonstrated to have an antibacterial effect and to prevent biofilm formation. It could interact with sulphydryl groups of proteins and with DNA29, thereby altering hydrogen bonding and inhibiting respiration, DNA unwinding, cell-wall synthesis and cell division12. Moreover, silver ions can interact with a reactive side chain of the collagenases involved in dentine degradation, thus inactivating their catalytic functions13. Second, fluoride plays a crucial role in the remineralisation process; calcium fluoride is an important product that is produced when fluoride is deposited onto the tooth surface. Calcium fluoride can act as a temporary fluoride reservoir and can release fluoride ions at a low pH30. The fluoride ion released facilitates formation of fluoroapatite and makes the tooth surface more resistant to acid dissolution. Fluoride enhances enamel remineralisation, increasing the speed of the remineralisation process and the mineral content of early carious lesions. The incorporation of fluoride also makes the deposited mineral less acid-soluble31. This synergistic effect of silver and fluoride ions could be the reason behind the promising caries-arresting effect of SDF.
The results of this study showed that the restorations with SDF conditioning were more resistant to development of secondary caries during a cariogenic challenge. SDF at 38% contains a relatively high concentration of fluoride ions (44,800 ppm) and silver ions (253,870 ppm)32. Ten per cent silver nitrate has been shown to enhance the concentration of fluoride released from glass ionomer cements and from a resin-modified glass ionomer cement33. This large amount of fluoride and silver ions might alter the micro-environment around the restoration and retard the caries process. The present study found that conditioning with SDF before placement of composite restorations can also be beneficial in preventing secondary caries. Quock et al.14 reported that SDF does not adversely affect the bond strength of composite resin. SDF is not known to produce pulpal damage34. Gotjamanos reported a favourable response in primary teeth treated with SDF, including the formation of reparative dentine15. A major concern with the use of SDF is aesthetics because SDF stains caries lesions with a dark colour34. In this study, a stained margin of the restoration was found after SDF treatment. Therefore, care should be taken when treating patients with a high demand for aesthetics. Studies have tried to use chemicals such as potassium iodide35 or nano-silver particles36 to improve the aesthetic outcome; however, further investigation is still needed. Another concern is the discoloration caused by SDF. Clinicians might misdiagnose the stained restoration margins as arrested, or even secondary, caries. It is important that clinicians use adjunctive tools, such as intra-oral dental radiography, before making a final diagnosis.
CONCLUSION
In this laboratory study, conditioning with 38% SDF increased the resistance of the glass ionomer cement and composite resin restorations to secondary caries. When used at a concentration of 38%, SDF can be incorporated into restorative therapy to improve the success rate of direct restorations.
Acknowledgements
The authors thank Ms Samantha Li for her statistical support. This study was supported by HKU Small Project Funding (No. 201309176058).
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Hsu CY, Donly KJ, Drake DR, et al. Effects of aged fluoride-containing restorative materials on recurrent root caries. J Dent Res. 1998;77:418–425. doi: 10.1177/00220345980770021101. [DOI] [PubMed] [Google Scholar]
- 2.Kuper NK, van de Sande FH, Opdam NJ, et al. Restoration materials and secondary caries using an in vitro biofilm model. J Dent Res. 2015;94:62–68. doi: 10.1177/0022034514553245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordan VV, Riley JL, 3rd, Geraldeli S, et al. Repair or replacement of defective restorations by dentists in The Dental Practice-Based Research Network. J Am Dent Assoc. 2012;143:593–601. doi: 10.14219/jada.archive.2012.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke FJ, Wilson NH, Cheung SW, et al. Influence of patient factors on age of restorations at failure and reasons for their placement and replacement. J Dent. 2001;29:317–324. doi: 10.1016/s0300-5712(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 5.Gama-Teixeira A, Simionato MR, Elian SN, et al. Streptococcus mutans-induced secondary caries adjacent to glass ionomer cement, composite resin and amalgam restorations in vitro. Braz Oral Res. 2007;21:368–374. doi: 10.1590/s1806-83242007000400015. [DOI] [PubMed] [Google Scholar]
- 6.Mjor IA, Toffenetti F. Secondary caries: a literature review with case reports. Quintessence Int. 2000;31:165–179. [PubMed] [Google Scholar]
- 7.Mei ML, Chu CH, Low KH, et al. Caries arresting effect of silver diamine fluoride on dentine carious lesion with S. mutans and L. acidophilus dual-species cariogenic biofilm. Med Oral Patol Oral Cir Bucal. 2013;18:e824–e831. doi: 10.4317/medoral.18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei ML, Li QL, Chu CH, et al. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 2013;12:4. doi: 10.1186/1476-0711-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenblatt A, Stamford TC, Niederman R. Silver diamine fluoride: a caries “silver-fluoride bullet”. J Dent Res. 2009;88:116–125. doi: 10.1177/0022034508329406. [DOI] [PubMed] [Google Scholar]
- 10.Chu CH, Lo EC, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81:767–770. doi: 10.1177/0810767. [DOI] [PubMed] [Google Scholar]
- 11.Llodra JC, Rodriguez A, Ferrer B, et al. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84:721–724. doi: 10.1177/154405910508400807. [DOI] [PubMed] [Google Scholar]
- 12.Chu CH, Mei L, Seneviratne CJ, et al. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomyces naeslundii biofilms. Int J Paediatr Dent. 2012;22:2–10. doi: 10.1111/j.1365-263X.2011.01149.x. [DOI] [PubMed] [Google Scholar]
- 13.Mei ML, Ito L, Cao Y, et al. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent. 2013;41:809–817. doi: 10.1016/j.jdent.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Quock RL, Barros JA, Yang SW, et al. Effect of silver diamine fluoride on microtensile bond strength to dentin. Oper Dent. 2012;37:610–616. doi: 10.2341/11-344-L. [DOI] [PubMed] [Google Scholar]
- 15.Gotjamanos T. Pulp response in primary teeth with deep residual caries treated with silver fluoride and glass ionomer cement (‘atraumatic’ technique) Aust Dent J. 1996;41:328–334. doi: 10.1111/j.1834-7819.1996.tb03142.x. [DOI] [PubMed] [Google Scholar]
- 16.Mei ML, Ito L, Chu CH, et al. Prevention of dentine caries using silver diamine fluoride application followed by Er:YAG laser irradiation: an in vitro study. Lasers Med Sci. 2014;29:1785–1791. doi: 10.1007/s10103-013-1329-y. [DOI] [PubMed] [Google Scholar]
- 17.Liu BY, Lo EC, Li CM. Effect of silver and fluoride ions on enamel demineralization: a quantitative study using micro-computed tomography. Aust Dent J. 2012;57:65–70. doi: 10.1111/j.1834-7819.2011.01641.x. [DOI] [PubMed] [Google Scholar]
- 18.Raggio DP, Sonego FG, Camargo LB, et al. Efficiency of different polyacrylic acid concentrations on the smear layer, after ART technique, by Scanning Electron Microscopy (SEM) Eur Arch Paediatr Dent. 2010;11:232–235. doi: 10.1007/BF03262753. [DOI] [PubMed] [Google Scholar]
- 19.Adebayo OA, Burrow MF, Tyas MJ. Resin-dentine interfacial morphology following CPP-ACP treatment. J Dent. 2010;38:96–105. doi: 10.1016/j.jdent.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Ozer L, Thylstrup A. What is known about caries in relation to rstorations as a reason for replacement? A review. Adv Dent Res. 1995;9:394–402. [Google Scholar]
- 21.Darvell BW, editor. Materials Science for Dentistry. Woodhead; Hong Kong: 2009. Resin restorative materials; p. 103. [Google Scholar]
- 22.Dhondt CL, De Maeyer EA, Verbeeck RM. Fluoride release from glass ionomer activated with fluoride solutions. J Dent Res. 2001;80:1402–1406. doi: 10.1177/00220345010800050301. [DOI] [PubMed] [Google Scholar]
- 23.Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials–fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–362. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Creanor SL, Carruthers LM, Saunders WP, et al. Fluoride uptake and release characteristics of glass ionomer cements. Caries Res. 1994;28:322–328. doi: 10.1159/000261996. [DOI] [PubMed] [Google Scholar]
- 25.Tan HP, Lo EC, Dyson JE, et al. A randomized trial on root caries prevention in elders. J Dent Res. 2010;89:1086–1090. doi: 10.1177/0022034510375825. [DOI] [PubMed] [Google Scholar]
- 26.Mei ML, Li QL, Chu CH, et al. The inhibitory effects of silver diamine fluoride at different concentrations on matrix metalloproteinases. Dent Mater. 2012;28:903–908. doi: 10.1016/j.dental.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Mei ML, Ito L, Cao Y, et al. The inhibitory effects of silver diamine fluorides on cysteine cathepsins. J Dent. 2014;42:329–335. doi: 10.1016/j.jdent.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Chu CH, Lo EC. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent. 2008;36:387–391. doi: 10.1016/j.jdent.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Wu MY, Suryanarayanan K, van Ooij WJ, et al. Using microbial genomics to evaluate the effectiveness of silver to prevent biofilm formation. Water Sci Technol. 2007;55:413–419. doi: 10.2166/wst.2007.285. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Wang J, Joiner A, et al. The remineralisation of enamel: a review of the literature. J Dent. 2014;42(Suppl 1):S12–S20. doi: 10.1016/S0300-5712(14)50003-6. [DOI] [PubMed] [Google Scholar]
- 31.Chu CH, Mei ML, Lo EC. Use of fluorides in dental caries management. Gen Dent. 2010;58:37–43. quiz 44-35, 79-80. [PubMed] [Google Scholar]
- 32.Mei ML, Chu CH, Lo EC, et al. Fluoride and silver concentrations of silver diammine fluoride solutions for dental use. Int J Paediatr Dent. 2013;23:279–285. doi: 10.1111/ipd.12005. [DOI] [PubMed] [Google Scholar]
- 33.Ariffin Z, Ngo H, McIntyre J. Enhancement of fluoride release from glass ionomer cement following a coating of silver fluoride. Aust Dent J. 2006;51:328–332. doi: 10.1111/j.1834-7819.2006.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 34.Chu CH, Lo EC. Promoting caries arrest in children with silver diamine fluoride: a review. Oral Health Prev Dent. 2008;6:315–321. [PubMed] [Google Scholar]
- 35.Knight GM, McIntyre JM, Craig GG, et al. Differences between normal and demineralized dentine pretreated with silver fluoride and potassium iodide after an in vitro challenge by Streptococcus mutans. Aust Dent J. 2007;52:16–21. doi: 10.1111/j.1834-7819.2007.tb00460.x. [DOI] [PubMed] [Google Scholar]
- 36.Santos VE, Jr, Vasconcelos Filho A, Targino AG, et al. A new “silver-bullet” to treat caries in children–nano silver fluoride: a randomised clinical trial. J Dent. 2014;42:945–951. doi: 10.1016/j.jdent.2014.05.017. [DOI] [PubMed] [Google Scholar]