Abstract
Purpose: To investigate the relative erosion protection potential of marketed dentifrices formulated with either stabilised stannous fluoride (SnF2), sodium fluoride (NaF) and/or sodium monofluorophosphate (SMFP) using an established laboratory erosion cycling model. Methods: Sound enamel cores from extracted, human enamel were cleaned, ground and polished, soaked in pooled saliva (pellicle formation) and treated with a 1:3 slurry of dentifrice and saliva. Specimens were subjected to daily challenges with 1% citric acid, a potentially damaging acid found in common food and drinks. Marketed dentifrices compared were: (1) a stabilised stannous fluoride product formulated with 1,100 ppm F as SnF2; (2) a cavity protection product containing 1,100 ppm F as NaF; (3) a cavity protection product comprising a mixed active fluoride system with 1,000 ppm F as SMFP + 450 ppm F as NaF; and (4) a sensitivity product containing 1,450 ppm F as SMFP + 8% arginine bicarbonate. Results: Specimens from Group 1 demonstrated an average loss of 5.5 (±1.2) μm of tooth surface enamel; Groups 2, 3 and 4 lost an average of 18.3 (±0.9) μm, 16.0 (±2.0) μm and 17.1 (±1.1) μm, respectively, of tooth surface enamel. Group 1 provided a statistically significant difference in protection compared with the other products. Conclusions: These results suggest that the marketed dentifrice formulated with stabilised SnF2 may provide enhanced protection of exposed tooth surfaces against dietary acid attack compared with the other products tested.
Key words: Erosion, dentifrice, fluoride, arginine bicarbonate, mixed fluoride, stannous fluoride
INTRODUCTION
Dental pellicle is a protein film that forms on the surface of human enamel owing to selective adsorption of proteins and glycoproteins from the saliva onto exposed tooth surfaces. The saliva-generated pellicle provides human enamel with a natural protective coating, shielding the exposed tooth surfaces from both aggressive abrasive forces and dissolution caused by excessive dietary acid attack1., 2.. In the absence of this pellicle coating, the sound structure of enamel will not endure for a long period of time. This is demonstrated by the rapid demise of tooth enamel and structure that can occur as a result of various diseases or procedures that lead to hyposalivation, such as in the case of Sjögren’s syndrome or head and neck radiation treatments3., 4.. Even when present, salivary pellicle has limitations with regard to its ability to protect against high levels of acid challenge. Dental erosion is one condition that can result from an overwhelming acid insult to the teeth, as excessive levels of acid are beyond the protective limits of the salivary pellicle. If left untreated, dental erosion can then lead to irreversible tooth surface loss5., 6.. There is evidence that the prevalence of dental erosion is increasing globally7.
The primary source of the acid insult most often considered responsible for the reported increase in dental erosion is acid-containing beverages8. Over the past 50 years, the consumption of these beverages has increased dramatically, with the average American consuming in excess of 50 gallons (189 l) of acid-containing beverages annually9. These include not only carbonated beverages, but also sports drinks, energy drinks and fruit juices, among others. While many consumers have listened to both dietary and dental concerns about the level of sugar found in many marketed beverages and have switched to drinking artificially sweetened beverages, there is little, if any, difference in the acid content of the natural and artificially sweetened beverages10. The flavour profile of most beverages is optimised through the use of acidulants, which not only add tartness to beverages but also enhance a product’s overall flavour profile and perception. Common acidulants include citric, phosphoric, malic, tartaric and acetic acids11. Any individual with intact, natural teeth, who also drinks a high level of acid-containing beverages, is at risk for developing symptoms associated with dental erosion12. Of particular concern are children who consume a combination of fruit juices, soft drinks and sports drinks rather than milk or water, as this combination can result in a substantial level of acid intake13.
The irreversible nature of dental erosion has generated substantial interest in the dental research community, where numerous models and various technologies to protect against its progression have been proposed14., 15., 16., 17., 18., 19., 20., 21.. The use of appropriate models to demonstrate erosion protection benefits is essential for understanding the relative performance of available products22 and assisting in the development of new products capable of providing even greater levels of protection against the effects of irreversible acid damage. The irreversible damage that can be caused by dental erosion highlights the need for products capable of providing significant protection against its initiation and progression. Hooper et al.12 state: ‘the clinical relevance of routine fluoride exposure to protection of the natural teeth against dental erosion has yet to be shown, particularly in light of the fact the reported incidence of this condition is on the rise globally. This is in spite of the essentially global use of fluoride containing toothpastes’. They further state: ‘An ideal treatment regimen would be for toothpaste, which the majority of the population use on a daily basis, to provide protection against dental erosion that is in addition to the anticaries benefits provided by the various fluoride sources. This would not be unlike toothpastes designed for the alleviation of hypersensitivity, tartar control, or other conditions beyond caries’12.
The objective of the present study was to determine the relative ability of several marketed dentifrices to protect human enamel against the initiation and progression of damage caused by dietary acid attack using an in vitro erosion cycling model16 that has been demonstrated to correlate well with an in situ erosion clinical trial when testing the same set of test products12. The test products in this current study included a stabilised SnF2 dentifrice that has demonstrated significant erosion protection benefits in both in vitro and in situ clinical studies12., 16.. Other products included in the study represent two widely available cavity prevention products [one formulated with NaF and the other a combination sodium monofluorophosphate (SMFP)/NaF formula] and a new product sold on the global market containing a combination of SMFP as the anticaries agent and arginine bicarbonate for sensitivity benefits. The majority of products currently claiming to provide erosion protection benefits are sensitivity-based products, as tooth sensitivity is often linked closely with dental erosion23., 24.. Stannous fluoride, NaF and SMFP are all accepted for their ability to both strengthen enamel (remineralisation) and to fight cavities (protection against demineralisation). In question in the present study was the relative ability of products formulated with these proven anticaries ingredients to strengthen and protect tooth enamel against the type of extrinsic acids commonly associated with dental erosion.
The methodology used for this study involved the use of sound, human pellicle-coated enamel specimens exposed to an erosion cycling regimen designed to simulate the in vivo environment as closely as possible under controlled, laboratory conditions16.
METHODS
The study was conducted following standards for good laboratory practice. Sound, human enamel specimens were prepared using standard procedures25., 26.. In this model, tooth specimens are exposed to twenty 2-minute treatment cycles over a 5-day period. Specimens received 10 minute erosive acid challenges applied 1 hour after each treatment with test product. Ten-minute acid challenges were chosen because this time-frame represents a reasonable time for consumption of a can of soft drink or juice beverage. By including the acid challenge 1 hour after product treatment, the model was designed to assess the ability of a test product to be retained on the tooth surface for at least 1 hour after treatment and still withstand an erosive acid challenge. When not in treatment or challenge, specimens remained in a pooled, human saliva bath to simulate normal oral conditions.
At the conclusion of the cycling phase, specimens were sectioned, and the cross-sectional samples analysed using a specialised software program (Inspektor Research Systems BV, Amsterdam, the Netherlands). The mean surface loss was reported for each treatment group as μm of enamel lost. The per cent change in surface loss versus the NaF reference control was calculated for each treatment group.
Products evaluated
Table 1 provides details on the products and controls included in these studies and the major ingredients in each formula. Test products included a stabilised SnF2 dentifrice, two cavity prevention products (one formulated with NaF and the other a combination SMFP/NaF formula) and one additional product that contained a combination of SMFP and arginine bicarbonate. The NaF-based cavity protection dentifrice served as the reference control. All products were used within the expiration dates listed on the individual product packages.
Table 1.
Dentifrice products tested and their major ingredients
| Type of formula tested | Active ingredient(s) |
|---|---|
| Stabilised SnF2* | 1,100 ppm F as SnF2 |
| Single active cavity protection† | 1,100 ppm F as NaF |
| Mixed-active cavity protection‡ | 1,000 ppm F as SMFP + 450 ppm F as NaF |
| SMFP/arginine bicarbonate§ | 1,450 ppm F as SMFP + 8% arginine bicarbonate |
Crest® Pro-Health®, The Procter & Gamble Company, Cincinnati, OH, USA.
Crest® Cavity Protection, The Procter & Gamble Company, Cincinnati, OH, USA.
Colgate® Cavity Protection, Colgate-Palmolive (UK) Ltd, Guildford, UK.
Colgate® Sensitive Pro-Relief™, Colgate-Palmolive (UK) Ltd, Guildford, UK.
Collection of human saliva
Eight to 10 healthy volunteers were recruited to provide human saliva for this study. Saliva samples were collected from the volunteers each day of the study, pooled and stored under refrigeration until use. All required precautions were in place to ensure proper handling of saliva from the point of collection to the ultimate use in the laboratory study. Pre-screened, healthy volunteers chewed paraffin wax and expectorated any stimulated saliva generated into a plastic collection vessel over a period that averaged 30–40 minutes per volunteer per collection period. Saliva was collected early in the morning from each volunteer on each day of the study in order to maintain a relatively constant pool of saliva for use in the study. Once completed, collection vessels were pooled together, mixed and stored under refrigeration at approximately 5 °C until use.
Specimen collection and preparation
Enamel samples were prepared from human teeth for all studies. Specimens were obtained from local oral surgeons who collected the teeth after removing them, typically for orthodontic reasons. Necessary precautions were in place to ensure proper handling of tooth samples from the point of collection to the ultimate use in this study. Available teeth were individually cleaned and checked for any visible surface cracks or other imperfections; those with any visible imperfections were discarded. Teeth were stored before use under refrigeration (approximately 5 °C) in a 1% thymol solution.
Enamel specimens were prepared by cutting 3 mm cores from the teeth using a diamond core drill. Enamel cores were mounted in ¼-inch (0.64 cm) diameter Lucite rods using dental acrylic (Durabase; Reliance Manufacturing Company, Worth, IL, USA) covering all sides except the natural facial surface. Grinding with 600 grit silicon carbide–water slurry was used to remove approximately 50 μm of the outer enamel. Following grinding, specimens were polished for 90 minutes with gamma alumina (Linde No. 3, AB Gamma Polishing Alumina; Buehler Limited, Lake Bluff, IL, USA). Enamel specimens found to have surface imperfections were rejected. Following this preparation, nail polish (#551; Revlon, New York, NY, USA) was applied to the surface, leaving a treatment window of unprotected enamel approximately 3.0 × 0.4 mm (Figure 1). Specimens were randomly assigned to treatment groups (four specimens per group).
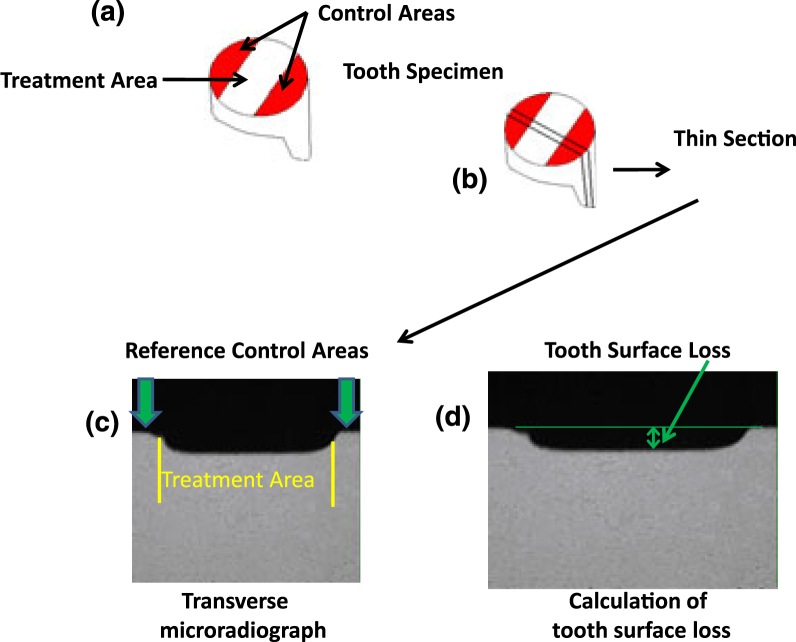
Figure 1.
Graphical representation of the analysis procedure. (a) depiction of human enamel specimen after the completion of erosion cycling; (b) a thin cross-section of the specimen is removed for X-ray analysis; (c) typical radiograph; and (d) calculation of surface loss as the difference between the treated and untreated control areas (measured in μm). [Adapted with permission from Faller et al., 2011]16.
Daily cycling protocol
Each group of four specimens was placed into 20 ml of fresh, pooled human saliva for 1 hour to initiate the formation of a pellicle layer on the enamel surfaces. For each treatment, dentifrice slurries were prepared by mixing 5 g of dentifrice with 15 g of fresh, pooled human saliva for a period of not less than 4 minutes or more than 5 minutes before use. A fresh slurry of each dentifrice was prepared for each treatment. Each treatment cycle consisted of: dentifrice slurry (2 minutes) → rinse in deionised, distilled (ddi)H2O → saliva (1 hour) → erosion challenge (10 minute) → rinse in ddiH2O → saliva. There were four treatments per day for a total of five treatment days.
Dentifrice treatments consisted of immersing the specimens into the dentifrice slurry for 2 minutes. Specimens were rotated in the slurries at a constant speed of 75 rpm. The erosion challenge consisted of soaking each treatment group in 12 ml of 1% citric acid at a neat pH of around 2.3 (at room temperature). When not undergoing treatment, specimens remained in 20 ml of pooled, human saliva, which was gently stirred. The saliva was refreshed three times per day. At night, each group of specimens remained immersed in pooled saliva, which was gently stirred at all times.
Post-treatment specimen handling
After 5 days of treatment, specimens were rinsed well in ddiH2O and stored refrigerated in a humid environment until analysis.
Analysis of specimens
Figure 1 provides a graphical description of the analysis procedure. Before analysis, a layer of nail polish was applied to each specimen to seal the surface and protect the eroded areas against further damage. Specimens were cut plano-parallel using a hard tissue sectioning saw (Silverstone-Taylor Hard Tissue Microtome; Scientific Fabrications, Littleton, CO, USA). Each section was cut to allow both control and treated portions to be presented for analysis. A thin section approximately 100 μm thick was removed from each specimen, positioned on a mount that was then fitted to a camera connected to an X-ray generator (Model #PW1830; Philips Analytical, Natick, MA, USA) and exposed to CuKα radiation. Microradiographs were taken using Kodak SO253 Holographic film (Eastman Kodak Company, Rochester, NY, USA) and processed using standard film developing methods. Radiographic images were analysed using transverse microradiography (TMR), a computer-based analysis system (Inspektor Research Systems BV, Amsterdam, The Netherlands). By comparing the original, untreated (control) surface with the post-treatment surface, the depth of the eroded area was measured and reported as μm of tooth surface mineral lost.
Statistical methods
Statistical analyses for each study were performed using Fisher’s least significant difference (LSD) test. All comparisons were performed at the 0.05 significance level.
RESULTS
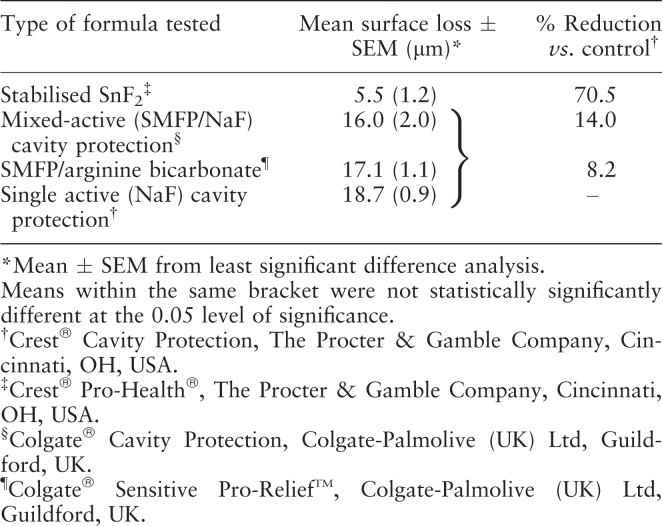
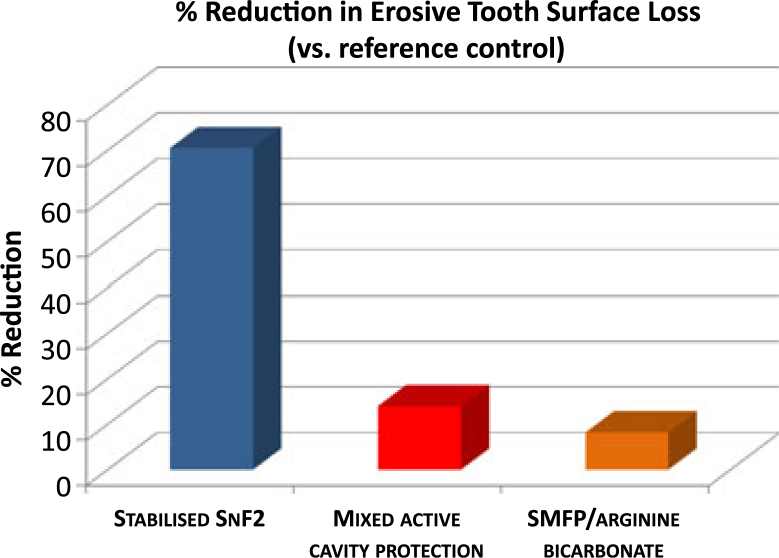
The marketed dentifrice formulated with stabilised SnF2 provided a 70.5% benefit in enamel surface protection compared with the reference control (Table 2, Figure 2). Under the same conditions, treatment with the SMFP/NaF and the SMFP + arginine bicarbonate dentifrices resulted in a net benefit of only 14.0% and 8.2%, respectively, compared with the reference control. There was no statistical difference in performance between the NaF reference control, SMFP/NaF or SMFP + arginine bicarbonate dentifrices in terms of their ability to protect the tooth enamel surface against acid attack (P < 0.05, anova).
Table 2.
Results and statistical analysis
Figure 2.
Graphical representation of data as a per cent change in enamel surface protection versus the 1,100 ppm F (NaF) reference control. Larger positive values indicate greater protection against tooth surface loss.
DISCUSSION
The in vitro erosion cycling model used in this study tests the relative ability of oral care products to reduce or inhibit damage to enamel surfaces caused by a common dietary acid. In this study, human tooth specimens were exposed to four treatment cycles per day per 5 days consisting of treatment (dentifrice slurry), followed by approximately 1 hour in saliva and followed by an erosive acid challenge (citric acid). While other dietary acids have certainly been used in other erosion model studies, citric acid represents an acid commonly found in both food and drink27. Between cycles, teeth remained in a pooled, human saliva bath to simulate normal oral conditions. Following the 5-day protocol, enamel surfaces were analysed to determine μm of total enamel mineral loss, relative to baseline. The microradiographic system used for analyses (Figure 1) provides an accurate means to measure the level of tooth surface damage that occurred over the course of study treatments. Appropriate statistical evaluations were then applied to the data.
Using this same erosion cycling model, Faller and co-workers16 recently demonstrated that the same stabilised SnF2 dentifrice tested here provided tooth surface protection against erosive acid attack that was not only superior to that of the NaF control, but was also superior to a product formulated with SMFP as the active agent, as well as to products formulated with NaF + triclosan, NaF + KNO3 and NaF + liquid calcium. Further, in an in situ clinical trial designed to measure the protective effect of fluoride dentifrice against an erosive acid challenge, this same stabilised SnF2 dentifrice provided a significant improvement in the ability of the product to protect tooth enamel against the onset and progression of dietary acid attack compared with the NaF control12. In both of these previous studies, all products contained a maximum of 1,100 ppm F.
The present study measured the relative effectiveness of the same F dentifrices compared in the previous in situ clinical trial12 against two marketed products formulated with higher levels of total F. In the present study, the SMFP/NaF dentifrice contained a total of 1,450 ppm F, with 1,000 ppm F as SMFP + 450 ppm F as NaF, and the SMFP/arginine bicarbonate formulation also contained 1,450 ppm F, with all of the fluoride coming from the SMFP active. As in the in vitro erosion cycling16 and the human in situ clinical studies12, treatment with the stabilised SnF2 dentifrice was found to significantly reduce the mean enamel surface loss after an erosive acid challenge compared with NaF and, in this case, also compared with the SMFP/NaF and SMFP/arginine bicarbonate dentifrices. There was no significant difference in surface loss observed for the SMFP/NaF dentifrice, the SMFP + arginine bicarbonate dentifrice, and the NaF dentifrice. Results from the present study are in agreement with the previously published in vitro studies in which the stabilised SnF2 dentifrice provided a 58% benefit in one study and 65% benefit in another, compared with the NaF reference control, when tested using a citric acid challenge16. In the present study, the measured benefit for the stabilised SnF2 dentifrice was 70.5%. As in the previous study, SMFP-containing dentifrices again performed at a level that was not significantly different from the NaF reference control, suggesting a general similarity in erosion protection for these dentifrices.
There are a limited number of additional studies available that have reported on the erosion protective benefits of SMFP-containing dentifrices. One paper demonstrated significantly lower performance for two similar calcium-based SMFP-containing formulations tested in that study28. As the hydrolysis of SMFP to free, available F can be influenced by the presence of calcium in a particular dentifrice formulation29., 30., it is likely that variations in performance for these types of products from one study to the next can be influenced by the levels of available F in a particular product at a particular point in time. In the present study, both SMFP products appear to have provided a level of performance that is consistent with the total level of F in their respective formulations. Both of these products contained a total of 1,450 ppm F and the performance for each was directionally higher, although not statistically greater, than the 1,100 ppm F (NaF) control. Neither the combination of NaF and SMFP into a single formula nor the addition of arginine bicarbonate into the SMFP-based formula appeared to have any significant impact on the erosion protection performance of these formulations. The directional benefits noted for these formulations compared with the 1,100 ppm F (NaF) reference control were likely a result of the total level of fluoride being somewhat higher in these formulations. Although most fluoride-based products do provide a measureable level of benefit against erosive acid damage31., 32., 33., 34., SnF2 appears to be unique among the anticaries active ingredients most often used in dentifrice formulations in its ability to provide enhanced protection to tooth surfaces against dietary, erosive acid challenges12., 16., 35., 36.. This enhanced, protective effect was clearly evident in the present study.
Stannous fluoride has been demonstrated to provide enhanced acid protection benefits in both mechanistic37., 38., 39. and predictive performance modelling12., 16., 28.. In addition to reducing the potential for dental erosion, protective benefits have also been noted in studies modelling the prevention of tooth sensitivity40, where deposition of acid-resistant minerals into exposed dentinal tubules has provided a strong rationale for the enhanced clinical performance of stabilised SnF2 formulations in full-scale sensitivity trials41. Enhancing the resistance of tooth minerals against all types of acid challenge may provide a means to increase overall tooth longevity, as the prevention of caries, of tooth surface demineralisation that can lead to wear and of sensitivity all combine to maximise the long-term function of the natural dentition.
CONCLUSION
These results demonstrate that the marketed dentifrice formulated with stabilised SnF2 provides significantly better protection for the tooth surface against dietary acid attack compared with the other products tested.
Acknowledgement
This study was funded by The Procter & Gamble Company, Mason, Ohio 45040, USA.
Conflicts of interest
R. V. Faller is a retired Principal Scientist from The Procter & Gamble Company, Mason, OH, USA and is now an Associate Professor at the Kornberg School of Dentistry, Temple University, Philadelphia, PA, USA. S. L. Eversole (Principal Researcher) and K. Saunders-Burkhardt (Researcher) are full-time employees at The Procter & Gamble Company.
REFERENCES
- 1.Zahradnik RT, Moreno EC, Burke EJ. Effect of salivary pellicle on enamel subsurface demineralization in vitro. J Dent Res. 1976;55:664–670. doi: 10.1177/00220345760550042101. [DOI] [PubMed] [Google Scholar]
- 2.Hannig M, Joiner A. In: The Teeth and Their Environment. Duckworth RM, editor. Karger; Basel: 2006. The structure, function and properties of the acquired pellicle; pp. 29–64. [Google Scholar]
- 3.Mathews SA, Kurien BT, Scofield RH. Oral manifestations of Sjögren’s syndrome. J Dent Res. 2008;87:308–318. doi: 10.1177/154405910808700411. [DOI] [PubMed] [Google Scholar]
- 4.Vissink A, Jansma J, Spijkervet FKL, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 5.Muerman JH, Frank RM. Scanning electron microscopic study of the effect of salivary pellicle on enamel erosion. Caries Res. 1991;25:1–6. doi: 10.1159/000261335. [DOI] [PubMed] [Google Scholar]
- 6.Amaechi BT, Higham SM, Edgar WM, et al. Thickness of acquired salivary pellicle as a determinant of the sites of dental erosion. J Dent Res. 1999;78:1821–1828. doi: 10.1177/00220345990780120901. [DOI] [PubMed] [Google Scholar]
- 7.Lussi A. In: Dental Erosion. From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Erosive tooth wear – a multifactorial condition of growing concern and increasing knowledge; pp. 1–8. [Google Scholar]
- 8.Dugmore CR, Rock WP. A multifactorial analysis of factors associated with dental erosion. Br Dent J. 2004;196:283–286. doi: 10.1038/sj.bdj.4811041. [DOI] [PubMed] [Google Scholar]
- 9.Putnam JJ, Allshouse JE. Food and Consumers Economics Division, Economic Research Service, Department of Agriculture; Washington DC: 1999. Food Consumption, Prices and Expenditures 1970–97. [Google Scholar]
- 10.von Fraunhofer JA, Rogers MM. Dissolution of dental enamel in soft drinks. Gen Dent. 2004;52:308–312. [PubMed] [Google Scholar]
- 11.Available from: http://www.univar.com/~/media/PDFs/food/Univar%20Food%20Function%20Library%20-%20Acidulants.ashx. [Accessed 6 June 2013]
- 12.Hooper SM, Newcombe RG, Faller R, et al. The protective effects of toothpaste against erosion by orange juice: studies in situ and in vitro. J Dent. 2007;35:476–481. doi: 10.1016/j.jdent.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Millward A, Shaw L, Smith AJ, et al. The distribution and severity of tooth wear and the relationship between erosion and dietary constituents in a group of children. Int J Paed Dent. 1994;4:151–157. doi: 10.1111/j.1365-263x.1994.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 14.Mok TB, McIntyre J, Hunt D. Dental erosion: in vitro model of wine assessor’s erosion. Aust Dent. 2001;46:263–268. doi: 10.1111/j.1834-7819.2001.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 15.Young A, Tenuta LMA. Initial erosion models. Caries Res. 2011;45(Suppl. 1):33–42. doi: 10.1159/000325943. [DOI] [PubMed] [Google Scholar]
- 16.Faller RV, Eversole SL, Tzeghai GE. Enamel protection: a comparison of marketed dentifrice performance against dental erosion. Am J Dent. 2011;24:205–210. [PubMed] [Google Scholar]
- 17.Fowler C, Willson R, Rees GD. In vitro microhardness studies on a new anti-erosion desensitizing toothpaste. J Clin Dent. 2006;17:100–105. [PubMed] [Google Scholar]
- 18.Fowler CE, Gracia L, Edwards MI, et al. Inhibition of enamel erosion and promotion of lesion rehardening by fluoride: a white light interferometry and microindentation study. J Clin Dent. 2009;20(Spec Iss):178–185. [PubMed] [Google Scholar]
- 19.Fowler CE, Gracia L, Edwards MI, et al. Fluoride penetration from toothpastes into incipient enamel erosive lesions investigated using dynamic secondary ion mass spectroscopy. J Clin Dent. 2009;20(Spec Iss):186–191. [PubMed] [Google Scholar]
- 20.Zero DT, Hara AT, Kelly SA, et al. Evaluation of a desensitizing test dentifrice using an in situ erosion remineralization model. J Clin Dent. 2006;17:112–116. [PubMed] [Google Scholar]
- 21.Barlow AP, Sufi F, Mason SC. Evaluation of different fluoride dentifrice formulations using an in situ erosion remineralization model. J Clin Dent. 2009;20(Spec Iss):192–198. [PubMed] [Google Scholar]
- 22.West NX, Davies M, Amaechi BT. In vitro and in situ erosion models for evaluating tooth substance loss. Caries Res. 2011;45(Suppl 1):43–52. doi: 10.1159/000325945. [DOI] [PubMed] [Google Scholar]
- 23.Dababneh RH, Khouri AT, Addy M. Dentine hypersensitivity – an enigma? A review of terminology, mechanisms, aetiology and management. Br Dent J. 1999;187:606–611. doi: 10.1038/sj.bdj.4800345. [DOI] [PubMed] [Google Scholar]
- 24.West N. In: Dental Erosion. From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Dentine Hypersensitivity; pp. 173–189. [Google Scholar]
- 25.Casals E, Boukpessi T, McQueen CM, et al. Anticaries potential of commercial dentifrices as determined by fluoridation and remineralization efficiency. J Cont Dent Prac. 2007;8:1–19. [PubMed] [Google Scholar]
- 26.Faller RV, Eversole SL, Yan J. Anticaries potential of a stabilized stannous-containing sodium fluoride dentifrice. Am J Dent. 2010;23(Spec Iss):32B–38B. [PubMed] [Google Scholar]
- 27.Featherstone JDB, Lussi A. In: Dental Erosion. From Diagnosis to Therapy. Lussi A, editor. Karger; Basel: 2006. Understanding the chemistry of dental erosion; pp. 66–76. [Google Scholar]
- 28.Faller RV, Eversole SL. Enamel protection from acid challenge – benefits of marketed fluoride dentifrices. J Clin Dent. 2013;24:25–30. [PubMed] [Google Scholar]
- 29.Conde NC, Rebelo MA, Cury JA. Evaluation of the fluoride stability of dentifrices sold in Manaus, AM, Brazil. Pesqui Odontol Braz. 2003;17:247–253. doi: 10.1590/s1517-74912003000300009. [DOI] [PubMed] [Google Scholar]
- 30.Tabchoury CPM, Cury JA. Accelerated aging of dentifrices to predict fluoride stability under normal conditions. [Original in Portuguese] Rev Bras Farm. 1994;75:67–71. [Google Scholar]
- 31.Hughes JA, West NX, Addy M. The protective effect of fluoride treatments against enamel erosion in vitro. J Oral Rehabil. 2004;31:357–363. doi: 10.1046/j.1365-2842.2003.01240.x. [DOI] [PubMed] [Google Scholar]
- 32.Larsen MJ. Prevention by means of fluoride of enamel erosion as caused by soft drinks and orange juice. Caries Res. 2001;35:229–239. doi: 10.1159/000047461. [DOI] [PubMed] [Google Scholar]
- 33.Larsen MJ, Richards A. Fluoride is unable to reduce dental erosion from soft drinks. Caries Res. 2002;36:75–80. doi: 10.1159/000057595. [DOI] [PubMed] [Google Scholar]
- 34.Ganss C, Klimek J, Schaffer U, et al. Effectiveness of two fluoridation measures on erosion progression in human enamel and dentine in vitro. Caries Res. 2001;35:325–330. doi: 10.1159/000047470. [DOI] [PubMed] [Google Scholar]
- 35.Magalhães AC, Wiegand A, Rios D, et al. In: Fluoride and the Oral Environment. Buzalaf M, editor. Karger; Basel: 2011. Fluoride in dental erosion; pp. 158–170. [Google Scholar]
- 36.Huysmans MCDNJM, Jager DHJ, Ruben JL, et al. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011;45:518–523. doi: 10.1159/000331391. [DOI] [PubMed] [Google Scholar]
- 37.Baig AA, Faller RV, Yan J, et al. Protective effects of SnF2 – Part I. Mineral solubilisation studies on powdered apatite. Int Dent J. 2014;64(Suppl. 1):4–10. doi: 10.1111/idj.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khambe D, Eversole SL, Mills T, et al. Protective effects of SnF2 – Part II. Deposition and retention on pellicle-coated enamel surfaces. Int Dent J. 2014;64(Suppl. 1):11–15. doi: 10.1111/idj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faller RV, Eversole SL. Protective Effects of SnF2 – Part III. Mechanism of barrier layer attachment. Int Dent J. 2014;64(Suppl. 1):16–21. doi: 10.1111/idj.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zsiska M, White DJ, Moore JA. Acid durability of rapid-onset smear layers produced by dentifrices. J Dent Res. 2011;90(Spec Iss A):2635. [Google Scholar]
- 41.He T, Chang J, Cheng R, et al. Clinical evaluation of the fast onset and sustained sensitivity relief of a 0.454% stannous fluoride dentifrice compared to an 8.0% arginine–calcium carbonate–sodium monofluorophosphate dentifrice. Am J Dent. 2011;24:336–340. [PubMed] [Google Scholar]