Abstract
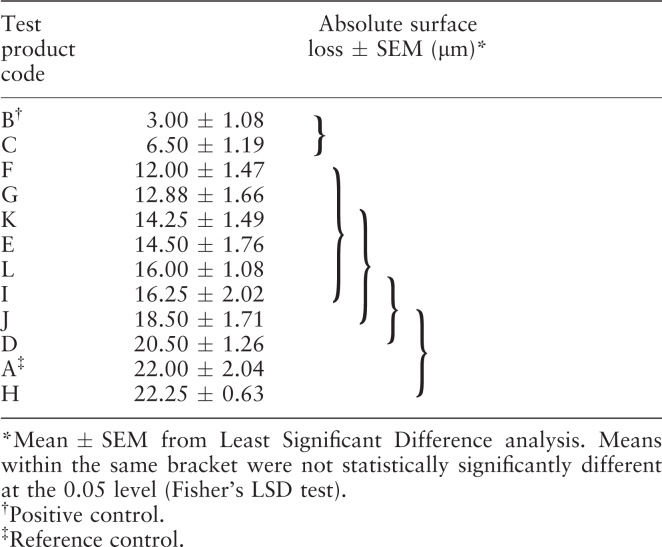
Purpose: To assess the potential of a stabilised stannous (Sn)-containing NaF dentifrice (Oral B/blend-a-Med® Pro-Expert), in addition to a number of other marketed European dentifrices formulated with various fluoride actives and two control dentifrices, to protect enamel against erosive acid damage. Methods: Cores of human enamel (four per group) were soaked in pooled human saliva, and then treated with a 1:3 slurry (dentifrice:saliva) using a standardised in vitro erosion model (5-day cycling) that includes 10-minute challenges with 1% citric acid applied 60 minutes after each dentifrice treatment. Enamel surface loss was measured using transverse microradiography (TMR). Results: Specimens treated with the Sn-containing NaF dentifrice showed 6.5 μm of surface loss ± 1.2 (SEM), which was not significantly different (P < 0.05, Fisher LSD) from that of a clinically proven, stabilised SnF2 positive control [Crest® Pro-Health, 1,100 ppm F as SnF2: 3.0 μm of surface loss ± 1.1 (SEM)]. The Sn-containing NaF dentifrice and the clinically proven positive control both provided significantly greater protection (P < 0.05, Fisher LSD) compared with all of the other products tested. Enamel loss (SEM) values for other European products and the reference control (active agents) were: Meridol®: (1,400 ppm F as AmF + SnF2) 12.0 μm (1.47); Colgate® Cavity Protection: (1,450 ppm F as SMFP + NaF) 12.9 μm (1.66); Odol med 3® (1,400 ppm F as NaF) 14.2 μm (1.49); Elmex® (1,400 ppm F as AmF) 14.5 μm (1.76); Colgate® Enamel Protect: (1,450 ppm F as NaF + KNO3) 16.3 μm (2.02); Lacalut® aktiv: (1,400 ppm F as AlF3) 18.5 μm (1.71); Sensodyne® ProNamel™: (1,450 ppm F as NaF + KNO3) 20.5 μm (1.26); Crest Cavity Protection (1,100 ppm F as NaF, reference control) 22.00 μm (2.04); and Mentadent®: (1,450 ppm F as NaF + Zn citrate) 22.3 μm (0.63). Conclusion: These results support the potential for the stabilised, Sn-containing NaF dentifrice to provide erosion protection benefits that are not significantly different from the positive control benchmark for erosion protection (stabilised SnF2), and are significantly better than a broad range of dentifrice formulations available on the European market.
Key words: Erosion, dentifrice, fluoride, stannous, Sn-containing
INTRODUCTION
Ongoing development of new and improved dentifrices designed to meet changing consumer needs and lifestyles often include incorporation of new ingredients into conventional formulations, with the goal of delivering enhanced benefits relative to the base formulation. The inclusion of anti-tartar and whitening benefits into conventional sodium fluoride (NaF) or sodium monofluorophosphate (SMFP) dentifrice formulations is an example of formulation development designed to meet changing consumer expectations1., 2., 3., 4., 5.. While not available in dentifrices from the 1960s or 1970s, these benefits have now come to be expected by most consumers, regardless of which product they use. Over the past few decades, consumers have become more aware of emerging oral health issues, such as tooth sensitivity and dental erosion6. Both of these issues have been the focus of a significant amount of scientific research and new product introductions. Although there are many approaches and formulations proven to be effective in treating or preventing tooth sensitivity7, dental erosion is an area with less substantiation of efficacy, despite many products making erosion-related claims8.
One obvious approach that has been studied for aiding in the prevention of dental erosion is the use of fluoride8., 9., an ingredient found in most over-the-counter dentifrices with a well-credentialled heritage for strengthening enamel against cariogenic acids10. Although dental erosion and dental caries share similar mineral processes, such as tooth surface softening and demineralisation, dental caries differs from dental erosion in that caries is the result of subsurface tooth mineral loss associated with bacterial fermentation of sugars in the oral cavity, resulting in a softened, yet intact tooth structure, whereas dental erosion is characterised by initial tooth surface softening, followed by layer-by-layer dissolution of the enamel crystals that eventually leads to a permanent loss of tooth volume11. While caries is the result of acids generated by plaque bacteria, dental erosion is associated with acid-containing foods and beverages. As such, the reported increase in prevalence of dental erosion is more commonly associated with changing patterns of consumer lifestyles12, which necessitates the need for development of products specifically designed to help counter new issues that may result. Studies have demonstrated that many of the currently available products are unable to provide a sufficient level of protection of enamel against dietary acid challenges13., 14., which can be significantly stronger than acid challenges associated with the caries process. Although fluoride is able to provide a certain amount of protection against dental erosion, consumption patterns of products associated with dental erosion have changed over the past few decades15, and this change in behaviour may have resulted in the issue of dental erosion increasing at a rate that has exceeded the ability of most oral care products to help keep the problem under control. One exception to this, however, is stannous fluoride (SnF2), an anticaries agent that is unique among fluoride sources commonly used in over-the-counter dentifrices. In addition to their ability to fight caries16, dentifrices formulated with SnF2 have also been proven to be clinically effective against gingivitis17., 18., sensitivity19., 20. and, more recently, dental erosion21., 22..
While SnF2 offers a broad range of oral care benefits, other dentifrices have been designed to provide a similar range of benefits using different formulation chemistries. For example, a stabilised stannous (Sn)-containing dentifrice was developed which contains NaF (1,450 ppm F−) as the active ingredient and stannous chloride (SnCl2) as a key excipient. During toothbrushing, NaF and SnCl2 combine synergistically to generate a stannous-fluoride complex23. The use of NaF, rather than SnF2, provides enhanced formulation flexibility, with the overall formulation designed to deliver the same range of benefits as the stabilised SnF2 dentifrice. Enhanced formulation flexibility is important, as this flexibility increases the potential to reach a greater number of consumers with a broad range of products. Although the anticaries potential of the Sn-containing NaF formula has been demonstrated23, its effectiveness in protecting against dental erosion was previously untested.
The aim of the current research was to evaluate the erosion protection potential of the Sn-containing NaF dentifrice as well as a number of marketed European dentifrices using a credentialled in vitro erosion cycling model14., 24.. As previous in vitro14., 24. and in situ21 studies included an 1,100 ppm F (NaF/silica) formulation as a benchmark control, that same product was included in the present study in addition to the stabilised SnF2 product demonstrated to provide significantly greater erosion protection benefits in each of the previous studies. Based on its performance in preceding studies, the stabilised SnF2 dentifrice is considered a positive control for providing erosion protection benefits. Products included in the present study comprise a wide range of fluoride sources [NaF, SMFP, SnF2, amine fluoride (AmF), aluminum fluoride (AlF3)] and, in some cases, combinations of these active ingredients.
METHODS
The study was conducted following standards for good laboratory practice. Standard procedures were used to prepare all human samples used in the study14. All products were over-tubed using coded, white tubes and all specimens were individually numbered before the start of the study. Laboratory personnel were blinded to both treatments and analyses, with the codes for both treatments and specimens being broken by the investigator only after all analyses were completed.
The methods followed for this study have been reported previously14., 24.. Briefly, tooth specimens were exposed to twenty 2-minute treatment cycles over a 5-day period. One hour after each treatment, specimens received 10-minute erosive acid challenges, simulating a reasonable time for consumption of a can of soft drink or juice beverage. This protocol assesses the ability of a test product to be retained on the tooth surface for at least 1 hour after treatment and still withstand an erosive acid challenge. When not in treatment or challenge, specimens remained in a pooled, human saliva bath that was refreshed three times daily.
At the conclusion of cycling, specimens were cross-sectioned and analysed using transverse microradiography (TMR) software (Inspektor Research Systems BV, Amsterdam, the Netherlands). The mean surface loss is reported for each treatment group as μm of enamel lost (from the tooth surface). The per cent change in surface loss versus the NaF reference control was then calculated for each treatment group14., 24..
Products evaluated
Table 1 provides a listing of all products and controls included in the study, along with the active ingredients, as reported by the individual manufacturer of each product. All products were used within the expiration dates listed on the individual product packages. In the present study, the ability of the test products to protect the enamel against the dietary acid challenge was measured against two reference controls: the positive control for delivering erosion protection benefits [a stabilised SnF2 formula containing 1,100 ppm F as SnF2 in a silica abrasive system (Crest Pro-Health dentifrice; The Procter & Gamble Company, Cincinnati, OH, USA)] and an 1,100 ppm F (NaF/silica) reference control dentifrice (Crest Cavity Protection dentifrice; The Procter & Gamble Company). The remaining products in the study were all marketed European formulations.
Table 1.
Dentifrice products tested and their key formulation ingredients
| Test code | Marketed product | Key formulation ingredients |
|---|---|---|
| A | Crest® Cavity Protection* (reference control) | 1,100 ppm F as NaF, silica abrasive |
| B | Crest® Pro-Health* (positive control) | 1,100 ppm F as SnF2, silica abrasive |
| C | Oral B/blend-a-med® Pro-Expert† | 1,450 ppm F as NaF, SnCl2, silica abrasive |
| D | Sensodyne® ProNamel™‡ | 1,450 ppm F as NaF, KNO3, silica abrasive |
| E | Elmex®§ | 1,400 ppm F as AmF, silica abrasive |
| F | Meridol®§ | 1,400 ppm F (AmF + SnF2), silica abrasive |
| G | Colgate® Cavity Protection¶ | 1,450 ppm F (1,000 ppm F as SMFP, 450 ppm F as NaF, calcium glycerophosphate, DCPD abrasive |
| H | Mentadent® (Microgranuli)** | 1,450 ppm F as NaF, zinc citrate, silica abrasive |
| I | Colgate® Enamel Protect¶ | 1,450 ppm F as NaF, KNO3, pyrophosphate, silica abrasive |
| J | Lacalut® aktiv†† | 1,400 ppm F as AlF3 (abrasive not identified) |
| K | Odol-med 3® (samt weiss)‡ | 1,100 ppm F as NaF, pentasodium triphosphate, silica abrasive |
| L | Crest® Decay Prevent† | 1,450 ppm F as NaF, silica abrasive |
The Procter & Gamble Company, Cincinnati, OH, USA.
Procter & Gamble Company, Egham, UK.
GlaxoSmithKline Consumer Healthcare, Brentford, UK.
Gaba, Lörrach, Germany.
Colgate-Palmolive (UK) Ltd, Guildford, UK.
Church & Dwight UK Ltd, Folkestone, UK.
Arcam GmbH, Homburg, Germany.
Collection of human saliva
Eight to 10 healthy volunteers were recruited to provide human saliva for this study. Restrictions (e.g. no use of chemotherapeutic oral care products, antibiotics, etc.) were in place to ensure that saliva samples did not influence results. Volunteers chewed paraffin wax, expectorating any stimulated saliva generated into a plastic collection vessel over a period that averaged 30–40 minutes per volunteer per collection period. Saliva, collected from each volunteer each day of the study, was pooled and stored under refrigeration (approximately 5 °C) until use, ensuring a relatively constant supply of fresh saliva for use in the study. All necessary precautions were in place to ensure proper handling of saliva from the point of collection to its use in the study.
Specimen collection and preparation
Specimens used for this study were all prepared from extracted, human teeth. Upper incisors, typically removed for orthodontic reasons, were obtained from local oral surgeons who had extracted them. Proper protocols were in place to ensure appropriate handling of teeth from the point of collection to preparation of specimens and use of them in the study. Each tooth was cleaned and checked for any visible surface cracks or other imperfections. Those with any visible imperfections were discarded. All teeth were stored before use at approximately 5 °C in a 1% thymol solution.
Study specimens were prepared from this pool of teeth by cutting a single 3-mm core from each tooth using a diamond core drill (Figure 1a). Enamel cores were mounted in ¼ inch (0.64 cm) diameter Lucite rods using dental acrylic (Durabase; Reliance Manufacturing Company, Worth, IL, USA), leaving only the natural facial surface exposed (Figure 1b). Each specimen was then polished with 600 grit silicon carbide-water slurry, removing approximately 50 μm of the outer enamel. Specimens were then polished for 90 minutes with gamma alumina (Linde No. 3, AB Gamma Polishing Alumina; Buehler Limited, Lake Bluff, IL, USA). Any specimen found to have surface imperfections was rejected. Nail polish (#551; Revlon, New York, NY, USA) was then applied to the surface, leaving a treatment window of unprotected enamel approximately 3.0 × 0.4 mm (Figure 1b). Specimens were randomly assigned to treatment groups of four specimens each (Figure 1c).
Figure 1.
Visual description of the model. (a) example of a 3 mm core of human enamel removed from an extracted, human tooth; (b) 3 mm cores of enamel after mounting in ¼ inch (0.64 cm) diameter Lucite rods, then ground and polished in preparation for the study (left) and after placement of nail polish on the tooth surface, leaving a treatment window approximately 0.4 mm wide; (c) each group of four specimens was mounted in a holding appliance that enabled treatment of all specimens in the group at the same time; (d) the holding appliance with four rod-mounted specimens is attached to the bit of a controlled-speed motor and suspended for treatment; (e) after treatment, each group of specimens is suspended in pooled, human saliva using gentle agitation (stirring bars) over a multi-place stir-plate.
Daily cycling protocol
Each group of four specimens was placed into 20 ml of fresh, pooled human saliva for 1 hour to initiate the formation of a pellicle layer on the enamel surfaces. For the treatment phase of the study, dentifrice slurries were prepared by mixing 5 g of dentifrice with 15 g of fresh, pooled human saliva (1:3 slurry, w/w) for a period of not less than 4 minutes or more than 5 minutes before use. A fresh slurry was prepared for each treatment. Each treatment cycle consisted of: dentifrice slurry (2 min) ⇒ rinse in deionised, distilled (ddi)H2O ⇒ saliva (1 hour) ⇒ erosion challenge (10 min) ⇒ rinse in ddiH2O ⇒ saliva. There were four treatments per day for a total of five treatment days. Dentifrice treatments consisted of immersing and rotating each group of four specimens in the dentifrice slurry for 2 minutes at 75 rpm (Figure 1d). The erosion challenge consisted of soaking each treatment group in 12 ml of 1% citric acid at a neat pH of around 2.3 (at room temperature). When not undergoing treatment, specimens remained in 20 ml of pooled, human saliva that was gently stirred (Figure 1e). The saliva was refreshed three times per day. At night, each group of specimens remained immersed in pooled saliva.
Post-treatment specimen handling
After 5 days of treatment, specimens were thoroughly rinsed using ddiH2O, then placed in a closed, humid environment and kept refrigerated (approximately 5 °C) to minimise the potential for any bacterial growth.
Analysis of specimens
Before analysis, a layer of nail polish was applied to the entire surface of each specimen to seal the surface and protect the fragile eroded areas against loss from vibration during sectioning. Specimens were cut plano-parallel using a hard tissue sectioning saw (Silverstone-Taylor Hard Tissue Microtome; Scientific Fabrications, Littleton, CO, USA). Each section was cut to allow the control and treated portion to be analysed together. A thin section (~100 μm) was removed from each specimen, placed flat on a specially designed mount that was then fitted to a camera connected to an X-ray generator (Model #PW1830; Philips Analytical, Natick, MA, USA) and exposed to CuKα radiation. Micrographs were taken using Kodak SO253 Holographic film (Eastman Kodak Company, Rochester, NY, USA). The film was processed using standard film developing methods. Radiographic images were analysed using TMR, a computer-based image analysis system (Inspektor Research Systems BV). By comparing the original surface, based on the control (untreated) area, with the post-treatment surface, the depth of the eroded area was measured (μm of mineral lost)14.
Statistical methods
Statistical analyses for each study were performed using Fisher’s least significant difference (LSD) Test. All comparisons were performed at the 0.05 level of significance.
RESULTS
Results are presented in Table 2, which include the mean surface loss or depth of erosion per treatment group (±SEM) and the statistical groupings based on the LSD analyses. Relative to the 1,100 ppm F (NaF/silica) control, products demonstrated a wide range of potential erosion protection benefits, with the stabilised SnF2 dentifrice and the stabilised Sn-containing NaF dentifrice both providing significantly better protection compared with all of the other products tested. There were no statistically significant differences in erosion protection between the stabilised SnF2 and the stabilised Sn-containing NaF dentifrices. While the 1,450 ppm F (NaF/silica) dentifrice performed significantly better than the 1,100 ppm F (NaF/silica) reference control, demonstrating the model’s sensitivity to fluoride dose, three of the marketed European dentifrices (J: 1,400 ppm F as AlF3; D: 1,450 ppm F as NaF + KNO3; and H: 1,450 ppm F as NaF + zinc citrate) performed at a level that was not significantly different from the 1,100 ppm F (NaF/silica) control. All of the remaining marketed products, formulated with 1,400/1,450 ppm F as AmF, SMFP/NaF or NaF, performed at a level that was not significantly different from the marketed, 1,450 ppm F (NaF/silica) dentifrice.
Table 2.
Results and statistical analysis
DISCUSSION
The purpose of the present study was to investigate the anti-erosion potential of a marketed, Sn-containing NaF dentifrice compared with a number of other commercially available European dentifrices containing a range of fluoride/active systems using a credentialled erosion cycling model14., 24..
The model mimics the dynamics of the dental erosion process, beginning with pellicle-coated human enamel and ends with significant levels of erosive surface tissue loss. Results from this model have been demonstrated to parallel those found in an in situ erosion clinical model that tested the same two products included in this study as positive and reference controls21. This model provides an efficient means to compare the ability of oral care products to protect exposed tooth surfaces against erosive acid damage. One study using this model demonstrated directionally better performance for products formulated with 1,450 ppm F compared with a control product formulated at 1,100 ppm F (NaF)24. In that study, products were not matched by F source, with the higher fluoride products containing either a combination of NaF and SMFP or SMFP alone. In the present study, two essentially identical products (except for the total level of F) were compared. In this case, the 1,450 ppm F (as NaF) version of the product performed significantly better than its 1,100 ppm F (NaF) counterpart, confirming the model’s sensitivity to fluoride dose, at least over the range of fluoride levels found globally in fluoride dentifrices sold in over-the-counter consumer markets. The model has demonstrated clear differences in previous studies between stabilised SnF2 and NaF, SMFP and combination NaF/SMFP-containing dentifrices14., 24.. However, the previous studies did not include other SnF2 formulations, AmF, AlF2 or other combination-active formulations other than one dentifrice that contained a combination of NaF and SMFP24.
The findings of the present study are in agreement with the previous studies in that the stabilised SnF2 dentifrice included in both the in vitro14., 24. and in situ clinical21 trials again performed significantly better than the 1,100 ppm F (NaF/silica) reference control. The stabilised, Sn-containing NaF dentifrice included in the current study provided a level of erosion protection that was not significantly different from this positive control benchmark, demonstrating enhanced performance over all of the other products formulated at the 1,400/1,450 ppm F level. This result strongly suggests the simultaneous delivery of both stannous and fluoride from a stabilised formulation containing NaF and SnCl2, which forms SnF2 in situ during brushing, can provide a level of protection that is not unlike that provided by the stabilised SnF2 formula. This is important because it increases the potential to develop a broader range of future products intended to deliver enhanced erosion protection benefits.
One of the other test products contained at least some level of SnF2. This product, formulated with a combination of AmF and SnF2, is reported by the manufacturer to contain a total of 1,400 ppm F, although the manufacturer does not report the individual level of each active ingredient. On the manufacturer’s website, a description of this product suggests that the SnF2 is semi-stabilised in the formula and carried to the tooth surface via an amine fluoride ‘cage’, with the SnF2 being released during brushing25. Although the performance of this dentifrice was directionally better than many of the other European products tested, it did not perform statistically better than the 1,400 ppm F (AmF), 1,450 ppm F (NaF), 1,450 ppm F (AlF3) or 1,450 ppm F (NaF + SMFP) dentifrices. This combination AmF + SnF2 product was approximately half as effective as the stabilised, Sn-containing NaF dentifrice. Specimens treated with the stabilised, Sn-containing NaF dentifrice resulted in an average surface loss of 6.5 μm, while specimens treated with the AmF + SnF2 product suffered an average of 12 μm of tooth surface loss. This suggests that although a product may contain SnF2, the ability of the SnF2 to release from the product and protect the enamel surface may be very different from one formulation to another, thus necessitating the need to confirm performance of each individual formulation rather than assume that the mere presence of a particular active ingredient will ensure effective erosion protection benefits.
Other products included in the study provided marginal levels of effectiveness, with a few demonstrating a statistically significant improvement in effect compared with the 1,100 ppm F (NaF/silica) reference control. However, none of these remaining products provided a statistically significant benefit that was greater than that provided by the conventional dentifrice that contained 1,450 ppm F (NaF/silica). This would suggest that the level of benefit provided by each of these formulations was more likely related to fluoride dose than any other component included in their respective formulations.
CONCLUSION
These results support the potential for the stabilised, Sn-containing NaF dentifrice to provide erosion protection benefits that are not significantly different from the positive control benchmark for erosion protection (stabilised SnF2), and are significantly better than a broad range of formulations available on the European market.
Acknowledgements
This study was funded by The Procter & Gamble Company, Mason, OH 45040, USA.
Conflicts of interest
R. V. Faller is a retired Principal Scientist from The Procter & Gamble Company, Mason, OH, USA and is now an Associate Professor at the Kornberg School of Dentistry, Temple University, Philadelphia, PA, USA. S. L. Eversole (Principal Researcher) and K. Saunders-Burkhardt (Researcher) are full-time employees at The Procter & Gamble Company.
REFERENCES
- 1.White D, Cox E, Suszcynskymeister E, et al. In vitro studies of the anticalculus efficacy of a sodium hexametaphosphate whitening dentifrice. J Clin Dent. 2002;13:33–37. [PubMed] [Google Scholar]
- 2.White DJ, Cox ER. In vitro studies of the anticalculus efficacy of an improved whitening dentifrice. J Clin Dent. 2001;12:38–41. [PubMed] [Google Scholar]
- 3.Featherstone JD, Shariati M, Brugler S, et al. Effect of an anticalculus dentifrice on lesion progression under pH cycling conditions in vitro. Caries Res. 1988;22:337–341. doi: 10.1159/000261133. [DOI] [PubMed] [Google Scholar]
- 4.Nathoo S, Mateo LR, Delgado E, et al. Extrinsic stain removal efficacy of a new dentifrice containing 0.3% triclosan, 2.0% PVM/MA copolymer, 0.243% NaF and specially-designed silica for sensitivity relief and whitening benefits as compared to a dentifrice containing 0.3% triclosan, 2% PVM/MA copolymer, 0.243% NaF and to a negative control dentifrice containing 0.243% NaF: a 6-week study. Am J Dent. 2011;24:28A–31A. Spec No A. [PubMed] [Google Scholar]
- 5.Yudhira R, Peumans M, Barker ML, et al. Clinical trial of tooth whitening with 6% hydrogen peroxide whitening strips and two whitening dentifrices. Am J Dent. 2007;20:32A–36A. Spec No A. [PubMed] [Google Scholar]
- 6.Faller RV. Meeting the challenges of tooth sensitivity and dental erosion with stannous fluoride. Cosmet Toiletr. 2012;127:362–371. [Google Scholar]
- 7.Kanapka JA. Over-the-counter dentifrices in the treatment of tooth hypersensitivity. Review of clinical studies. Dent Clin North Am. 1990;34:545–560. [PubMed] [Google Scholar]
- 8.Ganss C, Lussi A, Grunau O, et al. Conventional and anti-erosion fluoride toothpastes: effect on enamel erosion and erosion abrasion. Caries Res. 2011;56:581–589. doi: 10.1159/000334318. [DOI] [PubMed] [Google Scholar]
- 9.White AJ, Jones SB, Barbour ME, et al. Inhibition of erosive dissolution by sodium fluoride: evidence for a dose-response. J Dent. 2012;40:654–660. doi: 10.1016/j.jdent.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Featherstone JD. Dental caries: a dynamic disease process. Aust Dent J. 2008;53:286–291. doi: 10.1111/j.1834-7819.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 11.Lussi A, Schlueter N, Rakhmatullina E, et al. Dental erosion – an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011;45(Suppl. 1):2–12. doi: 10.1159/000325915. [DOI] [PubMed] [Google Scholar]
- 12.Packer CD. Cola-induced hypokalemia: a super-sized problem. Int J Clin Pract. 2009;63:833–835. doi: 10.1111/j.1742-1241.2009.02066.x. [DOI] [PubMed] [Google Scholar]
- 13.Faller RV, Eversole SL. Enamel protection from acid challenge – benefits of marketed fluoride dentifrices. J Clin Dent. 2013;24:25–30. [PubMed] [Google Scholar]
- 14.Faller RV, Eversole SL, Tzeghai GE. Enamel protection: a comparison of marketed dentifrice performance against dental erosion. Am J Dent. 2011;24:205–210. [PubMed] [Google Scholar]
- 15.Gambon DL, Brand HS, Veerman ECI. Dental erosion in the 21st century: what is happening to nutritional habits and lifestyle in our society? Br Dent J. 2012;213:55–57. doi: 10.1038/sj.bdj.2012.613. [DOI] [PubMed] [Google Scholar]
- 16.Stookey GK, Mau MS, Isaacs RL, et al. The relative anticaries effectiveness of three fluoride-containing dentifrices in Puerto Rico. Caries Res. 2004;38:542–550. doi: 10.1159/000080584. [DOI] [PubMed] [Google Scholar]
- 17.Mankodi S, Bartizek RD, Winston JL, et al. Anti-gingivitis efficacy of a stabilized 0.454% stannous fluoride/sodium hexametaphosphate dentifrice. J Clin Periodontol. 2005;32:75–80. doi: 10.1111/j.1600-051X.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 18.Mallatt M, Mankodi S, Bauroth K, et al. A controlled 6-month clinical trial to study the effects of a stannous fluoride dentifrice on gingivitis. J Clin Periodontol. 2007;34:762–767. doi: 10.1111/j.1600-051X.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 19.Schiff T, He T, Sagel L, et al. Efficacy and safety of a novel stabilized stannous fluoride and sodium hexametaphosphate dentifrice for dentinal hypersensitivity. J Contemp Dent Pract. 2006;7:1–8. [PubMed] [Google Scholar]
- 20.Schiff T, Saletta L, Baker RA, et al. Desensitizing effect of a stabilized stannous fluoride/sodium hexametaphosphate dentifrice. Compend Contin Educ Dent. 2005;26(Suppl. 1):35–40. [PubMed] [Google Scholar]
- 21.Hooper SM, Newcombe RG, Faller R, et al. The protective effects of toothpaste against erosion by orange juice: studies in situ and in vitro. J Dent. 2007;35:476–481. doi: 10.1016/j.jdent.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Huysmans MCDNJM, Jager DHJ, Ruben JL, et al. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011;45:518–523. doi: 10.1159/000331391. [DOI] [PubMed] [Google Scholar]
- 23.Faller RV, Eversole SL, Yan J. Anticaries potential of a stabilized stannous-containing sodium fluoride dentifrice. Am J Dent. 2010;23(Sp Is B):32B–38B. [PubMed] [Google Scholar]
- 24.Eversole SL, Saunders-Burkhardt K, Faller RV. Erosion protection comparison of stabilised SnF2, mixed fluoride active and SMFP/arginine-containing dentifrices. Int Dent J. 2014;64(Suppl. 1):22–28. doi: 10.1111/idj.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.http://www.gaba.com/htm/417/en/Amine-fluoride-stannous-fluoride.htm?Subnav2=AmineFluoride_StannousFluoride. Accessed 11 November 2013