Abstract
Aim: To characterise and measure the Schneiderian membranes of individuals with periodontal diseases in China and to analyse the factors impacting maxillary sinus mucosal thickness using cone-beam computed tomography (CBCT). Material and method: A cohort of 221 patients with periodontal disease was subjected to cross-sectional CBCT examination. Various parameters, including age, sex, alveolar bone loss, furcation lesions and vertical infrabony pockets, were analysed as correlates of mucosal thickening (MT). Sinus mucosal thickness ≥2 mm qualified as MT. Results: MT was detected in 103 (48.9%) patients, increasing in frequency as the degree of alveolar bone loss advanced (mild, 14.5%; moderate, 29.5%; severe, 87.9%). The association between MT and vertical infrabony pockets was statistically significant (P < 0.001). The likelihood of MT increased with moderate [odds ratio (OR) = 1.02] and severe (OR = 4.62) periodontal bone loss (P < 0.001), as well as with furcation lesions (OR = 2.76) and vertical infrabony pockets (OR = 13.58). Conclusions: Relative to the case in patients with periodontitis and normal mucosa, the probability of MT increased dramatically as alveolar bone loss worsened. Periodontal pathologies (i.e. furcation lesions and vertical infrabony pockets) were also more likely to coincide with MT.
Key words: Periodontitis, mucosal thickening, alveolar bone loss, cone-beam computed tomography
INTRODUCTION
Periodontal disease is the most prevalent infectious disease in humans and it can considerably impact systemic health1. The most destructive form of periodontal disease is periodontitis, which has a high prevalence in China. According to a targeted epidemiological investigation, only 14.5% of Chinese people older than 35 years of age enjoy periodontal health2.
Periodontitis is a chronic oral infection generated and sustained by a polymicrobial biofilm in the mouth. The resultant immunoinflammatory response alters both the mucosa and the supportive connective tissue elements, stimulating net resorption of alveolar bone3., 4., 5., 6.. Maxillary sinusitis, which can arise from bacterial, fungal or viral infections7, is often attributed to periodontal disease8., 9.. Recent efforts have actually shown that most instances of maxillary sinusitis can be traced to odontogenic origins10. Mucosal thickening (MT) commonly develops in conjunction with chronic maxillary sinusitis11 and it is probably pathologic when it is >2 mm. However, even MT of up to 4–5mm can be asymptomatic, going unnoticed by those in whom it is present.
The close anatomical proximity of the posterior maxilla to the maxillary sinuses renders the maxilla vulnerable to periodontal inflammation. Furcation lesions and vertical infrabony pockets of the posterior teeth are also common accompaniments of periodontitis, especially severe periodontitis. Because conventional diagnostics (i.e. intraoral and panoramic radiographs) in the past showed limited reliability12, cone-beam computed tomography (CBCT) is now widely used for imaging studies of the oral cavity and the maxillofacial region. In comparison with conventional diagnostics, CBCT provides superior diagnostic accuracy in defining periodontal bone defects and the soft-tissue morphology of the maxillary sinus floor13. CBCT imaging is also a better means of assessing crater and furcation defects. Three-dimensional (3D) views are ideal for evaluating infrabony abnormalities. In addition, CBCT has lower radiation requirements and is less costly than traditional computed tomography (CT)14, making it the preferred non-invasive and quantitative technique for studying both the periodontal tissues and the maxillary sinus.
In the current study, advanced CBCT was used to measure periodontal bone height, to evaluate furcation lesions and vertical infrabony pockets and to assess the maxillary sinus mucosa in patients under periodontal care. Along with clinical input regarding patient age, sex and other relevant parameters, a full analysis of the relationship between MT and periodontitis was undertaken, focusing on MT as a potential risk factor for periodontitis. In drawing attention to MT, we also aimed to raise awareness of prevention and treatment measures for periodontitis, thus reducing the incidence of secondary maxillary sinusitis.
MATERIALS AND METHODS
Recruitment of patients
Between May 2012 and November 2012, all CBCT images (n = 290) taken to diagnose periodontitis in the Department of Periodontics, Stomatological Hospital of the China Medical University, were analysed. Patient sex and age were recorded, and the following eligibility criteria were used: (i) maxillary premolar or molar teeth free of dental caries and periapical lesions; (ii) no missing premolar and molar teeth in the maxilla; and (iii) no maxillary implants. The criteria for exclusion were as follows: (i) pregnancy; (ii) nursing; (iii) antibiotic use in the 2 months before recruitment; (iv) common cold or flu accompanied by fever, stuffiness, nasal discharge, pain and tenderness to pressure or swelling over the sinus in the 4 weeks before recruitment15; (v) a predisposition to active seasonal (e.g. pollen) or perennial (e.g. mould and dust) allergies; (vi) use of nasal drops; (vii) active asthma; (viii) use of vasoconstrictive medications or cocaine16; and (ix) partial opacification, total opacification and polypoidal MT demonstrated by CBCT. Ultimately, 221 patients (113 male and 108 female) seeking periodontal care qualified for inclusion in the study. The mean age of the patients was 30.1 years (range, 17–71 years).
To evaluate the impact of age on the prevalence and severity of maxillary sinus MT, the patients were stratified as follows17: 0–18 years of age (juveniles); 19–25 years of age (young adults); 26–40 years of age (adults); 41–60 years of age (middle-aged adults); and >60 years of age (geriatric adults).
The research was permitted with approval from the Local Research Ethics Committee of China Medical University (no. 2012-03). All candidates agreed to periodontal and imaging examinations, and provided signed informed consent before trial enrolment. Parents or guardians provided consent for the juvenile study participants (i.e. those <18 years of age). The consent procedure consisted of a written form and was approved by the Ethics Committee. Our research was conducted in full accordance with the World Medical Association Declaration of Helsinki.
Periodontal examination
Each patient was subjected to a professional oral examination to determine probing depth (PD), clinical attachment loss (CAL) and sulcus bleeding index (SBI, scored 0–5)18 from six sites (mesiobuccal, buccal, distobuccal, mesiolingual, lingual and distolingual) of the posterior teeth. The PD (mm) was defined as the distance from the gingival margin to the base of the periodontal pocket, and CAL (mm) was defined as the distance from the cemento–enamel junction (CEJ) to the base of the pocket. Furcation lesions were categorised as follows19: Class 0, no horizontal loss of periodontal tissue support (in addition to the original scoring scale); Class I, ≤3 mm of horizontal loss of periodontal tissue support; Class II, >3 mm of horizontal loss of periodontal tissue support, with no through-and-through furcation; and Class III, through-and-through furcation (requiring seeing the tip of the probe at the contralateral furcation opening)19. Information on smoking history was simply recorded as never smoked (i) or daily/occasional smoking (ii).
All clinical measurements were determined by two professionally trained periodontal dentists (H.J.Z. and S.R.; kappa > 0.85). The range of kappa coefficients (±1 mm) between examiners was 0.81–0.93 for PD (serial readings, 0.89–0.95) and 0.83–0.94 for CAL (serial readings, 0.83–0.93).
Imaging procedures
CBCT images of the patients were acquired using a 3D CBCT scanner (QR-NIM s.r.l., Verona, Italy) with standard parameters (110 kVp, 5.0 mA 160 μm, 3.6 s). The field of view was 20 cm × 25 cm and the images were reconstructed using the system’s NNT software (QR-NIM s.r.l.). Slices at 0.25-mm intervals were reconstructed on the sagittal and coronal planes for overall evaluation and measurements. To ensure data reliability, all images were reconstructed and assessed under standardised conditions at a single workspace by two general dentists, each of whom was trained (1 week apart) by experienced radiologists. The kappa coefficient for observations was 0.91, and the range of the coefficients for repeated assessment was 0.88–0.92.
Diagnosis and classification of MT
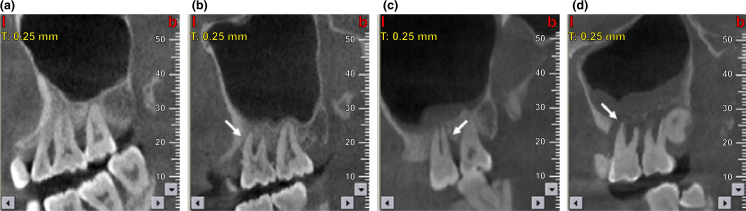
Maxillary sinus mucosal thickness of ≥2 mm served as the cut-off for MT20. Mucosal thickness (mm) was measured at the highest thickness from the sinus floor. Classification of MT was performed as follows21: (i) none; (ii) <2 mm, normal; (iii) 2–4 mm, mild; (iv) 4–10 mm, moderate; and (v) >10 mm, severe (Figure 1). In each patient, MT was considered to be present if at least one sinus qualified.
Figure 1.
Cone beam computed tomography (CBCT) images of maxillary sinus mucosa: (a) normal mucosa in patients with periodontitis; (b) mild mucosal thickness (MT), left maxillary sinus (a 28-year-old woman, with furcation lesion of Tooth 26); (c) moderate MT, left maxillary sinus (a 41-year-old man, with a vertical infrabony pocket of Tooth 26 and peak-type MT); and (d) severe MT, left maxillary sinus (a 32-year-old man, with the sinus floor gap penetrated by inflammation caused by periodontitis).
Classification of alveolar bone loss
The measurements of bone loss at the maxillary premolar and molar areas were computer generated (using NNT software). Normal periodontal bone height was determined by measuring from the CEJ to the top of the alveolar bone crest (ABC)22, adjusting the images on the axial plane and placing the necks of the teeth in cross-section. The sites measured included the mesial, distal, buccal and lingual surfaces, selecting three points at each surface23. The average values at each site served as alveolar bone loss decreases for statistical analysis. The results were accurate to 0.1 mm.
We classified the degree of alveolar bone loss as follows24: (i) mild (the distance from the CEJ to the top of ABC was >2 mm but not beyond one-third of the root at the crown); (ii) moderate (the distance from the CEJ to the ABC was within the middle one-third of the root); and (iii) severe (the distance from the CEJ to the ABC reached the apical one-third).
Imaging analysis and classification of lesions associated with periodontitis
Furcation lesions (radiolucencies at furcations of the maxillary molars; Figure 1b) and vertical infrabony pockets (Figure 1c) were also examined during the course of our study. Vertical infrabony pockets were categorised as follows25: (1) no pocket; (2) pocket ≥3 mm deep, extending to the middle third of the root; or (3) pocket extending to the apical third of the tooth25 (Vallo et al. 2010 Page: e82). All aspects of each tooth were assessed on sagittal, coronal and axial views. Any sextant affected had at least one potential site for such lesions26.
Statistical analysis
Date were analysed using spss 13.0 (Chicago, IL, USA). The χ2 test was performed to analyse the prevalence of MT between groups with varying degrees of alveolar bone loss. Comparison of clinical parameters in patients with and without MT was achieved using the independent-sample t-test. The relationships between MT and periodontal pathologies, such as furcation lesions, vertical infrabony pockets and penetrated sinus floors, are expressed as odds ratios (ORs) with 95% confidence interval (95% CI). Analysis of variance (ANOVA) was applied to compare the risk factors for furcation lesions and vertical infrabony pockets. All statistical tests were two-tailed, with the level of significance (P value) <0.05.
RESULTS
Prevalence of MT according to degree of alveolar bone loss
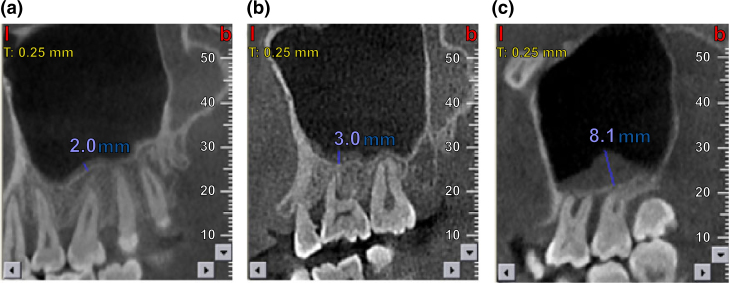
Among the 221 periodontal patients studied, 103 (48.9%) displayed MT. When analysed with regard to extent of alveolar bone loss, the prevalence of MT paralleled the degree of alveolar bone loss, with 14.5%, 29.5%, and 87.9% of patients showing mild, moderate, and severe alveolar bone loss, respectively (CBCT images shown in Figure 2). These results indicate a dramatic increase in MT as the degree of alveolar bone loss advances (P < 0.001).
Figure 2.
Alveolar bone loss in patients with maxillary sinus mucosal thickening (MT): (a) mild loss (a 35-year-old man, with 2 mm left maxillary sinus MT); (b) moderate loss (a 45-year-old woman, with 3 mm left maxillary sinus MT); and (c) severe loss (a 52-year-old man, with 8.1 mm left maxillary sinus MT).
Prevalence of mucosal thickening according to sex, age and smoking
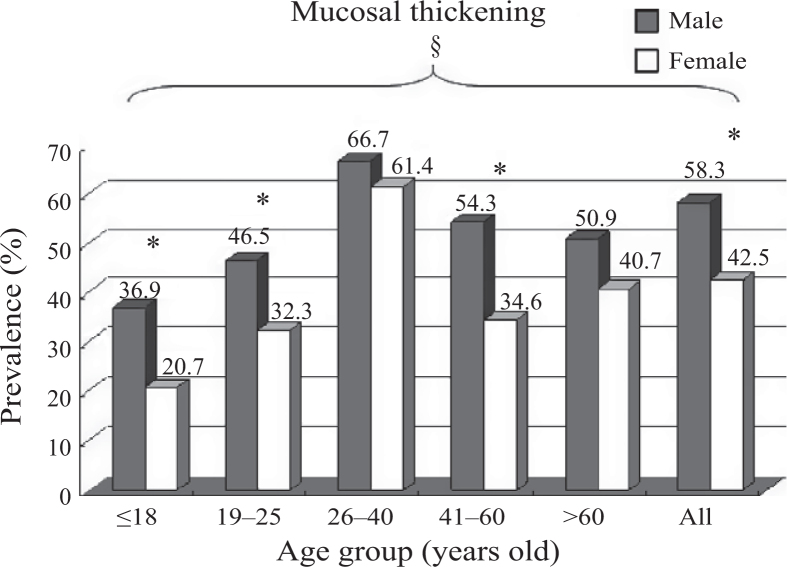
The prevalence of MT was 58.3% in male patients and 42.5% in female patients. According to age, the prevalence of MT was 22.2% in juveniles (≤18 years of age), 38.5% in young adults (19–25 years of age), 58.6% in adults (26–40 years of age), 45.2% in middle-aged adults (41–60 years of age) and 53.3% in geriatric adults (>60 years of age). The prevalence of MT was significantly higher in patients >26 years of age than in younger patients (<25 years of age) and was significantly higher in male patients than in female patients (both groups, P < 0.05) (Figure 3). The prevalence of smoking was 46% (52/113) in male patients and 4.9% (5/108) in female patients.
Figure 3.
Maxillary sinus mucosal thickening (MT), stratified by age and sex (male predominance) *Sex differences, P < 0.05. §Age differences, P < 0.05.
Comparison of clinical parameters in patients with and without MT
The mean PD and CAL measurements were significantly higher in patients with MT (PD, 3.65 mm; CAL, 3.81 mm) than in patients with normal mucosa (PD, 3.05 mm; CAL, 3.25 mm) (P < 0.05). There were no significant differences in SBI with and without MT (P > 0.05). These results suggest a worse periodontal status in patients with MT than in those with normal mucosa (Table 1).
Table 1.
Probing depth (PD) and clinical attachment loss (CAL) determinations, with and without mucosal thickening (MT) (note the significantly higher PD and CAL values in patients with MT)
| Maxillary sinus with no MT | Maxillary sinus with MT | t | P | |
|---|---|---|---|---|
| PD | 3.06 ± 0.88 | 3.65 ± 0.81 | 0.326 | 0.027* |
| CAL | 3.25 ± 0.76 | 3.81 ± 0.73 | 0.253 | 0.004* |
Values are given as mean ± standard deviation.
P < 0.05.
Severity and prevalence of maxillary sinus MT in association with adjuncts of periodontitis
The relationships between maxillary sinus MT and furcation lesions or vertical infrabony pockets were also analysed. Patients with furcation lesions were more likely to display mild MT (mean ± SE, 2.5 ± 0.26; range, 0–5.5 mm; prevalence, 69.8%), whereas MT tended to be moderate in instances of vertical infrabony pockets (mean ± SE, 5.2 ± 0.46; range, 0.5–19 mm; prevalence, 90.2%). Intergroup differences in MT, stratified according to lesions associated with periodontitis (furcation lesions and vertical infrabony pockets), were significant (P < 0.001), as were differences in MT among furcation lesions (Table 2).
Table 2.
Mucosal thickening, stratified according to lesions associated with periodontitis (furcation lesions and vertical infrabony pockets)
| Variable | Furcation lesions | Vertical infrabony pockets |
|---|---|---|
| n | 43 | 51 |
| Minimum (mm) | 0 | 0.5 |
| Maximum (mm) | 5.5* | 19.0* |
| Mean ± SE | 2.48 ± 0.26† | 5.25 ± 0.46† |
| Prevalence of mucosal thickening (%) | 69.8‡ | 90.2‡ |
n, number of sinuses; SE, standard error.
Statistically significant intergroup differences in maximal (mm) mucosal thickening, P < 0.001.
Statistically significant intergroup differences in prevalence of mucosal thickening, P < 0.001.
Statistically significant intergroup differences in mean (mm) mucosal thickening, P < 0.001.
Risk factors for MT of the maxillary sinus
Using binary logistic analysis, adults (26–40 years of age) were more likely to have MT (OR = 2.96, 95% CI: 1.29–6.78; P < 0.05) than were patients <25 years of age (Table 3). The likelihood of MT also increased commensurate with more severe periodontal bone loss (moderate: OR = 1.02, 95% CI: 1.07–1.36; severe: OR = 4.62, 95% CI: 3.37–6.33; P < 0.001). The associations of MT with furcation lesions (OR = 2.76, 95% CI: 1.73–4.41; P < 0.001) and with vertical infrabony pockets (OR = 13.58, 95% CI: 6.26–29.49; P < 0.001) were pronounced (Table 3).
Table 3.
Mucosal thickening, stratified according to age, periodontal bone loss, furcation lesions, vertical infrabony pockets, and penetrated sinus floors
| Variable | Category | OR† | 95% CI | P-value‡ |
|---|---|---|---|---|
| Sex | Male vs. female | 1.74 | 1.05–3.00 | 0.041* |
| Age (years) | 26–40 vs. <25 | 2.96 | 1.29–6.78 | 0.013* |
| 41–60 vs. <25 | 1.28 | 0.57–2.87 | 0.684 | |
| ≥60 vs. <25 | 1.97 | 0.55–7.16 | 0.339 | |
| 41–60 vs. 26–40 | 1.71 | 0.94–3.12 | 0.095 | |
| ≥60 vs. 26–40 | 1.42 | 0.46–4.39 | 0.572 | |
| ≥60 vs. 41–60 | 0.97 | 0.30–3.12 | 1.000 | |
| Periodontal bone loss | Moderate vs. mild | 1.02 | 1.07–1.36 | 0.000** |
| Severe vs. mild | 4.62 | 3.37–6.33 | 0.000** | |
| Furcation lesions | Yes vs. no | 2.76 | 1.73–4.41 | 0.000** |
| Class I vs. Class 0 | 0.32 | 0.21–1.98 | 0.761 | |
| Class II vs. Class 0 | 1.56 | 0.93–2.69 | 0.011* | |
| Class III vs. Class 0 | 3.48 | 1.11–3.92 | 0.008* | |
| Vertical infrabony pockets | Yes vs. no | 13.58 | 6.26–29.49 | 0.000** |
| 2 vs. 1 | 2.36 | 1.51–3.13 | 0.026* | |
| 3 vs. 1 | 5.58 | 2.56–5.31 | 0.018* |
Values are given as odds ratio (OR) and 95% confidence interval (95% CI).
OR scale of disease risk: OR = 1.0–1.1, no risk; OR = 1.2–1.4, low risk; OR = 1.5–2.9, moderate risk; OR = 3.0–9.9, high risk; OR ≥ 10, extremely high risk.
Binary logistic regression analysis.
P < 0.05.
P < 0.001.
DISCUSSION
This study demonstrated that MT is more pervasive in Chinese patients with periodontal disease than has previously been believed. A complex disorder, MT is fuelled by smouldering inflammation, apical periodontitis and other odontogenic infections. The likelihood that the infection is of dental origin increases with the degree of MT27. However, there have been few known reports linking the severity of periodontitis with maxillary sinus MT. Moreover, the early studies all lacked clinical examinations. The present investigation demonstrated positive correlations of MT and periodontitis in periodontal patients with the ages of patients, their sex and various periodontal parameters.
The prevalence of maxillary sinus MT in our study was 14.5%, 29.5% and 87.9% in patients with mild, moderate and severe alveolar bone loss, respectively. The above outcomes are in agreement with those of previously published studies25., 26., establishing the contribution of periodontal disease to maxillary sinus MT. As in a study by Phothikhun et al.26, which demonstrated a three-fold increase in the likelihood of MT with severe periodontitis (OR = 3.02), we found that patients with severe (OR = 4.62) alveolar bone loss were at significantly higher risk for MT than were those with mild alveolar bone loss.
The PD and CAL values recorded were similarly somewhat higher in patients with MT than in those with normal mucosa. Periodontitis is marked by inflammation of the tooth-supporting tissues (periodontal ligament fibres and alveolar bone), caused by bacterial infection1. PD and CAL values are clinical indices of periodontitis. PD increases as the fibres of the periodontal ligaments are progressively destroyed, and alveolar bone is resorbed, in tandem with CAL. Higher PD and CAL values reflect more serious periodontitis. The maxillary sinuses can also become inflamed as a result of bacterial infection, possibly arising in adjacent periodontitis. An earlier study suggested that the bony floor of the maxillary sinus is not continuous but rather is perforated by a number of vessels, allowing for close approximation of the maxillary sinus mucosa and periodontal ligament in the endosteal bony space adjacent to the maxillary molar teeth28. Another factor promoting inflammation is the main blood supply of the dental and periodontal structures: the intra-alveolar branches of the anterior and posterior superior alveolar arteries. Collateral branches of these vessels merge with vessels of the basilar maxillary sinus, forming an intricate vascular network29. Finally, the roots of the maxillary premolar and molar teeth are normally separated from the sinus floor by dense cortical bone of variable thickness, but this separation consists only of mucoperiosteum in some individuals. The latter circumstance is believed to encourage the spread of inflammation to the Schneiderian membrane30. Indeed, there has been abundant evidence to support the notion that inflammation of the maxillary sinus develops from adjacent periodontitis.
Our findings similarly indicated a significant association between MT in sinusitis of periodontal origin and furcation lesions (OR = 2.76) and vertical infrabony pockets (OR = 13.58). These results compared favourably with those of Vallo et al.25, in which furcation lesions (OR = 4.4) and vertical infrabony pockets (OR = 6.3) corresponded with a significantly higher risk of MT, relative to edentulous subjects. Given that furcation lesions and vertical infrabony pockets are characteristic of severe periodontitis, the inherently higher levels of pathogenic bacteria, bacterial products and inflammatory cytokines might easily explain this heightened risk31. A case study by Lane and O’Neal8 suggested that sinusitis could develop from the extension of periodontal disease into the maxillary sinus. Moreover, Moskow and Polson32 did not believe that periodontitis is a localised and marginal disease, and they suggested that its effects might be much more pervasive than previously believed. Based on a histopathological study, Moskow9 also found an apparently direct relationship between moderate and severe periodontitis of the maxillary molar teeth and pathological changes resulting in thickening of the maxillary sinus mucosa.
In our investigation, MT proved to be male predominant (male patients, 58.3%; female patients, 42.5%), more than in similar data generated by Vallo et al.25 (male patients, 18%; female patients, 8%) and by Phothikhun et al.26 (male patients, 50.9%; female patients, 35%), perhaps because of patient selection. All our subjects were referred for periodontal treatment planning, whereas Vallo et al.25 analysed recruits for clinical oral health examinations, and Phothikhun et al.26 studied patients required dental implants. Another potential explanation is that Chinese men are more likely to be smokers (male patients: 46%, 52/113; female patients: 4.9%, 5/108). Although some studies have linked sinusitis to cigarette smoking33., 34., there are currently no compelling data to support this argument. Aside from our simple analysis, further research addressing the factors that might influence the physiology/anatomy of the oral cavity in smokers is clearly needed.
The maxillary sinus varies in size, depending not only on the constitution, but also on the age, of an individual. Our study showed that the prevalence of MT increased in patients 26–40 years of age and declined thereafter. This phenomenon was inconsistent with a study by Shanbhag et al.35, which reported a significant association between MT and age >60 years. As a possible coincidence, it was suggested that the presence of periodontal disease, especially aggressive periodontitis occurring chiefly in young people, might contribute to the prevalence of MT.
We elected to study the relationship between periodontitis and maxillary sinus MT using CBCT. This approach provides better diagnostic and quantitative information about periodontitis than does conventional radiography, particularly in terms of periodontal bone levels, furcation lesions and vertical infrabony pockets. In traditional imaging, which is limited by the projection geometry and the superposition of adjacent anatomic structures, the state of the maxillary sinus floor in patients with periodontitis has generally been ignored. CT scans have become the gold standard in medicine for maxillary sinus visualisation because of the capacity of CT to delineate both bone and soft tissue in thin sections on multiple views36. Nevertheless, it has been difficult to apply CT widely in the oromaxillofacial region. Compared with traditional CT, the radiation exposure and cost of CBCT are lower37, and it can help to clarify the origin and extent of maxillary sinus involvement in periodontitis38. The use of CBCT has clearly depicted the importance of the maxillary sinus in guiding implant treatment in some studies39., 40., 41.. This method might thus be useful in assessing periodontitis-related maxillary sinus problems in candidates for implants.
Although we endeavoured to analyse the relationship between periodontitis and maxillary sinus MT, other pathologic states, such as mucosal cysts, partial opacification and total opacification, performed in the maxillary sinus, were not included. Moreover, owing to the limitations of our facility, only radiographic evidence of the relationship between periodontal disease and MT was pursued. No pathophysiological, microbiological or histopathological assessments of thickened Schneiderian membranes were attempted during this study.
CONCLUSIONS
Within the scope of our work, the prevalence of MT paralleled the degree of alveolar bone loss, with 87.9% of patients displaying MT when alveolar bone loss of the maxillary posterior teeth was severe. Similarly, the periodontal status of patients was worse with MT than with normal mucosa. Furcation lesions and vertical infrabony pockets were more likely to be associated with MT. Further clinical investigations are warranted to determine the benefits of treating sinusitis related to periodontal disease. A close collaborative effort between periodontologists and medical specialists is essential for accurate diagnosis and optimal patient treatment.
Acknowledgements
This research was supported by the 2015 Public Welfare Industry Special Research Project (2015SQ00084), Shenyang, Liaoning, China, and the National Nature Science Foundation of China (81271153). Y. P. Pan was mainly responsible for the design of the study, the assurance of the quality of this paper, and supervised the manuscript as the corresponding author. S. Ren was in charge of the collection and the analysis of the data, drafted the manuscript and conducted the literature review. H. J. Zhao designed the study, collected the data and revised the manuscript. J. B. Liu was in charge of the structure and the rationality of this paper. Q. X. Wang prepared the tables and the figures. All authors have reached an agreement in terms of the final manuscript.
Competing interest
None of the authors declare any competing interest related to this study.
REFERENCES
- 1.Brito F, Almeida S, Figueredo CM, et al. Extent and severity of chronic periodontitis in chronic kidney disease patients. J Periodontal Res. 2012;47:426–430. doi: 10.1111/j.1600-0765.2011.01449.x. [DOI] [PubMed] [Google Scholar]
- 2.Qin XQ. 1st ed. People’s Medical Publishing House; Beijing: 2008. Report About the Third Time Periodontal Status Epidemiological Investigation; p. 24. [Google Scholar]
- 3.Kinane DF, Mark Bartold P. Clinical relevance of the host responses of periodontitis. Periodontol 2000. 2007;43:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000. 2006;42:180–218. doi: 10.1111/j.1600-0757.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 6.Timmerman MF, van der Weijden GA. Risk factors for periodontitis. Int J Dent Hyg. 2006;4:2–7. doi: 10.1111/j.1601-5037.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 7.Jousimies-Somer HR, Savolainen S, Ylikoski JS. Bacteriological findings of acute maxillary sinusitis in young adults. J Clin Microbiol. 1988;26:1919–1925. doi: 10.1128/jcm.26.10.1919-1925.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane JJ, O’Neal RB. The relationship between periodontitis and the maxillary sinus. J Periodontol. 1984;55:477–481. doi: 10.1902/jop.1984.55.8.477. [DOI] [PubMed] [Google Scholar]
- 9.Moskow BS. A histomorphologic study of the effects of periodontal inflammation on the maxillary sinus mucosa. J Periodontol. 1992;63:674–681. doi: 10.1902/jop.1992.63.8.674. [DOI] [PubMed] [Google Scholar]
- 10.Obayashi N, Ariji Y, Goto M, et al. Spread of odontogenic infection originating in the maxillary teeth: computerized tomographic assessment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:223–231. doi: 10.1016/j.tripleo.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Kolo ES. The role of plain radiographs in the diagnosis of chronic maxillary rhinosinusitis in adults. Afr Health Sci. 2012;12:459–463. doi: 10.4314/ahs.v12i4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eickholz P, Hausmann E. Accuracy of radiographic assessment of interproximal bone loss in intrabony defects using linear measurements. Eur J Oral Sci. 2000;108:70–73. doi: 10.1034/j.1600-0722.2000.00729.x. [DOI] [PubMed] [Google Scholar]
- 13.Janner SF, Caversaccio MD, Dubach P, et al. Characteristics and dimensions of the schneiderian membrane: a radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin Oral Implant Res. 2001;22:1446–1453. doi: 10.1111/j.1600-0501.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- 14.Fuhrmann RA, Wehrbein H, Langen HJ, et al. Assessment of the dentate alveolar process with high resolution computed tomography. Dentomaxillofac Radiol. 1995;24:50–54. doi: 10.1259/dmfr.24.1.8593909. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeld RM. Clinical practice guideline on adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:365–377. doi: 10.1016/j.otohns.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Williams JW, Simel DL. Dose this patient have sinusitis? Diagnosing acute sinusitis by history and physical examination. JAMA. 1993;270:1242–1246. [PubMed] [Google Scholar]
- 17.Lu Y, Liu Z, Zhang L, et al. Associations between maxillary sinus mucosal thickening and apical periodontitis using cone-beam computed tomography scanning: a retrospective study. J Endod. 2012;38:1069–1074. doi: 10.1016/j.joen.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Benamghar L, Penaud J, Kaminsky F, et al. Comparison of gingival index and sulcus bleeding index as indicators of periodontal status. Bull World Health Organ. 1982;60:147–151. [PMC free article] [PubMed] [Google Scholar]
- 19.Hamp SE, Nyman S, Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2:126–135. doi: 10.1111/j.1600-051x.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 20.Cagici CA, Yilmazer C, Hurcan C, et al. Appropriate interslice gap for screening coronal paranasal sinus tomography for mucosal thickening. Eur Arch Otorhinolarynol. 2009;266:519–525. doi: 10.1007/s00405-008-0786-6. [DOI] [PubMed] [Google Scholar]
- 21.Rak KM, Newell JD, Yakes WF, et al. Paranasal sinuses on MR images of the brain: significance of mucosal thickening. AJR Am J Roentgenol. 1991;156:381–384. doi: 10.2214/ajr.156.2.1898819. [DOI] [PubMed] [Google Scholar]
- 22.Kim TS, Obst C, Zehaczek S, et al. Detection of bone loss with different X-ray techniques in periodontal patients. J Periodontol. 2008;79:1141–1149. doi: 10.1902/jop.2008.070578. [DOI] [PubMed] [Google Scholar]
- 23.Feijo CV, Lucena JG, Kurita LM, et al. Evaluation of cone beam computed tomography in the detection of horizontal periodontal bone defects: an in vivo study. Int J Periodontics Dent. 2012;32:162–168. [PubMed] [Google Scholar]
- 24.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Vallo J, Suominen-Taipale L, Huumonen S, et al. Prevelence of mucosal abnormalities of the maxillary sinus and their relationship to dental disease in panoramic radiography: results from the Health 2000 Health Examination Survey. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:80–87. doi: 10.1016/j.tripleo.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Phothikhun S, Suphanantachat S, Chuenchompoonut V, et al. Cone bean computed tomographic evidence of the association between periodontal bone loss and mucosal thickening of the maxillary sinus. J Periodontol. 2012;83:557–564. doi: 10.1902/jop.2011.110376. [DOI] [PubMed] [Google Scholar]
- 27.Bomeli SR, Branstetter BF, Ferguson BJ. Frequency of a dental source for acute maxillary sinusitis. Laryngoscope. 2009;119:580–584. doi: 10.1002/lary.20095. [DOI] [PubMed] [Google Scholar]
- 28.Hauman CH, Chandler NP, Tong DC. Endodontic implications of the maxillary sinus: a review. Int Endon J. 2002;35:127–141. doi: 10.1046/j.0143-2885.2001.00524.x. [DOI] [PubMed] [Google Scholar]
- 29.Sicher H. 5th ed. Health Sciences; Mosby: 1962. Orban’s Oral Histology and Embryology; pp. 344–351. [Google Scholar]
- 30.Pagin O, Centurion BS, Rubira-Bullen IR, et al. Maxillary sinus and posterior teeth: accessing close relationship by cone-beam computed tomographic scanning in a Brazilian population. J Endod. 2013;7:748–751. doi: 10.1016/j.joen.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Feng Z, Weinberg A. Role if bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 32.Moskow BS, Polson AM. Histologic studies on the extension of the inflammatory infiltrate in human periodontitis. J Clin Periodontol. 1991;18:534–542. doi: 10.1111/j.1600-051x.1991.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 33.Lieu JE, Feinstein AR. Confirmation and surprises in the association of tobacco use with sinusitis. Arch Otolaryngol Head Neck Srug. 2000;126:940–946. doi: 10.1001/archotol.126.8.940. [DOI] [PubMed] [Google Scholar]
- 34.Reh DD, Higgins TS, Smith TL. Impact of tobacco smoke on chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol. 2012;2:362–369. doi: 10.1002/alr.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddharth S, Prabodh K, Prashant S, et al. Cone-beam computed tomographic analysis of sinus membrane thickness, ostium patency, and residual ridge heights in the posterior maxilla: implications for sinus floor elevation. Clin Oral Implants Res. 2014;25:755–760. doi: 10.1111/clr.12168. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura T, Iizuka T. Evaluation of odontogenic maxillary sinusitis after conservative therapy using CT and bone SPECT. Clin Imaging. 2002;26:153–160. doi: 10.1016/s0899-7071(01)00390-4. [DOI] [PubMed] [Google Scholar]
- 37.Ludlow JB, Ivanovic M. Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:106–114. doi: 10.1016/j.tripleo.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Cymerman JJ, Cymerman DH, O’Dwyer RS. Evaluation of odontogenic maxillary sinusitis using cone-beam computed tomography: three case reports. J Endod. 2011;37:1465–1469. doi: 10.1016/j.joen.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Shokri A, Khajeh S. In vitro comparison of the effect of different slice thicknesses on the accuracy of linear measurements on cone beam computed tomography images in implant sites. J Craniofac Surg. 2015;26:157–160. doi: 10.1097/SCS.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 40.Yepes JF, Al-Sabbagh M. Use of cone-beam computed tomography in early detection of implant failure. Dent Clin North Am. 2015;59:41–56. doi: 10.1016/j.cden.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Vogiatzi T, Kloukos D, Scarfe WC, et al. Incidence of anatomical variations and disease of the maxillary sinuses as identified by cone beam computed tomography: a systematic review. Int J Oral Maxillofac Implants. 2014;29:1301–1314. doi: 10.11607/jomi.3644. [DOI] [PubMed] [Google Scholar]