Abstract
Purpose
Gasdermin D (GSDMD) is a cytoplasmic protein that is encoded by the gasdermin family GSDMD gene and is the ultimate executor of pyroptosis. Pyroptosis is a mode of lysis and inflammation that regulates cell death, ultimately leading to cell swelling and rupture. In sepsis, a dysregulated host response to infection frequently results in hyperinflammatory responses and immunosuppression, eventually leading to multiple organ dysfunction. Pyroptosis regulates innate immune defenses and plays an important role in the process of inflammatory cell death, and the absence of any link in the entire pathway from GSDMD to pyroptosis causes bacterial clearance to be hampered. Under normal conditions, the process of pyroptosis occurs much faster than apoptosis, and the threat to the body is also much greater.
Materials and methods
We conducted a systematic review of relevant reviews and experimental articles using the keywords sepsis, Gasdermin D, and Pyroptosis in the PubMed, Scopus, Google Scholar, and Web of Science databases.
Conclusion
Combined with the pathogenesis of sepsis, it is not difficult to find that pyroptosis plays a key role in bacterial inflammation and sepsis. Therefore, GSDMD inhibitors may be used as targeted drugs to treat sepsis by reducing the occurrence of pyroptosis. This review mainly discusses the key role of GSDMD in sepsis.
Keywords: Gasdermin D, Pyroptosis, Sepsis, Multiple organ dysfunction syndrome
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. It is usually a common complication following infection, surgery, severe burns, poisoning and cardiopulmonary resuscitation, and is an important cause of multiple organ dysfunction syndromes and septic shock [2]. Anyone can become infected, and almost any infection including COVID-19, can lead to sepsis. Septic shock is also one of the main clinical symptoms of severe COVID-19 patients [3]. But sepsis is distinguished from infection by the presence of an abnormal or dysregulated host response and organ dysfunction. Sepsis-induced organ dysfunction can be subtle. As a result, its presence should be considered in any patient who has an infection. Conversely, unrecognized infections may be cause new-onset organ dysfunction. Thus, any unexplained organ dysfunction should raise the possibility of an underlying infection [1]. With the characteristics of high incidence, rapid disease progression, and difficulty of cure, sepsis has gradually become one of the global public health problems due to changes in causative and host factors (e.g., sex, race, and other genetic determinants, age, comorbidities, environment) [4]. According to statistics, there are about 31.5 million sepsis patients worldwide, of which about 5.3 million die from sepsis, and in the United States, more than 750,000 people are hospitalized and 215,000 people died from sepsis [5, 6]. Mervyn explained that the new emphasis on organ dysfunction rather than infection stems from a growing understanding of sepsis pathophysiology, which includes both inflammatory and anti-inflammatory responses [7].
In sepsis, the dysregulation of the body's inflammatory response culminates in a major inflammatory outbreak that is fatal. Recent studies have identified that an inflammatory cell death process known as pyroptosis, a programmed cell death mechanism commonly found in pathogenic infection states, plays an important role in sepsis [8]. During pyroptosis, immune cells release pro-inflammatory cytokines to recruit other immune cells to fight infection, enhance the host's defense response, and facilitate the elimination of invading pathogens [9]. Overactivation of this process, however, is a major cause of immune dysregulation in sepsis. A large number of immune cell pyroptosis in a short period of time will cause the cells to swell and lyse, leading to tissue inflammation. Once unbalanced, it will cause a strong inflammatory storm and organ dysfunction, at the same time immune cell exhaustion [10]. Studies have shown that host-expressed members of the gasdermin family are the final effector proteins of pyroptosis. Among them, the N-terminal and C-terminal structures of Gasdermin D (GSDMD) are in an auto-inhibited state, which can be cleaved by the inflammatory caspases activated by the host inflammasome to release the activated N-terminal GSDMD. It binds to phospholipids located on the inner side of cell membranes, forming pores in the membrane, leading to the release of inflammatory factors and cell rupture. Therefore, GSDMD simultaneously regulates the death of immune cells and the release of related inflammatory factors [11–13]. It was shown that Gsdmd−/− macrophages stimulated by LPS and Gram-negative bacteria do not cause cellular scorching, while Gsdmd−/− mice have a higher survival rate after sepsis induction [14, 15]. Therefore, GSDMD is regarded as a novel and ideal target for drug development in sepsis.
Sepsis and pyroptosis
Pyroptosis was first observed by Sansonetti's team in J774 macrophages that died after being infected by Shigella flexneri. Their electron micrograph results found that cell death had "chromatin condensation, membrane blebbing, cytoplasmic vacuolization, endoplasmic reticulum expansion, but still retains the organelle structure", so it is considered apoptosis [16]. Until 2000, Brennan and Cookson found that the death of macrophages after infection with Salmonella was significantly different from traditional apoptosis [17]. Subsequently, this mode of cell death was officially named "pyroptosis", a term derived from the Greek word pyro, which means "fire", to describe pro-inflammatory programmed cell death [18]. Pyroptosis is a mode of dissolution and inflammation that regulates cell death. Its morphology is the formation of pores with a diameter of 12–14 nm in the cell membrane, and the exchange of ions inside and outside the cell membrane leads to intracellular osmotic pressure increase, cell swelling, and lysis. The signaling pathways are mainly it relies on the inflammasome to activate some proteins of the caspase family, causing it to cleave the gasdermin protein, activate the gasdermin protein, and translocate the activated gasdermin protein to the membrane, forming pores, cell swelling, cytoplasmic outflow, and finally leading to cell membrane rupture, accompanied by inflammatory content. The release of substances induces the occurrence of an inflammatory storm, and at the same time, cell pyroptosis. Pyroptosis can also be divided into canonical and non-canonical pathways [19]. Pyroptosis is also an important natural immune response of the body, helping to fight infections and endogenous danger signals.
Recently, extensive studies have shown that pyroptosis plays an important role in the occurrence and development of sepsis and septic shock [20–22]. Pyroptosis dominates the innate immune defense, and the loss of any link in the entire pathway from GSDMD to pyroptosis will result in obstacles to bacterial clearance [23]. Pyroptosis is usually faster than apoptosis, and the cells are already pyroptotic before apoptosis. The process of pyroptosis is much faster than apoptosis and threatens the body much more [24]. The survival rate of caspase-11 and Gsdmd knockout mice was nearly 100% in an LPS-induced mouse sepsis model [13]. Combined with the pathogenesis of sepsis, it is not difficult to find that pyroptosis plays a key role in bacterial inflammation and sepsis (Fig. 1).
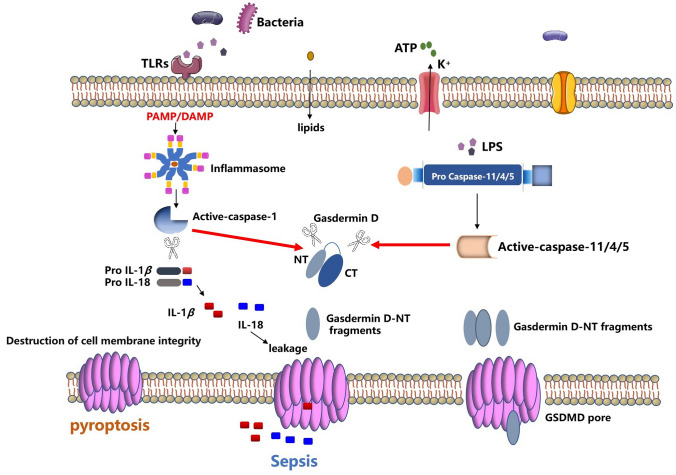
Fig. 1.
An overview of the molecular mechanisms of pyroptosis. Pyroptosis is a type of cell death mediated by caspase-specific proteases (caspase-specific proteases). The canonical pathway of pyroptosis mainly depends on caspase-1. PAMPs and DAMPs associated molecular patterns in the cytoplasm can stimulate inflammasome assembly, leading to the processing and activation of caspase-1, which converts pro-IL-1β and pro-IL-18 cleavage to mature form and cleavage of gasdermin D. The non-canonical pathway of pyroptosis mainly depends on caspase-11 (the human homologous caspase-4/5), activation of caspase 11/4/5 does not normally require PRR-mediated inflammasome, caspase-11 can directly recognize LPS in the cytoplasm for oligomerization and activation, disrupt the membrane structure by cleaving and activating GSDMD. GSDMD has two conserved domains: an N-terminal effector domain and a C-terminal inhibitory domain. The N-terminal is the main functional domain and is involved in the occurrence of pyroptosis, while the C-terminal has an auto-inhibitory function. In the resting state, the N-terminal and C-terminal domains are connected by a long loop. When stimulated by an external signal, Caspase-1/4/5/11 activates the cleavage of GSDMD protein, and GSDMD-NT is activated to bind to the cell membrane. The phospholipid protein binds, multimerizes, and forms a hole in the cytoplasmic membrane, which damages the cytoplasmic membrane and induces the occurrence of pyroptosis. At the same time, activated forms of pro-inflammatory cytokines such as IL-1β and IL-18 are released through the hole, leading to a massive inflammatory response
GSDMD and pyroptosis
In 2018, the Committee on Nomenclature for Cell Death (NCCD) proposed to redefine pyroptosis as the programmed death that plasma membrane pores formed by members of the Gasdermin family of proteins, mostly as a result of inflammatory caspases activation [25]. Therefore, how closely is the relationship between GSDMD and pyroptosis? With the discovery of pyroptosis, the only thing clear about its signaling pathway is that caspase-1 can activate IL-1β after infection and cause cell death. The in-depth study of the inflammasome has clarified the signaling pathway upstream of caspase-1. During the process, the inflammasome receptor forms an inflammatory complex and caspase-1 in the cell by recognizing different infection and injury stimuli and makes it self-hydrolyzed (Autoproteolysis) and forms a heterodimer to complete the response to IL-1β and IL-18 activation and pyroptosis [26]. However, new research found that caspase-4/5/11 can directly recognize the LPS produced by binding intracellular bacteria, but not through other intracellular receptors (such as NLRP3, etc.), which can directly activate caspase-11 without caspase-1. Caspase-1 directly causes pyroptosis [27]. Of note, GSDMD was discovered in 2015 to be a downstream molecule of caspase-4/11, the final effector protein in the onset of focal death. Shao et al. conducted a genome-wide screen by CRISPR–Cas9 technology and found GSDMD, its existence mediates the occurrence of GSDMD, and if it exists, cell pyroptosis occurs. Dixit et al. conducted a forward genetic screen with ethyl-N-nitrosourea-mutagenized and found that the mutant mice were insensitive to LPS treatment, targeting the molecule GSDMD. Shi et al. constructed GSDMD-deficient mice (Gsdmd−/−) through CRISPR/CAS9 genome-wide technology and found that the classical pyroptosis process mediated by NLRC4, NLRP3 and AIM2 inflammasomes in Gsdmd−/− mice was all unable to start [11, 28, 29]. These studies have clarified that pyroptosis is essentially inflammatory necrosis of cells mediated by GSDMD protein, which is closely related to a variety of pathophysiological processes. How does GSDMD work in pyroptosis? At this time, the GSDMD-NT protein is activated and transferred to the cytoplasmic membrane, where it joins the membrane to form a hole, and a large number of cell contents are released including IL-1β, and the cell gradually expands until it ruptures, and finally amplifies the inflammatory response.
Pyroptosis can be divided into canonical and non-canonical pathway according to the different types of caspases (cysteinyl aspartate specific proteinase, caspase) it depended on [30]. The canonical pathway of pyroptosis is mainly depended on caspase-1, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs)-associated molecular patterns in the cytoplasm can stimulate inflammasome assembly while leading to processing and activation of caspase-1, cleavage of pro-IL-1β and pro-IL-18 to their mature forms, and cleavage of Gasdermin D (encoded by GSDMD) to induce pyroptosis [31]. In 2002, the concept of “Inflammasome” was first proposed [32], which mainly includes NLRP1, NLRP3, NLRC4 of the NLR family, AIM2 of the HIN200 family, and Pyrin of the TRIM family. Studies have found that the intracellular receptor NALP1 (i.e., NLRP1) can form a complex with the adaptor proteins ASC and caspase-1 in both intracellular and cell-free systems, and participate in the activation of IL-1β [33, 34]. Non-canonical pathway pyroptosis mainly depends on caspase-11 (human homologous caspase-4/5), Gram-negative bacteria can induce caspase11-dependent pyroptosis in mouse macrophages, while the activation of caspase 11/4/5 usually does not require the PRR-mediated inflammasome, and caspase-11 can directly recognize the LPS in the cytoplasm for oligomerization and activation, destroy the membrane structure by cleaving and activating GSDMD. Caspases 11/4/5, unlike caspase-1, do not process interleukins and instead induce pyroptosis (Fig. 1) [35].
Gasdermin D (GSDMD)
GSDMD, also known as GSDMDC1 or DFNA5L, is a cytoplasmic protein encoded by the GSDMD gene of the gasdermin family that is widely expressed in different cells and tissues [36]. The GSDMD family contains six paralogous genes in humans: gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), gasdermin D (GSDMD), gasdermin E (GSDME) (also known as DFNA5), and PJVK (also known as DFNB59). These proteins have been implicated in many inflammatory diseases and cancers, and they also promise therapeutic targets. GSDMD is the final executor of pyroptosis. It consists of 242 amino acids with a full length of 53 kDa and contains a characteristic gasdermin domain. GSDMD has two conserved domains: an effector domain at the N-terminus and an inhibitory domain at the C-terminus. The N-terminus is the main functional domain, which is involved in the occurrence of pyroptosis, while the C-terminus has the function of autoinhibition. In the resting state, the N-terminal and C-terminal domains are connected by a long loop, when stimulated by external signals, Caspase-1/4/5/11 is activated to cleave the GSDMD protein, and the cleaved GSDMD is divided into two independent domains of N-terminal and C-terminal fragments. The GSDMD-N-terminal domain can target the cell membrane, bind to the phospholipid protein on the cell membrane, multimerize, and form a hole in the cytoplasmic membrane, and the cytoplasmic membrane is damaged, thereby inducing the occurrence of pyroptosis [37]. At the same time, activated caspase-1 cleaves IL-1β and IL-18 precursors to form active IL-1β and IL-18, and the activated forms of pro-inflammatory cytokines such as IL-1β and IL-18 are released through the pores to the outside of the cell, the extracellular release of a large number of inflammatory factors leads to the occurrence of an inflammatory response [12]. At present, the crystal structure of GSDMD is still in the exploratory stage. Fortunately, Xia et al. recently reported the cryo-electron microscopy structures of the GSDMD pore and prepore, finding that the GSDMD pore conduit is predominantly negatively charged, and pro-IL-1 has an acidic domain that can be proteolytically removed by caspase-1. When permeated by GSDMD pores, uncleaved liposomes release both positively and neutrally charged species faster than similarly sized negatively charged species, and they preferentially release mature IL-1β. Mutation of acidic residues of GSDMD impairs this preference, hindering retention within precursor cells and secretion of mature cytokines [29].
IL-1α is also a pro-inflammatory cytokine, and maturation of IL-1α occurs in a caspase-1-dependent manner after inflammasome activation, but pro-IL-1α is not a substrate of caspase-1 [38]. New research shows that during inflammasome activation, caspase-1-treated GSDMD forms plasma membrane pores that mediate Ca2+ influx, resulting in calpain-dependent maturation of IL-1α [39]. Alphacoronavirus TGEV can trigger pyroptosis and upregulate the expression of GSDMD, which broadly inhibits the infection of enteric coronaviruses TGEV and PDCoV through its pore-forming activity by promoting the unconventional release of IFN-β [40]. GSDMA3 is 70% homologous to GSDMD.
The study found that caspase-3 can recognize and hydrolyze GSDME molecules, focusing on the pyroptosis process in a GSDMD-like pattern after Caspase-3 hydrolysis [41]. GSDMB was found to be highly expressed in the leukocytes of patients with septic shock, which was associated with increased release of the N-terminus of GSDMD. Downregulation of GSDMB alleviates division and cell death in GSDMD. Consistently, overexpression of GSDMB promotes the division of GSDMD while increasing the release of LDH. On the other hand, GSDMB promotes atypical pyroptosis by enhancing caspase-4 activity [42]. Simultaneous silencing of GSDME in mice attenuated acute kidney injury and inflammation [43]. These studies provide a potential new strategy for the treatment of inflammatory diseases.
The role of GSDMD in sepsis
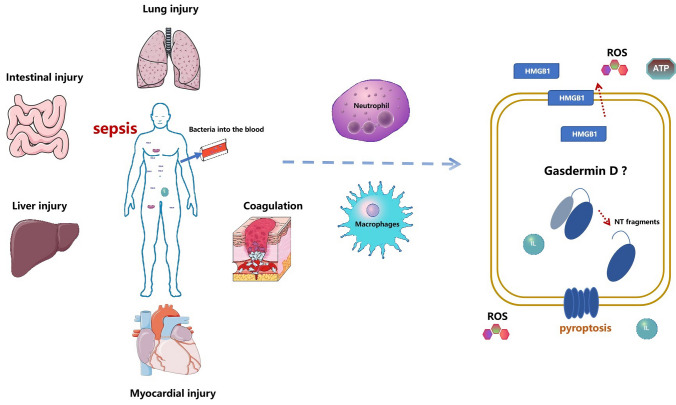
GSDMD is an executor of pyroptosis. Studies have shown that it mediates different lesions in sepsis and the expression of related molecules [44]. Therefore, we summarize the organ damage, cellular regulation, and molecular mediation of the dominant role of GSDMD in the development of sepsis (Fig. 2).
Fig. 2.
GSDMD dominates organ damage, cellular and molecular regulation in sepsis. During sepsis, bacteria enter the blood, neutrophils increase the release of pro-inflammatory factors, reactive oxygen species and HMGB1, and the body produces coagulation dysfunction, lung injury, myocardial injury, liver injury, and intestinal injury. GSDMD, which functions as a motor, is usually involved in the damage of these molecules and the body
GSDMD vs. coagulation
Coagulation dysfunction is a common and serious complication of sepsis, with early manifestations of thrombocytopenia and prolonged clotting time, aggregation of other inflammatory cells (such as neutrophils and lymphocytes), and vascular endothelial cell damage, which can eventually develop into disseminated intravascular coagulation (disseminated intravascular coagulation, DIC), leading to increased mortality [45]. The abnormal coagulation system plays an important role in the occurrence and development of sepsis, but its specific triggering mechanism is still unclear. It was found that in GSDMD-deficient mice, E.coli-induced coagulation activation was attenuated [46]. Meanwhile, EprJ-induced coagulation was abolished after caspase-1 or GSDMD deficiency, suggesting that EprJ-induced coagulation activation is dependent on inflammasome activation and pyroptosis [47]. Another study found that Caspase-11-induced disseminated intravascular coagulation in sepsis by activating its substrate GSDMD [48]. Combined together, pyroptosis is at least one of the main mechanisms of bacterial infection coagulation, which provides a research direction for the early diagnosis and treatment of septic DIC in the future and also provides a theoretical basis for the development of GSDMD-related molecules as potential drug intervention targets.
GSDMD vs. oxidative stress
Oxidative stress plays a key role in the regulation of various biological processes, including cell death and innate immunity. It is a concomitant phenomenon in the inflammatory process, exacerbating the inflammatory response through oxidation, which in turn promotes oxidation by mediators of the inflammatory response [49]. Early antioxidant intervention during the onset of sepsis, which is often accompanied by oxidative stress, may be beneficial in sepsis control.
Kang et al. found that deletion of glutathione-dependent lipid hydroperoxidase 4 (GPX4) increased GSDMD-N formation, proteolytic IL-1β (p17), caspase-1 maturation, and the activation of caspase-11 (p26) in BMDM following LPS electroporation or E. coli infection, and when cystatin-11 is absent, this process is blocked. They also found that mice with Gpx4 and GSDMD deficiency (which has a GSDMD cleavage site mutation that makes the protein resistant to proteolytic activation of caspase-1 or -11) in backcrossed bone marrow cells are resistant to CLP-induced lethality septicemia. In addition, knockdown of Phospholipase C (PLC) also prevented GSDMD-N-mediated cellular cytotoxicity in THP-1 (human monocytic leukemia cell line), HL-60 (human promyelocytic leukemia cell line) and HeLa (human cervical cancer cell line). Taken together, these studies demonstrate that the caspase-11-GSDMD-PLC pathway leads to multi-organ failure in septic mice. This indicated that the lipid peroxides produced by phospholipid oxidation promoted GSDMD-N-mediated pyroptosis in BMDMs [22]. This suggests that whether inhibiting activation of GSDMD-N can reduce the occurrence of cellular lipid peroxidation, thereby reducing the dual response of oxidative stress and inflammation, and reducing the lethality of sepsis? At the same time, it also provides a new idea for the screening of targeted drugs.
GSDMD vs. HMGB1
High mobility group box-1 (HMGB1) is a ubiquitous nuclear and cell membrane protein that is released into the circulation during sepsis and is a pro-inflammatory mediator of late sepsis lethality, maintaining and prolonging sepsis pathology [50]. Hepatocytes are often the main source of increased circulating HMGB1 in sepsis and many liver-based diseases. In 2018, studies found that HMGB1 and RAGE released by hepatocytes were critical for infection with caspase-11-dependent lethality in a "double-whammy" model of endotoxemia and bacterial sepsis [51]. However, studies have shown that in mice given a single high dose of LPS, hepatocyte Hmgb1 deletion does not protect hepatocytes [52]. This suggests that HMGB1 mediates caspase-11-dependent pyroptosis in lethal sepsis. Therefore, does HMGB1 have the same link to GSDMD? Recent studies have found that deletion or knockout of GSDMD inhibits LPS-induced HMGB1 release from hepatocytes. Hepatocytes sense LPS via extracellular TLR4 and intracellular caspase-11 on one hand, and intracellular LPS leads to caspase-11-dependent cleavage of GSDMD on the other hand, which promotes an increase in intracellular free calcium, activation of camkkβ, and nuclear HMGB1 relocates to the cytoplasm, thereby efficiently releasing HMGB1[53].
GSDMD vs. neutrophil
Neutrophils are the most numerous cells involved in the innate immune response. The death of neutrophils plays a key role in regulating neutrophil numbers during infection and inflammation [54]. Aging neutrophils undergo programmed death and are then recognized, phagocytosed and safely removed by professional phagocytes such as macrophages [55]. In the early stages of sepsis, when the host is attacked by pathogens, neutrophils in the bone marrow are rapidly mobilized, and a large number of neutrophils migrate to the lesion to remove pathogens under the action of chemokines to function by phagocytosis, degranulation, and the formation of extracellular traps. Although neutrophils are very important for pathogen clearance, studies have shown that neutrophils are closely related to organ dysfunction [56].
When neutrophils increase in large numbers after infection or tissue damage, IL-1β is mainly released. It has been reported that neutrophils release IL-1β without pyroptosis during canonical NLRP3 inflammasome activation. However, studies have also shown that neutrophil IL-1β release is reduced in Gsdmd−/− mice, similar to macrophages [46]. Interestingly, a recent study identified another mechanism by inflammasome-activated neutrophils resisting pyroptosis, unlike macrophages, does not accumulate functional N-GSDMD pores in the plasma membrane and transport N-GSDMD proteins to the plasma membrane, nor activating calcium-regulated plasma membrane repair, but instead transporting N-GSDMD to cyanophilic (primary) granules and the autophagosome releases IL-1β through an autophagy machinery-dependent pathway. Furthermore, N-GSDMD permeability of cyanophilic granules releases neutrophil elastase into the cytoplasm, thereby mediating a secondary cascade of serine protease-dependent GSDMD processing [57].
However, GSDMD was found to be pleiotropic, with pro- and anti-inflammatory. In both acute peritoneal infection (peritonitis) and lipopolysaccharide (LPS)-induced sepsis models in mice, Gsdmd−/−-deficient mice were also found to have significantly improved survival, fertility, and size compared to wild-type (WT) mice. Peripheral blood differential counts were normal, and blood smears showed no hematopoietic abnormalities. This is primarily due to GSDMD deficiency delaying neutrophil death, paradoxically enhancing the host response to extracellular E. coli. The study found that the division and activation of neutrophils are caspase-independent and mediated by the neutrophil-specific serine protease neutrophil elastase (ELANE), and ELANE-mediated cleavage of GSDMD is located half upstream of the caspase cleavage site and yields a fully active ELANE-derived NT fragment (GSDMD-eNT) that induces lytic cell death as efficiently as GSDMD-cNT. Therefore, GSDMD is pleiotropic with pro- and anti-inflammatory effects, making it a potential target for antibacterial and anti-inflammatory therapy [58].
GSDMD VS sepsis-induced cardiomyopathy (SIC)
The heart has gradually received extensive attention as an important organ damaged in the development of sepsis. In 1984, Parker et al. first proposed the concept of sepsis-induced cardiomyopathy (SIC) [59]. They observed that 65% of septic shock patients developed LV systolic dysfunction (defined as ejection fraction < 45%). According to statistics, about 40–50% of sepsis patients will be complicated with cardiac insufficiency, including severe cardiac failure (heart failure) [60]. Sepsis-induced cardiomyopathy can be seen one of the main complications of sepsis, but its specific mechanism is still unclear.
Studies in recent years have revealed that, at the cellular and molecular levels, in addition to inflammation and apoptosis, pyroptosis is considered an important pathophysiological phenomenon in sepsis and SIC. Shi et al. found that the expression of GSDMD-N was significantly increased in cardiomyocytes after stimulation by I/R injury, and the cells underwent pyroptosis. Induction of myocardial I/R injury in cardiac-specific knockout male mice (GSDMDflox/flox; CreαMHC [GSDMD-CKO]) resulted in decreasing GSDMD protein levels in the mouse myocardium, but not in other tissues tested. Meanwhile, oxidative stress induces cardiomyocyte pyroptosis through a GSDMD-dependent pathway. In addition, GSDMD can also be detected in the serum of patients undergoing percutaneous coronary intervention and I/R injury. Overall, GSDMD may be a potential biomarker and therapeutic target for the identification and treatment of cardiomyocyte thermal ptosis and subsequent myocardial I/R injury [61]. Another study on SIC found that myocardial levels of NLRP3 and ASC were significantly increased in the hearts of mice injected with LPS. In LPS-induced Neonatal rat cardiomyocytes (NRCMs), inhibition of cellular reactive oxygen species (ROS) blocked LPS-induced export cytoplasmic translocation of NLRP3 from the nucleus to the cytoplasm [62]. Activation in the classical pyroptosis pathway depends on caspase-1 cleavage of the key mediator GSDMD, and the N-terminal domain of GSDMD in pyroptosis can trigger a series of inflammatory cascades by activating the NLRP3 inflammasome reaction [63, 64]. Therefore, is there a necessary targeted link between GSDMD and SIC? The study found that after intraperitoneal injection of LPS (10 mg/kg) into wild-type (WT) and Gsdmd knockout (Gsdmd−/−) mice, GSDMD-NT was up-regulated in the cardiac tissue of WT mice, accompanied by decreased cardiac function. In contrast, Gsdmd−/− mice significantly reduced myocardial dysfunction, improved inflammatory cell infiltration, and inhibited NF-κB signaling pathway and NOD-like receptor protein 3 (NLPR3) inflammasome activation [65]. This is the point that needs to be explored urgently after GSDMD is found to be the executive protein of pyroptosis, and it will also be a direction of our future research.
GSDMD vs. liver injury
The liver plays a critical role in sepsis, as it is an important line of defense against microorganisms and a common target of dysregulated inflammation [66, 67]. The study concluded that pyroptosis plays an important role in the development of liver disease, including sepsis-induced liver injury [68]. Overexpression of the p10/p20 activation domain of caspase-1 or caspase-11 induces typical GSDMD-dependent pyroptosis in hepatocytes but not cell rupture after LPS stimulation in vitro and in vivo death is different [69]. In addition, Escherichia coli can also enter liver tissue and cause bacterial liver injury, and liver injury and pyroptosis are more severe in CD38−/− mice, because CD38 deficiency triggers extracellular molecule recognition to activate inflammasome NLRP3-GSDMD-mediated Pyroptosis leads to severe bacterial liver damage [70].
GSDMD vs. intestinal injury
In sepsis, the intestinal microenvironment is destroyed, the gut microbiota is imbalanced, and a large number of ROS and inflammatory factors are released, which increases the intestinal permeability, damages the intestinal mucosa, promotes cell apoptosis, and finally induces intestinal failure [71, 72]. In recent years, studies have found that GSDMD play an important role in intestinal injury, especially intestinal inflammation. In a dextran sulfate sodium (DSS)-induced colitis model, GSDMD was activated, and GSDMD-deficient mice have less severe colitis than wild-type mice. In addition, the massive overgrowth of E. coli during colitis mediates the activation of GSDMD, and activated GSDMD promotes the release of interleukin-18 (IL-18), but not the transcriptional or maturation levels of IL-18, which in turn induces the loss of goblet cells, thereby inducing the occurrence of colitis [73]. Both caspase-8 and GSDMD are required for the development of mixed lineage kinase-like MLKL-independent ileitis in mice deficient in epithelial Fas associated with death domain FADD [74]. In DSS colitis mice and IBD patients, GSDMD is increased and localized in testinal epithelial cells (IECs), and its deficiency leads to reduce intestinal inflammation during experimental colitis [75]. Colonic GSDMD was increased in CD patients compared with healthy controls. Meanwhile, studies have shown that LPS can also induce the expression of IL-1β and GSDMD in colonic epithelial cells [76]. However, the study of GSDMD in gut-derived sepsis has not been reported, but the study of GSDMD-mediated intestinal inflammation provides new ideas for the treatment of sepsis-induced intestinal damage.
Disease therapeutic drug candidate-based on GSDMD inhibition
So far, the clinical treatment of sepsis has mainly relied on the use of antibiotics and clinical intensive care [77]. The initial drug exploration for sepsis was a polyclonal antibody targeting the J5 strain of Escherichia coli LPS [78], and subsequent development of related targeted drugs focused on pattern recognition receptors-Toll Like Receptors (Toll Like Receptors) and the targeted neutralization of pro-inflammatory factors [79], but the clinical effect is not significant, and there is still no specific drug. In recent years, with the research on the role of pyroptosis in sepsis, GSDMD inhibitors have also been extensively developed to alleviate the release of pyroptosis, ROS, and inflammation, and also provide molecular targets for the treatment of related diseases (Table 1).
Table 1.
Regulators of GSDMD activity
| Mediator | Description | Effect | Reference |
|---|---|---|---|
| Disulfiram / NSA | Disulfiram modified the Cys191 residue of GSDMD, thereby inhibiting the pore-forming activity and liposome leakage of GSDMD | Disulfiram can inhibit IL-1β secretion in iBMDM and THP-1 cells, and improve the survival rate of mice with LPS-induced sepsis | [80, 81, 84] |
| DMF | DMF reacts with GSDMD at critical cysteine residues to form S-(2-succinyl)-cysteine | In mice, the administration of DMF protects against sepsis shock | [83] |
| TPNs | TPNs inhibit pyroptosis by blocking GSDMD-NT oligomerization | TPNs block canonical and noncanonical inflammasomes-induced pyroptosis in primary macrophages and THP-1 cells in a dose-dependent manner. TPNs treatment increases survival and body temperature in septic mice | [8] |
| Mg2+ | Mg2+ blocks Ca2 + influx by inhibiting the ATP-gated Ca2 + channel P2X7, thereby impeding the function of GSDMD-NT | In HEK293T cells, Mg2 + inhibits GSDMD-NT membrane binding and oligomerization and protects against LPS-induced septic shock | [85] |
| IL-6 | IL-6 inhibits caspase-3-GSDME and caspase-1-GSDMD | IL-6 protects streptococcus pneumoniae-induced pyroptosis and inflammatory injury in lung macrophages | [86] |
| TRIM21 | The PRY-SPRY domain of TRIM21 interacts with GSDMD, maintains stable expression of GSDMD in the resting state, and induces GSDMD-N aggregation during pyroptosis | TRIM21-deficient cells have a reduced inflammasome-activated response, and mice with TRIM21 gene ablation are protected from LPS-induced inflammation and DSS-induced colitis | [87] |
GSDMD Gasdermin D, DMF dimethyl fumarate, TPNs tea polyphenols nanoparticles, Cys191 191-position cysteine, HEK293T human embryonic kidney cells, IL-6 Interleukin 6, TRIM21 tripartite motif 21, DSS dextran sulfate sodium salt, LPS lipopolysaccharide
Disulfiram, an alcohol abstinence drug, was found to be a potent inhibitor of GSDMD-induced liposome leakage, and this molecule can modify the cysteine 191 (Cys191) residue of GSDMD, thereby inhibiting the pore-forming activity of GSDMD. Experiments found that disulfide can inhibit nigericin-induced IL-1β secretion in THP-1 and can also inhibit LPS transfection-induced IL-1β secretion in iBMDM. Disulfiram treatment significantly improved survival and significantly decreased serum IL-1 and IL-6 concentrations in the LPS-induced sepsis mouse model. And disulfiram has proved to be the only direct and effective GSDMD inhibitor so far. Other studies have found that inhibition of GSDMD with disulfiram or gene deletion abolishes neutrophil extracellular traps (NETs) formation and reduces multiple organ dysfunction and sepsis mortality [80]. Notably, disulfiram will be a promising target for future sepsis treatment [81].
Dimethyl fumarate (DMF), a derivative of fumarate in the Krebs cycle, is commonly used in immunomodulation to treat multiple sclerosis and psoriasis [82]. It was found that DMF can react with GSDMD at critical cysteine residues to form S-(2-succinyl)-cysteine regardless of its intracellular/extracellular abundance. GSDMD succinylation prevents its interaction with caspases, limiting its ability to process, oligomerize, and induce cell death. Administration of DMF can prevent LPS shock and alleviates familial Mediterranean fever and experimental autoimmune encephalitis in mice by targeting GSDMD [83].
Unlike Disulfiram, necrosulfonamide (NSA) inhibits p30 GSDMD pore formation by directly binding to GSDMD via Cys191 on GSDMD, and it inhibits pyroptotic cell death in immortalized mouse macrophages and human monocytes while improving the survival of GSDMD-induced sepsis mice. Therefore, chemical inhibition of two forms of inflammatory cell death, pyroptosis and necroptosis, has high therapeutic potential in inflammatory disease patients [84].
TPNs are epigallocatechin-3-gallate (EGCG) derived from tea polyphenol nanoparticles. Mechanistic studies suggest that TPNs can effectively inhibit pyroptosis by blocking GSDMD-NT oligomerization, which complements RONS clearance and thus synergizes the therapeutic effect of sepsis. In a sepsis model induced by intraperitoneal injection of LPS, the TPNs group had a 50% survival rate within 96 h of modeling, decreased IL-1α, IL-1β, IL-6, thickened alveolar walls, and pneumonitis in mice. There was less neutrophil infiltration and fewer inflammatory cells in the liver blood vessels. Additionally, TPNs block pyroptosis of NLRP3 inflammasome and non-inflammasome activators in a dose-dependent manner. This suggests that TPNs have the potential to treat sepsis and deserve more systematic preclinical studies for clinical translation [8].
Mg2+ inhibited canonical pyroptosis by preventing membrane localization of GSDMD-NT, but did not affect the activation of the NLRP3 inflammasome, but inhibited typical pyroptosis in LPS- and ATP-induced iBMDMs. It also inhibited GSDMD-NT membrane binding and oligomerization in HEK293T cells and attenuated LPS-induced lung inflammation and lung injury. These results suggest that Mg2+ may have a protective effect against LPS-induced septic shock [85].
Interleukin 6 (IL-6), a cytokine with pleiotropic activity, protects streptococcus pneumoniae-induced pulmonary macrophages by inhibiting caspase-3-GSDME and caspase-1-GSDMD-mediated pyroptosis death and inflammatory damage to the lungs [86]. In addition, the PRY-SPRY domain of tripartite motif 21 (TRIM21) interacts with GSDMD, keeping it stable expression of GSDMD in the resting state, and inducing GSDMD-N aggregation during pyroptosis. TRIM21 gene ablation in mice protects them from LPS-induced inflammation and dextran sulfate sodium-induced colitis. Therefore, TRIM21 plays an important role in GSDMD-mediated pyroptosis and may be a useful tool for the control and treatment of inflammation-related diseases [87].
Summary and perspective
In the emergency ICU, sepsis usually has a higher mortality rate. Both clinical and basic research are trying to find the key points of sepsis at different stages and different times, to find corresponding therapeutic targets to reduce the mortality of sepsis and improve the prognosis. However, due to the heterogeneity of individual differences in sepsis, there is still a lack of effective and specific therapeutic drugs. With COVID-19 sweeping the world today, critically ill patients infected by it have a higher risk of developing viral sepsis, making treatment more complicated [3, 88]. GSDMD simultaneously regulates the death of immune cells and the release of related inflammatory factors; both immunity and inflammation are also dysregulated in sepsis. Both cell and animal experiments show that the deletion of GSDMD can significantly inhibit the pyroptosis induced by LPS or infection and improve the severity of sepsis. These findings open new doors for targeted therapy in sepsis, and GSDMD is regarded as a novel ideal target for drug development in sepsis.
Author contributions
LJ conceived the current study. RS and GC designed and wrote the manuscript. XL analyzed the feasibility of the article and searched the literature. JX and DN searched the literature and revised the article. Data authentication is not applicable. All the authors have read and approved the final version of the manuscript.
Funding
This study was supported by Yunnan Key Laboratory of Traditional Chinese Medicine Transformation and Application (202105AG070032), Yunnan Clinical Medical Center Open Project (2021LCZXXF-HX03) and National Natural Science Foundation of China (No. 81960068, No. 82160366).
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guobing Chen, Email: rocktom38chen@163.com.
Lihong Jiang, Email: jlh15198763375@163.com.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):775–787. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maloney PJ. Sepsis and septic shock. Emergency medicine clinics of North America. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2. [DOI] [PubMed] [Google Scholar]
- 3.Munoz AC, Nawaratne U, Mcmann D, Ellsworth M, Boukas K. Late-onset neonatal sepsis in a patient with covid-19. N Engl J Med. 2020;382(19):e49. doi: 10.1056/NEJMc2010614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson S, Varpula M, Ruokonen E, Pettil V, Parviainen I, Ala-Kokko TI, Kolho E, Rintala EM. Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med. 2007;33(3):435–443. doi: 10.1007/s00134-006-0504-z. [DOI] [PubMed] [Google Scholar]
- 5.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Reinhart K. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 6.Walkey AJ, Kirkpatrick AR, Summer RS. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;373(9):1629–1638. doi: 10.1056/NEJMoa1415236. [DOI] [PubMed] [Google Scholar]
- 7.Jacob JA. New sepsis diagnostic guidelines shift focus to organ dysfunction. JAMA. 2016;315(8):739. doi: 10.1001/jama.2016.0736. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Luo R, Li J, Wang S, Ding J, Zhao K, Lu B, Zhou W. Intrinsic radical species scavenging activities of tea polyphenols nanoparticles block pyroptosis in endotoxin-induced sepsis. ACS Nano. 2022;16(2):2429–2441. doi: 10.1021/acsnano.1c08913. [DOI] [PubMed] [Google Scholar]
- 9.Miao E, Leaf I, Treuting P, Mao D, Sarkar A, Wewers M, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X, Chen W, Gong F, Chen Y, Chen E. The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: a review. Front Immunol. 2021;12:711939. doi: 10.3389/fimmu.2021.711939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 12.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25(12):1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jianjin S, Yue Z, Kun W, Xuyan S, Yue W, Huanwei H, Yinghua Z, Tao C, Fengchao W, Feng S. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 14.Cierra N, Casson J, Yu V, Reyes M, Frances O. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci USA. 2015;112(21):6688–6693. doi: 10.1073/pnas.1421699112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 17.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Molecular Microbiology Mol Microbiol. 2000;38(1):31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 18.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 19.Muendlein HI, David J, Connolly WM, Eidell KP, Zoie M, Irina S, Alexander P. cFLIPL protects macrophages from LPS-induced pyroptosis via inhibition of complex II formation. Science. 2020;367(6484):1379–1384. doi: 10.1126/science.aay3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R, Zeng L, Zhu S, Liu J, Kang R. cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis. Sci Adv. 2019 doi: 10.1126/sciadv.aav5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattori Y, Takano KI, Teramae H, Yamamoto S, Yokoo H, Matsuda N. Insights into sepsis therapeutic design based on the apoptotic death pathway. J Pharmacol Sci. 2010;114(4):354–365. doi: 10.1254/jphs.10r04cr. [DOI] [PubMed] [Google Scholar]
- 22.Kang R, Ling Z, Shan Z, Xie Y, Liu J, Wen Q, Cao L, Min X, Ran Q, Guido K. Lipid peroxidation drives gasdermin d-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24(1):97–108.e4. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Wang Y, Ding J, Wang C, Liu Z. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579(7799):421–426. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 24.Melanie F, Günther S, Robin S, Marie-Christine A, Fabian S, Paul WJ, Schiffmann LM, Neil S, Hannah S, Seeger JM. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575(7784):683–687. doi: 10.1038/s41586-019-1770-6. [DOI] [PubMed] [Google Scholar]
- 25.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Kroemer G. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Yang T, Xiao J, Xu C, Alippe Y, Sun K, Kanneganti T, Monahan J, Abu-Amer Y, Lieberman J, Mbalaviele G. NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci Immunol. 2021 doi: 10.1126/sciimmunol.abj3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Peng L, Hu L, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 28.Feng S. Gasdermins: making pores for pyroptosis. Nat Rev Immunol. 2021;21(10):620–621. doi: 10.1038/s41577-021-00602-2. [DOI] [PubMed] [Google Scholar]
- 29.Xia S, Zhang Z, Magupalli VG, Pablo JL, Wu H. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature. 2021;593(7860):607–611. doi: 10.1038/s41586-021-03478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao YL, Zhai JH, Chai YF. Recent advances in the molecular mechanisms underlying pyroptosis in sepsis. Mediators Inflamm. 2018;2018:5823823. doi: 10.1155/2018/5823823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karki R, Kanneganti T. Diverging inflammasome signals in tumorigenesis and potential targeting. Nat Rev Cancer. 2019;19(4):197–214. doi: 10.1038/s41568-019-0123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes-Alnemri T, Yu J, Datta P, Wu J, Alnemri E. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey D, Latz E, Fitzgerald K. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding J, Shao F. SnapShot: The noncanonical inflammasome. Cell. 2017;168(3):544–544. doi: 10.1016/j.cell.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Katoh M, Katoh M. Identification and characterization of human DFNA5L, mouse Dfna5l, and rat Dfna5l genes in silico. Int J Oncol. 2004;25(3):765–770. [PubMed] [Google Scholar]
- 37.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli V, Wu H, Lieberman J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gro O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36(3):388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya K, Hosojima S, Hara H, Kushiyama H, Mahib M, Kinoshita T, Suda T. Gasdermin D mediates the maturation and release of IL-1α downstream of inflammasomes. Cell Rep. 2021;34(12):108887. doi: 10.1016/j.celrep.2021.108887. [DOI] [PubMed] [Google Scholar]
- 40.Zhao L, Li L, Xue M, Liu X, Jiang C, Wang W, Tang L, Feng L, Liu P. Gasdermin D inhibits coronavirus infection by promoting the noncanonical secretion of beta interferon. MBio. 2022 doi: 10.1128/mbio.03600-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 42.Chen Q, Shi P, Wang Y, Zou D, Wu X, Wang D, Hu Q, Zou Y, Huang Z, Ren J. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2019;11(6):496–508. doi: 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia W, Li Y, Wu M, Jin Q, Jia Z. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021;12(2):139. doi: 10.1038/s41419-021-03431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding X, Kambara H, Guo R, Kanneganti A, Luo HR. Inflammasome-mediated GSDMD activation facilitates escape of Candida albicans from macrophages. Nat Commun. 2021;12(1):6699. doi: 10.1038/s41467-021-27034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDonald B, Davis R, Kim S, Tse M, Esmon C, Kolaczkowska E, Jenne C. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129(10):1357–1367. doi: 10.1182/blood-2016-09-741298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monteleone M, Stanley AC, Chen KW, Brown DL, Bezbradica JS, von Pein JB, et al. Interleukin-1β maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Rep. 2018;24(6):1425–1433. doi: 10.1016/j.celrep.2018.07.027. [DOI] [PubMed] [Google Scholar]
- 47.Wu C, Lu W, Zhang Y, Zhang G, Li Z. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity. 2019;50(6):1401–1411. doi: 10.1016/j.immuni.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X, Cheng X, Tang Y, Qiu X, Lu B. Bacterial endotoxin activates the coagulation cascade through gasdermin D-dependent phosphatidylserine exposure. Immunity. 2019;51(6):983–996.e6. doi: 10.1016/j.immuni.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12(6):646–652. doi: 10.1097/MCO.0b013e3283312956. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Liao H, Ochani M, Justiniani M, Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10(11):1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 51.Deng M, Tang Y, Li W, Wang X, Lu B. The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis. Immunity. 2018;49(4):740–753. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huebener P, Pradere JP, Hernandez C, Gwak GY, Caviglia JM, Mu X, Loike JD, Jenkins RE, Antoine DJ, Schwabe RF. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2019;130(4):1802. doi: 10.1172/JCI126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W, Deng M, Loughran PA, Yang M, Scott MJ. LPS induces active HMGB1 release from hepatocytes into exosomes through the coordinated activities of TLR4 and caspase-11/GSDMD signaling. Front Immunol. 2020;11:229. doi: 10.3389/fimmu.2020.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starling S. Neutrophils: interfering with intestinal inflammation. Nat Rev Immunol. 2017;17(10):594. doi: 10.1038/nri.2017.113. [DOI] [PubMed] [Google Scholar]
- 55.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 56.Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O’’Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20(5):524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karmakar M, Minns M, Greenberg EN, Diaz-Aponte J, Pearlman E. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat Commun. 2020;11(1):2212. doi: 10.1038/s41467-020-16043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kambara H, Fei L, Zhang X, Peng L, Bajrami B, Yan T, Li Z, Zhou S, Yu H, Zhou W. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 2018;22(11):2924–2936. doi: 10.1016/j.celrep.2018.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parker M. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984;100(4):483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- 60.Vieillard-Ba RA, Cecconi M. Understanding cardiac failure in sepsis. Intensive Care Med. 2014;40(10):1560–1563. doi: 10.1007/s00134-014-3367-8. [DOI] [PubMed] [Google Scholar]
- 61.Shi H, Gao Y, Dong Z, Yang J, Ge J. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ Res. 2021;129(3):383–396. doi: 10.1161/CIRCRESAHA.120.318629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li N, Zhou H, Wu H, Wu Q, Tang Q. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. doi: 10.1016/j.redox.2019.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, Sun H, Wang DC, Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535(7610):111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 64.Gao L, Gong W, Huang W, Xue J, Zhu Q, Ma N, Chen W, Fu X, Gao X, Lin Z. Acinar cell NLRP3 inflammasome and GSDMD activation mediates pyroptosis and systemic inflammation in acute pancreatitis. Br J Pharmacol. 2021;178(17):3533–3552. doi: 10.1111/bph.15499. [DOI] [PubMed] [Google Scholar]
- 65.Dai S, Ye B, Zhong L, Chen Y, Hong G, Zhao G, Su L, Lu Z. GSDMD mediates LPS-induced septic myocardial dysfunction by regulating ROS-dependent NLRP3 inflammasome activation. Front Cell Dev Biol. 2021 doi: 10.3389/fcell.2021.779432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Protzer U, Maini MK, Knolle PA. Living in the liver: hepatic infections. Nat Rev Immunol. 2012;12(3):201–213. doi: 10.1038/nri3169. [DOI] [PubMed] [Google Scholar]
- 67.Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology. 2014;146(6):1513–1524. doi: 10.1053/j.gastro.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mamun AA, Wu Y, Jia C, Munir F, Xiao J. Role of pyroptosis in liver diseases. Int Immunopharmacol. 2020;84:106489. doi: 10.1016/j.intimp.2020.106489. [DOI] [PubMed] [Google Scholar]
- 69.Sun P, Zhong J, Liao H, Loughran P, Mulla J, Fu G, Tang D, Fan J, Billiar TR, Gao W. Hepatocytes are resistant to cell death from canonical and non-canonical inflammasome-activated pyroptosis. Cell Mol Gastroenterol Hepatol. 2022;13(3):739–757. doi: 10.1016/j.jcmgh.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H, Du Y, Guo Y, Wang Z, Li R. TLR4-NLRP3-GSDMD-mediated pyroptosis plays an important role in aggravated liver injury of CD38-/- sepsis mice. J Immunol Res. 2021;2021:6687555. doi: 10.1155/2021/6687555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alverdy J, Holbrook C, Rocha F, Seiden L, Licheng R, Musch M, Chang E, Ohman D, Suh S. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host. Ann Surg. 2000;232(4):480–489. doi: 10.1097/00000658-200010000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.My A, Bb B, Mn A, Amz A, Ddo A, Ear A, Jn A, Mm A, Ap A, Db A. Gut microbiota, endotoxemia, inflammation, and oxidative stress in patients with heart failure, left ventricular assist device, and transplant - ScienceDirect. J Heart Lung Transplant. 2020;39(9):880–890. doi: 10.1016/j.healun.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao H, Cao M, Yao Y, Hu W, Sun H, Zhang Y, Zeng C, Tang J, Luan S, Chen P. Dysregulated microbiota-driven gasdermin D activation promotes colitis development by mediating IL-18 release. Front Immunol. 2021;12:750841. doi: 10.3389/fimmu.2021.750841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwarzer R, Jiao H, Wachsmuth L, Tresch A, Pasparakis M. FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity. 2020;52(6):978–993.e6. doi: 10.1016/j.immuni.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Bulek K, Zhao J, Liao Y, Rana N, Li X. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. J Clin Invest. 2020;130(8):4218–4234. doi: 10.1172/JCI138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price AE, Kiarash S, Lugo KA, Jacques D, Roberts AW, Lee BL, Barton GM. A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity. 2018;49(3):560–575. doi: 10.1016/j.immuni.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sevransky, Investigators. Brokowski C. Effect of vitamin C, thiamine, and hydrocortisone on ventilator- and vasopressor-free days in patients with sepsis: the VICTAS randomized clinical trial. JAMA. 2021;325(8):742–750. doi: 10.1001/jama.2020.24505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharjee AK, Opal SM, Palardy JE, Drabick JJ, Hugh C, Robert T, Amos C, Cross AS. Affinity-purified escherichia coli j5 lipopolysaccharide-specific igg protects neutropenic rats against gram-negative bacterial sepsis. J Infect Dis. 1994;170(3):622–629. doi: 10.1093/infdis/170.3.622. [DOI] [PubMed] [Google Scholar]
- 79.Pomerenke A, Lea S, Herrick S, Lindsay M, Singh D. Characterization of TLR-induced inflammatory responses in COPD and control lung tissue explants. Int J Chron Obstruct Pulmon Dis. 2016;11:2409–2417. doi: 10.2147/COPD.S105156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silva CM, Wanderley CW, Veras FP, Sonego F, Nascimento DC, Gonalves AV, Martins TV, Cólon D, Borges VF, Brauer VS. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood. 2021;138(25):2702–2713. doi: 10.1182/blood.2021011525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, Ruan J, Luo X, Lou X, Bai Y. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21(7):736–745. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kornberg MD, Bhargava P, Kim PM, Putluri V, Snowman AM, Putluri N, Calabresi PA, Snyder SH. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. 2018;360(6387):449–453. doi: 10.1126/science.aan4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Humphries F, Shmuel-Galia L, Ketelut-Carneiro N, Li S, Fitzgerald KA. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369(6511):1633–1637. doi: 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rathkey JK, Zhao J, Liu Z, Chen Y, Jie Y, Kondolf HC, Benson BL, Chirieleison SM, Huang AY, Dubyak GR. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018 doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang D, Zheng J, Hu Q, Zhao C, Lin Z. Magnesium protects against sepsis by blocking gasdermin D N-terminal-induced pyroptosis. Cell Death Differ. 2020;27(2):466–481. doi: 10.1038/s41418-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gou X, Xu W, Liu Y, Peng Y, Xu W, Yin Y, Zhang X. IL-6 prevents lung macrophage death and lung inflammation injury by inhibiting GSDME- and GSDMD-mediated pyroptosis during pneumococcal pneumosepsis. Microbiol Spectr. 2022;10(2):e0204921. doi: 10.1128/spectrum.02049-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao W, Li Y, Liu X, Wang S, Li J. TRIM21 regulates pyroptotic cell death by promoting Gasdermin D oligomerization. Cell Death Differ. 2022;29(2):439–450. doi: 10.1038/s41418-021-00867-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carlet J, Payen D, Opal SM. Steroids for sepsis and ARDS: this eternal controversy remains with COVID-19. Lancet. 2020;396(10259):e61–e62. doi: 10.1016/S0140-6736(20)32132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]