Abstract
Traditionally, the dental profession has primarily treated periodontitis using a mechanical/surgical, rather than a pharmaceutical, approach. However, based on experiments several decades ago which demonstrated that tetracyclines, unexpectedly, inhibit collagen- and bone-destructive mammalian-derived enzymes (e.g. the collagenases), and through non-antibiotic mechanisms, the concept of host-modulation therapy (HMT) was developed. Accordingly, two drug-development strategies evolved: (i) the development of non-antimicrobial formulations of doxycycline; and (ii) the chemical modification of tetracyclines to eliminate their antibiotic activity but retain (or even enhance) their anti-collagenase properties. Regarding the latter, these chemically modified tetracyclines (CMTs) showed efficacy in vitro, in animal models of periodontal (and relevant systemic) disease, and in preliminary clinical trials on patients with Kaposi’s sarcoma (however, at the high doses used, photosensitivity was a significant side-effect). In the first strategy, subantimicrobial-dose doxycycline (SDD) demonstrated safety and efficacy in human clinical trials and was approved by the U S Food and Drug Administration (U S FDA) and in other countries for the treatment of periodontitis (20 mg, twice daily, i.e. once every 12 hours) adjunctive to scaling and root planing, and for chronic inflammatory skin diseases (40-mg sustained-release ‘beads’). SDD also showed efficacy in patients with systemic diseases relevant to periodontitis, including diabetes mellitus and arthritis, and in postmenopausal women with local and systemic bone loss. Importantly, long-term administration of SDD, of up to 2 years, in clinical trials did not produce antibiotic side-effects. SDD (and in the future, new HMTs, such as low-dose CMT-3, resolvins and chemically modified curcumins) may shift the paradigm of periodontal therapy from a predominantly surgical approach to the greater use of medicinal/pharmacologic strategies, ultimately to benefit larger numbers of patients.
Key words: Periodontitis, non-antibacterial tetracyclines, host-modulation therapy, systemic disease
INTRODUCTION
Historically, the dental profession has not treated the most common chronic inflammatory disease, periodontitis, using a pharmacological/biological approach, even adjunctively. With this view in mind, plus the ever-increasing sophistication of modern dental research and its translation into clinical practice, the current review addresses the biological and clinical evidence, accumulated over several decades, supporting the use of a unique pharmacological (non-antibacterial) approach, called ‘host-modulation therapy’, as an adjunct to the universally used mechanical debridement, scaling and root planing (SRP) in the long-term management of periodontal patients. This treatment strategy is relevant considering the impact of periodontitis on overall health1., 2., 3., 4., 5., 6., 7.. Moreover, evidence-based guidelines, recently published by the American Dental Association, support the use of host-modulation therapy [subantimicrobial-dose doxycycline (SDD); see below] as an adjunct to SRP8. The reader is also referred to several recently published meta-analyses demonstrating the efficacy of this adjunct to non-surgical periodontal therapy37., 38., 39..
HOST-MODULATION THERAPY: A BRIEF HISTORY
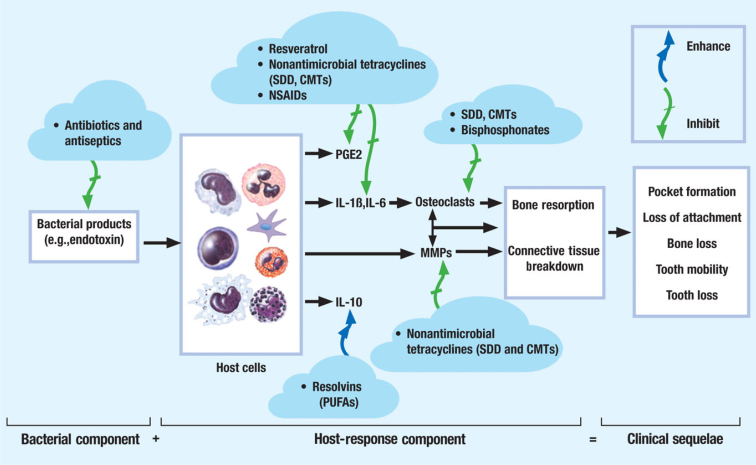
In the past, several approaches have been proposed to modulate the host response using adjunctive medications in patients with chronic periodontitis (Figure 1). Often, this strategy has involved the use of drugs or compounds that either inhibit or, as discussed more recently, ‘resolve’ the inflammatory response to the bacterial aetiological factors9., 10.. These approaches have included the use of compounds ranging from bisphosphonates (such as alendronate), which inhibit osteoclast-mediated bone resorption11, to natural products (such as polyunsaturated fatty acids, as found in fish oil rich in omega-3 and docosahexaenoic acid derivatives), which preserve the acute inflammatory response required to combat infection and for normal wound healing, but which prevent its prolongation (i.e. through the ‘resolvins’)12., 13. and, finally, the non-steroidal anti-inflammatory drugs (NSAIDs) – regarding this last group of compounds, perhaps the greatest attention has been paid to the propionic acid derivatives (e.g. ibuprofen), particularly flurbiprofen. This NSAID has been shown, in a number of studies on periodontitis in the Beagle dog model and in clinical trials over long periods of time (2 years), to reduce alveolar bone loss when used adjunctively to non-surgical periodontal therapy14. However, the well-known side effects (cardiovascular and gastrointestinal, and on the kidneys and liver) of the long-term use of these compounds, plus the ‘rebound’ effect after terminating flurbiprofen treatment (i.e. accelerated bone loss), have precluded their use clinically.
Figure 1.
The therapeutic target of various host-response modulators including the government ‘approved’ non-antibacterial doxycycline formulation (SDD), as well as CMTs, such as inhibitors of matrix metalloproteinase (MMP) and of inflammatory mediators. IL, interleukin; PGE2, prostaglandin E2; PUFAs, polyunsaturated fatty acids. Significantly modified from Golub LM, Ryan ME and Williams RC (18). Modulation of the host response in the treatment of periodontitis. Dent. Today 17:102-109.
Historically, the first use of the term ‘host-modulation’ as a novel therapeutic strategy for periodontal disease was based on the unexpected non-antibiotic, anti-collagenolytic properties of tetracyclines15, which were described over two decades ago. This is the theme of the current review.
NON-ANTIBACTERIAL TETRACYCLINES AS HOST-MODULATORS: THE EARLY ‘DISCOVERY’ EXPERIMENTS
The discovery of the previously unrecognised ability of a common antibiotic family, the tetracyclines (particularly doxycycline), to function as a host-modulating drug, not only for bacteria-induced periodontitis but also for other ‘collagenolytic’ diseases (many unrelated to any microbial insult), has been described in detail16., 17., 18., 19. and is well known in the periodontal literature (see Kornman et al.20). In brief, Ramamurthy et al.21 were the first to demonstrate that experimental induction of diabetes increases the production and activity of gingival (and other) tissue (i.e. host-derived) collagenolytic matrix metalloproteinases (MMPs). They proposed that this metabolic abnormality, which was reversed by insulin therapy to reduce the severity of hyperglycaemia, could explain, at least in part, the increased severity of periodontitis as well as other diabetic complications associated with this all-too-common disease17., 18.. During follow-up experiments using systemic tetracycline therapy in an attempt to suppress the periodontal pathogens present in the microbial biofilm of diabetic rats, it was discovered that this pathologically excessive gingival collagenase activity was reduced by this antibiotic, not only in conventional rats harbouring a typical oral microflora, but also in germ-free rats16. Moreover, they found the same pattern of change, caused by tetracycline treatment, in extra-oral connective tissues not associated with any diabetes-induced alteration in the microbial flora. That is, diabetes increased the MMP activity and collagen loss in skin (as well as in gingiva) and, once again, tetracycline (i.e. minocycline or doxycycline) therapy reduced collagenolysis, even in the absence of any effect on the severity of hyperglycaemia, and by non-antibacterial mechanisms16., 18..
This series of studies: (i) demonstrated, for the first time, that tetracyclines possess an unexpected ability to inhibit host-derived MMP activity and connective tissue destruction in various tissues, and by mechanisms unrelated to their antibiotic properties; and (ii) led to the concept of host-modulation therapy in the management of periodontal patients15. [The biology of the host-derived MMPs, and their potential importance for the development of new diagnostic approaches for periodontal disease, was recently detailed by Sorsa et al.22].
THE DEVELOPMENT OF GOVERNMENT REGULATORY AGENCY-APPROVED, TETRACYCLINE-BASED, MMP-INHIBITOR DRUGS FOR DENTAL AND MEDICAL DISEASES
After decades of research that resulted in the identification of several mechanisms by which tetracyclines inhibit pathologically excessive MMPs (including suppression of the synthesis, activation and activity of these host-generated proteinases), two pathways of drug development were pursued to reduce connective tissue breakdown during periodontitis and in other dental and medical diseases: non-antibacterial tetracycline formulations and novel compositions.
Subantimicrobial dose doxycycline
The first strategy involved the systematic and progressive reduction of the amount of doxycycline per capsule, from 100 mg to 20 mg. This formulation, referred to as SDD, given orally at 12-hour intervals (i.e. twice daily), yielded peak serum levels of 0.3–0.7 μg/mL. Doxycycline serum levels of less than 1 μg/mL are considered too low to have an antibacterial effect17., 18., 19., 23.. In contrast, the doses of doxycycline traditionally recommended – 100 mg, once or twice daily – produce peak serum levels of 2–5 μg/mL and have been demonstrated to result in the rapid emergence of bacteria, both intra- and extra-orally, with resistance to >4 μg/mL of doxycycline23., 24., 25., 26.. Based on the data from these studies, clinical trials were conducted on subjects with chronic periodontitis using SDD as an adjunct to non-surgical periodontal therapy. As reviewed by Golub et al.17., 18. and others19., 27., 28., the novel SDD formulation (20 mg, twice daily) in contrast to the traditional antibiotic dose (100 mg, once or twice daily), has been shown not to induce the development of antibiotic-resistant bacteria27., 29., 30., 31., 32., or other antibiotic side-effects, even after long-term administration of up to 2 years for the management of diseases such as periodontitis, rheumatoid arthritis (RA) and acne33., 35..
To satisfy the requirements of the U S Food and Drug Administration (U S FDA), and the regulatory agencies in other countries (the UK and Canada), a number of safety studies on humans were required for SDD (20 mg, twice daily). These double-blind placebo-controlled clinical trials were conducted on the subgingival flora, the intestinal flora, the vaginal flora and the skin flora. In all cases, there were no statistically or microbiologically significant differences between the subjects who received SDD and those who received placebo. In addition, there were no significant differences in the composition of the bacterial biofilm or its susceptibility to doxycycline or other antibiotics at the end of the study relative to baseline values27., 29., 30., 31., 32.. More recently, a once-daily formulation of SDD has been developed, tested for safety and efficacy, and approved by the government agencies of several countries (e.g. the U.S. FDA) for the treatment of the chronic inflammatory skin disease, acne rosacea.35 This formulation of doxycycline consists of a novel, sustained-release capsule (10-mg slow-release doxycycline ‘beads’ coating 30 mg of traditional doxycycline) designed to release slowly, over a 24-hour time period, low non-antimicrobial levels of the drug (so that ‘peak’ blood levels are less than 1 μg/mL), to prevent the development of antibiotic-resistant bacteria.
In addition to the safety issues just addressed, the use of the SDD formulation has been found to produce significant evidence of efficacy in reducing the severity of periodontitis, in a number of randomised clinical trials in the USA and other countries18., 19., 34., 36., a conclusion confirmed by several review publications that carried out meta-analyses of the data37., 38., 39.. The measurements used to assess clinical efficacy included gingival bleeding scores, probing depth, clinical attachment loss/gain and radiographic measurements of loss of alveolar bone height (subtraction radiography) and bone density (computer-assisted densitometric image analysis). The biological mechanisms involved in these observed beneficial clinical responses have been addressed in numerous studies on human subjects and in animal models. In this regard, administration of SDD has been found to result in a significant reduction in the levels of inflammatory mediators [e.g. cytokines such as interleukin (IL)-1β and tumour necrosis factor-alpha (TNF-α)], markers of alveolar bone resorption (ICTP, a bone type I collagen degradation fragment) and mediators of collagenolysis and connective tissue destruction (collagenase activity; MMP-8, MMP-9 and other proteinases) in human gingiva and in the gingival exudate [gingival crevicular fluid (GCF)] in the periodontal pocket17., 18., 19.. Recently, SDD has also shown an ability to reduce oxidative stress in gingival tissues, including the inhibition of lipid peroxidation and the ‘normalisation’ of antioxidant enzymes, such as superoxide dismutase, providing additional mechanisms by which this host-modulating therapy can inhibit alveolar bone loss during periodontitis40.
As a result of their systematic analysis of this topic, the American Dental Association recently published an ‘evidence-based’ multiauthor/expert paper, assessing the safety and efficacy of non-surgical periodontal therapeutic regimens including SRP alone and SRP combined with various adjuncts, including topical (sustained-release antibiotics and antiseptics; photodynamic and laser therapy) and systemic (antibiotics such as azithromycin and amoxicillin; and SDD as a non-antibiotic host-modulator) treatments8. Based on their review, they reported that SRP alone, and SRP combined with SDD as a host modulator, were the most evidence-based treatments regarding safety and efficacy. For clinical attachment loss measurements described in their analysis, SDD improved the clinical results by 71% compared with SRP without this adjunctive therapy.
Chemically modified tetracyclines
The second strategy used to develop non-antibacterial tetracyclines as host-modulating MMP inhibitors was to modify the chemical structure of these compounds by removing the side-chain, the dimethylamino group at carbon-4, which is known to be responsible for its antibiotic activity. This strategy has been described in detail17., 18., 19., 41. and our experiments resulted in: (i) identification of the site on the tetracycline molecule responsible for its anti-MMP activity, namely the metal ion (calcium and zinc)-binding, β-diketone moiety on carbon-11 and carbon-12; and (ii) development of a series of non-antibiotic, chemically modified tetracyclines, called CMTs or COLs, which show efficacy as inhibitors of MMPs, collagenolysis and bone loss in various in vitro and in vivo models of periodontitis, diabetes (including impaired wound healing), rheumatoid and osteoarthritis, acute lung disease and cancer4., 17., 18., 19..
The most potent of these CMTs or COLs, CMT-3 (COL-3) (i.e. 6-demethyl 6-deoxy 4-dedimethyamino tetracycline), has been tested in FDA-required Phase I and Phase II clinical trials on patients with Kaposi’s sarcoma42., 43.. These trials demonstrated that this experimental non-antibacterial tetracycline compound, administered orally once per day (this compound was rapidly absorbed into the bloodstream after oral administration and exhibited a long serum half-life), produced significant reductions in both angiogenic lesions in the skin of these patients and in their serum biomarkers of the disease, i.e. MMP-2 and -9 and vascular endothelial growth factor (VEGF). However, a side-effect of this experimental drug, significant photosensitivity at the high oral doses used (100–150 mg/day), precluded further clinical development.
At similar high doses, this compound has also shown evidence of efficacy in a lung disease with 40% mortality, namely acute respiratory distress syndrome (ARDS), in a Yorkshire pig model44. However, more recently, much lower doses (one 10-mg capsule per day) administered adjunctive to SRP, versus placebo capsules plus SRP, to humans with periodontal disease, showed preliminary evidence of efficacy by reducing pathologically excessive levels of MMP-8 and collagenase activity, as well as IL-1β, in the GCF of periodontal pockets45. At this low dose, no side-effects, such as photosensitivity, were observed in this pilot study. These studies, both in vitro (not described here) and in preliminary clinical trials, suggest that a ‘low-dose’ formulation of CMT-3 (or one of its analogues) could, in the future, emerge as a safe and effective treatment for a variety of chronic inflammatory diseases, including periodontitis.
EFFICACY OF SDD IN ‘COLLAGENOLYTIC’ MEDICAL DISEASES
When tetracyclines were first discovered to function as MMP-inhibitor drugs, and by mechanisms unrelated to their conventional antibiotic activity, it was immediately proposed that this unexpected property would benefit not only patients with periodontitis but also those with various ‘collagenolytic’ medical diseases16., 17., 18.. The rationale was: (i) collagen is the major structural protein of all the connective tissues in the body, not only in the gingiva, periodontal ligament, cementum and alveolar bone, but also in the skin, skeleton, cartilage, tendons and ligaments, cornea of the eye, and cardiovascular and pulmonary systems; and (ii) the only enzymes produced by body tissues (the host) that can degrade collagen, and also attack other connective tissue constituents, are the collagenases and other MMPs. With this overview in mind, the therapeutic efficacy of SDD formulations in a number of medical diseases has been studied and detailed previously4., 17., 18., 46.. The evidence for dermatology, ophthalmology, rheumatology, diabetes mellitus and postmenopausal bone loss is summarised.
Dermatology and ophthalmology
These two medical specialties were among the first to study, systematically, the efficacy of non-antibiotic properties of tetracyclines, notably doxycycline, in their clinical disciplines17., 18., 19., 28., 35., 47.. In brief, chronic inflammatory skin diseases, such as acne vulgaris and rosacea, involve an abnormal host response, as occurs in periodontitis, including pathological collagenolysis, and a number of randomised clinical trials have demonstrated safety and efficacy in both dermatological diseases. In fact, a recently developed sustained-release one-capsule-per-day formulation of non-antibiotic doxycycline, described above, now an FDA- and international government-approved treatment for rosacea, is widely prescribed as a systemic medication (Oracea®; Galderma Laboratories, L.P., Ft. Worth, TX, USA) for this chronic inflammatory skin disease35, and safely and effectively reduces erythema lesions and telangiectasia in these patients. [Of interest, regarding the common mechanisms for inflammatory skin disease and periodontitis, Preshaw48 described the significant efficacy of this dermatological non-antibiotic low-dose doxycycline formulation, Oracea®, as adjunctive treatment for human periodontitis in their double-blind placebo-controlled study.]
Ophthalmological applications were also among the earliest disorders to be studied using this dental research discovery, and Federici47 recently summarised the animal and clinical studies demonstrating the efficacy of non-antibiotic properties of tetracyclines, notably doxycycline, in successfully healing sterile corneal ulcers (corneal ‘melts’) associated with immune diseases and chemical burns mediated by excess MMP activity, and in blepharitis of the eyelid in patients with rosacea.
Rheumatology
The proposed therapeutic use of tetracyclines, including non-antibiotic chemically modified analogues (the CMTs), for both rheumatoid arthritis (RA) and osteoarthritis (OA), has a long history which has been reviewed extensively17., 18., 19., 49., 50., 51., 52.. Of particular interest, a recent paper by Payne et al.7 addressed the link between RA and periodontitis, including the adjunctive use of non-antimicrobial doxycycline (SDD) therapy for both diseases. This publication is of particular interest for several reasons: (i) it was a collaborative effort involving both academic periodontists and academic rheumatologists; (ii) it highlighted the ‘two-hit’ model of periodontitis53 that is defined by the interaction, in the development of progressive periodontitis, of an altered host response locally in the periodontal tissues and systemic inflammation; and (iii) it described the synergistic therapeutic response, during both periodontitis and RA, when administered as a combination of a non-antibiotic tetracycline (e.g. SDD or a CMT) plus an NSAID (low-dose flurbiprofen). An early incorrect rationale, based on a bacterial origin of RA for this medical use of tetracyclines, has long been abandoned based on the results of numerous animal and clinical studies17., 46.. However, the potential efficacy of these medications in RA, including minocycline, and, more recently, doxycycline and the CMTs, has been confirmed based on their non-antimicrobial, host-modulating properties. In this regard, Greenwald et al.52 reported ‘a profound decline in (host) collagenase activity (average 67%)’ in the synovial tissues surgically excised from patients with RA who were administered doxycycline preoperatively. More recently, O’Dell’s group described a significant reduction in clinically assessed RA severity in response to SDD adjunctive therapy in a long-term (2-year) double-blind placebo-controlled study33.
Regarding OA, a series of studies by Brandt and his colleagues (and others) demonstrated, in tissue culture, in animal models and in humans, that doxycycline administration reduces cartilage breakdown and the severity of OA lesions, effects clearly unrelated to its antibiotic activity, and associated with the inhibition of host-derived MMPs including the collagenases and gelatinases49., 50., 51..
Diabetes
The links between periodontitis and this systemic disease, one of the most common that the dentist has to manage, have been repeatedly addressed in review articles1., 6., 54., 55., 56., including our own17., 18., 19., 57.. Both type 1 and type 2 diabetes, when the severity of hyperglycaemia is not well controlled, often present numerous complications and include those in the oral cavity, such as increased severity of periodontal disease and impaired wound healing. As is well known in the field, the management of the non-enzymatic glycation end-product in the patient’s circulation, glycated haemoglobin (HbA1c), reflects prolonged exposure to elevated glucose levels and is the current ‘gold standard’ for clinical glycaemic control.
Although earlier clinical studies indicated that reducing periodontal severity by SRP can improve glycaemic control in diabetic patients, as reflected by a reduction in the HbA1c levels58., 59., a recent major multi-institutional study by Engebretson and his colleagues did not find any significant improvement in HbA1c levels as a result of non-surgical periodontal therapy (SRP plus the use of an antiseptic mouthrinse)60. Earlier, however, the same author, in a preliminary study on patients with diabetes treated with systemic host-modulation therapy (i.e. SDD 20 mg, twice daily) over a 3-month time period adjunctive to SRP, found that this treatment did appear to show a clinically significant improvement in glycaemic control. In contrast, SRP alone, or in combination with a 2-week regimen of antibiotic therapy, had no effect on the levels of HbA1c61. Additional longer-term studies on the clinical potential of adjunctive host-modulation therapy in diabetic patients are indicated particularly because this therapeutic strategy has a strong basic scientific rationale. As examples, the excess MMP activity and the pathological alterations in bone remodelling (decreased bone formation and increased resorption) induced by experimental diabetes have all benefitted from treatment with non-antibiotic formulations of tetracyclines18., 19..
Postmenopausal skeletal and alveolar bone loss
Postmenopausal osteoporosis and its milder form of systemic bone deficiency, osteopenia, increase the risk for fracture, which, with advancing age, can have serious adverse effects. Postmenopausal skeletal deficiency has also been implicated as a risk factor for periodontal disease62., 63.. About 90% of bone matrix consists of type I collagen, and the destruction of this organic phase during bone resorption (which also, of course, involves the dissolution of the calcium phosphate mineral phase by acid secreted by osteoclasts) is mediated by various MMPs, particularly the collagenases and gelatinases (and osteoclast-produced metallo-elastase) produced by several types of bone cells. Based on this understanding, the therapeutic potential of the non-antibiotic tetracyclines as MMP inhibitors has been extensively explored for the management of pathological bone loss, both local and systemic34, using both in vitro (cell and organ culture) and in vivo animal models of periodontitis, diabetes and postmenopausal osteoporosis17., 18., 19., 34.. These successful experiments, followed by preliminary human studies, culminated in a major long-term double-blind placebo-controlled clinical study that has been published in various dental, medical and biological journals and summarised recently34. In this study, postmenopausal women who exhibited both local (periodontitis) and systemic (osteopenia) bone loss were administered SDD or placebo capsules daily for 2 years, adjunctive to regular periodontal maintenance therapy. This regimen was found to be safe and effective based on biochemical, clinical and quantitative X-ray measurements of periodontal disease severity. In addition, the beneficial effects on circulating biomarkers of skeletal bone resorption (ICTP and CTX) generated the hypothesis that SDD treatment in postmenopausal women could decrease the risk of osteoporosis development64.
Cardiovascular and pulmonary diseases
A number of publications in the dental and medical literature have described evidence, at both the basic science and clinical levels, that non-antibiotic tetracyclines (e.g. SDD) can improve these all-too-common conditions (Figure 2). Accordingly, the reader is referred to recent and earlier reviews of these topics17., 18., 19., 65., 66., 67., 68., 69., 70., 71., 72., 73., 74., 75., 76., 77., 78., 79..
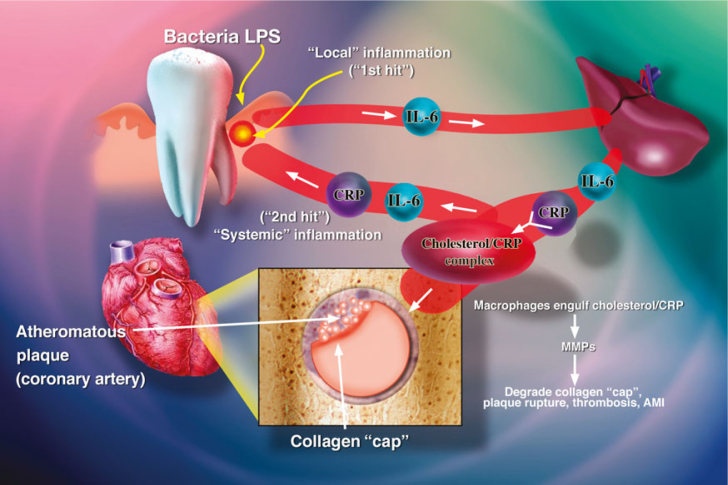
Figure 2.
The ‘Two-Hit’ model of periodontal disease and its systemic implications. The ‘1st Hit’ represents local gingival inflammation induced by the subgingival biofilm. The inflammatory mediators generated by this ‘Hit’ reach the liver via the circulation to induce an acute-phase response and systemic inflammation. These circulating mediators [e.g. C-reactive protein (CRP) and interleukin-6 (IL-6)] then target other organs (e.g. the heart, to promote cardiovascular disease), including the already-inflamed gingiva, thus exacerbating periodontal disease severity (the ‘2nd Hit’). LPS, lipopolysaccharide. Modified from Golub et al.53
ON THE HORIZON
Several related innovative categories of potential host-modulation therapies have recently emerged and include the following:
-
•
A low dose of CMT-3, a novel derivative of tetracycline that has no antibiotic activity at any dose but which is a potent MMP inhibitor. In earlier clinical trials, this experimental drug at relatively high oral doses (e.g. 100 mg/day) did show efficacy in patients with Kaposi’s sarcoma as an angiogenesis inhibitor. However, a side-effect at these doses was significant photosensitivity42., 43.. Of particular interest, a low-dose formulation of CMT-3, of 10 mg/day, also administered orally, did show evidence of efficacy in patients with chronic periodontitis as a suppressor of MMP-8 and IL-1β22., 45.; no evidence of adverse events were seen in this limited and preliminary clinical study
-
•
A novel combination of SDD plus ‘low-dose’ NSAID (flurbiprofen) which, in preliminary human studies, dramatically reduced pathological levels of MMPs and elastase in gingival tissues of patients with periodontitis requiring surgical intervention80. This host-modulation therapy formulation is currently being tested in the management of patients with either refractory periodontitis or with peri-implantitis81. This experimental therapeutic rationale is based, in part, on the pathological levels of MMP-7 and MMP-8 in these lesions22.
CONCLUSIONS
We have reviewed the translational research which resulted in the first government-approved oral/systemically administered drugs as host-modulation therapies. This research provides evidence and a rationale for a shift from the traditional ‘surgical’ model of periodontal therapy, to one that increasingly incorporates a ‘medical/pharmaceutical’ approach. This new strategy, which manages periodontitis as an inflammatory and collagenolytic disorder, not just an anatomical defect, may result in an increasing number of patients receiving treatment for this most common chronic disease.
Acknowledgements
LMG acknowledges the following grants which supported early and more recent studies discussed in this article, including early grants from the Medical Research Council of Canada (#MA-3516, MA-3627, MA-5073), the N.Y. Diabetes Assoc., the Kroc foundation for Medical research, Johnson and Johnson, Collagenex Pharmaceuticals, Inc. and the NIDR (#DE-03987; DE-04234; DE-07788; DE-09576), and more recent grants from the NIDCR (#R37 DE-03987 and RO1 DE-12872).
Disclosure
Lorne M. Golub is listed as an inventor in patents on medications described in this paper and these have been fully assigned to his institution, Stony Brook University, S.U.N.Y. The following co-authors declare no conflicts of interest and no acknowledgements: Y. Gu, C. Walker, M. Wolff, M. Elburki, M. Ryan, T. Sorsa, H. Tenenbaum, M. Goldberg.
References
- 1.Löe H. Periodontal disease: the sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329–334. [PubMed] [Google Scholar]
- 2.Beck J, Garcia R, Heiss G, et al. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 3.Katz J, Chaushu G, Sharabi Y. On the association between hypercholesterolemia, cardiovascular disease and severe periodontal disease. J Clin Periodontol. 2001;28:865–868. doi: 10.1034/j.1600-051x.2001.028009865.x. [DOI] [PubMed] [Google Scholar]
- 4.Golub LM, Greenwald RA. Clinical applications of non-antibacterial tetracyclines. Part II. Pharmacol Res. 2011;64:549–550. doi: 10.1016/j.phrs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 6.Paquette DW, Bell KP, Phillips C, et al. Dentists’ knowledge and opinions of oral-systemic disease relationships: relevance to patient care and education. J Dent Educ. 2015;79:626–635. [PubMed] [Google Scholar]
- 7.Payne JB, Golub LM, Thiele GM, et al. The link between periodontitis and rheumatoid arthritis: a periodontist’s perspective. Curr Oral Health Rep. 2015;2:20–29. doi: 10.1007/s40496-014-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smiley CJ, Tracy SL, Abt E, et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146:525–535. doi: 10.1016/j.adaj.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN. Novel pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenenbaum H, Shelemay A, Girard B, et al. Bisphosphonates and periodontics: potential applications for regulation of bone mass in the periodontium and other therapeutic/diagnostic uses. J Periodontol. 2002;73:813–822. doi: 10.1902/jop.2002.73.7.813. [DOI] [PubMed] [Google Scholar]
- 12.Naqvi AZ, Hasturk H, Mu L, et al. Docosahexaenoic acid and periodontitis in adults: a randomized controlled trial. J Dent Res. 2014;93:767–773. doi: 10.1177/0022034514541125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dyke TE. Commentary: Periodontitis is characterized by an immuno-inflammatory host-mediated destruction of bone and connective tissues that support the teeth. J Periodontol. 2014;85:509–511. doi: 10.1902/jop.2014.130701. [DOI] [PubMed] [Google Scholar]
- 14.Williams RC, Jeffcoat MK, Howard Howell T, et al. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J Periodontol. 1989;60:485–490. doi: 10.1902/jop.1989.60.9.485. [DOI] [PubMed] [Google Scholar]
- 15.Golub L, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent. 1992;2:80–90. [PubMed] [Google Scholar]
- 16.Golub LM, Lee HM, Lehrer G, et al. Minocycline reduces gingival collagenolytic activity during diabetes. J Periodontal Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 17.Golub LM, Ramamurthy NS, McNamara TF. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–322. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 18.Golub LM, Lee HM, Ryan M, et al. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 19.Gu Y, Walker C, Ryan ME, et al. Non-antibacterial tetracycline formulations: clinical applications in dentistry and medicine. J Oral Microbiol. 2012;4:1–14. doi: 10.3402/jom.v4i0.19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornman KS, Robertson PB, Williams RC. Commentary: The literature that shaped modern periodontology. J Periodontol. 2014;85:3–9. doi: 10.1902/jop.2014.141003. [DOI] [PubMed] [Google Scholar]
- 21.Ramamurthy NS, Zebrowski EJ, Golub LM. Collagenolytic activity of gingivae from alloxan-diabetic rats. Diabetes. 1973;22:272–274. doi: 10.2337/diab.22.4.272. [DOI] [PubMed] [Google Scholar]
- 22.Sorsa T, Gursoy U, Nwhator S, et al. Analysis of matrix metalloproteinases in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000. 2016;70:142–163. doi: 10.1111/prd.12101. [DOI] [PubMed] [Google Scholar]
- 23.McNamara TF, Golub LM, Yu Z et al. Reduced doxycycline blood levels in humans fail to promote resistant organisms. In: Int Conf Periodont Dis Pathog Host Immune Responses 1990 Abstracts # B54, Osaka, Japan, p. 100.
- 24.Heimdahl A, Nord C. Influence of doxycycline on the normal flora and colonization of the oral cavity and colon. Swed Dent J. 1983;7:199–204. [Google Scholar]
- 25.Fiehn N-E. Doxycycline-resistant bacteria in periodontally diseased individuals after systemic doxycycline therapy and in healthy individuals. Oral Microbiol Immunol. 1990;5:219–222. doi: 10.1111/j.1399-302x.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 26.Rams TE, Babalola OO, Slots J. Subgingival occurrence of enteric rods, yeasts and staphylococci after systemic doxycycline therapy. Oral Microbiol Immunol. 1990;5:166–168. doi: 10.1111/j.1399-302x.1990.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 27.Walker C, Thomas J, Nango S, et al. Long-term treatment with subantimicrobial dose doxycycline exerts no antibacterial effect on the subgingival microflora associated with adult periodontitis. J Periodontol. 2000;71:1465–1471. doi: 10.1902/jop.2000.71.9.1465. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg M. New applications of doxycycline hyclate in medicine and dentistry. US Pharm. 2004;29:1–6. [Google Scholar]
- 29.Walker C, Preshaw PM, Novak J, et al. Long-term treatment with sub-antimicrobial dose doxycycline has no antibacterial effect on intestinal flora. J Clin Periodontol. 2005;32:1163–1169. doi: 10.1111/j.1600-051X.2005.00840.x. [DOI] [PubMed] [Google Scholar]
- 30.Thomas J, Walker C, Bradshaw MH. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol. 2000;71:1472–1483. doi: 10.1902/jop.2000.71.9.1472. [DOI] [PubMed] [Google Scholar]
- 31.Walker C, Puumala S, Golub LM, et al. Subantimicrobial dose doxycycline effects on osteopenic bone loss: microbiologic results. J Periodontol. 2007;78:1590–1601. doi: 10.1902/jop.2007.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skidmore R, Kovach R, Walker C, et al. Effects of subantimicrobial-dose doxycycline hyclate in the treatment of moderate acne. Arch Dermatol. 2003;139:459–464. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- 33.O’Dell JR, Elliott JR, Mallek JA, et al. Treatment of early seropositive rheumatoid arthritis: doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum. 2006;54:621–627. doi: 10.1002/art.21620. [DOI] [PubMed] [Google Scholar]
- 34.Payne JB, Golub LM. Using tetracyclines to treat osteoporotic/osteopenic bone loss: from the basic science laboratory to the clinic. Pharmacol Res. 2011;63:121–129. doi: 10.1016/j.phrs.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63:130–145. doi: 10.1016/j.phrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Ryan M. Host modulation: conceptualization to clinical trials and integration into clinical practice. J Calif Dent Assoc. 2002;30:285–293. [PubMed] [Google Scholar]
- 37.Reddy MS, Geurs N, Gunsolley J. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003;8:12–37. doi: 10.1902/annals.2003.8.1.12. [DOI] [PubMed] [Google Scholar]
- 38.Preshaw PM, Hefti AF, Jepsen S, et al. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. J Clin Periodontol. 2004;31:697–707. doi: 10.1111/j.1600-051X.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 39.Sgolastra F, Petrucci A, Gatto R, et al. Long-term efficacy of subantimicrobial-dose doxycycline as an adjunctive treatment to scaling and root planing: a systematic review and meta-analysis. J Periodontol. 2011;82:1570–1581. doi: 10.1902/jop.2011.110026. [DOI] [PubMed] [Google Scholar]
- 40.Yağan A, Kesim S, Liman N. Effect of low-dose doxycycline on serum oxidative status, gingival antioxidant levels, and alveolar bone loss in experimental periodontitis in rats. J Periodontol. 2014;85:478–489. doi: 10.1902/jop.2013.130138. [DOI] [PubMed] [Google Scholar]
- 41.Golub LM, McNamara TF, D’Angelo G, et al. A non-antibacterial chemically – modified tetracycline inhibits mammalian collagenase activity. J Dent Res. 1987;66:1310–1314. doi: 10.1177/00220345870660080401. [DOI] [PubMed] [Google Scholar]
- 42.Dezube BJ, Krown SE, Lee JY, et al. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi’s Sarcoma: an AIDS malignancy consortium study. J Clin Oncol. 2006;24:1389–1394. doi: 10.1200/JCO.2005.04.2614. [DOI] [PubMed] [Google Scholar]
- 43.Richards C, Pantanowitz L, Dezube BJ. Antimicrobial and non-antimicrobial tetracyclines in human cancer trials. Pharmacol Res. 2011;63:151–156. doi: 10.1016/j.phrs.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Roy SK, Kendrick D, Sadowitz BD, et al. Jack of all trades: pleiotropy and the application of chemically modified tetracycline-3 in sepsis and the acute respiratory distress syndrome (ARDS) Pharmacol Res. 2011;64:580–589. doi: 10.1016/j.phrs.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryan ME, Lee HM, Sorsa T et al. Effects of short-term COL-3 on local biomarkers of periodontitis. J Dent Res 2008 87; Abstract # 0040.
- 46.Greenwald RA. The road forward: the scientific basis for tetracycline treatment of arthritic disorders. Pharmacol Res. 2011;64:610–613. doi: 10.1016/j.phrs.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Federici TJ. The non-antibiotic properties of tetracyclines: clinical potential in ophthalmic disease. Pharmacol Res. 2011;64:614–623. doi: 10.1016/j.phrs.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Preshaw PM. Host response modulation in periodontics. Periodontol 2000. 2008;48:92–110. doi: 10.1111/j.1600-0757.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- 49.Smith G, Yu L, Brandt K, et al. Oral administration of doxycycline reduces collagenase and gelatinase activities in extracts of human osteoarthritic cartilage. J Rheumatol. 1998;25:532–535. [PubMed] [Google Scholar]
- 50.Brandt KD, Mazzuca SA, Katz BP, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52:2015–2025. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- 51.De Bri E, Lei W, Svensson O, et al. Effect of an inhibitor of matrix metalloproteinases on spontaneous osteoarthritis in Guinea pigs. Adv Dent Res. 1998;12:82–85. doi: 10.1177/08959374980120012601. [DOI] [PubMed] [Google Scholar]
- 52.Greenwald RA, Golub LM, Lavietes B, et al. Tetracyclines inhibit human synovial collagenase in vivo and in vitro. J Rheumatol. 1987;14:28–32. [PubMed] [Google Scholar]
- 53.Golub LM, Payne JB, Reinhardt RA, et al. Can systemic diseases co-induce (not just exacerbate) periodontitis? A hypothetical “two-hit” model. J Dent Res. 2006;85:102–105. doi: 10.1177/154405910608500201. [DOI] [PubMed] [Google Scholar]
- 54.Chrysanthakopoulos NA, Chrysanthakopoulos PA. Association between indices of clinically-defined periodontitis and self-reported history of systemic medical conditions. J Investig Clin Dent. 2014;5:1–10. doi: 10.1111/jicd.12119. [DOI] [PubMed] [Google Scholar]
- 55.Lalla E, Lamster IB. Assessment and management of patients with diabetes mellitus in the dental office. Dent Clin North Am. 2012;56:819–829. doi: 10.1016/j.cden.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Lamster IB, Lalla E, Borgnakke WS, et al. The relationship between oral health and diabetes mellitus. J Am Dent Assoc. 2008;139:19S–24S. doi: 10.14219/jada.archive.2008.0363. [DOI] [PubMed] [Google Scholar]
- 57.Walker S, Golub LM. Host modulation therapy for periodontal disease: subantimicrobial-dose doxycycline, medical as well as dental benefits. Oral Health J. 2012;10:24–35. [Google Scholar]
- 58.Aldridge JP, Lester V, Watts TLP, et al. Single-blind studies of the effects of improved periodontal health on metabolic control in Type 1 diabetes mellitus. J Clin Periodontol. 1995;22:271–275. doi: 10.1111/j.1600-051x.1995.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 59.Grossi SG, Skrepcinski F, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997;68:713–719. doi: 10.1902/jop.1997.68.8.713. [DOI] [PubMed] [Google Scholar]
- 60.Engebretson SP, Hyman LG, Michalowicz BS, et al. The effect of nonsurgical periodontal therapy on Hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310:2523–2532. doi: 10.1001/jama.2013.282431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engebretson SP, Hey-Hadavi J. Sub-antimicrobial doxycycline for periodontitis reduces hemoglobin A1c in subjects with type 2 diabetes: a pilot study. Pharmacol Res. 2011;64:624–629. doi: 10.1016/j.phrs.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol. 1994;65:1134–1138. doi: 10.1902/jop.1994.65.12.1134. [DOI] [PubMed] [Google Scholar]
- 63.Martínez-Maestre M, Gonzalez-Cejudo C, Machuca G, et al. Periodontitis and osteoporosis: a systematic review. Climacteric. 2010;13:523–529. doi: 10.3109/13697137.2010.500749. [DOI] [PubMed] [Google Scholar]
- 64.Golub LM, Lee H-M, Stoner JA, et al. Doxycycline effects on serum bone biomarkers in post-menopausal women. J Dent Res. 2010;89:644–649. doi: 10.1177/0022034510363367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro MM, Kandasamy AD, Youssef N, et al. Matrix metalloproteinase inhibitor properties of tetracyclines: therapeutic potential in cardiovascular diseases. Pharmacol Res. 2011;64:551–560. doi: 10.1016/j.phrs.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 66.Meier C, Derby L, Jick S, et al. Antibiotics and risk of subsequent first-time acute myocardial infarction. JAMA. 1999;281:427–431. doi: 10.1001/jama.281.5.427. [DOI] [PubMed] [Google Scholar]
- 67.Golub LM, Greenwald RA, Thompson RW. Antibiotic use and risk of myocardial infarction. JAMA. 1999;282:1997–1998. [PubMed] [Google Scholar]
- 68.Brown DL, Desai K, Vakili B, et al. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 69.Payne JB, Golub LM, Stoner JA, et al. The effect of subantimicrobial-dose-doxycycline periodontal therapy on serum biomarkers of systemic inflammation: a randomized, double-masked, placebo-controlled clinical trial. J Am Dent Assoc. 2011;142:262–273. doi: 10.14219/jada.archive.2011.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong M, Zhong L, Chen WQ, et al. Doxycycline stabilizes vulnerable plaque via inhibiting matrix metalloproteinases and attenuating inflammation in rabbits. PLoS ONE. 2012;7:e39695. doi: 10.1371/journal.pone.0039695. Aikawa E, editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salminen A, Pussinen PJ, Payne JB, et al. Subantimicrobial-dose doxycycline treatment increases serum cholesterol efflux capacity from macrophages. Inflamm Res. 2013;62:711–720. doi: 10.1007/s00011-013-0626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 73.Gu Y, Ryan ME, Tenzler R et al. Periodontal status in acute coronary syndrome patients: pilot study. J Dent Res. 2011 90; spec. issue A, Abstract # 2206.
- 74.Gotsman I, Lotan C, Soskolne WA, et al. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J Periodontol. 2007;78:849–858. doi: 10.1902/jop.2007.060301. [DOI] [PubMed] [Google Scholar]
- 75.Curci JA, Petrinec D, Liao S, et al. Pharmacologic suppression of experimental abdominal aortic aneurysms: a comparison of doxycycline and four chemically modified tetracyclines. J Vasc Surg. 1998;28:1082–1093. doi: 10.1016/s0741-5214(98)70035-7. [DOI] [PubMed] [Google Scholar]
- 76.Thompson RW, Baxter BT. MMP inhibition in abdominal aortic aneurysms: rationale for a prospective randomized clinical trial. Ann N Y Acad Sci. 1999;878:159–178. doi: 10.1111/j.1749-6632.1999.tb07682.x. [DOI] [PubMed] [Google Scholar]
- 77.Steinberg J, Halter J, Schiller H, et al. Chemically modified tetracycline prevents the development of septic shock and acute respiratory distress syndrome in a clinically applicable porcine model. Shock. 2005;24:348–356. doi: 10.1097/01.shk.0000180619.06317.2c. [DOI] [PubMed] [Google Scholar]
- 78.Moses MA, Harper J, Folkman J. Doxycycline treatment for lymphangioleiomyomatosis with urinary monitoring for MMPs. N Engl J Med. 2006;354:2621–2622. doi: 10.1056/NEJMc053410. [DOI] [PubMed] [Google Scholar]
- 79.Pimenta S, Baldi B, Kairalla R, et al. Doxycycline use in patients with lymphangioleiomyomatosis: biomarkers and pulmonary function response. J Bras Pneumol. 2013;39:5–15. doi: 10.1590/S1806-37132013000100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee HM, Ciancio SG, Tüter G, et al. Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal anti-inflammatory drug. J Periodontol. 2004;75:453–463. doi: 10.1902/jop.2004.75.3.453. [DOI] [PubMed] [Google Scholar]
- 81.Goldberg MB, Golub LM, Lee LM et al. Assessing the effects of subantimicrobial-dose doxycycline/flurbiprofen on refractory periodontal disease. J Dent Res 2010 89; abstract #1176.