Abstract
Tooth sensitivity is a common complaint of patients in dental practices. Studies have demonstrated dentinal hypersensitivity to affect 10–30% of the population. There are various potential causes of tooth sensitivity and a variety of available treatment options. This narrative review will discuss the possible aetiology of this condition, as well as the treatment modalities available. A tailor-made treatment plan that starts with the most non-invasive treatment options and escalates only when those options have proven insufficient in alleviating symptoms should be provided for each patient. Only after all non- and less-invasive methods have failed to reduce the symptoms should more invasive treatment options, such as root-coverage, be considered.
Key words: Plaque, gingival health, prevention, root coverage, gingival graft
INTRODUCTION
Tooth sensitivity is a common complaint of patients in a dental practices. Studies have demonstrated dentinal hypersensitivity to affect 10–30% of the population1., 2.. Although studies vary, the most common age range in which dentinal hypersensitivity is experienced is 20–50 years, with female patients predominantly being affected3., 4.. Canines and first premolars are found to be the teeth most commonly affected as a result of their prominent position in the maxillary and mandibular arches3., 4.. Dentinal hypersensitivity has varying degrees of pain; however, it can alter a patient’s daily activities, leading them to seek treatment from dental professionals. Therefore, it is important for the dental team to be aware of the different treatments available and to decide which treatments are appropriate and when they should be utilized.
The sensitivity experienced is mostly attributed to exposure of dentin. Dentinal tubules have a diameter of approximately 0.5 μm at the periphery and are normally protected by a layer of enamel5. When the enamel is removed or recession reveals the root surfaces of a tooth, these tubules are exposed and as a result can be highly sensitive to stimuli5. The pulp is richly innervated, but the dentin is not, creating controversy around the mechanism of tooth hypersensitivity. There are multiple theories regarding sensitivity; however, the most widely accepted is known as the ‘hydrodynamic theory’. Proposed by Brannstrom and Astrom, the hydrodynamic theory considers that thermal, osmotic or physical stimuli create movement of fluid within the dentinal tubules, causing the activation of nerve endings6., 7.. These nerve endings are thought to be at the border of the dentin and the pulp5. The activation of nerve endings causes a distinguishing sharp and rapid pain8 and many treatments have been created to relieve these symptoms.
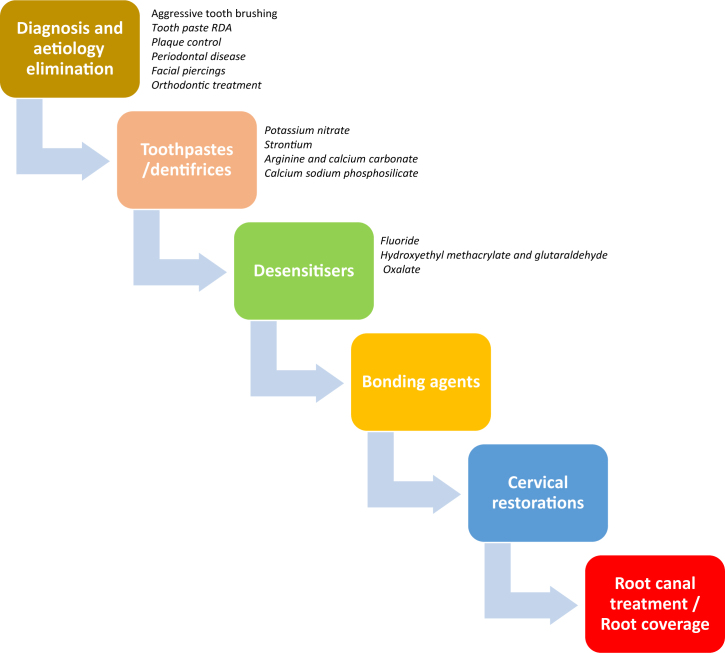
The aim of this review was to present a summary of the conventional acceptable treatment options for tooth hypersensitivity based on the available literature and common practice (Figure 1).
Figure 1.
It is important to implement appropriate treatment options for dentinal hypersensitivity that are based on individual situations. Nonetheless, consider the consequence of proceeding with surgical treatment before trying non-invasive options, such as eliminating the cause or changing the toothpaste. To treat the condition properly, consider the patient’s risk factors and the initial cause of the sensitivity. As in any condition, start with eliminating the cause, and then select the least invasive option available as opposed to one that is more invasive. RDA, relative dentin abrasivity.
METHODS
This was a narrative review conducted using data obtained from a literature search of the Medline and PubMed databases. Specific key words used to conduct the literature search were dentine hypersensitivity, treatment, gingival recession, prevention and aetiology. Other types of sensitivity, such as microcracks, occlusal fractures, and sensitivity after restoration, were beyond the scope of this review and were not included. The review focussed specifically on hypersensitivity caused by gingival recession and dentinal exposure.
RESULTS AND DISCUSSION
The first steps in treating a symptom such as tooth hypersensitivity should be diagnosis and identification of aetiology followed by an attempt to reduce or eliminate the factors contributing to the symptom.
Treatment options for tooth hypersensitivity
Elimination of aetiological factors
The most common aetiology of dentin exposure is gingival recession5. When the gingival margin recedes past the cemento–enamel junction it reveals cementum. This thin cementum covering is easily lost, as a result of which dentinal tubules become exposed to the external environment9. This exposure is thought to be influenced by aggressive toothbrushing, abrasive toothpastes, poor plaque control, facial piercings, periodontal disease, anatomical predisposition and orthodontic treatment. Avoiding and eliminating these known aetiological factors will help to prevent gingival recession and as a result will prevent dentinal hypersensitivity.
Aggressive Toothbrushing.
Aggressive toothbrushing is considered to be the use of excessive force with a hard-bristled toothbrush. A study published in 2013 indicated that most cases of dentinal hypersensitivity studied involved patients who were currently using a hard toothbrush10. However, it has also been argued that although aggressive toothbrushing may play a role in the abrasion of soft tissue, other factors, such as anatomical predisposition and toothpaste abrasiveness, should also be evaluated as potential co-contributors to tooth hypersensitivity11.
Toothpaste Relative Dentin Abrasavity.
Relative dentin abrasivity (RDA) is a method for measuring the abrasiveness of certain ingredients in toothpastes on the dentin surface. An in situ randomised trial published in 2012 determined that RDA was directly related to dentin loss and concluded that patients with dentin hypersensitivity should opt for a toothpaste with lower RDA12. Another study, published in 2009, differentiated the erosive effects of a moderately abrasive toothpaste and a highly abrasive toothpaste, and iterated the importance of implementing the use of low RDA toothpastes in patients susceptible to dentin hypersensitivity13. With RDA in mind, it is important to remember that dentin hypersensitivity is multifactorial and there may be other aspects impacting a patient’s sensitivity.
Plaque Control.
In a study published by Fukumoto et al.14, teeth devoid of plaque were more hypersensitive than teeth with plaque accumulation. This statement was disputed by another work that found a significant association between high plaque accumulation and gingival recession15. Nonetheless, there are few studies demonstrating a specific cause-and-effect relationship between plaque accumulation and dentin hypersensitivity. The relationship between plaque and recession can be attributed to attachment loss that might precede the plaque-induced gingival inflammation.
Periodontal Disease.
Periodontal disease can be considered as a risk factor or a cause of dentinal hypersensitivity as it involves gingival recession and therefore is associated with dentin exposure. A study performed on an adult and elderly population in Brazil stated that a reduction in the prevalence of tooth sensitivity may be accomplished by periodontal health improvements16. A systematic review published in 2013 examined whether periodontal therapy impacted tooth hypersensitivity17. It concluded that there was insufficient research to establish whether scaling and root planing procedures had any impact on tooth hypersensitivity and, as a result, more research needs to be performed before making recommendations specific to the correlation of periodontal disease and dentinal hypersensitivity17.
Facial Piercings.
Facial piercings are strongly correlated with the prevalence of recession18., 19., 20., 21., 22.. It is important to discuss with patients the potential outcomes of facial piercings and inform them of the association between recession and dentin hypersensitivity.
Orthodontic Treatment.
Depending on the extent and severity of orthodontic treatment, gingival recession may be an undesired outcome. A study published in 2008 examined 303 healthy patients and found that recession was strongly correlated with previous orthodontic treatment18. Therefore, orthodontic treatment is another potential risk factor for dentin hypersensitivity.
Following a diagnosis of tooth hypersensitivity, a thorough search for possible aetiology and contributing factors should be performed. An attempt to reduce or eliminate the contributing factors might be a helpful non-invasive and easy stage of treatment of tooth hypersensitivity. Upon elimination of these risk factors, the prevalence of tooth sensitivity and future sensitivity may be reduced.
Toothpastes/dentifrices
In efforts to treat dentinal hypersensitivity, certain ingredients are added to dentifrices. The purpose of these ingredients is to relieve dentinal hypersensitivity by either eliminating nerve conduction or occluding the dentinal tubules. Such ingredients include potassium nitrate, strontium acetate, arginine and calcium carbonate, and calcium sodium phosphosilicate.
Potassium Nitrate.
Potassium nitrate is added to toothpastes and marketed to decrease dentinal hypersensitivity (Pronamel: GlaxoSmithKline, Brentford, UK; Maximum Strength Sensitive Toothpaste: Toms of Maine, Kennebunk, ME, USA). One of its properties is nerve depolarisation. Potassium nitrate depolarises the nerves within the dentinal tubules and inhibits their ability to transmit pain23., 24., 25.. Some studies have found that this ingredient does not improve the symptoms of dentine hypersensitivity26., 27.. Other studies demonstrate that patients experiencing sensitivity have a reduction in their symptoms after a 2-week use of potassium nitrate-containing dentifrice28., 29..
Strontium.
Strontium is an ingredient commonly found in dentifrices directed towards reduction of dentinal hypersensitivity (Sensodyne Original; GlaxoSmithKline). Unlike potassium nitrate, strontium does not affect nerve polarisation. Instead, strontium has the ability to occlude dentinal tubules30. Strontium ions exchange with calcium ions, causing the formation of strontium crystals within dentinal tubules31., 32.. An in situ study published in 2015 found that strontium acetate occluded the dentin tubules to an average depth, below the surface, of 5 μm30. This study concluded that because strontium occluded tubules to a significant extent, its use in the management of dentinal hypersensitivity is warranted30.
Arginine and Calcium Carbonate.
The combination of arginine and calcium carbonate acts like strontium acetate in that it also occludes dentinal tubules and blocks the movement of fluid suspected to cause sensitivity (Colgate Sensitive Pro-Relief; Colgate Palmolive, New York City, NY, USA). A study by Kleinberg discussed the mechanism of occlusion as a process whereby the combination of calcium carbonate and arginine forms a positive complex with the negatively charged dentin surface, thus facilitating the occlusion of the tubules33. The alkalinity of the arginine and calcium carbonate also allows enhanced uptake of calcium and phosphate ions into saliva, which are then deposited onto the dentin surface33. According to a systematic review published in 2015, most studies demonstrated that the combination of arginine and calcium carbonate provided better alleviation of dentinal hypersensitivity than did strontium acetate34. According to this literature review, only one study described that strontium acetate had increased effectiveness compared to the combination of calcium carbonate and arginine; however, it was solely for tactile stimuli3. The review concluded that the combination of arginine and calcium carbonate was more effective than strontium acetate in treating dentinal hypersensitivity34.
Calcium Sodium Phosphosilicate.
The ingredient blend of calcium sodium phosphosilicate is designed to stimulate the remineralisation of enamel and simultaneously it occludes dentinal tubules (Sensodyne Complete Protection; GlaxoSmithKline). By the same mechanism as strontium acetate and the combination of arginine and calcium carbonate, calcium sodium phosphosilicate acts as a desensitising ingredient in toothpastes. When in the oral cavity, sodium ions exchange with hydrogen ions, allowing the release of calcium and phosphate from the dentifrice. These minerals deposit within the dentinal tubules until occlusion occurs35. The efficacy of these ingredients in reducing sensitivity has been proven in studies by Pradeepet et al.36 and Rajesh et al.37; however, there is controversy regarding the comparison of calcium sodium phosphosilicate with the arginine and calcium carbonate combination. A study performed in 2015, by Chen et al.35 demonstrated that arginine and calcium carbonate facilitated deeper tubule occlusion compared with sodium phosphosilicate. The authors of this study recognised that this result was inconsistent with the results of an in situ study performed by West et al.38, which demonstrated that sodium phosphosilicate was significantly more efficient at reducing dentin hypersensitivity compared with arginine and calcium carbonate35. An interesting difference within the studies was the method of application. The study by West et al. used an electrical toothbrush, which was speculated to enhance the uptake of the ingredients35., 38.. There is insufficient literature to conclude whether sodium phosphosilicate is superior to arginine and calcium carbonate; however, both ingredient combinations have been proven to be an effective intervention for the treatment of dentin hypersensitivity.
High fluoride concentration desensitisers.
High fluoride concentration toothpastes can also serve as part of this phase of treatment of hypersensitivity. These are usually prescription-only fluoride toothpastes that can deliver 5000–12,500 ppm fluoride (PreviDent® 5000; Colgate Palmolive/Elmex® gel, GABA International, Therwil, Switzerland). High levels of topical fluoride will help in remineralisation and can relieve dentinal hypersensitivity.
Once the aetiology has been identified, the symptoms of the dentinal hypersensitivity can be treated by using a variety of sensitivity toothpastes. However, because of the different mechanisms by which sensitivity toothpastes work, patients may need to use several different dentifrices before they find one that is successful in alleviating their symptoms.
Desensitisers
Dentin desensitisers are products used by dental professionals to treat dentinal hypersensitivity. Desensitisers have different ingredients, such as fluoride, hydroxyethyl methacrylate, glutaraldehyde, oxalate and potassium nitrate, as well as a combination of these ingredients. With the exception of potassium nitrate, these desensitising agents occlude the dentinal tubules to relieve sensitivity.
A further non-invasive stage for treatment of tooth hypersensitivity should focus on local administration of those agents.
Fluoride.
Fluoride varnish is a desensitiser, commonly used by dental professionals, which is applied by painting the solution onto the affected surfaces. The solution sets via interacting with saliva, thus allowing it to stay on the tooth surface and facilitating maximal uptake of fluoride. A study published in 2012 compared the efficacy of fluoride varnish with potassium nitrate regarding their desensitising abilities39. Instead of occluding dentin tubules, the potassium nitrate depresses nerve conduction to relieve sensitivity. Although both ingredients caused significant reduction in dentin hypersensitivity, the fluoride varnish provided longer relief than potassium nitrate39.
Hydroxyethyl Methacrylate and Glutaraldehyde.
The combination of glutaraldehyde and hydroxyethyl methacrylate is currently a popular desensitiser and is commonly referred to by its brand name, Gluma (Heraeus Kulzer, Hanau, Germany). The combination of ingredients from which Gluma is composed has been proven to be significantly effective in treating dentinal hypersensitivity, as studies have shown Gluma to occlude tubules by penetrating up to a depth of 50–200 μm40., 41., 42.. A study published in 2015 analysed different combinations of these ingredients23. The combinations included Gluma, Gluma plus a wetting agent intended to enhance the bond with the tooth (Gluma Comfort Bond) and a self-etching adhesive called Single Bond Universal43. All three desensitising agents were effective in relieving hypersensitivity43. The only difference found between the ingredients was between Gluma and Single Bond Universal and between Gluma Comfort Bond and Single Bond Universal43. Single Bond Universal was shown to be less effective in treating hypersensitivity43.
Oxalate.
Oxalate is another desensitiser used by dental professionals that works by combining with calcium ions present in saliva. The combination forms insoluble calcium oxalate crystals that precipitate within the tubules, eventually occluding them44. Studies have shown that this occlusion is sufficient to limit fluid movement and as a result reduces hypersensitivity45., 46.. An added benefit of oxalate use is its resistance in an acidic environment, making it more durable than other desensitising agents46.
Bonding agents
Bonding agents are used for a variety of dental applications, one of which is restorative dentistry. Bonding agents etch tooth surfaces in order to provide an adhesive layer for the application of a desired material45. Another use for dentin bonding agents, however, can be to treat hypersensitivity45. Self-etch bonding systems typically contain acidic ingredients that condition the dentin, as well as monomers that combine on the dentin, forming a hybrid layer45. This layer provides a coating over the dentin and significantly reduces hypersensitivity over a 4-week period45. Two-step systems are thought to be even more effective as they are proven to be less permeable and more durable45.
A randomised control trial compared the effects of dentine bonding agents, desensitising toothpaste and non-desensitising toothpastes46. The agents were evaluated at 2-week, 3-month and 6-month periods46. The study demonstrated that the greatest reduction in dentin hypersensitivity occurred following the use of dentine bonding agents46. Most importantly, the reduction in hypersensitivity was sustained throughout the 6-month study period with the use of these agents46.
The resin infiltration technique is considered a microinvasive approach for treating proximal caries lesions47. This new technique may be helpful in treating tooth hypersensitivity but has not been studied for this indication47.
When the previous options of non-invasive treatment of tooth hypersensitivity fail to improve the symptoms, bonding agents can be a recommended next step, which is essentially non-invasive and lacks significant adverse effects.
Cervical restorations
Cervical restorations are another option for treating dentinal hypersensitivity. Covering exposed dentin seals tubules, thus eliminating hypersensitivity symptoms. A study performed by Laybovich et al.48 compared the treatment of a tissue graft versus a Class V restoration in treating dentin hypersensitivity. Their results indicated no significant difference in the reduction of sensitivity; however, they found that patients preferred the tissue graft for aesthetic reasons48. Another study, published in 2013, discussed the effectiveness of potassium nitrate-containing toothpaste in reducing sensitivity49. However, in the article it was noted that restorative materials were significantly more effective in the treatment of sensitivity49. As a result of the extended wait time for sensitivity toothpaste to work, cervical restorations may provide a more immediate option for treating dentin hypersensitivity. Glass ionomer material is commonly used for cervical restorations as a result of its ability to bond with the dentin and enamel whilst simultaneously releasing fluoride50. A study focussing on the longevity of glass ionomer restorations over a period of 13 years revealed that the restorations sustained satisfactory qualities50. Therefore, and because of its well-known advantages, glass ionomer might be the restorative material preferred for this treatment option for tooth hypersensitivity. Depending on the extent of the symptoms and patient preference, cervical restorations may be an option in the treatment of hypersensitivity, particularly if the use of sensitivity toothpastes has been exhausted.
It should be remembered, however, that this treatment modality is invasive and irreversible. It should be saved for the stage when all other non-invasive treatments have failed to solve the problem.
Root canal treatment
The vital component of the tooth is the pulp, which contains the nerves responsible for the pain of dentin hypersensitivity. Therefore, endodontic treatment that involves the removal of pulp and its replacement with gutta percha eliminates all sensory feeling associated with that tooth. Dentinal hypersensitivity is not, and should not be a reason or indication for, root canal treatment. More often this procedure is performed to treat irreversible pulpitis and pulp necrosis49. However, the treatment would eliminate any dentinal hypersensitivity being experienced. Although root canal therapy would not be a first-line treatment, it is an option that might be considered in extreme cases when no other option can relieve the hypersensitivity.
Surgery and Laser
Regarding sensitivity caused by exposed root surfaces, a gingival graft may be desired to cover the exposed dentinal tubules. A study performed in 2013 demonstrated a statistically significant reduction of cervical dentinal hypersensitivity after treatment with a coronally positioned flap and connective tissue graft51. Periodontal flap surgery followed by the use of a 660 nm laser has also been found to decrease hypersensitivity by a considerable amount. A randomised controlled double-blind study of 30 individuals demonstrated statistically significant reductions in dentin hypersensitivity after periodontal flap surgery and low level laser irradiation were performed52. A preliminary in vitro study determined the use of the Nd:YAP laser to be effective in occluding dentin tubules without harming the tooth and suggested its use in future treatments53. Middle-output lasers, such as Nd:YAG, CO2 and Er:YAG, work by occluding dentinal tubules54 and lower level output lasers, such as He-Ne and GaAlAs, affect nerve activity55., 56., 57., 58.. A systematic review focussing on the effectiveness of lasers in treating dentinal hypersensitivity concluded that although lasers are effective, the evidence is considered weak because of the strong placebo effect54. Directly covering the dentin with a tissue graft has been shown be an effective treatment of dentin hypersensitivity; however, new technology, such as laser radiation, may be another last-resort option for patients.
Also, it should be remembered that periodontal surgery is not a risk-free procedure and should not serve as the first line of treatment for tooth hypersensitivity. Periodontal surgery should be the last resort for the resolution of tooth hypersensitivity and used only when other, less invasive, methods were unsuccessful as long as there is no other indication for tooth coverage. Only after all the above non-invasive and less-invasive methods have failed to reduce the symptoms should the root-coverage option be considered.
CONCLUSION
Dentinal hypersensitivity causes varying degrees of pain for patients. It is important to implement appropriate treatment options based on the individual. Nonetheless, the consequence of proceeding with a surgical treatment before trying non-invasive options, such as eliminating the cause or switching toothpastes, must be considered. To treat the condition properly, consider the patient’s risk factors and the initial cause of the sensitivity. When all the factors are considered, the dental professional and patient can agree on a treatment plan based on the desired outcome. As in any condition, begin by eliminating the causes and then select the least invasive option that is believed to provide the desired result.
Acknowledgement and competing interest
The authors declare that there are no competing interests regarding the manuscript. No funding was received for the work presented.
References
- 1.Bartold PM. Dentinal hypersensitivity: a review. Aust Dent J. 2006;51:212–218. [PubMed] [Google Scholar]
- 2.Ye W, Feng XP, Li R. The prevalence of dentine hypersensitivity in Chinese adults. J Oral Rehabil. 2010;39:182–187. doi: 10.1111/j.1365-2842.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- 3.Addy M. In: Tooth Wear and Sensitivity: Clinical Advances in Restorative Dentistry. Addy M, Embery G, Edgar WM, Orchardson R, editors. Martin Dunitz; London: 2000. Dentine hypersensitivity: definition, prevalence distribution and aetiology; pp. 239–248. [Google Scholar]
- 4.Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: recent trends in management. J Conserv Dent. 2010;13:218–224. doi: 10.4103/0972-0707.73385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West NX, Lussi A, Seong J, et al. Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Investig. 2013;17:9–19. doi: 10.1007/s00784-012-0887-x. [DOI] [PubMed] [Google Scholar]
- 6.Brannstrom M, Astrom A. The hydrodynamics of dentin and its possible relationship to dentinal pain. Int Dent J. 1972;22:219–227. [PubMed] [Google Scholar]
- 7.Petersson LG. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig. 2012;17:63–71. doi: 10.1007/s00784-012-0916-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canadian Advisory Board on Dentin Hypersensitivity Consensus-based recommendations for the diagnosis and management of Dentin Hypersensitivity. J Can Dent Assoc. 2003;69:221–226. [PubMed] [Google Scholar]
- 9.Bevenius J, Lindskog S, Hultenby K. The micromorphology in vivo of the buccocervical region of premolar teeth in young adults. A replica study by scanning electron microscopy. Acta Odontol Scand. 1994;52:323–334. doi: 10.3109/00016359409029030. [DOI] [PubMed] [Google Scholar]
- 10.Vijaya V, Sanjay V, Varghese RK, et al. Association of dentine hypersensitivity with different risk factors – a cross sectional study. J Int Oral Health. 2013;5:88–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Addy M, Hunter ML. Can tooth brushing damage your health? Effects on oral and dental tissues. Int Dent J. 2003;53:177–186. doi: 10.1111/j.1875-595x.2003.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 12.West NX, Hooper SM, O’Sullivan D, et al. In situ randomised trial investigating abrasive effects of two desensitising toothpastes on dentine with acidic challenge prior to brushing. J Dent. 2012;40:77–85. doi: 10.1016/j.jdent.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Giles A, Claydon NCA, Addy M, et al. Clinical in situ study investigating abrasive effects of two commercially available toothpastes. J Oral Rehabil. 2009;36:498–507. doi: 10.1111/j.1365-2842.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukumoto Y, Horibe M, Inagaki Y, et al. Association of gingival recession and other factors with the presence of dentin hypersensitivity. Odontology. 2014;102:42–49. doi: 10.1007/s10266-012-0099-5. [DOI] [PubMed] [Google Scholar]
- 15.Toker H, Ozdemir H. Gingival recession: epidemiology and risk indicators in an university dental hospital in Turkey. Int J Dent Hyg. 2009;7:115–120. doi: 10.1111/j.1601-5037.2008.00348.x. [DOI] [PubMed] [Google Scholar]
- 16.Costa RS, Rios FS, Moura MS, et al. Prevalence and risk indicators of dentin hypersensitivity in adult and elderly populations from Porto Alegre, Brazil. J Periodontol. 2014;85:1247–1258. doi: 10.1902/jop.2014.130728. [DOI] [PubMed] [Google Scholar]
- 17.Draenert ME, Jakob M, Kunzelmann KH, et al. The prevalence of tooth hypersensitivity following periodontal therapy with special reference to root scaling. A systematic review of the literature. Am J Dent. 2013;26:21–27. [PubMed] [Google Scholar]
- 18.Slutzkey S, Levin L. Gingival recession in young adults: occurrence, severity, and relationship to past orthodontic treatment and oral piercing. Am J Ortho Dentofac. 2008;134:652–656. doi: 10.1016/j.ajodo.2007.02.054. [DOI] [PubMed] [Google Scholar]
- 19.Rawal SY, Claman LJ, Kalmar JR, et al. Traumatic lesions of the gingiva: a case series. J Periodontol. 2004;75:762–769. doi: 10.1902/jop.2004.75.5.762. [DOI] [PubMed] [Google Scholar]
- 20.Levin L, Zadik Y, Becker T. Oral and dental complications of intra-oral piercing. Dent Traumatol. 2005;21:341–343. doi: 10.1111/j.1600-9657.2005.00395.x. [DOI] [PubMed] [Google Scholar]
- 21.Brooks JK, Hooper KA, Reynolds MA. Formation of mucogingival defects associated with intraoral and perioral piercing: case reports. J Am Dent Assoc. 2003;134:837–843. doi: 10.14219/jada.archive.2003.0281. [DOI] [PubMed] [Google Scholar]
- 22.Levin L. Alveolar bone loss and gingival recession due to lip and tongue piercing. N Y State Dent J. 2007;73:48–50. [PubMed] [Google Scholar]
- 23.Matis BA, Cochran MA, Eckert GJ, et al. In vivo study of two carbamide peroxide gels with different desensitizing agents. Oper Dent. 2007;32:549–555. doi: 10.2341/07-10. [DOI] [PubMed] [Google Scholar]
- 24.Leonard RH, Jr, Smith LR, Garland GE, et al. Desensitizing agent efficacy during whitening in an at-risk population. J Esthet Restor Dent. 2004;16:49–55. doi: 10.1111/j.1708-8240.2004.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 25.Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323–332. doi: 10.2334/josnusd.51.323. [DOI] [PubMed] [Google Scholar]
- 26.Gillam DG, Bulman JS, Jackson RJ, et al. Comparison of 2 desensitising dentifrices with a commercially available fluoride dentifrice in alleviating cervical dentine sensitivity. J Periodontol. 1996;67:737–742. doi: 10.1902/jop.1996.67.8.737. [DOI] [PubMed] [Google Scholar]
- 27.West NX, Addy M, Jackson RJ, et al. Dentine hypersensitivity and the placebo response. A comparison of the effect of strontium acetate, potassium nitrate and fluoride toothpastes. J Clin Periodontol. 1997;24:209–215. doi: 10.1111/j.1600-051x.1997.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 28.Ayad F, Berta R, De Vizio W, et al. Comparative study of two dentifrices containing 5% potassium nitrate on dentinal sensitivity: a twelve week clinical study. J Clin Dent. 1994;5:97–101. [PubMed] [Google Scholar]
- 29.Schiff T, Dotson M, Cohen S, et al. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: a twelve-week clinical study. J Clin Dent. 1994;5:87–92. [PubMed] [Google Scholar]
- 30.Olley RC, Moazzez R, Bartlett DW. Effects of dentifrices on subsurface dentin tubule occlusion: an in situ study. Int J Prosthodont. 2015;28:181–187. doi: 10.11607/ijp.4154. [DOI] [PubMed] [Google Scholar]
- 31.Kun L. Biophysical study of dental tissues under the effect of a local strontium application. Schweiz Monatsschr Zahnheilkd. 1976;86:661–676. [PubMed] [Google Scholar]
- 32.Mishima H, Sakae T, Kozawa Y. Scanning electron microscopy and energy dispersive spectroscopy analysis of calciotraumatic lines in rat labial dentin after acute exposure of strontium chloride. Scanning Microsc. 1995;9:797–803. [PubMed] [Google Scholar]
- 33.Kleinberg I. SensiStat a new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today. 2002;21:42–47. [PubMed] [Google Scholar]
- 34.Magno MB, Nascimento GC, Da Penha NK, et al. Difference in effectiveness between strontium acetate and arginine-based toothpastes to relieve dentin hypersensitivity. A systematic review. Am J Dent. 2015;28:40–44. [PubMed] [Google Scholar]
- 35.Chen CL, Parolia A, Pau A. Comparative evaluation of the effectiveness of desensitizing agents in dentin tubule occlusion using scanning electron microscopy. Aus Dent J. 2015;60:65–72. doi: 10.1111/adj.12275. [DOI] [PubMed] [Google Scholar]
- 36.Pradeep AR, Sharma A. Comparison of clinical efficacy of a dentifrice containing calcium sodium phosphosilicate to a dentifrice containing potassium nitrate and to a placebo on dentinal hypersensitivity: a randomized clinical trial. J Periodontol. 2010;81:1167–1173. doi: 10.1902/jop.2010.100056. [DOI] [PubMed] [Google Scholar]
- 37.Rajesh KS, Hedge S, Arun Kumar MS, Shetty DG. Evaluation of the efficacy of a 5% calcium sodium phosphosilicate (Novamin®) containing dentifrice for the relief of dentinal hypersensitivity: a clinical study. Indian J Dent Res. 2012;23:363–367. doi: 10.4103/0970-9290.102228. [DOI] [PubMed] [Google Scholar]
- 38.West NX, Macdonald EL, Jones SB, et al. Randomized in situ clinical study comparing the ability of two new desensitizing toothpaste technologies to occlude patent dentin tubules. J Clin Dent. 2011;22:82–89. [PubMed] [Google Scholar]
- 39.Pandit N, Gupta R, Bansal A. Comparative evaluation of two commercially available desensitizing agents for the treatment of dentinal hypersensitivity. Indian J Dent Res. 2012;23:778–783. doi: 10.4103/0970-9290.111259. [DOI] [PubMed] [Google Scholar]
- 40.Porto IC, Andrade AK, Montes MA. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323–332. doi: 10.2334/josnusd.51.323. [DOI] [PubMed] [Google Scholar]
- 41.Qin C, Xu J, Zhang Y. Spectroscopic investigation of the function of aqueous 2-hydroxyethylmethacrylate/glutaraldehyde solution as a dentin desensitizer. Eur J Oral Sci. 2006;114:354–359. doi: 10.1111/j.1600-0722.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 42.Schüpbach P, Lutz F, Finger WJ. Closing of dentinal tubules by Gluma desensitizer. Eur J Oral Sci. 1997;105:414–421. doi: 10.1111/j.1600-0722.1997.tb02138.x. [DOI] [PubMed] [Google Scholar]
- 43.Patil SA, Naik BD, Suma R. Evaluation of three different agents for in-office treatment of dentinal hypersensitivity: a controlled clinical study. Indian J Dent Res. 2015;26:38–42. doi: 10.4103/0970-9290.156796. [DOI] [PubMed] [Google Scholar]
- 44.Pashley DH. Dentin permeability, dentin sensitivity and treatment through tubule occlusion. J Endod. 1986;12:465–474. doi: 10.1016/S0099-2399(86)80201-1. [DOI] [PubMed] [Google Scholar]
- 45.Pashley DH, Livingston MJ, Reeder OW, et al. Effects of the degree of tubule occlusion on the permeability of human dentine in vitro. Arch Oral Biol. 1978;23:1127–1133. doi: 10.1016/0003-9969(78)90119-x. [DOI] [PubMed] [Google Scholar]
- 46.Pashley DH, Galloway SE. The effects of oxalate treatment on the smear layer of ground surfaces of human dentine. Arch Oral Biol. 1985;30:731–737. doi: 10.1016/0003-9969(85)90185-2. [DOI] [PubMed] [Google Scholar]
- 47.Dorri M, Dunne SM, Walsh T, et al. Micro-invasive interventions for managing proximal dental decay in primary and permanent teeth. Cochrane Database Syst Rev. 2015;11:1–51. doi: 10.1002/14651858.CD010431.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leybovich M, Bissada NF, Teich S, et al. Treatment of noncarious cervical lesions by a subepithelial connective tissue graft versus a composite resin restoration. Int J Periodontics Restorative Dent. 2014;34:649–654. doi: 10.11607/prd.2033. [DOI] [PubMed] [Google Scholar]
- 49.Veitz-Keenan A, Barna JA, Strober B, et al. Treatments for hypersensitive noncarious cervical lesions: a practitioners engaged in applied research and learning network randomized clinical effectiveness study. J Am Dent Assoc. 2013;144:495–506. doi: 10.14219/jada.archive.2013.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gordan VV, Blaser PK, Watson RE, et al. A clinical evaluation of a giomer restorative system containing surface prereacted glass ionomer filler: results from a 13-year recall examination. J Am Dent Assoc. 2014;145:1036–1043. doi: 10.14219/jada.2014.57. [DOI] [PubMed] [Google Scholar]
- 51.Douglas de Oliveira DW. Marques DP, Aguiar-Cantuária IC, et al. Effect of surgical defect coverage on cervical dentin hypersensitivity and quality of life. J Periodontol. 2013;84:768–775. doi: 10.1902/jop.2012.120479. [DOI] [PubMed] [Google Scholar]
- 52.Shreya D, Sanjay J, Rashmi H. Effect of Low-Level Laser Therapy in Reducing Dentinal Hypersensitivity and Pain Following Periodontal Flap Surgery. Photomed Laser Surg. 2014;32:700–706. doi: 10.1089/pho.2014.3802. [DOI] [PubMed] [Google Scholar]
- 53.Namour A, Nammour S, Peremans A, et al. Treatment of dentinal hypersensitivity by means of Nd:YAP Laser: a preliminary in vitro study. Sci World J. 2014;2014:323604. doi: 10.1155/2014/323604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sgolastra F, Petrucci A, Gatto R, et al. Effectiveness of laser in dentinal hypersensitivity treatment: a systematic review. J Endod. 2011;37:297–303. doi: 10.1016/j.joen.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 55.Rochkind S, Nissan M, Razon N, et al. Electrophysiological effect of HeNe laser on normal and injured sciatic nerve in the rat. Acta Neurochir. 1986;83:125–130. doi: 10.1007/BF01402391. [DOI] [PubMed] [Google Scholar]
- 56.Rochkind S, Nissan M, Barr-Nea L, et al. Response of peripheral nerve to He-Ne laser: experimental studies. Lasers Surg Med. 1987;7:441–443. doi: 10.1002/lsm.1900070512. [DOI] [PubMed] [Google Scholar]
- 57.Wakabayashi H, Hamba M, Matsumoto K, et al. Electrophysiological study of irradiation of semiconductor laser on the activity of the trigeminal subnucleues caudal neurons. J Jpn Soc Laser Dent. 1992;3:65–74. [Google Scholar]
- 58.Wakabayashi H, Hamba M, Matsumoto K, et al. Effect of irradiation by semiconductor laser on responses evoked in trigeminal caudal neurons by tooth pulp stimulation. Lasers Surg Med. 1993;13:605–610. doi: 10.1002/lsm.1900130603. [DOI] [PubMed] [Google Scholar]