Abstract
Objectives: Owing to its cost-effectiveness and operative convenience, dental amalgam remains in use as a restorative material for tooth caries in children in many countries. The aim of this study was to evaluate the relationship between dental amalgam exposure and urinary mercury (U-Hg) concentrations in children. Methods: In this longitudinal study, 463, 367 and 348 children, 8–11 years of age, were evaluated at baseline, and at the first and second follow-up visits, respectively. The interval between each survey was 6 months. For the oral examination and urine sample, the amalgam-filled tooth surface (TS), and U-Hg and creatinine concentrations of participants were determined, and the cumulative amalgam-filled TS and cumulative creatinine-adjusted U-Hg were calculated. To assess potential covariates, socio-demographic factors, oral health behaviour and dietary factors were surveyed by questionnaire. Data were analysed by the t-test, correlation analysis and mixed-model analysis. The statistical analyses were performed using SPSS 18.0. Results: Children with more than one amalgam-filled TS exhibited significantly higher creatinine-adjusted U-Hg concentrations than those without, in all three survey periods (P < 0.001). The results for the current and cumulative amalgam-filled TS significantly correlated with those for the current and cumulative creatinine-adjusted U-Hg concentration, respectively, in all surveys (P < 0.001). In the repeated-measures mixed model analysis, current and cumulative amalgam-filled TS was significantly related to current and cumulative creatinine-adjusted U-Hg concentration, respectively (P < 0.001). Conclusions: Amalgam-filled TS was significantly correlated with U-Hg concentrations in children. Therefore, dental amalgam exposure can affect the systemic mercury concentration in children.
Key words: Amalgam, dental practice, children
INTRODUCTION
Dental amalgam, utilised in the restoration of caries, releases small amounts of mercury, a heavy metal that exists in organic, inorganic and metallic forms in the natural world1. Organic mercury is mainly derived from the consumption of seafood, such as fish and shellfish, and is readily absorbed in the gastrointestinal tract. It dissolves into lipids and passes through the cerebrovascular wall or placenta, and, because of its long half-life, can accumulate in the body2., 3., 4., 5., 6.. Inorganic mercury is also absorbed through the gastrointestinal tract, although only 10% passes through the cerebrovascular wall or placenta. However, microbes convert inorganic mercury into an organic form that enters the food chain through the digestive system of small animals7. Although metallic mercury is almost unabsorbed by the body itself, it may be inhaled through the lung in a mercury vapor form and subsequently contact all organs through the bloodstream. Absorbed mercury accumulates in the body, mainly in the kidneys, and is excreted through urine and faeces8., 9..
Mercury is toxic to the central nervous system and kidneys, and may cause symptoms such as joint and muscle pain, gastrointestinal issues, fatigue and dizziness10., 11.. Mercury liberated from amalgam mainly accumulates in the kidneys and can increase the concentration of mercury in body fluids such as breast milk, blood, urine and saliva12. Nylander et al.13 reported that persons with amalgam restorations exhibited higher levels of mercury accumulation in their kidneys compared with amalgam-free individuals and also found that those with amalgam accumulated more mercury in the brain. Vimy et al.14 found that the filling and removal of dental amalgam in pregnant and lactating women increases the risk of mercury exposure to the fetus and neonate. Abraham et al.15 Khordi-Mood et al.16 and Leistevuo et al.17 reported higher concentrations of mercury in blood, urine and saliva, respectively, when amalgam fillings were present in the mouth. The urinary mercury (U-Hg) concentration is reported to be proportional to the number of amalgam fillings; it is approximately 6% greater per amalgam filling in posterior teeth, and approximately 0.6 μg/g of creatinine greater per 10 amalgam fillings18., 19..
Children, in particular, may be more sensitive to mercury exposure than adults; previous studies have investigated the relationship between dental amalgam and mercury exposure in children. Levy et al.20 found that dental amalgam fillings were associated with U-Hg concentrations approximately 50% higher than those among children with no amalgam fillings (after adjusting for fish intake) among 60 children, 4–8 years of age. DeRouen et al.21 and Woods et al.22 reported higher U-Hg concentrations in individuals with amalgam fillings compared with those with resin fillings among 507 Portuguese children, 8–10 years of age. In a clinical trial of 534 children, Bellinger et al.23., 24. found that the presence of amalgam fillings increased the intellectual quotient, memory index and glomerular function, without impacting albumin concentration.
As amalgam is inexpensive, cost-effective and convenient to use, it is the material of choice in some developing countries and is more frequently selected to treat the primary teeth of children than the permanent teeth.25., 26. Despite the numerous studies on the relationship between mercury contamination from dental amalgam and the health-risk assessment of children, there are still controversies relating to this issue. To the best of our knowledge, longitudinal studies on this topic are rare. Therefore, the aim of this study was to evaluate the association between mercury exposure from dental amalgam and U-Hg concentrations in children through a 1-year follow-up study.
MATERIALS AND METHODS
Study participants
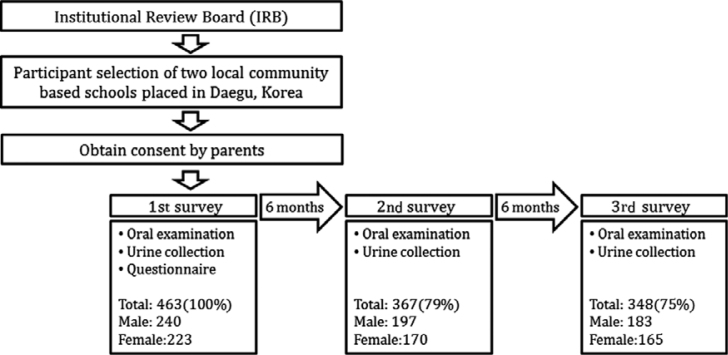
A total of 665 students from the first grade to the fourth grade, in two elementary schools located in one metropolitan city, were recruited between April 2010 and May 2011, according to the following inclusion criteria: parent/guardians provided written informed consent and completed questionnaires; and absence of hereditary diseases. A total of 463 students participated in the baseline survey, after excluding those who did not complete both the oral and urine sample examinations or those whose concentration of creatinine did not fall within the valid range (30–300 mg/dL). The first and the second follow-up surveys were conducted every 6 months after the baseline survey. Owing to some students transferring to other schools, 367 and 348 students participated in second and third follow-up surveys, respectively (Figure 1). This study was conducted in full accordance with the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of the Kyungpook National University Hospital in Daegu, Korea (KNUH_10-1056). All parents or guardians of the participants provided written informed consent.
Figure 1.
Study protocol and number of participants in the follow-up period.
Oral examination
Three trained dentists performed oral examinations to assess the decayed, missing and filled surfaces of both deciduous and permanent teeth, according to World Health Organization criteria, using a portable dental chair and artificial light at the baseline and two follow-up visits. The Cohen’s kappa coefficient value (0.9) indicated strong agreement between the three examiners. The filling material of each filled tooth surface and the number of amalgam-filled tooth surfaces (TS) were examined. However, the types of amalgam-filled TS were not recorded. The cumulative amalgam-filled TS of each participant was calculated by summing the amalgam-filled TS obtained at the present examination and those obtained at previous examinations (i.e. cumulative amalgam-filled TS of first follow-up survey = amalgam-filled TS of baseline survey + amalgam-filled TS of first follow-up survey; cumulative amalgam-filled TS of second follow-up survey = cumulative amalgam-filled TS of first follow-up survey + amalgam-filled TS of second follow-up survey). Cumulative amalgam-filled TS was calculated for the first and second follow-up surveys only.
Urinary mercury and creatinine measurements
At baseline and at the two follow-up surveys, which were separated by 6-month intervals, urine samples were collected in a plastic cup on the day of the oral examination. These were divided immediately into 2-mL polypropylene conical tubes suitable for heavy metal assessment, transferred into an icebox and stored in an ultra-low-temperature freezer at −70 °C until required for analysis. After the second survey, all urine samples were sent to the Department of Environmental Medicine at the Regional University Hospital for mercury analysis. Mercury concentration analysis was performed using a Direct Mercury Analyzer (DMA-80; Milestone Company, Sorisole, Italy) with a detection limit of 0.0015 ng of mercury. From the German External Quality Assessment Scheme, 46 specimens were utilised as the standard. For measurements of creatinine concentration, urine samples were sent to an analytical laboratory (Seegene Medical Foundation) in Seoul City. The cumulative U-Hg concentration of each participant was calculated by summing the U-Hg concentration obtained at the current examination and those obtained from previous examinations (i.e. cumulative U-Hg concentration of first follow-up survey = U-Hg concentration of baseline survey + U-Hg concentration of first follow-up survey; cumulative U-Hg concentration of second follow-up survey = cumulative U-Hg concentration of first follow-up survey + U-Hg concentration of second follow-up survey). Cumulative U-Hg concentrations were calculated for the first and second follow-up surveys only.
Confounding factors
Information on sociodemographic, oral health behavioural and dietary factors was obtained from the parents or guardians. Sociodemographic factors included gender (male or female), parents’ educational level (≤high school or >high school), parents’ profession (unemployed, manual worker or non-manual worker) and monthly income (<2, 2–3 or >3, in million won). Oral health behavioural factors included the frequency of toothbrushing per day (≤1, 2 or ≥3), visited a dentist within the previous 6 months (no/yes) and the frequency of gum-chewing per week (hardly ever or more than once). Dietary factors included the frequency of fish intake per week (hardly ever, 1–2 or ≥3).
Statistical analysis
All statistical analyses were performed using Statistical Package for Social Sciences software (SPSS version 18.0K for Windows, SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at 0.05. To compare mean values of the creatinine-adjusted U-Hg concentration between participants with and without amalgam-filled TS, a t-test was utilised. A correlation analysis was performed to identify the association between amalgam-filled TS and the creatinine-adjusted U-Hg concentration. Finally, a repeated-measures mixed model was used to analyse the factors affecting current and cumulative creatinine-U-Hg concentrations (log-converted). Because there were no effect modifications on the relationship between the numbers of amalgam-filled TS and the U-Hg concentration, according to chewing gum and/or toothbrushing frequency, these two variables were adjusted as confounders.
RESULTS
The average age ± standard deviation of participants in the baseline survey was 8.66 ± 1.13 (range: 7–10) years. Of the 463 participants, 240 (51.8%) were male (Table 1). The overall mean numbers of amalgam-filled deciduous and permanent TS were 2.83 and 0.29, 2.73 and 0.40, and 2.09 and 0.41 at the baseline, first and second follow-up surveys, respectively. In deciduous teeth, the mean number of amalgam-filled TS was approximately four to six times greater than that of composite resin-filled TS. In contrast, in permanent teeth, the mean number of amalgam-filled TS was approximately the same as that of composite resin-filled TS (Table 2). The overall mean ± standard deviation creatinine-adjusted U-Hg concentration was 1.04 ± 0.02 μg/g of creatinine, 1.18 ± 0.02 μg/g of creatinine and 1.23 ± 0.05 μg/g of creatinine at the baseline, first and second follow-up surveys, respectively. The mean creatinine-adjusted U-Hg concentration in participants with and without amalgam-filled TS were 1.15 ± 0.03 μg/g of creatinine and 0.90 ± 0.03 μg/g of creatinine, 1.26 ± 0.03 μg/g of creatinine and 1.09 ± 0.03 μg/g of creatinine, and 1.53 ± 0.09 μg/g of creatinine and 0.91 ± 0.05 μg/g of creatinine at the baseline, first and second follow-up surveys, respectively. In all of the surveys, participants with amalgam-filled TS showed a significantly higher creatinine-adjusted U-Hg concentration compared with those without amalgam-filled TS (P < 0.001; Table 3).
Table 1.
General characteristics of participants in the baseline survey
| Characteristics | n (%) | |
|---|---|---|
| Gender | Male | 240 (51.8) |
| Female | 223 (48.2) | |
| Monthly income (million won per month) | <2 | 126 (27.2) |
| 2–3 | 102 (22.0) | |
| >3 | 127 (37.4) | |
| Educational level of father | ≤High school | 148 (32.0) |
| >High school | 202 (43.6) | |
| Educational level of mother | ≤High school | 350 (43.4) |
| >High school | 175 (31.3) | |
| Job of father | Unemployed | 11 (2.4) |
| Manual worker | 113 (24.4) | |
| Non-manual worker | 211 (45.6) | |
| Job of mother | Unemployed | 173 (37.4) |
| Manual worker | 25 (5.4) | |
| Non-manual worker | 145 (31.3) | |
| Frequency of brushing per day | ≤1 | 32 (6.9) |
| 2 | 189 (40.8) | |
| ≥3 | 152 (32.8) | |
| Visited a dentist within the previous 6 months | No | 141 (30.5) |
| Yes | 221 (47.7) | |
| Frequency of chewing gum per week | Hardly ever | 220 (47.5) |
| More than once | 141 (30.5) | |
| Frequency of fish intake per week | Hardly ever | 65 (9.5) |
| 1–2 | 266 (39.1) | |
| ≥3 | 63 (9.3) | |
Table 2.
Mean number of amalgam-filled tooth surfaces and resin-filled tooth surfaces of participants
| Survey | Total | Deciduous tooth surface |
Permanent tooth surface |
||||
|---|---|---|---|---|---|---|---|
| Amalgam | fs* | Resin | Amalgam | FS† | Resin | Amalgam | |
| Baseline | 3.12 | 6.81 | 0.46 | 2.83 | 0.75 | 0.27 | 0.29 |
| First follow up | 3.13 | 6.53 | 0.55 | 2.73 | 1.05 | 0.43 | 0.40 |
| Second follow up | 2.50 | 5.13 | 0.49 | 2.09 | 1.03 | 0.50 | 0.41 |
fs, filled tooth surface of deciduous teeth; †FS, filled tooth surface of permanent teeth.
Table 3.
Creatinine-adjusted urinary mercury concentration of participants with and without amalgam-filled tooth surfaces
| Survey | Creatinine-adjusted urinary mercury concentration |
||||||
|---|---|---|---|---|---|---|---|
| Total (n, mean ± SE*) | Participants without amalgam-filled TS (n, mean ± SE*) | Participants with amalgam-filled TS (n, mean ± SE*) | P-value | ||||
| Baseline | 463 | 1.04 ± 0.02 | 207 | 0.90 ± 0.03 | 256 | 1.15 ± 0.03 | <0.001 |
| First follow up | 367 | 1.18 ± 0.02 | 169 | 1.09 ± 0.03 | 198 | 1.26 ± 0.03 | <0.001 |
| Second follow up | 348 | 1.23 ± 0.05 | 170 | 0.91 ± 0.05 | 178 | 1.53 ± 0.09 | <0.001 |
Unit: μg/g of creatinine; SE, standard error.
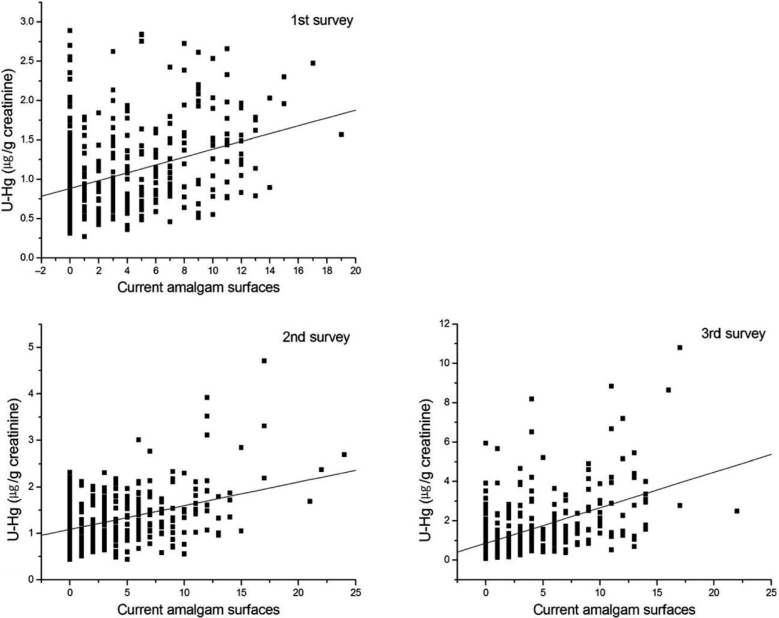
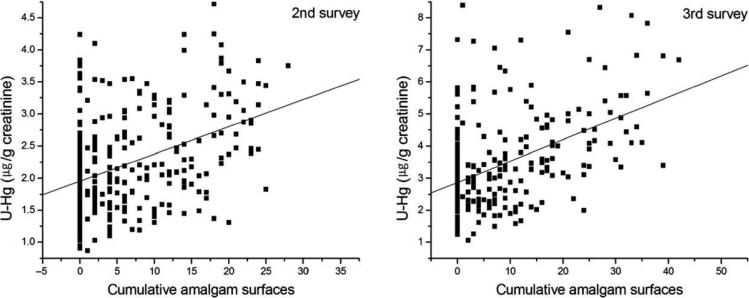
The correlation coefficients between current amalgam-filled TS and current creatinine-adjusted U-Hg concentration was 0.351, 0.283 and 0.454 at the baseline and first and second follow-up surveys, respectively (P < 0.001). The cumulative creatinine-adjusted U-Hg concentration was also significantly correlated with cumulative amalgam-filled TS in all surveys (P < 0.001; Table 4). Two scatter diagram distributions demonstrated a linear relation between both the current and cumulative amalgam-filled TS and the creatinine-adjusted U-Hg concentration (Figures 2 and 3). In the multivariate repeated-measures mixed model for current and cumulative creatinine-adjusted U-Hg, amalgam-filled TS was positively associated with U-Hg concentration (P < 0.001; Table 3). In contrast, fish ingestion was negatively associated with U-Hg concentration at accumulated exposure (P = 0.033; Table 5).
Table 4.
Correlation coefficients between amalgam-filled tooth surfaces and creatinine-adjusted urinary mercury concentration
| Survey | Correlation coefficients |
|
|---|---|---|
| Current* | Cumulative† | |
| Baseline | 0.351 | – |
| First follow up | 0.283 | 0.342 |
| Second follow up | 0.454 | 0.424 |
Pearson correlation coefficient between urinary mercury concentration (U-Hg) and amalgam-filled tooth surface examined at each survey; P < 0.001 for all correlations.
Pearson correlation coefficient between cumulative U-Hg concentration and cumulative amalgam-filled tooth surface examined at each survey; P < 0.001 for all correlations. The cumulative U-Hg concentration is the sum of the U-Hg concentration measured at each survey and those of previous surveys. Cumulative U-Hg was calculated for first and second follow-up surveys only. The cumulative amalgam-filled tooth surface is the sum of the amalgam-filled tooth surface examined at each survey and those of previous surveys. Cumulative amalgam-filled tooth surface was calculated for first and second follow-up surveys only.
Figure 2.
Current urinary mercury concentration (U-Hg) according to current amalgam-filled tooth surfaces.
Figure 3.
Cumulative urinary mercury concentration (U-Hg) according to cumulative amalgam-filled tooth surfaces.
Table 5.
Repeated-measures mixed model for log-transformed creatinine-adjusted urinary mercury concentration (U-Hg) at baseline and at first and second follow-up surveys
| Characteristic | Current U-Hg concentration* |
Cumulative U-Hg concentration† |
||
|---|---|---|---|---|
| β (SE) | P-value | β (SE) | P-value | |
| Amalgam-filled tooth surface | 0.028 (0.004) | <0.001 | 0.022 (0.002) | <0.001 |
| Gender | ||||
| Male | ref. | ref. | ||
| Female | −0.033 (0.037) | 0.371 | −0.058 (0.041) | 0.164 |
| Monthly income (million won per month) | ||||
| <2 | ref. | ref. | ||
| 2–3 | 0.042 (0.048) | 0.391 | 0.014 (0.054) | 0.799 |
| >3 | −0.014 (0.050) | 0.775 | −0.010 (0.057) | 0.854 |
| Education | ||||
| Father | ||||
| ≤High school | ref. | ref. | ||
| >High school | −0.020 (0.044) | 0.645 | −0.029 (0.049) | 0.559 |
| Mother | ||||
| ≤High school | ref. | ref. | ||
| >High school | 0.001 (0.044) | 0.980 | 0.003 (0.050) | 0.959 |
| Job | ||||
| Father | ||||
| Unemployed | ref. | ref. | ||
| Manual worker | −0.182 (0.108) | 0.093 | −0.184 (0.121) | 0.130 |
| Non-manual worker | −0.198 (0.108) | 0.069 | −0.193 (0.121) | 0.112 |
| Mother | ||||
| Unemployed | ref. | ref. | ||
| Manual worker | 0.088 (0.077) | 0.250 | 0.099 (0.083) | 0.234 |
| Non-manual worker | 0.061 (0.038) | 0.104 | 0.063 (0.042) | 0.135 |
| Frequency of brushing per day | ||||
| ≤1 | ref. | ref. | ||
| 2 | 0.139 (0.074) | 0.059 | 0.093 (0.081) | 0.255 |
| ≥3 | 0.100 (0.075) | 0.181 | 0.057 (0.083) | 0.494 |
| Visited a dentist within the previous 6 months | ||||
| No | ref. | ref. | ||
| Yes | 0.046 (0.038) | 0.218 | 0.013 (0.042) | 0.752 |
| Frequency of chewing gum per week | ||||
| Hardly ever | ref. | ref. | ||
| More than once | 0.062 (0.037) | 0.094 | 0.071 (0.041) | 0.086 |
| Frequency of fish intake per week | ||||
| Hardly ever | ref. | ref. | ||
| 1–2 | −0.087 (0.047) | 0.068 | −0.114 (0.053) | 0.033 |
| ≥3 | −0.069 (0.064) | 0.277 | −0.126 (0.071) | 0.078 |
U-Hg concentration and amalgam-filled tooth surface were examined at each survey.
Sum of the current U-Hg concentration measured at each survey and the U-Hg concentrations of previous surveys. Cumulative U-Hg was calculated for first and second follow-up surveys only.
SE, standard error.
DISCUSSION
In a 1-year follow-up study, we evaluated the relationship between dental amalgam exposure and U-Hg concentration in children. A significant relationship was observed between U-Hg concentration and amalgam-filled TS at each of the three survey periods. Furthermore, there was a significant relationship between cumulative U-Hg concentration and cumulative amalgam-filled TS at the first and second follow-up surveys; this was calculated to establish the cumulative effect of mercury exposure from amalgam fillings. To the best of our knowledge, this is the first study to investigate the relationship between dental amalgam exposure and U-Hg concentration in Korea.
The mean U-Hg concentration in the participants of this study was 1.04–1.23 μg/g of creatinine; this is comparable to that previously reported in a study of British children, 6–10 years of age (of 0.9–1.2 μg/g of creatinine).27 In the current study, the mean U-Hg concentration was higher in participants with amalgam-filled teeth than in those without, in all of the surveys. This finding supports the results of a study by Olstad et al.28, of a group of 12-year-old children, that reported a mean U-Hg concentration of 0.31 μg/g for 14 children without amalgam-filled teeth and a mean U-Hg concentration of 1.0 μg/g for 73 children with an average of 5.8 amalgam-filled teeth. In addition, a study by Levy et al.20 reported a U-Hg concentration of 0.61 μg/g for 86 children without amalgam-filled teeth and a U-Hg concentration of 1.70 μg/g for 93 children with amalgam-filled teeth.
Our findings demonstrate that amalgam-filled teeth have a strong influence on the U-Hg concentration, even after adjusting for other variables. This supports the findings of other cross-sectional research. According to Levy et al.20 after adjusting for fish intake, the amalgam-filled surface index was correlated with the U-Hg concentration, and the accumulated exposure of amalgam-filled surfaces of the molars exhibited the strongest relationship with U-Hg concentration. Link et al.29 reported that the U-Hg concentration increases by approximately 0.3 μg per amalgam-filled tooth. The findings from the repeated-measures mixed model analysis in the present study confirmed that current and accumulated amalgam-filled TS are the most influential factors on the current and accumulated U-Hg concentration in children, respectively. These findings correspond to the repeated-measures mixed model analysis results of a longitudinal 5-year study of 240 children, 6–10 years of age, of Maserejian et al.27 which demonstrated amalgam-filled TS to be the most influential factor on mercury concentration.
In our multivariable model, fish intake was negatively correlated with U-Hg concentration. This contradicts findings from previous studies that demonstrated a positive correlation between fish intake and U-Hg. As the species and the amount of ingested fish may vary regionally, such direct comparison with other studies may not be valid. In addition, our study targeted participants residing in a city located inland and therefore the diets of these individuals may not be tailored towards fish. However, further studies are required to clarify this.
This study has several limitations. First, our results are not generalisable because all study participants lived in a metropolitan area and we utilised convenience sampling. Second, a large amount of mercury is released immediately after a restorative procedure using amalgam; as time passes, the total amount of mercury released is reduced. This study did not investigate mercury release from amalgam fillings over time; instead, we analysed the cumulative mercury exposure from amalgam fillings over a 1-year follow-up period. Finally, this study did not investigate additional sources of mercury exposure other than fish intake and dental amalgam. Mercury exposure can arise from a variety of other sources.
Despite these limitations, this study investigated a relatively large number of participants and conducted a longitudinal analysis for 1 year involving three survey intervals. Our findings confirm that amalgam-filled TS is significantly correlated with U-Hg concentration and that the U-Hg concentration increases by approximately 0.02 μg/g of creatinine per one amalgam-filled TS. The biological effect of this level of Hg on the general health of children is not clear.
CONCLUSIONS
Amalgam-filled TS was significantly correlated with U-Hg concentrations in children. Therefore, dental amalgam fillings may release mercury and increase U-Hg concentrations in children. Further epidemiological studies, focusing on the biological impact of the long-term effects of low level exposure of mercury from dental amalgam on children are needed.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2010-0012893).
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Berry TG, Summit JB, Chung AK, et al. Amalgam at the new millennium. J Am Dent Assoc. 1998;129:1547–1556. doi: 10.14219/jada.archive.1998.0101. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- 3.Yamamura Y, Yoshinaga Y, Arai F, et al. Background levels of total mercury concentrations in blood and urine. Sangyo Igaku. 1994;36:66–69. doi: 10.1539/joh1959.36.2_66. [DOI] [PubMed] [Google Scholar]
- 4.Myers GJ, Davidson PW, Cox C, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- 5.Grandjean P, White RF, Weihe P, et al. Neurotoxic risk caused by stable and variable exposure to methylmercury from seafood. Ambul Pediatr. 2003;3:18–23. doi: 10.1367/1539-4409(2003)003<0018:nrcbsa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.Okati N, Sari AE, Ghasempouri SM. Hair mercury concentrations of lactating mothers and breastfed infants in iran (fish consumption and mercury exposure) Biol Trace Elem Res. 2012;149:155–162. doi: 10.1007/s12011-012-9424-7. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration, HHS Dental devices: classification of dental amalgam, reclassification of dental mercury, designation of special controls for dental amalgam, mercury, and amalgam alloy. Final rule. Fed Reg. 2009;74:38685–38714. [PubMed] [Google Scholar]
- 8.Skare I, Engqvist A. Human exposure to mercury and silver released from dental amalgam restorations. Arch Environ Health. 1994;49:384–394. doi: 10.1080/00039896.1994.9954991. [DOI] [PubMed] [Google Scholar]
- 9.Drasch G, Schupp I, Höfl H, et al. Mercury burden of human fetal and infant tissues. Eur J Pediatr. 1994;153:607–610. doi: 10.1007/BF02190671. [DOI] [PubMed] [Google Scholar]
- 10.Sweeney M, Creanor SL, Smith RA, et al. The release of mercury from dental amalgam and potential neurotoxicological effects. J Dent. 2002;30:243–250. doi: 10.1016/s0300-5712(02)00040-4. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . World Health Organization; Geneva: 2003. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects; pp. 4–5. [Google Scholar]
- 12.Kasraei S, Mortazavi H, Vahedi M, et al. Blood mercury level and its determinants among dental practitioners in Hamadan, Iran. J Dent. 2010;7:55–63. [PMC free article] [PubMed] [Google Scholar]
- 13.Nylander M, Friberg L, Lind B. Mercury concentrations in the human brain and kidneys in relation to exposure from dental amalgam fillings. Swed Dent J. 1987;11:179–187. [PubMed] [Google Scholar]
- 14.Vimy MJ, Hooper DE, King WW, et al. Mercury from maternal ““silver”“tooth fillings in sheep and human breast milk. A source of neonatal exposure. Biol Trace Elem Res. 1997;56:143–152. doi: 10.1007/BF02785388. [DOI] [PubMed] [Google Scholar]
- 15.Abraham JE, Svare CW, Frank CW. The effect of dental amalgam restorations on blood mercury levels. J Dent Res. 1984;63:71–73. doi: 10.1177/00220345840630011801. [DOI] [PubMed] [Google Scholar]
- 16.Khordi-Mood M, Sarraf-Shirazi AR, Balali-Mood M. Urinary mercury excretion following amalgam filling in children. J Toxicol Clin Toxicol. 2001;39:701–705. doi: 10.1081/clt-100108510. [DOI] [PubMed] [Google Scholar]
- 17.Leistevuo J, Leistevuo T, Helenius H, et al. Dental amalgam fillings and the amount of organic mercury in human saliva. Caries Res. 2001;35:163–166. doi: 10.1159/000047450. [DOI] [PubMed] [Google Scholar]
- 18.Kingman A, Albertini T, Brown LJ. Mercury concentrations in urine and whole blood associated with amalgam exposure in a US military population. J Dent Res. 1998;77:461–471. doi: 10.1177/00220345980770030501. [DOI] [PubMed] [Google Scholar]
- 19.Dye BA, Schober SE, Dillon CF, et al. Urinary mercury concentrations associated with dental restorations in adult women aged 16-49 years: United States, 1999-2000. Occup Environ Med. 2005;62:368–375. doi: 10.1136/oem.2004.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy M, Schwartz S, Dijak M, et al. Childhood urine mercury excretion: dental amalgam and fish consumption as exposure factors. Environ Res. 2004;94:283–290. doi: 10.1016/j.envres.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 21.DeRouen TA, Martin MD, Leroux BG, et al. Neurobehavioral effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1784–1792. doi: 10.1001/jama.295.15.1784. [DOI] [PubMed] [Google Scholar]
- 22.Woods JS, Martin MD, Leroux BG, et al. The contribution of dental amalgam to urinary mercury excretion in children. Environ Health Perspect. 2007;115:1527–1531. doi: 10.1289/ehp.10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellinger DC, Trachtenberg F, Barregard L, et al. Neuropsychological and renal effects of dental amalgam in children: a randomized clinical trial. JAMA. 2006;295:1775–1783. doi: 10.1001/jama.295.15.1775. [DOI] [PubMed] [Google Scholar]
- 24.Bellinger DC, Trachtenberg F, Daniel D, et al. A dose-effect analysis of children’s exposure to dental amalgam and neuropsychological function: the New England Children’s Amalgam Trial. J Am Dent Assoc. 2007;138:1210–1216. doi: 10.14219/jada.archive.2007.0345. [DOI] [PubMed] [Google Scholar]
- 25.Espelid I, Tveit AB. Restorative treatment decisions on occlusal caries in Scandinavia. Acta Odontol Scand. 2001;59:21–27. doi: 10.1080/000163501300035724. [DOI] [PubMed] [Google Scholar]
- 26.Fuks AB. The use of amalgam in pediatric dentistry. Pediatr Dent. 2002;24:448–455. [PubMed] [Google Scholar]
- 27.Maserejian NN, Trachtenberg FL, Assmann SF, et al. Dental amalgam exposure and urinary mercury levels in children: the New England Children’s Amalgam Trial. Environ Health Perspect. 2008;116:256–262. doi: 10.1289/ehp.10440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olstad ML, Holland RI, Wandel N, et al. Correlation between amalgam restorations and mercury concentrations in urine. J Dent Res. 1987;66:1179–1182. doi: 10.1177/00220345870660061701. [DOI] [PubMed] [Google Scholar]
- 29.Link B, Gabrio T, Piechotowski I, et al. Environmental Health Survey (BW-EHS) from 1996 to 2003: toxic metals in blood and urine of children. Int J Hyg Environ Health. 2007;210:357–371. doi: 10.1016/j.ijheh.2007.01.031. [DOI] [PubMed] [Google Scholar]