Abstract
Background
There have been several controversies about the correlation between vitamin D and depression. This study aimed to investigate the relationship between vitamin D supplementation and the incidence and prognosis of depression and to analyze the latent effects of subgroups including population and supplement strategy.
Methods
A systematic search for articles before July 2021 in databases (PubMed, EMBASE, Web of Science, and the Cochrane Library) was conducted to investigate the effect of vitamin D supplementation on the incidence and prognosis of depression.
Results
This meta-analysis included 29 studies with 4,504 participants, indicating that the use of vitamin D was beneficial to a decline in the incidence of depression (SMD: −0.23) and improvement of depression treatment (SMD: −0.92). Subgroup analysis revealed that people with low vitamin D levels (<50 nmol/L) and females could notably benefit from vitamin D in both prevention and treatment of depression. The effects of vitamin D with a daily supplementary dose of >2,800 IU and intervention duration of ≥8 weeks were considered significant in both prevention and treatment analyses. Intervention duration ≤8 weeks was recognized as effective in the treatment group.
Conclusion
Our results demonstrate that vitamin D has a beneficial impact on both the incidence and the prognosis of depression. Whether suffering from depression or not, individuals with low vitamin D levels, dose >2,800 IU, intervention duration ≥8 weeks, and all females are most likely to benefit from vitamin D supplementation.
Keywords: depression, vitamin D supplementation, incidence, prognosis, meta-analysis
Introduction
Depression is a worldwide health issue for people that commonly coexists with other debilitating chronic illnesses. According to the World Health Organization, approximately 350 million people worldwide suffer from depression. It has been regarded as the leading cause of significant disability and mortality (1–3). Despite decades of intensive neurobiologically focused psychiatric study, the etiology of depression still remains a mystery. Classical antidepressants mainly increase the concentration of monoamines in the synaptic space by blocking the reuptake of serotonin and norepinephrine at presynaptic nerve terminals (4). However, approximately half of the patients fail to respond to the first-line treatment of antidepressants (5). Besides, the side effects of antidepressants are hard to be ignored. Hence, finding other clinical programs to optimize the treatment of depression appears extremely urgent.
Vitamin D has been investigated to be vital in the normal development and functionalization of the brain. The insufficient and deficiency of vitamin D are related to neurological disorders (6, 7). Also, the correlation between low-level vitamin D and depression has been proved (8). Epidemiological surveys demonstrated that the incidence of depression was 8–14% higher in people with vitamin D deficiency (9–11). Therefore, the implementation of vitamin D in anti-depression treatment has raised awareness among healthcare professionals. Compared with antidepressants, vitamin D is still believed by some to have more favorable safety and better patient compliance despite the adverse effect caused by excessive vitamin D. Nevertheless, the related results still remain controversial.
Previously, a meta-analysis exploring the effects of vitamin D supplements in the management of depression showed that vitamin D supplementation was therapeutically effective in relieving the symptoms of depression (2). However, in two subsequent meta-analyses, there was no evidence that vitamin D supplementation was always desirable in relieving depressive symptoms in people with different health problems (12). A recent meta-analysis demonstrated that vitamin D supplementation could reduce the occurrence of negative emotions, including depression and anxiety, to some extent (13). However, due to the small number of studies, absence of clear distinction between the prevention and treatment consequences of vitamin D for depression, and no clear requirements for the placebo control of the trial, these findings should be interpreted with caution. Based on the shortcomings above and in the hope of providing some guiding significance for clinical application, we intend to review the relevant randomized controlled trials (RCTs) and conduct an updated meta-analysis to investigate the effect of vitamin D supplementation on the incidence and prognosis of depression.
Methods
Search strategy
According to the preferred reporting items for Systematic Review and Meta-Analysis (PRISMA) 2015 (14), two authors independently ran a systematic search of PubMed, EMBASE, Web of Science, and the Cochrane Library, and covered all potentially relevant articles to appraise the effect of vitamin D supplementation on the incidence and prognosis of depression. And the time scope of the literature search was limited from the establishment of the database to July 2021. Literature retrieval was carried out through the random combinations of the following search terms: “vitamin D” OR “vitamin D supplementation” OR “25-hydroxyvitamin D” OR “25-hydroxyvitamin D supplementation” OR “25(OH)D” OR “cholecalciferol” AND “depression” OR “depressive” OR “negative emotion” OR “major depressive disorder” AND “placebo.” The references in the primary articles and relevant reviews were also reviewed manually to avoid the omission of any potentially relevant research.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) RCTs were included in the study; (2) studies in which the experimental groups were supplied with vitamin D supplementation, and the control groups were in absence of vitamin D or only supplemented with placebo in both at baseline and terminal of the intervention; (3) no psychological symptoms in depression or a diagnosis of depression was in the population (two groups of the population could not be mixed into the same study); (4) the participants in each group were categorized under the depression assessment scale in both at baseline and terminal of the intervention, based on their psychological symptoms, to assess the impact of vitamin D on the incidence and prognosis of depression.
The exclusion criteria were as follows: (1) study that was not published in English; (2) study in which the effects of vitamin D supplementation on the baseline and terminal of were only compared in the experimental groups without a control group; (3) study that was just a study protocol or without outcome reported; (4) the intervention contained nutritional supplements/medications; (5) study that unable to obtain the full text or extract available data; (6) study that was a duplicate publication (the latest published or most complete studies would be selected for inclusion).
Data extraction
The retrieved articles were independently screened by two authors, and the data were extracted following a pre-designed data extraction table. Any divergence was resolved through a third-party discussion. Data extracted from each study included: author; year of publication; country; follow up duration; participants' characteristics (e.g., mean age, sample size, body mass index (BMI), serum level of 25(OH)D at baseline and terminal); the dose of vitamin D supplementation; trial registration number; the mean and standard deviation in participants' depression assessment scale scores at baseline, terminal, and the difference between. Different depression assessment scales that were used for the evaluation of the psychological symptoms in different studies include the Beck Depression Inventory (BDI), BDI-II, Hospital Anxiety and Depression Scale (HADS), Hamilton Depression Rating Scale (HAM-D), etc. When multiple depression assessment scales were used in the same study, priority was given to the more well-known and the more commonly used ones (13).
Quality evaluation and outcome measures
The Cochrane Collaborative Risk of Bias Assessment Tool was used to assess the potential risk of bias in RCTs (15). It contains six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. The risk was classified into three levels: high risk, unclear risk, and low risk.
The efficacy outcome measure of this meta-analysis was the differences in the depression scores from baseline to terminal both in the experimental groups and the control groups, which were assessed by multiple depression assessment scales.
Statistical analysis
By using Revman 5.3 software, each study on the total differences in changes in depressive symptoms between vitamin D groups and control groups was all combined to evaluate the overall effect of vitamin D supplementation on the incidence and prognosis of depression. Through the data extracted from each study, for continuous variables, the effect size was calculated as standardized mean differences with a 95% confidence interval (CI). For the entire sample, the heterogeneity test was estimated using the Cochran chi-square test and quantified using the inconsistency test (I2) (16). Considering the differences in the type and dose of vitamin D supplements, a random-effects model was applied. All probabilities (p-values) of data were two-sided, with p < 0.05 regarded as being statistically significant.
The potential variables leading to sources of heterogeneity were investigated via the subgroup analysis. The potential variables included: participants' level of serum 25(OH)D, the dose of vitamin D supplementation, the depression assessment scale that was well-known and commonly used, BMI of participants, mean age of participants, participant's gender, duration of intervention. When included studies ≥10, the publication bias would be assessed using a funnel plot (17). In addition, the software was also used for the sensitivity analysis, which was conducted to estimate whether it would affect the pooled effect by excluding each study successively.
Results
Study selection
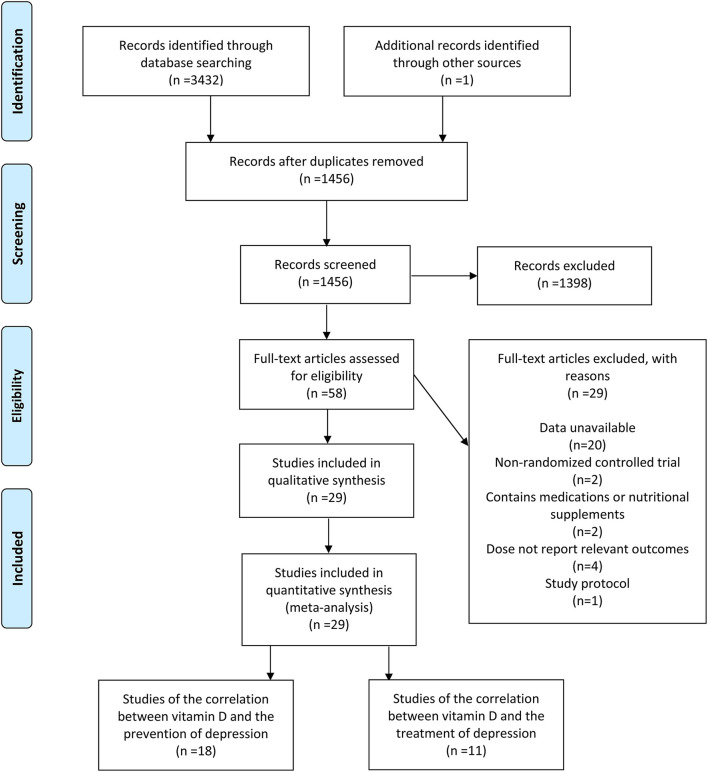
A total of 3,432 relevant studies were reviewed after the database search. In addition, one study was identified through other origins. After removing 1,977 duplicate studies, 1,398 studies were ruled out through the screening of their title and abstract. Additionally, at the back of the full-text review and evaluation of the remaining 58 studies, one study protocol was excluded, and 20 were excluded due to unavailable data. Furthermore, two studies were excluded because of non-RCT, four articles were excluded because they failed to report relevant outcomes, and two articles containing medications or nutritional supplements were also excluded. Ultimately, in accordance with inclusion and exclusion criteria, 29 eligible studies (7, 18–45) were included (Figure 1), 18 studies (18–35) for the correlation between vitamin D and the incidence of depression, and 11 studies (7, 36–45) for the correlation between vitamin D and the development of depression.
Figure 1.
Flow diagram describing inclusion and exclusion criteria.
Study characteristics
The basic characteristics of 18 studies illustrating the correlation between vitamin D and the occurrence of depression are displayed in Table 1 and Supplementary Table 1. The locations of the studies were classified as North America, Europe, Asia, and Oceania. Participants in these studies ranged in age from 13 to 85 years old with 2,111 cases in the experimental groups and 2,147 cases in the control groups. Dosage of vitamin D ranged from 200 IU per day to about 10,714 IU per day. The follow-up duration ranged from 6 to 144 weeks. The basic characteristics of 11 studies demonstrating the correlation between vitamin D and the development of depression are shown in Table 2 and Supplementary Table 2. The locations of the studies were classified as Europe and Asia. Participants in these studies varied in age from 18 to 79 years old with 878 cases in both the experimental groups and the control groups. Dosage of vitamin D ranged from 1,500 IU per day to about 7,143 IU per day. The follow-up duration varied between 4 and 52 weeks. On the basis of the Cochrane Collaboration tool, particulars concerning the quality assessment of 29 studies are shown in Supplementary Figures 1, 2.
Table 1.
The characteristics of studies included in this meta-analysis (the correlation between vitamin D and the incidence of depression).
| Author, year | Country | Follow-up (weeks) | Dose of experimental group | Sample size | Participants inclusion criteria | Depression assessment scale | |
|---|---|---|---|---|---|---|---|
| Experiment | Control | ||||||
| Jalali-Chimeh et al. (24) | Iran | 8 | 300,000 IU/4 weeks | 38 | 38 | BMI: 18.5–24.9 kg/m2, Age: 18–45 years for women with sexual dysfunction | BDI |
| Jorde et al. (27) | Norway | 48 | 40,000 IU/week 20,000 IU/week |
116 106 |
112 | Obese adults | BDI |
| Dean et al. (20) | Australia | 6 | 5,000 IU/day | 63 | 65 | Age ≥ 18 years for healthy adults | BDI |
| Kjærgaard et al. (28) | Norway | 24 | 40,000 IU/week | 120 | 110 | Age: 30 to 75 years with low 25(OH)D levels | BDI-II, HADS, MADRS |
| Bertone-Johnson et al. (18) | USA | 96 | 400 IU/day | 1109 | 1143 | Age: 50–79 years for postmenopausal women | Burnam scale |
| Frandsen et al. (21) | Denmark | 12 | 2,800 IU/day | 22 | 21 | Healthy adults | SIGH-SAD |
| Mason et al. (30) | USA | 48 | 2,000 IU/day | 109 | 109 | BMI ≥ 25 kg/m2, Age: 50–75 years for postmenopausal womenwith low 25(OH)D levels | BSI-18 |
| Vaziri et al. (34) | Iran | 12 | 2,000 IU/day | 75 | 78 | Age ≥ 18 years for healthy pregnant women | EPDS |
| Rolf et al. (33) | the Netherlands | 48 | 14,000 IU/day | 20 | 20 | Age: 18–55 years with multiple sclerosis | HADS-D |
| Grung et al. (23) | Norway | 21-28 | 1,600 IU/day | 23 | 23 | Age: 13–14 years for healthy adolescents | YSR-CBCL |
| Ghaderi et al. (22) | Iran | 12 | 50,000 IU/fortnight | 34 | 34 | Age: 25–70 years with methadone treatment | BDI |
| Jorde and Kubiak (26) | Norway | 16 | 100,000 IU bolus and 20,000 IU/week 100,000 IU bolus and 20,000 IU/week (+psychopharmaca) |
192 14 |
193 9 |
Age > 40 years with low 25(OH) D levels | BDI-II |
| Mousa et al. (31) | Australia | 16 | 100,000 IU bolus and 4,000 IU/day | 26 | 22 | BMI ≥ 25 kg/m2 or BMI≥ 29 kg/m2, Age: 18–60 years with low 25(OH) D levels | BDI-II |
| Raygan et al. (32) | Iran | 12 | 50,000 IU/fortnight | 30 | 30 | Age: 45–85 years for diabetics with CHD | BDI |
| Jamilian et al. (25) | Iran | 12 | 50,000 IU/fortnight | 30 | 30 | Age: 18–40 years for women with polycystic ovary syndrom | BDI-II, DASS |
| Choukri et al. (19) | New Zealand | 24 | 50,000 IU/months | 76 | 74 | Age: 18–40 years for healthy adult women | CES-D |
| Krivoy et al. (29) | Israel | 8 | 14,000 IU/week | 24 | 23 | Age: 18–65 years for schizophrenic patients with clozapine treatment and low 25(OH)D levels | CDS |
| Fazelian et al. (35) | Iran | 16 | 50,000 IU/fortnight | 26 | 25 | Age: 20–60 years for T2DM women with low 25(OH) D levels | DASS-21 |
BMI, body mass index; BDI, Beck Depression Inventory; HADS, Hospital Anxiety and Depression Scale; MADRS, Montgomery-Asberg Depression Rating Scale; GDS, Geriatric Depression Scale; SIGH-SAD, The Structured Interview Guide for the Hamilton Rating Scale for Depression-Seasonal Affective Disorder version; BSI, Brief Symptom Inventory Depression Subscale; EPDS, Edinburgh Postnatal Depression Scale; YSR-CBCL, Youth Self-report version of the Child Behavior Checklist; DASS, Depression Anxiety and Stress Scales; CES-D, Center for Epidemiological Studies Depression Scale; CDS, Calgary Depression Scale; CHD, coronary heart disease; T2DM, type 2 diabetes; HT, hormone therapy.
Table 2.
The characteristics of studies included in this meta-analysis (the correlation between vitamin D and the prognosis of depression).
| Author, year | Country | Follow-up (weeks) | Dose of experimental group | Sample size | Participants inclusion criteria | Depression assessment scale | |
|---|---|---|---|---|---|---|---|
| Experiment | Control | ||||||
| Yalamanchili and Gallagher (36) | USA | 144 | 200 IU/day (+HT) 200 IU/day |
122 123 |
120 123 |
Age: 65–77 years for postmenopausal women | GDS-30 |
| Khoraminya et al. (40) | Iran | 8 | 1,500 IU/day | 20 | 20 | Age: 18–65 years with MDD and deficiency of vitamin D | BDI-II, HAM-D |
| Mozaffari-Khosravi et al. (42) | Iran | 12 | 300,000 IU/3 months 150,000 IU/ 3 months |
39 36 |
34 | Age: 18–65 years with MDD, Free from other psychiatric diagnoses, any antidepressant drugs or dietary supplements | BDI-II |
| Wang et al. (44) | China | 52 | 50,000 IU/week | 362 | 364 | Age: 20 to 60 years for dialysis patients with MDD and deficiency of vitamin D, Free from other psychiatric diagnoses,any antidepressant drugs or dietary supplements | BDI-II |
| Sepehrmanesh et al. (43) | Iran | 8 | 50,000 IU/week | 18 | 18 | Age: 18–65 years with MDD | BDI-II |
| Hansen et al. (39) | Denmark | 24 | 2,800 IU/day | 28 | 34 | Age: 50–79 years with MDD | HAM-D17, MDI |
| Alavi et al. (37) | Iran | 8 | 50,000 IU/week | 39 | 39 | Age > 60 years with MDD and deficiency of vitamin D, Free from other psychiatric diagnoses | GDS-15 |
| Zhang et al. (45) | China | 8 | 100,000 IU/week | 58 | 65 | Age ≥ 18 years for recurrent pulmonary tuberculosis patients with MDD | BDI-II |
| Amini et al. (38) | Iran | 8 | 50,000 IU/fortnight 50,000 IU/fortnight(+Ca) |
26 26 |
24 | BMI ≤ 35 kg/m2 for women with PPD | EPDS |
| Libuda et al. (41) | Germany | 4 | 2,640 IU/day | 56 | 57 | Patients with MDD and low 25(OH)D levels | BDI-II, DISYPS-II |
| Abiri et al. (7) | Iran | 8 | 50,000 IU/week (+Mg) 50,000 IU/week |
25 26 |
26 25 |
BMI: 35 kg/m2, Age > 20 years for women with MDD and low 25(OH)D levels | BDI-II |
MDD, Major depressive disorder; PPD, Postpartum depression; BMI, body mass index; NA, no available; BDI, Beck Depression Inventory; HAM-D, Hamilton Depression Rating Scale; MDI, Major depression inventory; GDS, Geriatric Depression Scale; EPDS, Edinburgh Postnatal Depression Scale; DISYPS-II, Diagnostic System for Mental Disorders in Children and Adolescents according to ICD-10 and DSM-IV.
Overall effects and subgroup analysis of vitamin D and the incidence of depression
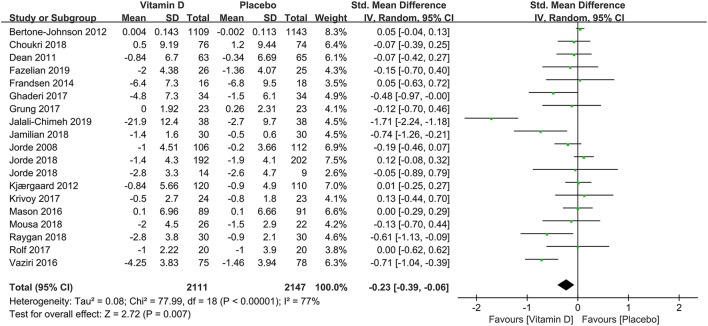
A pooled analysis of 18 studies demonstrated the overall effects of the correlation between vitamin D supplementation and the occurrence of depression. Despite the high heterogeneity, the depression assessment scale scores from baseline to terminal in the experimental groups have decreased compared with those in the control groups (SMD: −0.23) (Figure 2), which revealed that the usage of vitamin D supplements might reduce the incidence of depression.
Figure 2.
Forest plot of the correlation between vitamin D and the incidence of depression.
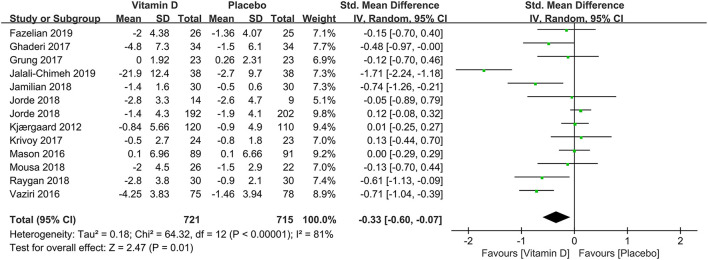
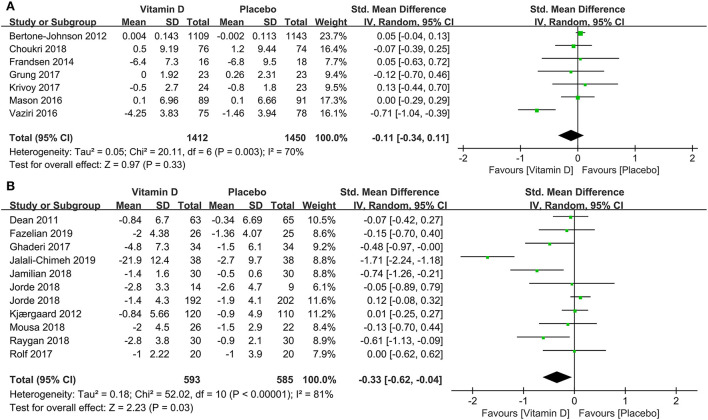
In subgroup analysis, the correlation between vitamin D supplementation and the occurrence of depression based on 1,436 participants with low levels of serum 25(OH)D at baseline were conducted in 12 studies. Compared to the control group, the experimental group had a significant effect on reducing the incidence of depression [SMD: −0.33; 95%CI: (−0.60, −0.07); p = 0.01] (Figure 3). To compare the effects of low doses (≤2,800 IU/day) and high doses (>2,800 IU/day) of vitamin D supplementation, seven and 10 studies were examined, respectively. The pooled effects (SMD) for low doses and high doses of vitamin D supplements were −0.11 (Figure 4A) and −0.33 (Figure 4B), respectively. The results revealed that vitamin D supplementation in high doses might promote a decrease in the incidence of depression, while low doses of vitamin D supplements were futile.
Figure 3.
Forest plot of the correlation between vitamin D and the incidence of depression for population with low vitamin D levels (<50 nmol/L).
Figure 4.
Forest plot of the correlation between supplementary doses of vitamin D and the incidence of depression. (A) low doses (≤2,800 IU/day); (B) high doses (>2,800 IU/day).
In nine studies where the BDI or BDI-II was used as the depression assessment scale, the use of vitamin D might also be helpful to the reduction of the incidence of depression compared with the control group (SMD: −0.36). Vitamin D supplements were shown in 13 RCTs to have effectively decreased the incidence of depression among people with normal weight (SMD: −0.28). On the contrary, it was useless for the overweight people in 4 RCTs (SMD: −0.11). Also, seven studies reported a significant diminution compared to the control group in the incidence of depression in females among 2,922 participants with the vitamin D supplementation (SMD: −0.44). Nevertheless, no apparent change relating to vitamin D supplementation was discovered neither in the elderly (SMD: 0.04) through two studies nor in the older female (SMD: 0.04) through other two studies. Finally, among those who received vitamin D supplements, there was no significant difference between the two groups for the intervention duration ≤8 weeks (SMD: −0.55). However, it was worth noting that when the intervention duration is ≥8 weeks, vitamin D supplementation might be conducive to the decrease in the occurrence of depression compared with the control group (SMD: −0.15) (Table 3).
Table 3.
Subgroup analysis of the correlation between vitamin D and the incidence/prognosis of depression.
| Subgroup | No. of studies | Participants | Changes in depression | SMD | 95%CI | p | Heterogeneity(I2) (%) | |
|---|---|---|---|---|---|---|---|---|
| Occurrence | ||||||||
| BDI | 9 | 1,305 | ↓ | −0.36 | −0.65–(−0.07) | 0.02 | 83 | |
| Overweight (BMI >24 kg/m2) | 4 | 497 | - | −0.11 | −0.29–0.06 | 0.21 | 0 | |
| Nomal weight (BMI: 18–24kg/m2) | 13 | 3,721 | ↓ | −0.28 | −0.49–(−0.07) | 0.01 | 83 | |
| The elderly (≥50 years old) | 2 | 2,432 | - | 0.04 | −0.04–0.12 | 0.29 | 0 | |
| Female | 7 | 2,922 | ↓ | −0.44 | −0.81–(−0.06) | 0.02 | 91 | |
| Older female | 2 | 2,432 | - | 0.04 | −0.04–0.12 | 0.29 | 0 | |
| Intervention duration | ≤ 8 weeks | 3 | 251 | - | −0.55 | −1.61–0.52 | 0.32 | 93 |
| ≥8 weeks | 16 | 4,054 | ↓ | −0.15 | −0.28–(−0.01) | 0.03 | 60 | |
| Development | ||||||||
| BDI | 7 | 1,236 | - | −1.04 | −2.29–0.21 | 0.10 | 99 | |
| Overweight (BMI >24 kg/m2) | 2 | 178 | ↓ | −0.79 | −1.34–(−0.24) | 0.005 | 72 | |
| Nomal weight (BMI: 18–24 kg/m2) | 4 | 445 | ↓ | −0.34 | −0.68–0.00 | 0.05 | 60 | |
| Female | 3 | 448 | ↓ | −0.61 | −1.16–(−0.06) | 0.03 | 84 | |
| Intervention duration | ≤ 8 weeks | 7 | 555 | ↓ | −0.69 | −1.08–(−0.30) | <0.001 | 80 |
| ≥8 weeks | 10 | 1,656 | ↓ | −1.00 | −1.90–(−0.09) | 0.03 | 98 | |
SMD, standard mean difference; BMI, body mass index; BDI, Beck Depression Inventory.
-, There was no statistical difference in the change of depression scores between the experimental and control groups.
↓, The depression scores of the experimental group decreased compared with control groups.
Overall effects and subgroup analysis of vitamin D and the prognosis of depression
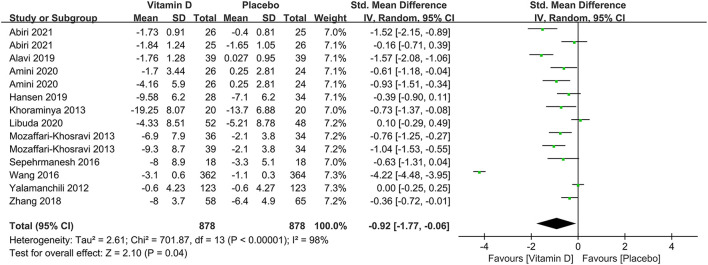
To probe the overall effects of the correlation between vitamin D supplementation and the prognosis of depression, 11 studies were included in a pooled analysis. From baseline to terminal, although there was also high heterogeneity, depression assessment scale scores showed a significant decline (SMD: −0.92) (Figure 5), indicating that the interventions with vitamin D supplements might be beneficial to the treatment of depression.
Figure 5.
Forest plot of the correlation between vitamin D and the prognosis of depression.
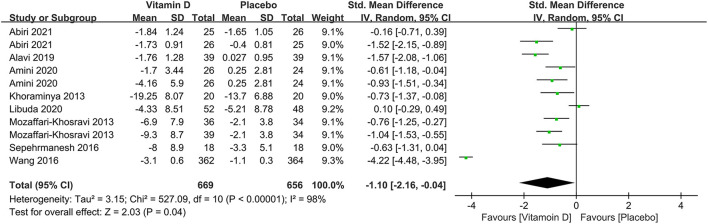
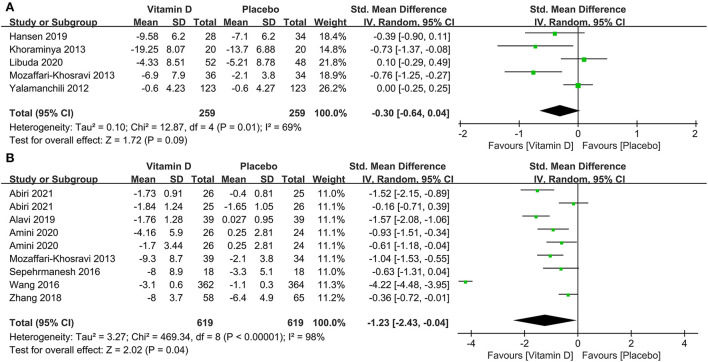
In subgroup analysis, eight studies compared the effects among 1,325 participants with a low level of serum 25(OH)D at baseline between the two groups. The results illustrated that compared to the control group, those with depression and low serum vitamin D levels might benefit from vitamin D supplementation (SMD: −1.10) (Figure 6). The correlation between vitamin D usage and the development of depression among low doses (≤2,800 IU/day) and high doses (>2,800 IU/day) of vitamin D supplementation were examined in five and seven studies, separately. The results demonstrated that high-dose vitamin D supplementation might be more favorable in the depression therapy, whereas low-dose vitamin D supplementation was relatively ineffective by comparing parameters with the control group, the pooled effects (SMD) for low-dose analysis was −0.30 (Figure 7A) and high-dose supplementation was −1.23 (Figure 7B), respectively.
Figure 6.
Forest plot of the correlation between vitamin D and the prognosis of depression for patients with low vitamin D levels (<50 nmol/L).
Figure 7.
Forest plot of the correlation between supplementary doses of vitamin D and the prognosis of depression. (A) low doses (≤2,800 IU/day); (B) high doses (>2,800 IU/day).
However, in the subset of seven studies where the BDI or BDI-II was used as the depression assessment scale, no significant difference was found (SMD: −1.04). With additional consideration of patients' BMI, vitamin D supplementation was effective in the treatment of depression both in people with normal weight (SMD: −0.34) in four studies and those who were overweight (SMD: −0.79) in another two studies. In three RCTs where the vitamin D was supplied to 448 females, compared to the control group, a significant therapeutic effect on depression was also observed (SMD: −0.61). Last but not least, vitamin D supplementation was beneficial as an adjunct to the treatment of depression, compared with the control group, not only for the intervention duration ≥8 weeks (SMD: −1.00) but also for the intervention duration ≤8 weeks (SMD: −0.69; 95%CI: [−1.08, −0.30]; p < 0.001) (Table 3).
Publication bias and sensitivity analysis
To evaluate publication bias, the funnel plots of the publication bias of vitamin D and the incidence/prognosis of depression was derived (Supplementary Figures 3, 4). All the funnel plots in our results seem to be symmetrical with the effect estimates, which indicated that there was no significant publication bias in our studies. Sensitivity analysis was also conducted to estimate the stability of the pooled effects by excluding each study successively, and the results were supportive to prove the stability of our results.
Discussion
In the present systematic review, we summarized the published outcomes related to the effect of vitamin D supplements on depression. A meta-analysis based on 18 studies was conducted to evaluate the correlation between vitamin D supplementation and the incidence of depression whereas the correlation between vitamin D supplementation and the prognosis of depression from 11 studies was estimated. Congruent with the previous systematic reviews and meta-analysis (13, 46, 47), in our main study, vitamin D supplementation was found to be associated with a decrease in the occurrence of depression as well as an improvement in the treatment of depression. The impact was also observed in subgroup analyses of serum 25(OH)D levels, doses of vitamin D supplementation, BMI, gender, and intervention duration. Consequently, our results may be supportive to consider vitamin D supplementation to be effective in lowering the risk and improving the treatment of depression.
So far, the correlation between vitamin D and mental health has been investigated mostly in the context of depression or depressive symptoms (2, 12, 46–50), with several putative mechanisms for the effect of vitamin D being proposed (51). Vitamin D has been discovered to have effects on the central nervous system. Some studies have shown that vitamin D receptors are widely distributed in areas of the brain, such as the amygdala and substantia nigra (52). Vitamin D is able to cross the blood-brain barrier, activate vitamin D receptors, and play a role in human behavior control (53).
In addition, in recent years, the cyclo-AMP responsive element-binding protein (CREB) cascade and its effect on the brain-derived neurotrophic factor (BDNF) have made some rationales for the pathogenesis and treatment of depression (54). The hypothesis of catecholamine and serotonin deficiency suggests that depression may be largely caused by the lack of monoamines (norepinephrine and serotonin) in the synaptic space (55). Vitamin D, however, weighs on the synthesis of monoamines and may regulate the activity of GABA-A receptors to some extent (52, 56, 57). By regulating the release of neurotransmitters and the synthesis of neurotrophic factors, vitamin D is relevant in ameliorating mood and behavior in humans (58). Meanwhile, the function of vitamin D may be attributed to its neuroprotective effect on the brain, as it has been proved that vitamin D is able to lower plasma C-reactive protein in patients with psychiatric disorders and modulate inflammation by suppressing proinflammatory cytokines (59, 60).
Although the relationship between vitamin D and depression has been extensively discussed, the evidence in terms of data and mechanism appears to be logical. Several studies (12, 49) have found that taking vitamin D supplements does not result in a substantial reduction in depression. These might stem from the biological defects of the preliminary studies, two studies (13, 50) revealed that those unflawed trials contributed to a statistically prominent improvement in depression. Similarly, a significant association between the use of vitamin D and depression by Anglin agreed with the previous finding (2). Moreover, in the meta-analysis by Shaffer (46), vitamin D was shown to be beneficial in relieving depressive symptoms in patients with clinically significant depression as well.
As for the assessment scale that was used to measure symptoms of depression, in this study, when taking into account the most frequently used assessment scale BDI and BDI-II only, vitamin D supplementation was only effective in the prevention of depression, not in the treatment of depression. This issue might be owing to the fact that the level of depression was incapable of being estimated with meta-regression when under different measurements (13). It served as a reminder to pay attention to the impact of various measurement tools.
In line with prior meta-analyses that have been reported (2, 61), in terms of the impact of the serum vitamin D level on depression, the subgroup analysis showed that patients with low vitamin D levels (<50 nmol/L) benefit from supplementation in both prevention and treatment of depression. Vitamin D deficiency is defined as serum 25(OH)D levels of <50 nmol/L, with levels of 50–75 nmol/L being deemed insufficiency (62). The prevalence of neurodegenerative, neuroinflammatory, and neuropsychological disorders has been linked to hypo-vitamin D in studies based on humans. In a large cohort study (63), higher serum vitamin D levels were authenticated to be associated with a lower incidence of depression at follow-up, suggesting that vitamin D deficiency may signify a latent biological vulnerability for depressive disorder. Hence, it comes as no surprise that depressive patients with hypovitaminosis D are more prone to be relieved from adequate vitamin D supplementation. Furthermore, patients with hypovitaminosis D are supposed to be more alert to the incidence of depression to some extent. It is advised that individuals with vitamin D deficiency should take suitable vitamin D supplements, including dietary sources or pills.
Concerning the BMI, our results revealed that supplementing with vitamin D worked well in both prevention and treatment in people with normal weight, whereas only treatment and not prevention in those who were overweight. According to previous studies (27, 64), BMI appeared to have an essential mediating role in the relationship between vitamin D and depression, and vitamin D supplements might improve depressive symptoms in obese patients. As mentioned before, in the overweight population, no significant difference was observed in the prevention group. The different findings might be ascribed to the truth that only a small proportion of the studies in subgroup analysis included assessed the effect of vitamin D supplementation on overweight patients in the aspect of prevention. Moreover, another contributing factor might contain differences in the study population, variable vitamin D supplementation dosages, and differences in baseline serum 25 (OH) D concentrations levels. A recent comparative observational study showed that the decrease in serum 25 (OH) D level in obese adults was related to incident depression, while the proportion of having vitamin D deficiency in depressed people was higher than that in non-depressed people (65). Vitamin D deficiency was also proved to be less common in healthy individuals (66). Due to the large body and adipose mass, overweight people might have a low increase in serum vitamin D level and require more vitamin D than normal (67), a regular dose of vitamin D would possibly be insufficient for them. Besides, the adipose mass could also lead to higher systemic inflammation, so higher doses of vitamin supplements were also considered necessary. As a result, the regular dose of vitamin D supplementation might not be as effective in overweight people without a depressive disorder.
Intriguingly, the subgroup analysis of gender demonstrated that vitamin D supplements significantly alleviated depression scores in all females, regardless of whether they were depressed or not. A previous study (68) found that female patients with depressive symptoms improved significantly more following vitamin D administration than male patients, which was in accordance with our findings. The mechanism might involve that vitamin D has a bearing on the synthesis of serotonin and a moderate amount of vitamin D may elevate the level of extracellular serotonin (69), particularly in females, so as to improve the symptoms of depressive disorder. Concerning vitamin D supplementation was ineffective among the elderly population in our results, due to the small number of studies and the unavailability of research data, the outcomes were tough to explain. Additionally, according to a pilot study (70), even though there was a correlation between an increase in vitamin D levels and a decrease in depressive symptom scores, the association vanished as the research population over 65 years old. Furthermore, since most included studies defined depression as being above the dividing point of the depression scale with no adjustment for physical frailty, the outcome could be easily influenced by the confounding factors as well and should be treated with more caution.
When it comes to the strategy of vitamin D supplement, considering the supplementary dosage, the replenishment threshold was set at 2,800 IU each day. Our findings illustrated that high doses (>2,800 IU/day) were beneficial in the prevention and treatment of depression, while low doses (≤2,800 IU/day) were regarded as ineffective. The common dose of vitamin D supplements for adults is 800 IU per day, with a range from 400 to 2,000 IU per day depending on age, weight, illness status, and race (71). Besides, 50,000 IU is acknowledged to be the upper limit of the recommended daily intake (72–75), excessive intake might result in toxicity and some adverse reactions such as hypercalciuria (76). Yet owing to the limited number of studies included in the treatment group in terms of low-dose supplementation, the relevant results should be interpreted with caution either. As for the intervention duration, “8 weeks” was recognized as the time point that might trigger the response to vitamin D, whether in the prevention or treatment groups. Although a previous study showed that the response to vitamin D could not be observed even at 20 weeks, this was based on a status of relative vitamin D repletion (77). As a secosteroid hormone, vitamin D functions through transcription in the nucleus, which takes a long time to work. This might be a plausible explanation for why observing the response to vitamin D took so long. Unexpectedly, we also observed a vitamin D response in the treatment group under the circumstances that the intervention duration was <8 weeks. This phenomenon can also be interpreted by the fact that the mean level of vitamin D in depressed people is more likely to be lower than that in non-depressed people, making depressed ones more sensitive to vitamin D administration. Anyway, our analysis indicated the effects of vitamin D with daily supplementary doses >2,800 IU and an intervention duration of 8 weeks was significant. High heterogeneity was also observed in some subgroups in our meta-analysis, which could be attributed to several reasons. Initially, due to the differences in race, outdoor activity intensity, sunshine time, diet, etc., the baseline and terminal serum vitamin D levels between participants also exist lot of discrepancies. Besides, on account of the differences in the cognition of mental diseases among different populations, there was also a discrepancy in the results of the depression assessment scale to some extent.
Strengths and limitations
To our knowledge, this is the first comprehensive meta-analysis to assess the correlation between vitamin D and the incidence as well as the prognosis of depression. And there were several strengths to our study. First, more recent RCTs were incorporated into this study, thereby updating previous findings. Additionally, although the tool evaluation constructs that were used to measure the psychological symptoms of subjects varied slightly, compared with previous studies (13, 59), they were limited within the depression assessment scale, which decreased the impact of other emotional or physical symptoms on confounding factors of outcomes.
Nonetheless, some associated limitations of our study should be mentioned. First, we have not pre-registered a protocol for this meta-analysis, which might introduce potential bias to the review. Second, due to the race, the intensity of outdoor activity, sun exposure time, dietary differences, and cognition of mental diseases in different ethnic groups and other factors, the high heterogeneity in some subgroups of our meta-analysis was predictable. Third, detailed information such as simultaneous psychosocial interventions, use of calcium supplementation, and antidepressants were not provided in a few studies, our results might be disturbed by overlapping factors. As a consequence, the possibility that the observed amelioration of psychological state in respondents might be the result of overlapping elements acting together should be taken into account. Fourth, the relationship between serum vitamin D levels and the severity of depression was not examined in this study. Also, vitamin D supplementation cannot be fully equivalent to an increase in serum vitamin D levels. Hence, relevant findings should be treated with more care.
Conclusion
To conclude, this meta-analysis demonstrates that vitamin D has a positive impact on both the decreased incidence of depression and a better prognosis of depression. The impact is consistently observed in subgroup analyses of serum 25(OH)D levels, doses of vitamin D supplementation, BMI, gender, and intervention duration. Whether suffering from depression or not, individuals with low vitamin D levels and females are most likely to benefit from vitamin D supplementation. A daily supplementary dose of 2,800 IU and an intervention duration of 8 weeks are considered as the point that may cause the observational effect of vitamin D. Besides, our results also reveal that vitamin D supplementation works well in both prevention and treatment in normal-weight people, whereas only in treatment not prevention for those who are overweight.
Author contributions
LR designed the research process. FX and TH searched the database for corresponding articles and drafted the meta-analysis. DL extracted useful information from the articles above. RF used statistical software for analysis. CN and JH polished this article. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.903547/full#supplementary-material
The characteristics of studies included in this meta-analysis (the correlation between vitamin D and the incidence of depression).
The characteristics of studies included in this meta-analysis (the correlation between vitamin D and the prognosis of depression).
Quality assessment of studies included in this meta-analysis (risk of bias graph).
Quality assessment of studies included in this meta-analysis (Risk of bias summary).
Funnel plot for publication bias (the correlation between vitamin D and the incidence of depression).
Funnel plot for publication bias (the correlation between vitamin D and the prognosis of depression).
References
- 1.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. (2007) 370:851–8. 10.1016/S0140-6736(07)61415-9 [DOI] [PubMed] [Google Scholar]
- 2.Anglin RES, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. (2013) 202:100–7. 10.1192/bjp.bp.111.106666 [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Cheng S, Xu H. Systematic review and meta-analysis of the relationship between sleep disorders and suicidal behaviour in patients with depression. BMC Psychiatry. (2019) 19:303. 10.1186/s12888-019-2302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. J Clin Psychiatry. (1998) 59(Suppl. 18):23–9. [PubMed] [Google Scholar]
- 5.Sinyor M, Schaffer A, Levitt A. The sequenced treatment alternatives to relieve depression (STAR*D) trial: a review. Can J Psychiatry. (2010) 55:126–35. 10.1177/070674371005500303 [DOI] [PubMed] [Google Scholar]
- 6.Kerr DCR, Zava DT, Piper WT, Saturn SR, Frei B, Gombart AF. Associations between vitamin D levels and depressive symptoms in healthy young adult women. Psychiatry Res. (2015) 227:46–51. 10.1016/j.psychres.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abiri B, Sarbakhsh P, Vafa M. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutr Neurosci. (2021) 2:1–13. 10.1080/1028415X.2021.1945859 [DOI] [PubMed] [Google Scholar]
- 8.Belvederi Murri M, Respino M, Masotti M, Innamorati M, Mondelli V, Pariante C, et al. Vitamin D and psychosis: mini meta-analysis. Schizophr Res. (2013) 150:235–9. 10.1016/j.schres.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 9.Ganji V, Milone C, Cody MM, McCarty F, Wang YT. Serum vitamin D concentrations are related to depression in young adult US population: the third national health and nutrition examination survey. Int Arch Med. (2010) 3:29. 10.1186/1755-7682-3-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May HT, Bair TL, Lappé DL, Anderson JL, Horne BD, Carlquist JF, et al. Association of vitamin D levels with incident depression among a general cardiovascular population. Am Heart J. (2010) 159:1037–43. 10.1016/j.ahj.2010.03.017 [DOI] [PubMed] [Google Scholar]
- 11.Hoang MT, Defina LF, Willis BL, Leonard DS, Weiner MF, Brown ES. Association between low serum 25-hydroxyvitamin D and depression in a large sample of healthy adults: the cooper center longitudinal study. Mayo Clin Proc. (2011) 86:1050–5. 10.4065/mcp.2011.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gowda U, Mutowo MP, Smith BJ, Wluka AE, Renzaho AMN. Vitamin D supplementation to reduce depression in adults: meta-analysis of randomized controlled trials. Nutrition. (2015) 31:421–9. 10.1016/j.nut.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 13.Cheng Y-C, Huang Y-C, Huang W-L. The effect of vitamin D supplement on negative emotions: a systematic review and meta-analysis. Depress Anxiety. (2020) 37:549–64. 10.1002/da.23025 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 17.Gu L, Huang X, Li S, Mao D, Shen Z, Khadaroo PA, et al. A meta-analysis of the medium- and long-term effects of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass. BMC Surg. (2020) 20:30. 10.1186/s12893-020-00695-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bertone-Johnson ER, Powers SI, Spangler L, Larson J, Michael YL, Millen AE, et al. Vitamin D supplementation and depression in the women's health initiative calcium and vitamin D trial. Am J Epidemiol. (2012) 176:1–13. 10.1093/aje/kwr482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choukri MA, Conner TS, Haszard JJ, Harper MJ, Houghton LA. Effect of vitamin D supplementation on depressive symptoms and psychological wellbeing in healthy adult women: a double-blind randomised controlled clinical trial. J Nutr Sci. (2018) 7:e23. 10.1017/jns.2018.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dean AJ, Bellgrove MA, Hall T, Phan WM, Eyles DW, Kvaskoff D, et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults–a randomised controlled trial. PLoS ONE. (2011) 6:e25966. 10.1371/journal.pone.0025966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frandsen TB, Pareek M, Hansen JP, Nielsen CT. Vitamin D supplementation for treatment of seasonal affective symptoms in healthcare professionals: a double-blind randomised placebo-controlled trial. BMC Res Notes. (2014) 7:528. 10.1186/1756-0500-7-528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghaderi A, Banafshe HR, Motmaen M, Rasouli-Azad M, Bahmani F, Asemi Z. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 79:84–9. 10.1016/j.pnpbp.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 23.Grung B, Sandvik AM, Hjelle K, Dahl L, Froyland L, Nygard I, et al. Linking vitamin D status, executive functioning and self-perceived mental health in adolescents through multivariate analysis: a randomized double-blind placebo control trial. Scand J Psychol. (2017) 58:123–30. 10.1111/sjop.12353 [DOI] [PubMed] [Google Scholar]
- 24.Jalali-Chimeh F, Gholamrezaei A, Vafa M, Nasiri M, Abiri B, Darooneh T, et al. Effect of vitamin D therapy on sexual function in women with sexual dysfunction and vitamin D deficiency: a randomized, double-blind, placebo controlled clinical trial. J Urol. (2019) 201:987–93. 10.1016/j.juro.2018.10.019 [DOI] [PubMed] [Google Scholar]
- 25.Jamilian M, Samimi M, Mirhosseini N, Afshar Ebrahimi F, Aghadavod E, Talaee R, et al. The influences of vitamin D and omega-3 co-supplementation on clinical, metabolic and genetic parameters in women with polycystic ovary syndrome. J Affect Disord. (2018) 238:32–8. 10.1016/j.jad.2018.05.027 [DOI] [PubMed] [Google Scholar]
- 26.Jorde R, Kubiak J. No improvement in depressive symptoms by vitamin D supplementation: results from a randomised controlled trial. J Nutr Sci. (2018) 7:e30. 10.1017/jns.2018.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorde R, Sneve M, Figenschau Y, Svartberg J, Waterloo K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. (2008) 264:599–609. 10.1111/j.1365-2796.2008.02008.x [DOI] [PubMed] [Google Scholar]
- 28.Kjaergaard M, Waterloo K, Wang CE, Almas B, Figenschau Y, Hutchinson MS, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. (2012) 201:360–8. 10.1192/bjp.bp.111.104349 [DOI] [PubMed] [Google Scholar]
- 29.Krivoy A, Onn R, Vilner Y, Hochman E, Weizman S, Paz A, et al. Vitamin D supplementation in chronic schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled clinical trial. EBioMedicine. (2017) 26:138–45. 10.1016/j.ebiom.2017.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason C, de Dieu Tapsoba J, Duggan C, Wang CY, Korde L, McTiernan A. Repletion of vitamin D associated with deterioration of sleep quality among postmenopausal women. Prev Med. (2016) 93:166–70. 10.1016/j.ypmed.2016.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mousa A, Naderpoor N, de Courten MPJ, de Courten B. Vitamin D and symptoms of depression in overweight or obese adults: a cross-sectional study and randomized placebo-controlled trial. J Steroid Biochem Mol Biol. (2018) 177:200–8. 10.1016/j.jsbmb.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 32.Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 84:50–5. 10.1016/j.pnpbp.2018.02.007 [DOI] [PubMed] [Google Scholar]
- 33.Rolf L, Muris AH, Bol Y, Damoiseaux J, Smolders J, Hupperts R. Vitamin D3 supplementation in multiple sclerosis: symptoms and biomarkers of depression. J Neurol Sci. (2017) 378:30–5. 10.1016/j.jns.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 34.Vaziri F, Nasiri S, Tavana Z, Dabbaghmanesh MH, Sharif F, Jafari P, et al. randomized controlled trial of vitamin D supplementation on perinatal depression: in Iranian pregnant mothers. BMC Pregnancy Childbirth. (2016) 16:239. 10.1186/s12884-016-1024-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fazelian S, Amani R, Paknahad Z, Kheiri S, Khajehali L. Effect of vitamin D supplement on mood status and inflammation in vitamin D deficient type 2 diabetic women with anxiety: a randomized clinical trial. Int J Prev Med. (2019) 10:17. 10.4103/ijpvm.IJPVM_174_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yalamanchili V, Gallagher JC. Treatment with hormone therapy and calcitriol did not affect depression in older postmenopausal women: no interaction with estrogen and vitamin D receptor genotype polymorphisms. Menopause. (2012) 19:697–703. 10.1097/gme.0b013e31823bcec5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alavi NM, Khademalhoseini S, Vakili Z, Assarian F. Effect of vitamin D supplementation on depression in elderly patients: a randomized clinical trial. Clin Nutr. (2019) 38:2065–70. 10.1016/j.clnu.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 38.Amini S, Amani R, Jafarirad S, Cheraghian B, Sayyah M, Hemmati AA. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: a randomized double-blind clinical trial. Nutr Neurosci. (2020) 25:22–32. 10.1080/1028415X.2019.1707396 [DOI] [PubMed] [Google Scholar]
- 39.Hansen JP, Pareek M, Hvolby A, Schmedes A, Toft T, Dahl E, et al. Vitamin D3 supplementation and treatment outcomes in patients with depression (D3-vit-dep). BMC Res Notes. (2019) 12:203. 10.1186/s13104-019-4218-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khoraminya N, Tehrani-Doost M, Jazayeri S, Hosseini A, Djazayery A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust N Z J Psychiatry. (2013) 47:271–5. 10.1177/0004867412465022 [DOI] [PubMed] [Google Scholar]
- 41.Libuda L, Timmesfeld N, Antel J, Hirtz R, Bauer J, Fuhrer D, et al. Effect of vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients: results of a randomized controlled trial. Eur J Nutr. (2020) 59:3415–24. 10.1007/s00394-020-02176-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mozaffari-Khosravi H, Nabizade L, Yassini-Ardakani SM, Hadinedoushan H, Barzegar K. The effect of 2 different single injections of high dose of vitamin D on improving the depression in depressed patients with vitamin D deficiency: a randomized clinical trial. J Clin Psychopharmacol. (2013) 33:378–85. 10.1097/JCP.0b013e31828f619a [DOI] [PubMed] [Google Scholar]
- 43.Sepehrmanesh Z, Kolahdooz F, Abedi F, Mazroii N, Assarian A, Asemi Z, et al. Vitamin D supplementation affects the beck depression inventory, insulin resistance, and biomarkers of oxidative stress in patients with major depressive disorder: a randomized, controlled clinical trial. J Nutr. (2016) 146:243–8. 10.3945/jn.115.218883 [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Liu Y, Lian Y, Li N, Liu H, Li G. Efficacy of high-dose supplementation with oral vitamin D3 on depressive symptoms in dialysis patients with vitamin D3 insufficiency: a prospective, randomized, double-blind study. J Clin Psychopharmacol. (2016) 36:229–35. 10.1097/JCP.0000000000000486 [DOI] [PubMed] [Google Scholar]
- 45.Zhang L, Wang S, Zhu Y, Yang T. Vitamin D3 as adjunctive therapy in the treatment of depression in tuberculosis patients: a short-term pilot randomized double-blind controlled study. Neuropsychiatr Dis Treat. (2018) 14:3103–9. 10.2147/NDT.S183039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaffer JA, Edmondson D, Wasson LT, Falzon L, Homma K, Ezeokoli N, et al. Vitamin D supplementation for depressive symptoms: a systematic review and meta-analysis of randomized controlled trials. Psychosom Med. (2014) 76:190–6. 10.1097/PSY.0000000000000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vellekkatt F, Menon V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J Postgrad Med. (2019) 65:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Głabska D, Kołota A, Lachowicz K, Skolmowska D, Stachoń M, Guzek D. The influence of vitamin D intake and status on mental health in children: a systematic review. Nutrients. (2021) 13:952. 10.3390/nu13030952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li G, Mbuagbaw L, Samaan Z, Falavigna M, Zhang S, Adachi JD, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol Metab. (2014) 99:757–67. 10.1210/jc.2013-3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spedding S. Vitamin D and depression: a systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients. (2014) 6:1501–18. 10.3390/nu6041501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menon V, Kar SK, Suthar N, Nebhinani N. Vitamin D and depression: a critical appraisal of the evidence and future directions. Indian J Psychol Med. (2020) 42:11–21. 10.4103/IJPSYM.IJPSYM_160_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertone-Johnson ER. Vitamin D and the occurrence of depression: causal association or circumstantial evidence? Nutr Rev. (2009) 67:481–92. 10.1111/j.1753-4887.2009.00220.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farhangi MA, Mesgari-Abbasi M, Nameni G, Hajiluian G, Shahabi P. The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neurosci. (2017) 18:81. 10.1186/s12868-017-0400-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. (1997) 54:597–606. 10.1001/archpsyc.1997.01830190015002 [DOI] [PubMed] [Google Scholar]
- 55.Burke MJ, Preskorn SH. Short term treatment of mood disorders with standard antidepressants. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. New York, NY: Raven Press; (1995). p. 1053–65. [Google Scholar]
- 56.Humble MB. Vitamin D, light and mental health. J Photochem Photobiol B. (2010) 101:142–9. 10.1016/j.jphotobiol.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 57.Marsh WK, Penny JL, Rothschild AJ. Vitamin D supplementation in bipolar depression: a double blind placebo controlled trial. J Psychiatr Res. (2017) 95:48–53. 10.1016/j.jpsychires.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 58.Máčová L, Bičíková M, Ostatníková D, Hill M, Stárka L. Vitamin D, neurosteroids and autism. Physiol Res. (2017) 66:S333–S40. 10.33549/physiolres.933721 [DOI] [PubMed] [Google Scholar]
- 59.Jamilian H, Amirani E, Milajerdi A, Kolahdooz F, Mirzaei H, Zaroudi M, et al. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: a systematic review and meta-analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 94:109651. 10.1016/j.pnpbp.2019.109651 [DOI] [PubMed] [Google Scholar]
- 60.Barker T, Martins TB, Hill HR, Kjeldsberg CR, Dixon BM, Schneider ED, et al. Circulating pro-inflammatory cytokines are elevated and peak power output correlates with 25-hydroxyvitamin D in vitamin D insufficient adults. Eur J Appl Physiol. (2013) 113:1523–34. 10.1007/s00421-012-2582-7 [DOI] [PubMed] [Google Scholar]
- 61.Ju SY, Lee YJ, Jeong SN. Serum 25-hydroxyvitamin D levels and the risk of depression: a systematic review and meta-analysis. J Nutr Health Aging. (2013) 17:447–55. 10.1007/s12603-012-0418-0 [DOI] [PubMed] [Google Scholar]
- 62.van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol. (2010) 162:805–11. 10.1530/EJE-09-0932 [DOI] [PubMed] [Google Scholar]
- 63.Milaneschi Y, Hoogendijk W, Lips P, Heijboer AC, Schoevers R, van Hemert AM, et al. The association between low vitamin D and depressive disorders. Mol Psychiatry. (2014) 19:444–51. 10.1038/mp.2013.36 [DOI] [PubMed] [Google Scholar]
- 64.Milaneschi Y, Simmons WK, van Rossum EFC, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry. (2019) 24:18–33. 10.1038/s41380-018-0017-5 [DOI] [PubMed] [Google Scholar]
- 65.Kamalzadeh L, Saghafi M, Mortazavi SS, Jolfaei AG. Vitamin D deficiency and depression in obese adults: a comparative observational study. BMC Psychiatry. (2021) 21:599. 10.1186/s12888-021-03586-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Józefowicz O, Rabe-Jabłońska J, Wozniacka A, Strzelecki D. Analysis of vitamin D status in major depression. J Psychiatr Pract. (2014) 20:329–37. 10.1097/01.pra.0000454777.21810.15 [DOI] [PubMed] [Google Scholar]
- 67.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. (2019) 380:33–44. 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alghamdi S, Alsulami N, Khoja S, Alsufiani H, Tayeb HO, Tarazi FI. Vitamin D supplementation ameliorates severity of major depressive disorder. J Mol Neurosci. (2020) 70:230–5. 10.1007/s12031-019-01461-2 [DOI] [PubMed] [Google Scholar]
- 69.Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. (2014) 28:2398–413. 10.1096/fj.13-246546 [DOI] [PubMed] [Google Scholar]
- 70.Elstgeest LEM, de Koning EJ, Brouwer IA, van Schoor NM, Penninx BWJH, Visser M. Change in serum 25-hydroxyvitamin D and parallel change in depressive symptoms in Dutch older adults. Eur J Endocrinol. (2018) 179:239–49. 10.1530/EJE-18-0187 [DOI] [PubMed] [Google Scholar]
- 71.Pludowski P, Holick MF, Grant WB, Konstantynowicz J, Mascarenhas MR, Haq A, et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. (2018) 175:125–35. 10.1016/j.jsbmb.2017.01.021 [DOI] [PubMed] [Google Scholar]
- 72.McCullough PJ, Lehrer DS, Amend J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J Steroid Biochem Mol Biol. (2019) 189:228–39. 10.1016/j.jsbmb.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 73.Shirvani A, Kalajian TA, Song A, Allen R, Charoenngam N, Lewanczuk R, et al. Variable genomic and metabolomic responses to varying doses of vitamin D supplementation. Anticancer Res. (2020) 40:535–43. 10.21873/anticanres.13982 [DOI] [PubMed] [Google Scholar]
- 74.Shirvani A, Kalajian TA, Song A, Holick MF. Disassociation of vitamin D's calcemic activity and non-calcemic genomic activity and individual responsiveness: a randomized controlled double-blind clinical trial. Sci Rep. (2019) 9:17685. 10.1038/s41598-019-53864-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Veugelers PJ, Pham TM, Ekwaru JP. Optimal vitamin D supplementation doses that minimize the risk for both low and high serum 25-hydroxyvitamin D concentrations in the general population. Nutrient. (2015) 7:10189–208. 10.3390/nu7125527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Billington EO, Burt LA, Rose MS, Davison EM, Gaudet S, Kan M, et al. Safety of high-dose vitamin d supplementation: secondary analysis of a randomized controlled trial. J Clin Endocrinol Metab. (2020) 105:1261–73. 10.1210/clinem/dgz212 [DOI] [PubMed] [Google Scholar]
- 77.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. (2003) 77:204–10. 10.1093/ajcn/77.1.204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The characteristics of studies included in this meta-analysis (the correlation between vitamin D and the incidence of depression).
The characteristics of studies included in this meta-analysis (the correlation between vitamin D and the prognosis of depression).
Quality assessment of studies included in this meta-analysis (risk of bias graph).
Quality assessment of studies included in this meta-analysis (Risk of bias summary).
Funnel plot for publication bias (the correlation between vitamin D and the incidence of depression).
Funnel plot for publication bias (the correlation between vitamin D and the prognosis of depression).