Abstract
Objective: The aim of this cross-sectional study was to evaluate the dental and periodontal health, as well as the microbiological and salivary conditions, of patients with and without diabetes mellitus (DM) who are receiving haemodialysis. Methods: One-hundred and fifty-nine haemodialysis patients were included and divided into groups according to the pre-existing diabetes status: DM or no DM. The oral examination included dental findings and assessment of the periodontal situation. The periodontal condition was classified as healthy/mild, moderate or severe periodontitis. Subgingival biofilm samples were analysed using the polymerase chain reaction. The salivary diagnostics included measurement of unstimulated and stimulated salivary flow, pH and buffer capacity. Statistical analyses used Fisher's test, the t-test and the Mann–Whitney U-test (α = 5%). Results: The dental findings showed no significant difference between patients with and without DM (P = 0.44). The prevalence of periodontitis was high (96% in patients with DM and 97% in patients who did not have DM) and there was no significant difference between the groups (P = 0.71). There was a higher prevalence of Porphyromonas gingivalis, Parvimonas micros, Eubacterium nucleatum and Capnocytophaga spp. in patients without DM (P < 0.05). The salivary pH was significantly higher in patients without DM (P < 0.01). Conclusion: While differences in the prevalence of periodontal pathogenic bacteria and in the salivary pH were detected between the groups, the dental and periodontal status was comparable between patients with and without DM. Accordingly, DM appears to have no decisive influence on the oral health in patients treated with haemodialysis who have well-controlled diabetes.
Key words: Haemodialysis, dental health, periodontitis, periodontal bacteria, saliva
Introduction
The number of patients with chronic renal failure (CRF) is increasing worldwide1. The most common reasons for CRF are diabetes mellitus (DM), primary glomerulonephritis, arterial hypertension and polycystic kidney diseases2. CRF is accompanied by an irreversible reduction in the number of functional nephrons, resulting in a decrease of the functional capacity of the kidneys3. When this capacity decreases below 5–10% of the normal efficiency, renal replacement therapy is necessary as a life-supporting measure4., 5.. In this context, three different forms of renal replacement therapies are available: haemodialysis (HD); peritoneal dialysis; and kidney transplantation. HD is the most common form of replacement therapy for CRF, improving the long-term survival of patients with end-stage renal disease6.
Compared with healthy individuals, however, HD patients have higher susceptibility to infectious complications as a result of general deficiencies and a compromised immune system7. This results in systemic changes as well as in oral complications8. It is known that patients undergoing HD show worse oral conditions compared with healthy controls9., 10.. Furthermore, the association between periodontitis and CRF in patients undergoing treatment with HD was confirmed by several other studies4., 7., 11.. Additionally, differences in the oral microbiology were detected12., 13.. Changes in the salivary flow and composition were particularly prominent, often resulting in xerostomia in addition to changes in the oral mucosa5., 14., 15., 16.. However, the fact that many patients treated with HD have DM as a major cause of HD or comorbidity was often not considered. The presence of DM might play an important role because it represents an important risk factor for the development of periodontitis17., 18.. Moreover, the literature indicates a bidirectional relationship between DM and periodontitis18. In this respect, DM leads to a two- to three-fold increase in the risk for periodontitis19. Consequently, poor periodontal health conditions in patients with DM and CRF, who are treated with HD, could be caused by both renal disease and DM. Only a few studies on oral health in patients with DM undergoing HD are available, and these focus mainly on clinical parameters16., 20., 21., 22.. However, apart from these parameters, additional factors which have an influence on oral health might be of relevance. Therefore, on one hand, the microbiological factors might be different between patients with and without DM23. On the other hand, salivary factors, such as salivary pH, might be of interest because the reduced salivary pH, which has been found in patients with DM compared with those without DM, could have an influence on dental caries and periodontitis24.
To the author's knowledge, a comprehensive investigation comparing patients with and without DM treated with HD, regarding clinical parameters, and microbiological and salivary findings, is not available in the literature; however, this subject would be of interest.
Therefore, following the previously published results of our group25, the aim of the present study was to investigate the dental and periodontal health conditions, microbiological differences and salivary parameters of patients with or without DM undergoing treatment with HD. Patients with DM undergoing treatment with HD were hypothesised to have greater periodontal involvement compared with patients without DM. Furthermore, the periodontal microflora could be influenced, and the salivary flow might be reduced to a greater extent by DM in patients treated with HD.
Methods
This multicentric clinical cross-sectional study was reviewed and approved by the Ethics Committee of the University Medical Center in Goettingen, Germany (No. 29/1/14), and the research was conducted in full accordance with the World Medical Association Declaration of Helsinki. Patients were informed verbally, as well as in writing, about the study and gave their written informed consent to participate.
Patients
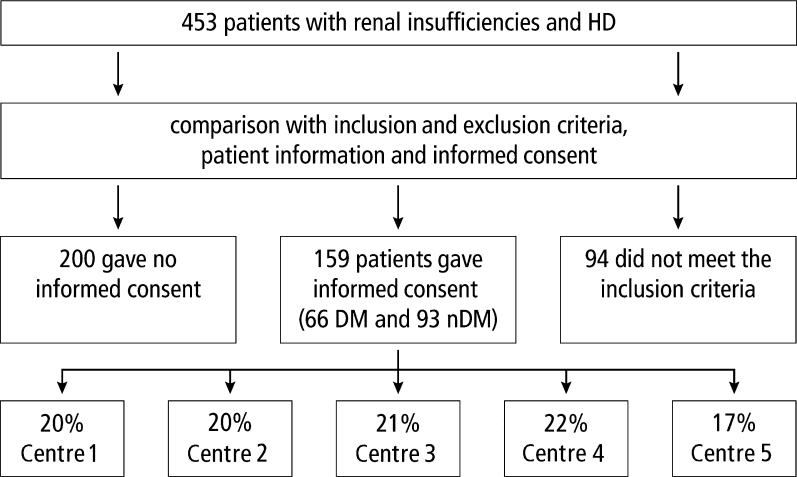
No preliminary power calculation was performed. The aim was to recruit as many patients as possible. Patients undergoing HD, including both men and women, with and without DM (type 2 diabetes), were recruited from five different facilities, as shown in Figure 1. Exclusion criteria were: organ transplantation (except for kidney); immune suppression; impossible oral examination as a result of poor overall health; addicts (drugs or alcohol); cerebral seizure disorders; infectious diseases (hepatitis A, B, C; tuberculosis; human immune-deficiency virus); pregnancy; lack of fine motor skills; other general diseases requiring medication; and inadequate German language skills.
Figure 1.
Flow chart of patient recruitment. DM, diabetes mellitus; HD, haemodialysis; nDM, no diabetes mellitus. Centre 1, Department of Nephrology and Rheumatology, University Clinic Goettingen; Centre 2, Kidney/Rheuma Center Goettingen; Centre 3, Medical Center Bad Bevensen; Centre 4, Internistic/nephrological group practice – dialysis-Uelzen; Centre 5, Dialysis and diabetes focused practice – Lueneburg.
Oral investigation
All patients received the following examinations during their dialysis therapy by two experienced and calibrated dentists (kappa > 0.8).
Dental examination
The decayed, missing and filled tooth (DMF-T) index was assessed visually using a mirror and a dental probe. The DMF-T index was determined as follows: teeth with a reasonable suspicion of or definitely showing a cavity in the dentine layer were categorised as decayed (D), teeth with fillings or crowns were categorised as filled (F) and missing teeth were categorised as missing (M). Based on the number of teeth categorised as D, F and M, the DMF-T was generated. Accordingly, the DMF-T generally reflects the caries experience of the person examined26.
Periodontal examination
The periodontal status, including the periodontal probing depth (PPD) and the presence of bleeding on probing (BOP positive), as well as the clinical attachment loss (CAL), was assessed based on six measurements per tooth made using a periodontal probe with a millimeter measurement scale (PCP 15; Hu-Friedy, Chicago, IL, USA). Periodontitis was classified into three categories according to the definition of the American Academy of Periodontology/Centers for Disease Control and Prevention (AAP/CDC) case definitions of 2007: (i) severe periodontitis; (ii) moderate periodontitis; or (iii) no/mild periodontitis27. Additionally, the papillary bleeding index (PBI) was used to classify the gingival inflammation. The PBI ranges from 0 (no bleeding/inflammation-free gingiva) to 4 (profuse bleeding/severe inflammation)28.
Microbiological analysis of periodontal pathogenic bacteria
Sulcular fluid samples from the deepest periodontal pocket were taken using sterile paper tips. Two (one each from the maxilla and the mandible) to four samples were examined for each patient. Microbiological analysis of the periodontal pathogens was carried out with the polymerase chain reaction (PCR) using a commercial test kit (Micro-IDentplus; HainLifescience, Nehren, Germany), in the clinical laboratory of the Department of Preventive Dentistry, Periodontology, and Cariology, University Medical Center Goettingen. The samples were screened for the following 11 species of periodontal pathogenic bacteria: Aggregatibacter actinomycetemcomitans (detection threshold >102), Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Prevotella intermedia, Parvimonas micra, Fusobacterium nucleatum, Campylobacter rectus, Eubacterium nodatum, Eikenella corrodens and Capnocytophaga spp. (detection threshold >103).
Salivary investigation
Salivary analysis was conducted during the HD therapy. Patients were advised to refrain from eating, drinking, chewing gum, smoking and mouth rinsing for 1 hour before saliva collection. Patients sat up straight for 5 minutes during which time saliva was collected in a calibrated vessel. The amount of unstimulated saliva collected was measured, and the pH was assessed using pH indicator strips 0–14 (Merck, Darmstadt, Germany). At this point, the result was determined according to the colour change after 5 minutes and was compared with the colour-scale template. Then, stimulated saliva was collected after 5 minutes of chewing on a paraffin pellet. The amount of stimulated saliva collected was measured, and the buffer capacity was assessed using CRT® buffer tests (Ivoclar Vivadent, Ellwangen, Germany). Here, the testing strips were evaluated after 5 minutes and were classified, according to the colour change, as high (blue), medium (green) or low (yellow) buffering capacity.
Statistical analysis
The data were analysed in ‘STATISTICA’ (Version 9.0; Statsoft, Tulsa, OK, USA) and the open source program ‘R’ (Version 3.1.0; The R Foundation for Statistical Computing, Auckland, New Zealand). For numerical data, a comparison of mean values was performed using the Student's t-test or the Mann–Whitney U-test, depending on the normal distribution of the data, respectively. The categorical variables were analysed using Fisher's exact test or the chi-square test. The significance level was α = 5%.
Results
Patients
Of the 453 potential patients, 159 (35.1%) took part in the study (Figure 1). Patients with DM were significantly older than patients without DM (P < 0.05). No significant differences regarding gender, smoking habits and underlying causual diseases was found (P > 0.05, Table 1).
Table 1.
Patients’ characteristics
| Characteristic/Parameter | Non-DM (n = 93) | DM (n = 66) | Significance level (P) |
|---|---|---|---|
| Gender (male) | 59 (63) | 43 (65) | >0.05* |
| Age (years) | 66.7 ± 13.0 | 70.5 ± 10.2 | <0.05** |
| Smoking habits | |||
| Smoker | 17 (18) | 10 (15) | >0.05*** |
| Former smoker | 4 (4) | 2 (3) | |
| Non-smoker | 72 (78) | 54 (82) | |
| Causal underlying disease | |||
| Diabetes mellitus | 0 (0) | 66 (100) | – |
| Nephrosclerosis | 13 (14) | 0 (0) | |
| Inflammatory renal disease | 28 (30) | 0 (0) | |
| Kidney transplant failure | 6 (7) | 4 (6) | |
| Other disease | 27 (29) | 0 (0) | |
| Unknown | 19 (20) | 0 (0) | |
| HbA1c value | 6.3 ± 1.2 | – | |
| Time under haemodialysis (years) | 4.4 ± 4.1 | 3.3 ± 2.7 | – |
Values are given as n (%) or mean ± standard deviation.
CRP, C-reactive protein; DM, diabetes mellitus; non-DM, no diabetes mellitus; HbA1c, glycated haemogobin; n, number of patients.*Fisher's exact test, **t-test, ***chi-square test. Significant results (P < 0.05) are highlighted in bold.
Oral investigation
Dental examination
Thirty (13 DM, 17 non-DM) patients undergoing HD were edentulous and were therefore not included in the dental and periodontal examination. The mean DMF-T showed no statistically significant difference between patients with and without DM (20.4 ± 6.0 vs. 21.2 ± 5.4, respectively; P = 0.44). No significant differences in D-T, M-T and F-T values were found between the groups (Table 2).
Table 2.
Comparison of the dental health parameters in both patient groups
| Parameter | Non-DM (n = 93) | DM (n = 66) | Significance level (P) |
|---|---|---|---|
| DMF-T, all patients | 22.3 ± 5.5 | 21.9 ± 6.1 | 0.62 |
| Edentulous patients | 17 (18) | 13 (20) | – |
| DMF-T patients with teeth | 21.2 ± 5.4 | 20.4 ± 6.0 | 0.44 |
| D-T patients with teeth | 1.4 ± 2.1 | 2.1 ± 3.0 | 0.14 |
| M-T patients with teeth | 12.8 ± 8.6 | 10.8 ± 7.8 | 0.18 |
| F-T patients with teeth | 7.0 ± 5.0 | 7.7 ± 5.5 | 0.62 |
Values are given as n (%) or mean ± standard deviation.
DM, diabetes mellitus; DMF-T, number of carious, missing, and filled teeth (caries index); D-T, carious teeth; F-T, filled teeth; M-T, missing teeth; non-DM, no diabetes mellitusP-values were determined using the Mann–Whitney U-test (significance level: P < 0.05).
Periodontal examination
The prevalence of moderate or severe periodontitis was high (96% in patients with DM; 97% in patients without DM) but there were no significant differences between the groups (P = 0.71). Further periodontal findings (PPD, CAL) were not significantly different, either overall or in the different disease categories (mild/moderate or severe periodontitis) between non-DM and DM (Table 3). Additionally, the values for BOP (non-DM: 0.10 ± 0.11; DM: 0.10 ± 0.14; P = 0.79) and PBI (non-DM: 0.36 ± 0.29; DM: 0.34 ± 0.27; P = 0.72) were not significantly different between the groups (Table 3).
Table 3.
Periodontal findings of diabetes mellitus (DM) and no DM (non-DM) groups
| Parameter | Non-DM (n = 76) | DM (n = 53) | Significance level (P) |
|---|---|---|---|
| PBI | 0.36 ± 0.29 | 0.34 ± 0.27 | 0.72 |
| PPD (mm) | |||
| Overall | 3.43 ± 1.24 (3.00) | 3.47 ± 1.25 (3.00) | 0.40 |
| Mild and moderate periodontitis | 2.86 ± 0.80 (3.00) | 2.87 ± 0.82 (3.00) | |
| Severe periodontitis | 3.82 ± 1.33 (4.00) | 3.74 ± 1.31 (4.00) | |
| CAL (mm) | |||
| Overall | 5.06 ± 2.1 (5.00) | 5.30 ± 2.14 (5.00) | 0.58 |
| Mild and moderate periodontitis | 4.14 ± 1.84 (4.00) | 4.52 ± 1.73 (4.00) | |
| Severe periodontitis | 5.69 ± 2.02 (5.00) | 5.65 ± 2.22 (5.00) | |
| BOP (%) | 0.10 ± 0.11 | 0.10 ± 0.14 | 0.79 |
| Periodontal condition | |||
| No/mild | 2 (3) | 2 (4) | 0.71 |
| Moderate | 32 (42) | 19 (36) | |
| Severe | 42 (55) | 32 (60) | |
Values are given as n (%) or mean ± standard deviation (median). CAL, clinical attachment loss; PBI, papillary bleeding index; PPD, pocket probing depth.P-values were determined using the Mann–Whitney U-test and for PBI the chi-square test was used. Significance level: P < 0.05.
Microbiological analysis
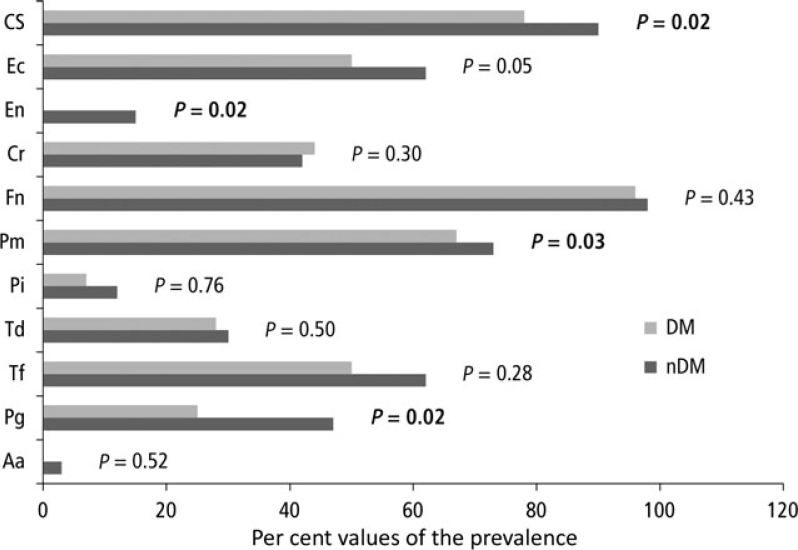
Of the 11 periodontal pathogens investigated, F. nucleatum (non-DM: 98%, DM: 96%), Capnocytophaga spp. (non-DM: 90%, DM: 78%), P. micra (non-DM: 73%, DM: 67%), and T. forsythia (non-DM: 62%, DM: 50%) were those most frequently detected. Significant differences were found in the prevalence of bacteria between patients with and without DM for P. gingivalis (non-DM: 47%, DM: 24%, P = 0.02), P. micra (non-DM: 72.5%, DM: 67.4%, P = 0.03), E. nodatum (non-DM: 62%, DM: 50%, P = 0.02) and Capnocytophaga spp. (non-DM: 89.9%, DM: 78.3%, P = 0.02) (Figure 2).
Figure 2.
Findings of the microbiological analysis. Diabetes mellitus (light bars), no diabetes mellitus (dark bars). Aa, Aggregatibacter actinomycetemcomitans; Cr, Campylobacter rectus; Cs, Capnocytophaga spp.; Ec, Eikenella corrodens; En, Eubacterium nodatum; Fn, Fusobacterium nucleatum; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Pm, Parvimonas micra; Td, Treponema denticola; Tf, Tannerella forsythia. Detection threshold >102, significant results are presented in bold type. P-values were determined using the Mann–Whitney U-test (significance level: P < 0.05).
Salivary analysis
The pH was significantly higher for the non-DM group (non-DM: 7.0 ± 0.9, DM: 6.7 ± 0.7; P < 0.01). No significant differences were found regarding the buffering capacity of the stimulated saliva (P = 1.0; Table 4).
Table 4.
Results of salivary diagnostics
| Parameter | Non-DM (n = 93) | DM (n = 66) | Significance level (P) |
|---|---|---|---|
| Unstimulated salivary flow rate (mL/minute) | 0.23 ± 0.23 | 0.16 ± 0.20 | 0.15 |
| Stimulated salivary flow rate (mL/minute) | 0.50 ± 0.40 | 0.42 ± 0.42 | 0.20 |
| pH of unstimulated saliva | 7.0 ± 0.9 | 6.7 ± 0.7 | <0.01 |
| Buffering capacity of stimulated saliva | |||
| Low | 56.2 | 41.2 | 1.0 |
| Medium | 28.1 | 27.9 | |
| High | 15.7 | 14.8 | |
Values are given as mean ± standard deviation or %.
DM, diabetes mellitus; mL/minute, milliliters per minute; non-DM, no diabetes mellitus.
P-values were determined using the Mann–Whitney U-test, and significant results (P < 0.05) are given in bold type.
Discussion
Moderate and severe periodontitis were detected in the majority of patients in both groups regardless of the diabetes status. Porphyromonas gingivalis, P. micra, E. nodatum and Capnocytophaga spp. showed a significantly higher prevalence in patients without DM. While the salivary flow was comparable in both groups, the pH was significantly higher in patients without DM.
The clinical examination showed no significant differences in the DMF-T of all patients treated with HD (including edentulous and dentulous patients) between the groups (DM: 21.9 ± 6.1, non-DM: 22.3 ± 5.5, P = 0.62). To discuss these results, the fourth German health study (DMS IV), a representative study of the German population29, can be used. In DMS IV, similar DMF-T values compared to the current study (14.5 ± 5.7 for age range 35–44 years; and 22.1 ± 5.9 for age range 65–74 years) were found29. Another publication by our group showed similar DMF-T of 22.1 ± 6.5 for patients treated with HD25. The international literature on the influence of DM on DMF-T in patients treated with HD is contradictory. While three studies showed a significantly higher DMF-T in patients with DM16., 20., 21., another study showed no difference22.
Taking into account the findings of DMS IV (moderate periodontitis: 45.3% for age range 35–44 years and 54.1% for age range 65–74 years; severe periodontitis: 46.9% for age range 35–44 years and 24.0% for age range 65–74 years), the current study found that patients undergoing HD had a higher prevalence of severe periodontitis regardless of their DM status, while moderate periodontitis was more prevalent in DMS IV29. In the international literature, the prevalence of severe periodontitis ranges from 0% to 24%, with periodontitis being more severe in patients with DM16., 20., 21., 22.. For moderate periodontitis, the prevalence ranges from 10% to 62%16., 20., 21., 22.. These results do not correspond to those of the current study, which may be because of different classification criteria and the fact that the patients in the current study had well-controlled DM [glycated haemoglobin (HbA1c): 6.3 ± 1.2%]. Furthermore, poor overall health could be a modifying factor in the current study30. Another aspect should be mentioned within the interpretation of the periodontal findings. The mean PPD was 3.43 mm in the non-DM group and 3.47 mm in the DM group. Therefore, it is possible that the classification scheme used27 might lead to overestimation of periodontal disease severity. The PPD might reflect that the current periodontal treatment need was relatively low; however, when taking into account the higher CAL values, the incidence of periodontal disease appears to be high. While the classification scheme used is validated, a periodontal case definition does not represent an overall measure of periodontal damage. The case definition chosen does not represent the clinical situation of the study participants but gives an overview of the periodontal disease burden in the two groups. In addition, a wide range in values appears to be present. However, PPD and CAL values in DM and non-DM groups were determined for the different severities of periodontal disease and compared between groups (Table 3), and no statistically significant differences were detected. Thus, neither in clinical parameters nor in case definitions was a difference in periodontal diseases found between patients with and without DM undergoing HD.
Other classification schemes are available. For example, whether the periodontal damage was localised versus generalised could be assessed31. The study protocol, however, used the classification recommended by the AAP/CDC for the analysis and interpretation of the results. Currently, updated case definitions are recommended32., 33., which were not available when the current study began.
In addition, low PBI and low BOP values indicate only mild gingival inflammation. Neither DMS IV29 nor comparable studies took these parameters into consideration16., 20., 21., 22..
The results of the microbiological analysis must be considered. Porphyromonas gingivalis, P. micra, E. nodatum and Capnocytophaga spp. showed a significantly higher prevalence in patients without DM. To the authors’ best knowledge, no studies have investigated the microbiological differences between patients with and without DM who are treated with HD. Only a few studies have examined microbiological findings in patients with renal insufficiency or undergoing treatment with HD, and they showed inconsistent results for patients treated with HD12., 30., 34..
Studies using different detection methods to compare the periodontal microflora between patients with and without DM have also produced contradictory results35., 36., 37., 38.. Most studies were not able to detect differences between the groups37., 38.. Castrillon et al. performed a PCR analysis and detected a lower concentration of red complex bacteria (P. gingivalis, T. forsythia and T. denticola) in patients with DM, which is in agreement with the findings of the current study23. The pathogenesis of periodontitis is not different between patients with and without DM; however, their host response may differ19. Thus, changes in the host response could be a reason for the different concentrations of bacteria in patients with DM36. Nevertheless, the absence of any meaningful differences in the dental and periodontal health between patients with and without DM undergoing HD makes the benefit of microbiological diagnostics questionable. Furthermore, it must be mentioned that a standardised PCR test only provides information on several selected periodontal pathogenic bacteria, whereas different test systems may show different results. Additionally, microbiological parameters should only be interpreted while taking clinical findings into consideration39.
Saliva plays an important role in oral diseases; thus, a lack of saliva increases the risk of caries and other infections40. In the salivary flow analysis, both the unstimulated and stimulated salivary flow rates were low compared with the normal values given in the literature for the stimulated flow rate (1.5–2.0 mL/minute) and the unstimulated flow rate (0.3–0.4 mL/minute)41., 42.. The reasons for a reduced salivary flow in patients treated with HD are the insufficient fluid supply and the large number of medications21., 43.. In recent studies, a significantly lower unstimulated salivary flow was found for DM patients with renal insufficiency16., 21., 44.. This is not confirmed by the current study, albeit the investigation of different patients complicates a comparison. Moreover, the mean age, which also has an influence on the salivary flow45, was considerably lower than that in the current study.
Higher pH values were recorded for patients with no DM, which is comparable with the findings of other studies20., 21.. Higher pH values, in combination with a low salivary flow, might contribute to the formation of calculus. This parameter was not assessed; however, the low PBI values suggest minimal gingival inflammation, which may suggest no increased calculus formation.
Strengths and Weaknesses
To the authors’ knowledge, this is the first study to investigate microbiological differences between patients with and without DM who are undergoing HD. Moreover, the complex assessment of the parameters adds to the uniqueness of this investigation. The time point at which the salivary diagnostic test was performed is a weakness of the study because it varied according to the time of the dialysis. While this might influence the results of the salivary diagnostic test, it could not be logistically overcome as it would have been difficult to standardise methods, used during the examination, in different dialysis centres. Also, the fact that no plaque index was assessed is a limitation of the study; however, only a short time span was available for the examination and therefore the focus was on the parameters investigated. No sample size estimation and power analysis were performed as the aim of the study was to recruit as many patients as possible. Therefore, the examination of 159 patients, is a clear advantage. The significant age difference between the groups may affect the results, especially with regard to the salivary conditions. Recruitment of a healthy control group might have strengthened the findings of the study; however, as the focus was on detection of differences between patients with and without DM undergoing HD, no healthy control group was necessary. A further problem was the large number of patients contacted who did not wish to participate in the study. Similar problems were experienced in our previous study25. This seems to be a general problem in patients undergoing HD in Germany. This clinical cross-sectional study serves to provide insights regarding differences between patients with and without DM receiving treatment with HD, but was not designed to show causal relationships. However, the findings provide a solid basis for further study.
Conclusion
A high prevalence of periodontitis was detected in patients undergoing treatment with HD, irrespective of their diabetes status. Differences in microbiological and salivary findings were detected between patients with and without DM undergoing HD. However, this seems to have no influence on the dental and periodontal status of the patients. Accordingly, diabetes status appears to have no decisive influence on the dental and periodontal health in HD patients. The poor periodontal health of patients with and without DM in the current study might be related to renal failure, to dialysis therapy, or to both.
Acknowledgements
We would like to thank the ‘Kidney/Rheuma Center’ Goettingen, the Medical Center Bad Bevensen, the ‘Internistic/nephrological group practice – dialysis’ Uelzen, and the ‘Dialysis and diabetes focused practice’ Lueneburg for supporting this investigation and providing us with the opportunity to examine their patients. Moreover, the authors would like to thank Mrs M. Hoch for her help with the microbiological analyses. The study did not receive any financial support.
References
- 1.Caskey FJ, Schober-Halstenberg HJ, Roderick PJ, et al. Exploring the differences in epidemiology of treated ESRD between Germany and England and Wales. Am J Kidney Dis. 2006;47:445–454. doi: 10.1053/j.ajkd.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Proctor R, Kumar N, Stein A, et al. Oral and dental aspects of chronic renal failure. J Dent Res. 2005;84:199–208. doi: 10.1177/154405910508400301. [DOI] [PubMed] [Google Scholar]
- 3.Snyder S, Pendergraph B. Detection and evaluation of chronic kidney disease. Am Fam Physician. 2005;72:1723–1732. [PubMed] [Google Scholar]
- 4.Bots CP, Poorterman JHG, Brand HS, et al. The oral health status of dentate patients with chronic renal failure undergoing dialysis therapy. Oral Dis. 2006;12:176–180. doi: 10.1111/j.1601-0825.2005.01183.x. [DOI] [PubMed] [Google Scholar]
- 5.Jover Cerveró A, Bagán JV, Jiménez Soriano Y, et al. Dental management in renal failure: patients on dialysis. Med Oral Patol Oral Cir Bucal. 2008;13:419–426. [PubMed] [Google Scholar]
- 6.Himmelfarb J, Ikizler TA. Hemodialysis. N Engl J Med. 2010;363:1833–1845. doi: 10.1056/NEJMra0902710. [DOI] [PubMed] [Google Scholar]
- 7.Bayraktar G, Kurtulus I, Kazancioglu R, et al. Evaluation of periodontal parameters in patients undergoing peritoneal dialysis or hemodialysis. Oral Dis. 2008;14:185–189. doi: 10.1111/j.1601-0825.2007.01372.x. [DOI] [PubMed] [Google Scholar]
- 8.Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3:71–78. doi: 10.1159/000350046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruospo M, Palmer SC, Craig JC, et al. Prevalence and severity of oral disease in adults with chronic kidney disease: a systematic review of observational studies. Nephrol Dial Transplant. 2014;29:364–375. doi: 10.1093/ndt/gft401. [DOI] [PubMed] [Google Scholar]
- 10.Palmer SC, Ruospo M, Wong G, et al. Dental health and mortality in people with end-stage kidney disease treated with hemodialysis: a multinational cohort study. Am J Kidney Dis. 2015;66:666–676. doi: 10.1053/j.ajkd.2015.04.051. [DOI] [PubMed] [Google Scholar]
- 11.Bayraktar G, Kurtulus I, Duraduryan A, et al. Dental and periodontal findings in hemodialysis patients. Oral Dis. 2007;13:393–397. doi: 10.1111/j.1601-0825.2006.01297.x. [DOI] [PubMed] [Google Scholar]
- 12.Bastos JA, Diniz CG, Bastos MG, et al. Identification of periodontal pathogens and severity of periodontitis in patients with and without chronic kidney disease. Arch Oral Biol. 2011;56:804–811. doi: 10.1016/j.archoralbio.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Castillo A, Mesa F, Liébana J, et al. Periodontal and oral microbiological status of an adult population undergoing haemodialysis: a cross-sectional study. Oral Dis. 2007;13:198–205. doi: 10.1111/j.1601-0825.2006.01267.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaushik A, Reddy SS, Umesh L, et al. Oral and salivary changes among renal patients undergoing hemodialysis: a cross-sectional study. Indian J Nephrol. 2013;23:125–129. doi: 10.4103/0971-4065.109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brotto RS, Vendramini RC, Brunetti IL, et al. Lack of correlation between periodontitis and renal dysfunction in systemically healthy patients. Eur J Dent. 2011;5:8–18. [PMC free article] [PubMed] [Google Scholar]
- 16.Swapna LA, Reddy RS, Ramesh T, et al. Oral health status in haemodialysis patients. J Clin Diagn Res. 2013;7:2047–2050. doi: 10.7860/JCDR/2013/5813.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 18.Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7:738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 19.Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br Dent J. 2014;217:433–437. doi: 10.1038/sj.bdj.2014.907. [DOI] [PubMed] [Google Scholar]
- 20.Asha V, Latha S, Pai A, et al. Oral manifestations in diabetic and nondiabetic chronic renal failure patients on hemodialysis. J Indian Acad Oral Med Radiol. 2012;24:274–279. [Google Scholar]
- 21.Chuang SF, Sung JM, Kuo SC, et al. Oral and dental manifestations in diabetic and nondiabetic uremic patients receiving hemodialysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:689–695. doi: 10.1016/j.tripleo.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 22.Murali P, Narasimhan M, Periasamy S, et al. A comparison of oral and dental manifestations in diabetic and non-diabetic uremic patients receiving hemodialysis. J Oral Maxillofac Pathol. 2012;16:374–379. doi: 10.4103/0973-029X.102490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castrillon CA, Hincapie JP, Yepes FL, et al. Occurrence of red complex microorganisms and Aggregatibacter actinomycetemcomitans in patients with diabetes. J Investig Clin Dent. 2015;6:25–31. doi: 10.1111/jicd.12051. [DOI] [PubMed] [Google Scholar]
- 24.Seethalakshmi C, Reddy RC, Asifa N, et al. Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: a cross-sectional study. J Clin Diagn Res. 2016;10:ZC12-4. doi: 10.7860/JCDR/2016/16310.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziebolz D, Fischer P, Hornecker E, et al. Oral health of hemodialysis patients: a cross-sectional study at two German dialysis centers. Hemodial Int. 2012;16:69–75. doi: 10.1111/j.1542-4758.2011.00606.x. [DOI] [PubMed] [Google Scholar]
- 26.WHO . 4th ed. WHO; Oral Health Unit; Geneva: 1997. World Health Organization: Oral Health Surveys, Basic Methods. [Google Scholar]
- 27.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 28.Lange DE, Plagmann HC, Eenboom A, et al. Clinical methods for the objective evaluation of oral hygiene. Germ Dent J. 1977;32:44–47. [in German]. [PubMed] [Google Scholar]
- 29.Micheelis W, Schiffner U. Deutscher Zahnärzte Verlag DÄV; Köln: 2006. The Fourth German Oral Health Study (DMS IV) [in German]. [Google Scholar]
- 30.Linden GJ, Lyons A, Scannapieco F. Periodontal systemic associations: review of the evidence. J Periodontol. 2013;84:8–19. doi: 10.1902/jop.2013.1340010. [DOI] [PubMed] [Google Scholar]
- 31.Tonetti MS, Claffey N, European Workshop in Periodontology group C Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. Group C consensus report of the 5th European Workshop in Periodontology. J Clin Periodontol. 2005;32(Suppl 6):210–213. doi: 10.1111/j.1600-051X.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 32.Eke PI, Page RC, Wei L, et al. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holtfreter B, Albandar JM, Dietrich T, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J Clin Periodontol. 2015;42:407–412. doi: 10.1111/jcpe.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paula H, Artese C. Effect of non-surgical periodontal treatment on the subgingival microbiota of patients with chronic kidney disease. Braz Oral Res. 2012;26:366–372. doi: 10.1590/s1806-83242012005000008. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi Y, Ishikawa H, Inada M, et al. Study of the oral microbial flora in patients with renal disease. Nephrology. 2007;12:182–190. doi: 10.1111/j.1440-1797.2007.00767.x. [DOI] [PubMed] [Google Scholar]
- 36.Ciantar M, Gilthorpe MS, Hurel SJ, et al. Capnocytophaga spp. in periodontitis patients manifesting diabetes mellitus. J Periodontol. 2005;76:194–203. doi: 10.1902/jop.2005.76.2.194. [DOI] [PubMed] [Google Scholar]
- 37.da Cruz GA, de Toledo S, Sallum EA, et al. Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus. J Periodontol. 2008;79:1150–1157. doi: 10.1902/jop.2008.070503. [DOI] [PubMed] [Google Scholar]
- 38.Field CA, Gidley MD, Preshaw PM, et al. Investigation and quantification of key periodontal pathogens in patients with type 2 diabetes. J Periodontal Res. 2012;47:470–478. doi: 10.1111/j.1600-0765.2011.01455.x. [DOI] [PubMed] [Google Scholar]
- 39.Untch M, Schlagenhauf U. Inter- and intra-test agreement of three commercially available molecular diagnostic tests for the identification of periodontal pathogens. Clin Oral Investig. 2015;19:2045–2052. doi: 10.1007/s00784-015-1418-3. [DOI] [PubMed] [Google Scholar]
- 40.Napeñas JJ, Brennan MT, Fox PC. Diagnosis and treatment of xerostomia (dry mouth) Odontology. 2009;97:76–83. doi: 10.1007/s10266-008-0099-7. [DOI] [PubMed] [Google Scholar]
- 41.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 42.Pedersen AM, Bardow A, Jensen SB, et al. Saliva and gastrointestinal functions of taste, mastication, swallowing and digestion. Oral Dis. 2002;8:117–129. doi: 10.1034/j.1601-0825.2002.02851.x. [DOI] [PubMed] [Google Scholar]
- 43.Klassen JT, Krasko BM. The dental health status of dialysis patients. J Can Dent Assoc. 2002;68:34–38. [PubMed] [Google Scholar]
- 44.Eltas A, Tozoğlu U, Keleş M, et al. Assessment of oral health in peritoneal dialysis patients with and without diabetes mellitus. Perit Dial Int. 2012;32:81–85. doi: 10.3747/pdi.2010.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Dion MR, Jurasic MM, et al. Xerostomia and salivary hypofunction in vulnerable elders: prevalence and etiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012;114:52–60. doi: 10.1016/j.oooo.2011.11.014. [DOI] [PubMed] [Google Scholar]