Abstract
Background: Although there is increasing evidence to suggest an association between periodontal disease and adverse pregnancy outcomes, the issue remains controversial. Study objective: This study tested the hypothesis that periodontal disease is a risk indicator for preterm delivery of low-birthweight infants. Materials and methods: The study sample comprised 443 pregnant women with a mean (± standard deviation) age of 24.13 (±5.30) years. At first visit, maternal oral health status was assessed by the measurement of probing pocket depth and clinical attachment loss, and periodontal status was graded as absent, mild, moderate or severe. An association was sought between pregnancy outcomes and maternal periodontal status. Results: While controlling for other factors, significant associations were found between pregnancy outcomes and maternal periodontal index scores. Conclusion: This study provides further evidence that periodontal disease is a risk indicator for adverse pregnancy outcomes.
Key words: Periodontal disease, low birthweight, preterm birth
Introduction
During pregnancy, changes in hormone levels promote an inflammatory response that increases the risk of developing gingivitis and periodontitis, two forms of periodontal disease involving the gingivae and alveolar bone, respectively1. Several risk factors for preterm birth of low-birthweight infants have been identified, including extremes of maternal age, ethnicity, low socio-economic status, nutritional status during pregnancy and infection2.
Among the factors implicated in periodontal disease, many (such as smoking, ethnicity and socio-economic conditions3) are also shared risk factors for preterm delivery, and it has been suggested that these risk factors work in synergy to induce adverse pregnancy outcomes3., 4..
Many of the suspected periodontopathogens are Gram-negative bacteria and it has been reported that that systemic exposure to derived endotoxin in women with generalised periodontal infection increases the risk for preterm birth5., 6..
It is believed that in the periodontal pocket, periodontal bacteria and their virulence factors induce a local periodontal host immune response that includes the production of inflammatory cytokines, such as interleukin-1β (IL-1β), prostaglandin E2 (PGE2), tumour necrosis factor-α (TNF-α) and antibodies against the bacteria7. If the immune response is not capable of keeping the infection localised, the bacteria and/or their virulence factors and the inflammatory cytokines may gain access systemically via the circulation, eventually reaching the placenta. In fact, about 40% of all pregnancies are associated with some fetal immunoglobulin M (IgM) response to organisms of maternal oral origin7., 8.. The increase in the production of inflammatory cytokines, such as IL-1β and PGE2, may contribute to preterm rupture of the membranes and uterine contraction, leading to miscarriage or preterm delivery7., 8..
Despite some studies that did not find an association between periodontal disease and adverse pregnancy outcomes9., 10., 11., 12., 13., 14., 15., evidence for such an association is increasing4., 16., 17., 18., 19., 20., 21., 22., 23., 24. with the prevalence reported to be between 30% and 100%25.
Aim and objectives
The aim of this study was therefore to investigate the oral health status and pregnancy outcomes of mothers attending an antenatal clinic in South Africa in order to assess periodontal disease as an independent risk indicator for preterm delivery of low-birthweight infants.
Materials and Methods
Subject selection
The study recruited 443 pregnant women during antenatal visits to maternal obstetric units in the area of Kwazulu-Natal, South Africa, over a period of 2 years. The sample size was determined according to estimates of the variance for epidemiological studies26. According to the calculation, it was estimated that 400 participants would be adequate given a 95% confidence interval and a standard deviation of 10. However, to account for attrition, a larger number of participants were enrolled, based on availability, during the first phase of the study.
Women were included in the study if they were pregnant and ≥18 years of age. The exclusion criteria were a history of medical problems that may have affected the study outcome, such as current use of systemic corticosteroids and/or antibiotics, existing heart disease, hypertension, diabetes, asthma, blood-cell disorders and chronic renal disease. Also excluded were mothers with induced labour, multiple pregnancies, smokers and those who used or abused alcohol.
Ethical considerations
The study complied with the Declaration of Helsinki27 and was ethically approved by the Research Ethics committee of the University of the Western Cape. The Provincial Department of Health and the Municipal health district managers granted permission for the research to be conducted at the various hospitals and clinics selected.
Individuals were approached and offered the opportunity to participate in the study. The nature of the study was explained to them verbally and in writing and they were requested to sign or indicate with an X that they had understood the nature of the study and voluntarily consented to participate. Their anonymity was ensured.
Data collection
An interviewer-administered questionnaire elicited demographic information about the participants and included age, race, educational level, stage of pregnancy and medical history28.
Clinical examination
Maternal oral health status was determined by a single calibrated examiner who recorded clinical indices and the presence of any oral lesions. Measurements of the clinical indices and periodontal recordings were taken at the mesial and distal locations of the Ramfjord teeth29 (specific teeth frequently used for studies of the epidemiology of periodontal disease).
Pocket probing depth (PD) was measured from the base of the pocket to the gingival margin and was scored as absent (<3 mm), mild (≥3–5 mm), moderate (>5–7 mm) and severe (>7 mm). Clinical attachment level (CAL) was measured from the cementoenamel junction to the base of the pocket and was scored as absent (<1 mm), mild (1–3 mm), moderate (>3–6 mm) and severe (>6 mm), using a North Carolina periodontal probe (Hu-Friedy®, Chicago, IL, USA).
Periodontal disease severity was graded according to the system adapted from Offenbacher et al.6, namely:
-
•
Absent: PD < 3 mm and CAL < 2 mm
-
•
Mild: PD ≥ 3 mm or CAL ≥ 2 mm
-
•
Moderate: two or more sites with PD ≥ 5 mm and two or more sites with CAL ≥ 2 mm
-
•
Severe: four or more sites with PD ≥ 5 mm and four or more sites with CAL ≥ 2 mm.
Other clinical indices included the decayed, missing and filled teeth (DMFT) index30, plaque index (PI) and gingival index (GI), both scored from 0 to 3 using the published criteria31.
Intra-examiner reliability
Intra-examiner reliability in using the dental-examination criteria was tested using the Kappa statistic. A 95% agreement on criteria for pocket depth was obtained.
Pregnancy outcomes
Post-delivery, the pregnancy outcomes were obtained from the maternal hospital folders of the participants and included gestational age, infant birthweight and method of delivery. Low birthweight was defined as birth weight < 2500 g and preterm was defined as birth <37 weeks' gestation.
Data analysis
SPSS was used to perform the statistical analyses. Statistical significance was defined as P < 0.05, using chi-square tests and analysis of variance (ANOVA). Predictive ratios were calculated using MedCalc (MedCalc Software bvba, Ostend, Belgium).
Results
Descriptive analyses
Four-hundred and forty-three mothers participated in the study. Race was described as white (<1%), Coloured (mixed race, 12.6%), Indian (5.19%) and African (81.26%), using the racial classification system to address national and regional inequities in South Africa.
All participants had some level of education, with the majority having at least secondary education (70.8%) followed by tertiary education (22.4%) and primary education (6.7%).
At the time of recruitment, the majority of mothers (81.7%) were in their second trimester of pregnancy, 14% were in their third and 4% in their first.
The mean age of the study group was 24 years (Table 1) with a range of 18–42 years. DMFT scores ranged between 0 and 20; the majority of scores were <10 and the mean was 7.18. The PI and GI scores ranged between 1 and 3 with the highest frequencies occurring between scores of 2 and 3. The mean values peaked between 3 and 5 months of pregnancy, after which they began to stabilise and eventually decrease. The mean [standard deviation (SD)] values of the age, PI, GI, DMFT and CAL of the study population are given in Table 1.
Table 1.
Maternal characteristics
| Variable | Mean (SD) |
|---|---|
| Age (years) | 24.13 (5.30) |
| Decayed, missing and filled teeth (DMFT) | 7.18 (4.22) |
| Plaque index (PI) | 2.17 (0.64) |
| Gingival index (GI) | 2.44 (0.58) |
| Clinical attachment level (CAL) mm | 1.69 (0.62) |
Using the classification system of Offenbacher et al.6, PD and CAL measurements were used to establish the presence and severity of periodontal disease and were graded as absent, mild, moderate and severe. Both PD and CAL were significantly associated with periodontal disease using chi-square analysis (Table 2). The significant associations with PD and CAL confirmed the accuracy of comparisons using only periodontal disease status in the remainder of this study.
Table 2.
Comparisons, using the chi-square test, of probing depth (PD) and clinical attachment level (CAL) classifications with periodontal disease severity
| Classification | PD | CAL | Periodontal disease severity | |||
|---|---|---|---|---|---|---|
| mm | % (n) | mm | n | Criteria | n | |
| Absent | <3 | 23.2 (103) | <1 | 39.5 (175) | PD < 3, CAL ≤ 2 | 27.8 (123) |
| Mild | ≥3–5 | 62.7 (277) | 1–3 | 52.4 (232) | PD ≥ 3 or CAL > 2; | 43.6 (193) |
| Moderate | >5–7 | 10.1 (45) | >3–6 | 7.7 (34) | ≥2 sites PD ≥ 5 and ≥2 sites CAL ≥ 2; | 18.5 (82) |
| Severe | >7 | 3.8 (17) | >6 | 0.5 (2) | ≥4 sites PD ≥ 5 and ≥4 sites CAL ≥ 2 | 10.1 (45) |
| Missing | 0.2 (1) | 0 | 0 | |||
| Total | 100 (442) | 100 (443) | 100 (443) | |||
| χ2 | 378.6 | 209.12 | ||||
| d.f. | 9 | 9 | ||||
| P | <0.0001 | <0.0001 | ||||
d.f., degrees of freedom.
Periodontal disease classification of Offenbacher et al.6
Of the 443 mothers who participated in the study, 73% had periodontal disease, which was classified as mild (44%), moderate (19%) or severe (10%). The remaining 27% showed no signs of periodontal disease. We were able to record the pregnancy outcomes of 442 mothers.
The mean and SD values for gestational age, birthweight and birthweight for gestational age are shown in Table 3.
Table 3.
Descriptive statistics for pregnancy outcomes
| Variable | n | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Gestational age (weeks) | 442 | 37.3 | 2.50 | 22 | 40 |
| Birthweight (g) | 442 | 2.99 | 0.53 | 1.5 | 4.2 |
| Birthweight for gestational age (g) | 442 | 0.08 | 0.01 | 0.4 | 0.15 |
Correlation coefficients and ANOVA revealed that DMFT was significantly associated with age (r = 0.220, P <0.001), race (F3,399 = 3.681, P = 0.012) and educational level (F2,440 = 5.591, P = 0.004), but not with pregnancy stage or any of the other clinical indices (Table 4).
Table 4.
Association of potential maternal risk indicators with pregnancy outcomes
| Variable | DMFT | PI | GI | PD | CAL | GA | BW |
|---|---|---|---|---|---|---|---|
| Age | <0.001 | NS | NS | NS | NS | NS | NS |
| Race | 0.012 | NS | NS | NS | <0.001 | NS | NS |
| Educational level | 0.004 | NS | NS | NS | NS | NS | NS |
| Pregnancy stage | NS | 0.001 | 0.008 | NS | NS | NS | NS |
| DMFT | – | NS | NS | NS | NS | NS | NS |
| PI | NS | – | NS | <0.001 | NS | NS | NS |
| GI | NS | NS | – | <0.001 | NS | NS | <0.001 |
| CAL | NS | NS | NS | <0.001 | – | 0.013 | 0.001 |
| PD | NS | <0.001 | <0.001 | – | <0.001 | <0.001 | 0.001 |
| Periodontal disease | - | NS | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
BW, birthweight; CAL, clinical attachment level; DMFT, Decayed, Missing and Filled Teeth; GA, gestational age; GI, gingival index; NS, not significant; PD, probing pocket depth; PI, plaque index.
PI and GI were significantly associated with pregnancy stage and PD (Table 4), but PI and GI scores did not differ significantly between race groups (F3,439 = 1.235, P = 0.297; F3,439 = 1.016, P = 0.05, respectively) or between the different educational levels (F2440 = 1.4, P = 0.233; F2.440 = 0.752, P = 0.472, respectively), as revealed by ANOVA.
PD showed a significant association (Table 4) with CAL [χ2 = 209.13, degrees of freedom (d.f.) = 9, P < 0.001] and periodontal disease (χ2 = 378.6, d.f. = 9, P < 0.001). No significant association was observed between PD and age (F3,439 = 0.835, P = 0.570), race (χ2 = 11.551, d.f. = 9, P = 0.239), educational level (χ2 = 5.655, d.f. = 6, P = 0.462) or pregnancy stage (χ2 = 25.892, d.f. = 21, P = 0.210).
CAL also showed a significant association with race (χ2 = 37.03, d.f. = 9, P < 0.0001) and periodontal disease (χ2 = 156.75, d.f. = 9, P < 0.0001) with no association between CAL and age (F3,439 = 0.835, P = 0.475), educational level (χ2 = 5.218, d.f. = 6, P = 0.516) or pregnancy stage (χ2 = 11.015, d.f. = 12, P = 0.528).
Pregnancy outcomes and associated risk factors
The mean gestational age at delivery decreased with an increase in CAL (data not shown) and, using ANOVA, this difference between means was found to be significant (Table 4). ANOVA demonstrated no association between gestational age (F3,438 = 1.33, P = 0.263) and race (F2,439 = 1.23, P = 0.294) or educational levels (Table 4), while a significant association was observed (Table 4) between gestational age and PD (F3,437 = 8.2, P < 0.0001), CAL (F3,438 = 3.583, P = 0.013) and periodontal disease (F3,438 = 11.54, P < 0.0001).
Most of the mothers were found to have mild or no periodontal disease. One-way ANOVA (F3,438 = 11.54, P < 0.0001) showed that mean gestational age (measured in weeks) decreased significantly with an increase in periodontal disease severity (Table 5).
Table 5.
Comparison of pregnancy outcomes with periodontal disease severity
| Periodontal disease severity | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Absent | Mild | Moderate | Severe | |||||||
| Mean GA (weeks) | 38.1 | 37.4 | 36.8 | 35.8 | ||||||
| Frequency (%) | 123 (27.8) | 193 (43.7) | 82 (18.6) | 45 (10.2) | 442 (100) | |||||
| PT | FT | PT | FT | PT | FT | PT | FT | PT | FT | |
| n | 24 | 99 | 56 | 136 | 36 | 46 | 26 | 19 | 142 | 300 |
| Row % | 16.9 | 33 | 39.4 | 45.3 | 25.4 | 15.3 | 18.3 | 6.3 | 100 | 100 |
| Column % | 19.5 | 80.5 | 29.2 | 70.8 | 43.9 | 56.1 | 57.8 | 42.2 | 32,1 | 67.9 |
| Significance | χ2= 28.54, d.f. = 3, P <0.0001 | |||||||||
| Mean BW (g) | 3.28 | 3.04 | 2.70 | 2.41 | ||||||
| Frequency (%) | 123 (27.8) | 193 (43.6) | 82 (18.5) | 45 (10.2) | 443(100) | |||||
| LW | NW | LW | NW | LW | NW | LW | NW | LW | NW | |
| n | 4 | 119 | 33 | 160 | 39 | 43 | 34 | 11 | 110 | 333 |
| Row % | 3.6 | 35.7 | 30 | 48 | 35.5 | 12.9 | 30.9 | 3.3 | 100 | 100 |
| Column % | 2.7 | 96.7 | 17 | 82.9 | 47.5 | 52.4 | 75.5 | 24.4 | 24.8 | 75.2 |
| Significance | χ2= 121.5, d.f. = 3, P <0.00001 | |||||||||
| Mean BWGA (g) | 0.086 | 0.079 | 0.072 | 0.068 | ||||||
| Frequency (%) | 123 (27.8) | 192 (43.4) | 82 (18.6) | 45 (10.2) | 442 (100) | |||||
| PT LW | FT NW | PT LW | FT NW | PT LW | FT NW | PT LW | FT NW | PT LW | FT NW | |
| n | 4 | 119 | 25 | 167 | 20 | 62 | 22 | 23 | 71 | 371 |
| Row % | 5.6 | 32.1 | 35.2 | 45 | 28.2 | 16.7 | 31 | 6.2 | 100 | 100 |
| Column % | 3.2 | 96.7 | 13 | 86.9 | 24.4 | 75.6 | 49 | 51 | 16.1 | 83.9 |
| Significance | χ2 = 56.470, d.f. = 3, P <0.00001 | |||||||||
BWGA, birthweight for gestational age; d.f., degrees of freedom; FT, full term; GA, gestational age; LW, low birthweight; NW, normal birthweight; PT, preterm.
One-hundred and forty-two (32%) mothers delivered preterm (i.e. <37 weeks' gestation) and 300 (68%) delivered full term (i.e. ≥37 weeks' gestation) (Table 5). No correlation was observed between maternal age and gestational age (r = 0.0016, P = 0.739).
Using chi-square comparisons, the preterm and full-term groups differed significantly, with 26 (58%) of the 45 mothers with severe periodontal disease delivering preterm and 99 (80.5%) of the 123 with no periodontal disease delivering full term (Table 5). Only 16.9% of mothers with no periodontal disease delivered preterm (Table 5).
Chi-square comparisons of preterm and full-term groups revealed significant differences between the two groups for periodontal disease (Table 5).
Infant birthweight
No significant correlation was observed between birthweight and maternal age (r = 0.0008, P = 0.872), although infant birthweight showed a strong correlation with PI (r = 0.217, P < 0.001) and GI (r = 0.257, P < 0.001). ANOVA showed no correlation between infant birthweight and race (F3439 = 0.146, P = 0.932) or education (F2,440 = 0.697, P = 0.499).
Chi-square comparison of low birthweight and normal birthweight showed a significant correlation between infant birthweight and maternal periodontal disease (Table 5). As periodontal disease increased from absent to severe, the mean birthweight of the infant decreased (Table 5). Only 2.7% of mothers with no periodontal disease delivered low-birthweight infants and 96.7% delivered infants with normal birthweight. Mothers with severe periodontal disease delivered low-birthweight infants in 75.5% of cases and normal-birthweight infants in 24.4% of cases (Table 5). Normal birthweight occurred predominantly when mothers had no or mild periodontal disease, with low birthweight and normal birthweight in almost similar proportions in mothers with moderate periodontal disease, and decreased infant birthweight observed in mothers with severe periodontal disease (Table 5).
Birthweight for gestational age
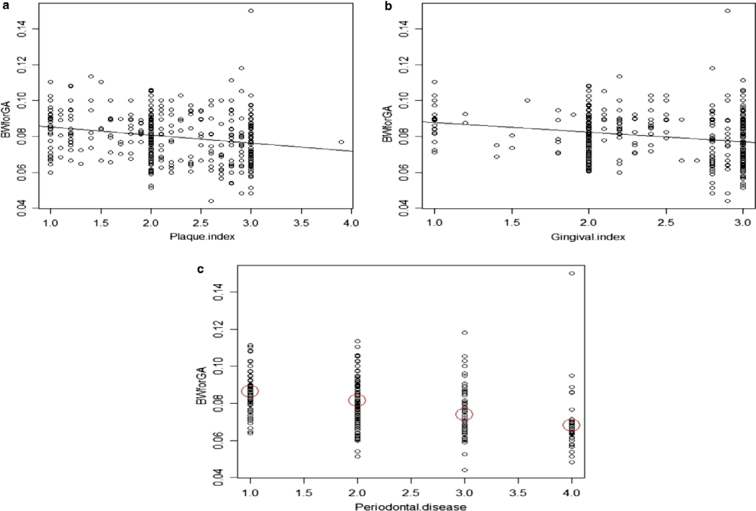
Birthweight for gestational age decreased significantly for increased PI (r = 0.220, P < 0.001), GI (r = 0.230, P < 0.001) and periodontal disease severity (F3,438 = 5.023, P = 0.002), as demonstrated in Figure 1 (a–c, respectively).
Figure 1.
Association of plaque index (a), gingival index (b) and periodontal disease (c) with birthweight (BW) for gestational age (GA).
Interpretation of likelihood ratios
Predictive values for periodontal disease and adverse pregnancy outcomes revealed that mothers with periodontal disease were likely to deliver preterm, low-birthweight and preterm low-birthweight infants with sensitivity values of 83.1%, 96.4% and 94.3% and likelihood ratios of 1.24, 1.50 and 1.39, respectively (Table 6). The probability that pregnancy outcomes of a mother with no periodontal disease would be normal, was demonstrated by negative predictive values of 80.5% for preterm, 96.8% for low birthweight and 96.5% for preterm low-birthweight infants (Table 6).
Table 6.
Predictive ratios for periodontal disease and adverse pregnancy outcomes
| Preterm | Low birthweight | Preterm low birthweight | |
|---|---|---|---|
| Sensitivity (%) | 83.1 (75.9–88.8) | 96.4 (90.9–99) | 94.3 (86.2–98.4) |
| Specificity (%) | 33 (27.7–38.6) | 35.7 (30.5–41.4) | 32.08 (27.3–37.1) |
| LR+ | 1.24 (1.11–1.38) | 1.50 (1.37–1.64) | 1.39 (1.27–1.52) |
| LR− | 0.51 (0.34–0.76) | 0.10 (0.04–0.27) | 0.18 (0.07–0.46) |
| PPV (%) | 36.9 (31.6–42.5) | 33.1 (27.9–38.5) | 21 (16.6–25.8) |
| NPV (%) | 80.5 (72.3–87) | 96.8 (91.8–99.1) | 96.5 (91.8–99.1) |
LR+, positive likelihood ratio; LR−, negative likelihood ratio; NPV, negative predictive value; PPV, positive predictive value.
Discussion
Studies on neonatal outcomes of preterm low-birthweight infants in South Africa revealed a relatively high mortality rate32., 33. but did not elaborate on the possible causes of these pregnancy outcomes.
Periodontal pathogens and their virulence factors have the ability to disseminate and induce both local and systemic inflammatory responses in the host. This has led to the hypothesis that periodontal disease may have effects beyond the periodontal tissues themselves34. The present study tested this hypothesis.
The average age of the study population was 24.13 (±5.30) years, falling within the 20- to 29-year age group, which represents a substantial portion of the most economically active individuals of childbearing age35., 36..
Of the 443 women recruited to this study, we managed to report on the pregnancy outcomes of 442. There are several possibilities which may explain why the records of the missing mother were not available, but all are based on speculation. It may be that she moved to a different area and her records were no longer filed in the clinic where she was recruited to the study; she may have given birth at home or in another hospital; or her records may simply have been misplaced or lost between clinics/hospitals. However, a follow up of 99.7%, the context in this study, is an outstanding achievement, considering the high rate of attrition normally reported in studies of this nature.
In South Africa, the black racial group constitutes the majority of the public health facility users35, thus explaining their increased frequency (81.26%) compared with the other race groups in this study, namely Coloured (mixed race, 12.64%), Asian (5.19%) and White (<1%). Despite other changes which may have occurred in South Africa, a vast proportion of the population still live in poverty, which predisposes them to infection and malnutrition, both of which have been recognised as risk factors for preterm delivery of low-birthweight infants. Race is considered to have a significant bearing on educational levels and socio-economic standards, which, in turn, impacts on access to adequate medical and dental care. In contrast to an earlier study37, no significant association was observed in this study between plaque index and age, race or education. The findings reported here are in agreement with previous reports31., 38.. No significant association was found between the different race groups and GI, PD measurements, education levels and pregnancy stage, although a significant difference was demonstrated between the different race groups and CAL. It is not clear whether this last finding was indeed the result of ethnic factors, as reported in other studies39., 40., or whether the notable difference in sample size between the African (n = 360), Coloured (mixed race, n = 56), Indian (n = 23) and White (n = 4) race groups may have influenced this outcome.
The mean gestational age at delivery showed a significant decrease with an increase in PD severity and an increase in CAL. Likewise, birthweight was found to decrease with increasing PD and CAL, and when birthweight was divided into low birthweight (<2500 g) and normal birthweight (≥2500 g), the distributions of PD and CAL at low birthweight and normal birthweight differed significantly (P < 0.0001) in both instances. This is in agreement with earlier studies, which associated PD and CAL with low birthweight19., 41.. Although we concede that low birthweight may not always be attributed to periodontal disease, in this study increased maternal periodontal-disease severity was correlated with a decrease in the number of infants delivered with normal birthweight, while the number of low-birthweight infants increased significantly in the presence of maternal periodontal disease, in accordance with the results of other studies4., 16., 17., 42..
The discrepancy between arguments for and against the association of periodontal disease with preterm and low-birthweight infant delivery may be attributed to differences in periodontal disease, preterm and low-birthweight definitions, as well as sampling techniques, diagnostic criteria, pregnancy stage at recruitment, ethnicity and geographical location43. Preterm is sometimes subclassified as moderate to late preterm (32 to <37 weeks), very preterm (28 to <32 weeks) or extremely preterm (<28 weeks), according to the definitions proposed by the World Health Organization44, and infant birthweight is sometimes reported as very-low birthweight (<1500 g) and extremely low birthweight (<1000 g)45. This complicates accurate comparison of results and development of finite conclusions.
Although many definitions of periodontitis have been used in the literature for population-based studies, there is no accepted standard46. Some researchers use the broad classification of periodontal disease, while others differentiate between gingivitis and periodontitis, along with their subclassifications, such as pregnancy-associated periodontal disease, etc.43., 47..
Generally, the clinical diagnosis of periodontitis is based largely on measurements of PD and CAL, the radiographic pattern and extent of alveolar bone loss, gingival inflammation (measured as bleeding on probing) or a combination of these measures46. In 2003, the Center for Disease Control and Prevention and the American Academy of Periodontology appointed a working group to develop further standardised clinical case definitions for population-based studies of periodontitis. This classification defines severe periodontitis and moderate periodontitis, in terms of PD and CAL, to improve case definitions46.
This study elected to employ the use of PD and CAL of the Ramfjord teeth to assess periodontal status. Ramfjord teeth measurements are frequently used in epidemiology studies and are reported to present as valid and reliable an assessment of periodontal status as full-mouth assessments48., 49.. Other authors have also reported on the convenience of partial-mouth protocols, considering them to be cost effective as well as timesaving, while assessing CAL and PD with the same reliability as full-mouth assessments50., 51.. However, there is data suggesting that partial-mouth examination may, in some instances, have a tendency to underestimate disease prevalence52. Furthermore, the categorisation of periodontal disease into absent, mild, moderate and severe, according to the criteria of Offenbacher et al.6, afforded a reasonable assessment of the oral health status of the mothers in this study, with pregnancy outcomes compared with periodontal disease status. Individual comparisons with PD and CAL were not deemed necessary, having previously established significant associations between these two indices and the classification of periodontal disease used in this study.
Annually, an estimated 13 million infants are born preterm, worldwide, with >15% occurring in developing countries and nine of 11 countries reported to be in sub-Saharan Africa53. An annual mortality rate of >1 million preterm neonates has been reported, with preterm accounting for 11.1% of live births globally and 60% of these occurring in South Asia and sub-Saharan Africa53. Although we achieved our objectives, a limitation of this study is that we did not follow up on the preterm infants to investigate morbidity or mortality.
Few countries have reliable national preterm birth prevalence data and there is a paucity of studies investigating periodontal disease as a risk indicator in the preterm delivery of low-birthweight infants in South Africa. In the present study, positive likelihood ratios demonstrated a strong probability of adverse pregnancy outcomes occurring when mothers were found to have periodontal disease and equally strong negative predictive values for normal pregnancy outcomes in the absence of periodontal disease. Thus, we can conclude that the outcomes of this study support an association between periodontal disease and adverse pregnancy outcomes.
Acknowledgements
This material is based upon work financially supported by the National Research Foundation (NRF) of South Africa. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors, and therefore the NRF does not accept any liability in regard thereto. We gratefully acknowledge those who consented to participate in the study.
Conflicts of interest
The authors declare no conflict of interest.
References
- 1.Jared H, Boggess KA. Periodontal diseases and adverse pregnancy outcomes: a review of the evidence and implications for clinical practice. J Dent Hygiene. 2008;82:3–21. [Google Scholar]
- 2.MacDorman MF, Callaghan WM. Trends in preterm-related infant mortality by race and ethnicity, United States, 1999-2004. J Health Serv. 2007;37:635–641. doi: 10.2190/HS.37.4.c. [DOI] [PubMed] [Google Scholar]
- 3.Lopez NJ, Da Silva I, Ipinza J, et al. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy-associated periodontitis. J Periodontol. 2005;76(11 Suppl):2144–2153. doi: 10.1902/jop.2005.76.11-S.2144. [DOI] [PubMed] [Google Scholar]
- 4.Jeffcoat MK, Geurs NC, Reddy MS, et al. Periodontal infection and preterm birth: results of a prospective study. JADA. 2001;132:875–880. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- 5.Dasanayake AP, Boyd D, Madianos PN, et al. The association between Porphyromonas gingivalis-specific maternal serum IgG and low birth weight. J Periodontol. 2001;72:1491–1497. doi: 10.1902/jop.2001.72.11.1491. [DOI] [PubMed] [Google Scholar]
- 6.Offenbacher S, Lieff S, Boggess KA, et al. Maternal periodontitis and prematurity. Part I: obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001;6:164–174. doi: 10.1902/annals.2001.6.1.164. [DOI] [PubMed] [Google Scholar]
- 7.Offenbacher S, Beck JD, Lieff S, et al. Role of periodontitis in systemic health: spontaneous preterm birth. J Dent Educ. 1998;62:852–858. [PubMed] [Google Scholar]
- 8.Li X, Kolltveit KM, Tronstad L, et al. Systemic disease caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davenport ES, Williams CE, Sterne JA, et al. Maternal periodontal disease and preterm low birth weight: case-control study. J Dent Res. 2002;81:313–318. doi: 10.1177/154405910208100505. [DOI] [PubMed] [Google Scholar]
- 10.Holbrook WP, Oskarsdottir A, Fridjonsson T, et al. No link between low-grade periodontal disease and preterm birth: a pilot study in a healthy Caucasian population. Acta Odontol Scand. 2004;62:177–179. doi: 10.1080/00016350410001522. [DOI] [PubMed] [Google Scholar]
- 11.Moore S, Randhawa M, Ide M. A case-control study to investigate an association between adverse pregnancy outcome and periodontal disease. J Clin Periodontol. 2005;32:1–5. doi: 10.1111/j.1600-051X.2004.00598.x. [DOI] [PubMed] [Google Scholar]
- 12.Rajapakse PS, Nagarathne M, Chandrasekra KB, et al. Periodontal disease and prematurity among non-smoking Sri Lankan women. J Dent Res. 2005;84:274–277. doi: 10.1177/154405910508400313. [DOI] [PubMed] [Google Scholar]
- 13.Hujoel PP, Lydon-Rochelle M, Robertson PB, et al. Cessation of periodontal care during pregnancy: effect on infant birthweight. Eur J Oral Sci. 2006;114:2–7. doi: 10.1111/j.1600-0722.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 14.Michalowicz BS, Hodges JS, DiAngelis AJ, et al. OPT study: treatment of periodontal disease and the risk of preterm birth. N Engl J Med. 2006;355:1885–1894. doi: 10.1056/NEJMoa062249. [DOI] [PubMed] [Google Scholar]
- 15.Abati S, Villa A, Cetin I, et al. Lack of association between maternal periodontal status and adverse pregnancy outcomes: a multicentric epidemiologic study. J Matern Fetal Neonatal Med. 2013;26:369–372. doi: 10.3109/14767058.2012.733776. [DOI] [PubMed] [Google Scholar]
- 16.Romero BC, Chiquito CS, Elejalde LE, et al. Relationship between periodontal disease in pregnant women and the nutritional condition of their newborns. J Periodontol. 2002;73:1177–1183. doi: 10.1902/jop.2002.73.10.1177. [DOI] [PubMed] [Google Scholar]
- 17.Goepfert AR, Jeffcoat MK, Andrews WW, et al. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol. 2004;104:777–783. doi: 10.1097/01.AOG.0000139836.47777.6d. [DOI] [PubMed] [Google Scholar]
- 18.Mokeem SA, Molla GN, Al-Jewair TS. The prevalence and relationship between periodontal disease and pre-term low birth weight infants at King Khalid University Hospital in Riyadh, Saudi Arabia. J Contemp Dent Pract. 2004;5:40–56. [PubMed] [Google Scholar]
- 19.Jarjoura K, Devine PC, Perez-Delboy A, et al. Markers of periodontal infection and preterm birth. Am J Obstet Gynecol. 2005;192:513–519. doi: 10.1016/j.ajog.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Offenbacher S, Lin D, Strauss R, et al. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot study. J Periodontol. 2006;77:2011–2024. doi: 10.1902/jop.2006.060047. [DOI] [PubMed] [Google Scholar]
- 21.Lin D, Moss K, Beck JD, et al. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol. 2007;78:833–841. doi: 10.1902/jop.2007.060201. [DOI] [PubMed] [Google Scholar]
- 22.Katz J, Chegini N, Shiverick K, et al. Localization of P. gingivalis in preterm delivery placenta. J Dent Res. 2009;88:575–578. doi: 10.1177/0022034509338032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persson R, Hitti J, Verhelst R, et al. The vaginal microflora in relation to gingivitis. BMC Infect Dis. 2009;22:6. doi: 10.1186/1471-2334-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shanti V, Vanka A, Bhambal A, et al. Association of pregnant women periodontal status to preterm and low-birth weight babies: a systematic and evidence-based review. Dent Res J (Isfahan) 2012;9:368–380. [PMC free article] [PubMed] [Google Scholar]
- 25.Laine MA. Effect of pregnancy on periodontal and dental health. Acta Odontol Scand. 2002;60:257–264. doi: 10.1080/00016350260248210. [DOI] [PubMed] [Google Scholar]
- 26.Hajian-Tilaki K. Sample size estimation in epidemiologic studies. Caspian J Intern Med. 2011;2:289–298. [PMC free article] [PubMed] [Google Scholar]
- 27.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 28.Marshall C, Rossman G. 2nd ed. SAGE publications; London: 1995. Designing Qualitative research. [Google Scholar]
- 29.Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30:51–59. [Google Scholar]
- 30.World Health Organization . 4th ed. World Health Organization; Geneva: 1997. Oral Health Surveys Basic Methods. [Google Scholar]
- 31.Silness J, Loe H. Periodontal disease in pregnancy. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 32.Cooper PA, Sandler DL. Outcome of very low birth weight infants at 12 to 18 months of age in Soweto, South Africa. Pediatrics. Pediatrics. 1997;99:537–544. doi: 10.1542/peds.99.4.537. [DOI] [PubMed] [Google Scholar]
- 33.Ballot DE, Potterton J, Chirwa T, et al. Developmental outcome of very low birth weight infants in a developing country. BMC Paediatr. 2012;12:11. doi: 10.1186/1471-2431-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465–1482. doi: 10.1902/jop.2006.060010. [DOI] [PubMed] [Google Scholar]
- 35.Dorrington R, Moultrie TA, Timæus IM. Estimation of mortality using the South African census 2001 data. S0020-6539(20)31702-0: 0-7992-2281-X, 2004.
- 36.Bachman MO, Booysen FLR. Health and economic impact of HIV/AIDS on South African households: a cohort study. BMC Public Health. 2003;3:14. doi: 10.1186/1471-2458-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilakarantne A, Soory M, Ranasinghe AW, et al. Periodontal disease status during pregnancy and 3 months postpartum, in a rural population of Sri-Lankan women. J Clin Periodontol. 2000;27:787–792. doi: 10.1034/j.1600-051x.2000.027010787.x. [DOI] [PubMed] [Google Scholar]
- 38.Taani DQ, Habashneh R, Hammad MM, et al. The periodontal status of pregnant women and its relationship with socio-demographic and clinical variables. J Oral Rehabil. 2003;30:440–445. doi: 10.1046/j.1365-2842.2003.01058.x. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki H, Yamashita Y, Goto-Kimura K, et al. Periodontal condition of pregnant women assessed by CPITN. J Clin Periodontol. 1991;18:751–754. doi: 10.1111/j.1600-051x.1991.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 40.Williams CE, Davenport ES, Sterne JA, et al. Mechanisms of risk in preterm low-birthweight infants. Periodontol 2000. 2000;23:142–150. doi: 10.1034/j.1600-0757.2000.2230115.x. [DOI] [PubMed] [Google Scholar]
- 41.Dortbudak O, Eberhardt R, Ulm M, et al. Periodontitis: a marker of risk in pregnancy for preterm birth. J Clin Periodontol. 2005;32:45–52. doi: 10.1111/j.1600-051X.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 42.Manau C, Echeverria A, Agueda A, et al. Periodontal disease definition may determine the association between periodontitis and pregnancy outcomes. J Clin Periodontol. 2008;35:385–397. doi: 10.1111/j.1600-051X.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organisation. Preterm birth. Fact Sheet No 363, 2014.
- 44.Subramanian KN, Rosenkrantz T. Extremely low birth weight infant. Medscape 2014. Available: http://emedicine.medscape.com/article/979717-overview.
- 45.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 46.Agueda A, Echeverria A, Manau C. Association between periodontitis in pregnancy and preterm or low birth weight: review of the literature. Med Oral Pathol Oral Cir Bucal. 2008;13:E609–E615. [PubMed] [Google Scholar]
- 47.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Mumghamba EG, Pitiphat W, Matee MI, et al. The usefulness of using Ramfjord teeth in predicting periodontal status of a Tanzanian adult population. J Clin Periodontol. 2004;31:16–18. doi: 10.1111/j.0303-6979.2004.00430.x. [DOI] [PubMed] [Google Scholar]
- 49.Najah A, Seham S, Fadhil R. The usefulness of Ramfjord teeth to represent the full-mouth pocket depth in epidemiological study. MDJ. 2010;7:272–275. [Google Scholar]
- 50.Dowsett SA, Eckert G, Kowolik MJ. The applicability of half-mouth examination to periodontal disease assessment in untreated adult populations. J Periodontol. 2002;73:975–981. doi: 10.1902/jop.2002.73.9.975. [DOI] [PubMed] [Google Scholar]
- 51.Owens JD, Dowsett SA, Eckert G, et al. Partial mouth assessment of periodontal disease in an adult population of the United States. J Periodontol. 2003;74:1206–1213. doi: 10.1902/jop.2003.74.8.1206. [DOI] [PubMed] [Google Scholar]
- 52.Eaton KA, Duffy S, Griffiths GS, et al. The influence of partial and full-mouth recordings on estimates of prevalence and extent of lifetime cumulative attachment loss: a study in a population of young male military recruits. J Periodontol. 2001;72:140–145. doi: 10.1902/jop.2001.72.2.140. [DOI] [PubMed] [Google Scholar]
- 53.Lawn JE, Gravett MG, Nunes TM, et al. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth. 2010;10(Suppl 1):S1. doi: 10.1186/1471-2393-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]