Abstract
Aim: This study aimed: (i) to identify and compare the prevalence of temporomandibular disorders (TMDs) and oral parafunctions among children living in child-protection institutions (CLCPI) with children living with their parents (CLWP); (ii) to determine whether or not there is an association between oral parafunctions and TMDs; and (iii) to examine the possible impact of stress on TMDs. Study design: The study was conducted on a total of 385 children who were divided into two groups: the CLCPI group (n = 184); and the CLWP group (control, n = 201). All children 8–18 years of age and living in protective-care facilities in Kocaeli, Turkey, were included in this study in the CLCPI group. The CLWP control group comprised children of the same age as those in the CLCPI group, but those in the CLWP group were living with their families and were randomly selected from one primary school, one elementary school and one high school in Kocaeli, Turkey. Each child in the study completed a questionnaire and underwent a clinical examination. Results: The overall prevalence of TMDs and oral parafunctions were higher in the CLCPI group than in the CLWP group (P < 0.05). The vast majority of participants reported at least one parafunction (CLCPI, n = 97.3%; CLWP, n = 93%). Problems related to family or friends were higher in the CLCPI group, whereas problems related to school lessons were higher in the CLWP group (P < 0.05). In both groups, positive associations were found between signs and symptoms of TMDs, oral parafunctions and stressful life events. Conclusion: The prevalence of signs and symptoms of TMDs and oral parafunctions differed significantly between CLCPI and CLWP groups, with children of the CLCPI group found to be significantly more prone to TMDs and oral parafunctions than children of the CLWP group.
Key words: Temporomandibular disorder, oral parafunctions, institutionalised children, orophanages
Introduction
Temporomandibular disorder (TMD) is defined as a functional disturbance of the masticatory system1., 2., 3.. The most prevalent clinical signs of TMD are temporomandibular joint (TMJ) sounds, limitation of mandibular movement, and TMJ and muscle tenderness. Symptoms include TMJ sounds, difficulty in mouth opening, jaw pain and facial pain4., 5..
While TMD generally affects adults, epidemiological studies have reported signs and symptoms of TMD in 6–68% of children and adolescents6., 7., 8.. Given these prevalence rates, additional research is needed to evaluate TMD in younger individuals, since early diagnosis may prevent the disease from progressing to a stage where it causes irreversible destruction of the intra-capsular structures of the TMJ and affect normal craniofacial growth9.
Multiple aetiological factors have been reported for TMD2. Although the majority of studies have shown poor correlations between any single aetiological factor and signs and symptoms of the disease10, associations have been reported between TMD and oral parafunctions, such as bruxism, thumb sucking, fingernail biting, tooth grinding, jaw clenching and lip/cheek biting, and between TMD and negative somatic and psychological factors and trauma to the mandible or TMJs11., 12., 13., 14., 15.. TMDs are also associated with myofunctional alterations, which usually cause compensatory muscle behaviours15.
The prevalence of TMD and oral parafunctions in the population of children living in protective care institutions has not been previously reported. However, studies have indicated that caries and oral diseases, as well as use of professional dental services, are worse among children living in protective-care facilities than among children living with their parents16., 17.. Moreover, some studies have reported institutionalised children to be a population at risk for abnormal psychological development because of constraining elements in their social environment, such as parental inadequacy, environmental deprivation and emotional disturbances18., 19., 20.. Therefore, the aim of the present study was: (i) to identify and compare the prevalence of the signs and symptoms of TMD and oral parafunctions in children living in child-protection institutions (CLCPI) with children living with their parents (CLWP); (ii) to determine whether oral parafunctions in either group of children are associated with signs and symptoms of TMD; and (iii) to examine the possible impact of stressful life events in both groups on the prevalence of oral parafunctions and signs and symptoms of TMD.
Material and method
The protocol for this cross-sectional study was approved by the Ethics Committee of Kocaeli University (KOU KAEK#92/2015) and the study was conducted in full accordance with the World Medical Association Declaration of Helsinki. Permission to conduct the study was obtained from public-school authorities and child-protection institutions run by the Government's Social Services and Child Protection Agency. The families of children attending public schools and the nursing staff of the government institutions were informed about the aim of the study, and all gave their written consent. Also, written consent was obtained from children in both CLWP and CLCPI groups. All children 8–18 years of age with permanent residency in the child-protection institutions in the province of Kocaeli were included in the study. Before the establishment of a control group, specific information, including the age, gender and number of children in the CLCPI group, was obtained. The control group comprised children of middle-class socio-economic status (SES) who were selected from one primary school, one elementary school and one high school in Kocaeli, based on the age, gender and number of children in the CLCPI group. Children were randomly selected from the class list using a computer-generated list. The age and gender distribution of the groups were as similar as possible.
Children who had difficulty understanding the questions, as well as children with congenital anomalies, a history of juvenile rheumatoid arthritis, psoriatic arthritis, muscle disease, toothache or upper respiratory infection, and children who were taking medication or who had received orthodontic treatment, were excluded from the study. In total, 385 children were included (CLCPI, n = 184; CLWP, n = 201).
Data associated with TMD were collected using both a questionnaire and clinical examination.
Questionnaire
The questionnaire used in this study was adapted from similar questionnaires used to examine TMD in children and adolescents6., 11. and was implemented through interviews. Before conducting the survey, the questionnaire was administered to 40 children who were not included in the study and their responses were evaluated for clarity and comprehensibility. Data were collected on the following topics:
-
•TMD symptoms (occurring at least once a week for the past 3 months)
-
○Pain or tiredness in facial muscles while chewing, talking or otherwise using the jaw
-
○Joint sticking, defined as a sudden, momentary and self-releasing locking of the jaw that prevents full opening, or a sense that the jaw is stuck and cannot be released with ease
-
○Joint noises (clicking, popping or grating) during jaw movement
-
○
-
•Oral parafunctions (occurring daily)
-
○Biting down on hard objects (pen, pencil, etc.)
-
○Crushing hard candies, ice, popsicles, etc. with the teeth
-
○Fingernail biting
-
○Taking apart toys/games with the teeth
-
○Opening bottles with the teeth
-
○Gum chewing
-
○Jaw-play (involuntary small mandibular movements without tooth contact)
-
○Bruxism (diurnal tooth grinding/clenching)
-
○
-
•Stressful life events
-
○Problems related to family
-
○Problems related to school
-
○Problems related to friends.
-
○
Clinical examination
Clinical examinations were performed by a single examiner who was blinded to the questionnaire findings. Children were examined whilst seated in an upright position in a regular chair. Calibration was performed previously as part of a pilot study in which 40 children were clinically examined and 15 were then re-examined, 1 day later, to calculate intra-examiner reliability. Cohen's kappa coefficient (κ = 0.86) showed excellent reliability. (Children in the pilot study were not included in the main study.)
Clinical examination for signs of TMD included the following:
-
•
Joint sounds. ‘Clicking’ (i.e. a single sound with a short duration) and ‘crepitation’ (i.e. multiple, grating sounds) that were clearly audible or could be felt by placing the fingertips over the lateral surface of the joint during mouth opening and closing21
-
•
Joint tenderness. TMJ tenderness was identified by bilateral palpation of the joints, with fingertips simultaneously placed over the lateral poles of the condyles21
-
•
Masticatory muscle tenderness. Tenderness was identified by digital palpation of the temporalis, masseter, sternocleidomastoid and posterior cervical muscles21
-
•
Mouth opening. Maximum vertical opening was recorded as the distance between the incisal edge of the maxillary and mandibular incisors plus any vertical overlap19. A distance of less than 30 mm was considered to represent restricted opening6., 22..
Statistical analysis
Statistical analysis was performed using a commercially available software program (SPSS 20.00; SPSS, Chicago, IL, USA). Differences in TMD signs and symptoms and oral parafunctions between groups were evaluated using the chi-square test. Differences between genders were analysed using chi-square and Fisher's exact tests. Associations between TMD signs and symptoms, oral parafunctions and stress were evaluated using chi-square, Spearman's correlation and Mann–Whitney U-tests. In all cases, the level of significance was set at P < 0.05.
Results
This cross-sectional study examined the prevalence of and correlations among TMD signs and symptoms, oral parafunctions and stress indicators in 385 children divided into two groups according to residency status (CLCPI, n = 184; CLWP, n = 201). The distribution of participants according to age, gender and dentition status is shown in Table 1.
Table 1.
Sample characteristics of the study (n = 385)
| Variable | Children living in child protection institutions | Children living with their parents |
|---|---|---|
| Gender (boys/girls) | 104/80 (56.5/43.5) | 87/114 (43.3/56.7) |
| Age (years) | 13.4 ± 2.82 | 13.5 ± 2.65 |
| Dentition (mixed/permanent) | 69/115 (37.5/62.5) | 78/123 (38.8/61.2) |
Values are given as n (%) or mean ± standard deviation.
Anamnestic and clinical findings of TMD (symptoms and signs)
The prevalence of TMD symptoms and signs were significantly higher (P < 0.05) in the CLCPI group than in the CLWP group, with 32.7% children in the CLCPI group showing at least one TMD symptom and 51.1% showing at least one TMD sign, compared with 23.9% and 22.4%, respectively, in the CLWP group (Table 2). In both groups, mean values for TMD symptoms and signs were higher in girls than in boys; however, this difference was statistically significant only for TMD signs in the CLWP group (Table 2).
Table 2.
Distribution of children according to the number of symptoms and signs
| Variable | CLCPI | CLWP | P values† | ||||
|---|---|---|---|---|---|---|---|
| Boys/Girls | Total (%) | Boys/Girls | Total (%) | CLCPI (Gender) | CLWP (Gender) | Difference between CLCPI and CLWP | |
| Symptoms | |||||||
| No symptom | 75/49 | 124 (67.4) | 70/83 | 153 (76.1) | 0.225 | 0.099 | 0.008* |
| One symptom | 23/20 | 43 (23.4) | 15/29 | 44 (21.9) | |||
| Two symptoms | 5/8 | 13 (7.1) | 0/2 | 2 (1) | |||
| Three symptoms | 1/3 | 4 (2.2) | 2/0 | 2 (1) | |||
| Signs | |||||||
| No sign | 57/33 | 90 (48.9) | 73/83 | 156 (77.6) | 0.198 | 0.023* | 0.000* |
| One sign | 32/28 | 60 (32.6) | 11/30 | 41 (20.4) | |||
| Two signs | 14/18 | 32 (17.4) | 2/1 | 3 (1.5) | |||
| Three signs | 1/1 | 2 (1.1) | 1/0 | 1 (0.5) | |||
CLCPI, children living in child protection institutions; CLWP, children living with their parents.
Significant difference (P < 0.05).
Chi-square test.
The prevalence of symptoms for both groups is given in Table 3. The prevalence of ‘joint noise’ was lower, and ‘masticatory muscle pain/tiredness’ and ‘joint sticking’ were higher in the CLCPI group compared with the CLWP group. However, a statistically significant difference was found only for ‘masticatory muscle pain/tiredness’ between the two groups (P < 0.05). The only statistically significant gender-based difference was found for symptoms in ‘masticatory muscle pain/tiredness’ in the CLCPI group, which was higher for girls. Neither age nor dentition status had any significant effect on self-reported TMD findings for either group (P < 0.05).
Table 3.
Anamnestic and clinical findings of temporomandibular disorder (TMD)
| CLCPI | CLWP | P values | |||||
|---|---|---|---|---|---|---|---|
| Girls/Boys | Total | Girls/Boys | Total (%) | CLCPI (Gender) | CLWP (Gender) | Difference between CLCPI and CLWP | |
| Anamnestic findings of TMD | |||||||
| Reported joint noise | 19/15 | 34 (18.5) | 25/16 | 41 (20.4) | 0.154 | 0.660 | 0.635 |
| Pain or tiredness in the masticatory muscles | 20/11 | 31 (16.8) | 3/3 | 6 (3) | 0.017* | 1,000 | 0.000* |
| Joint sticking | 6/9 | 15 (8.2) | 6/2 | 8 (4) | 0.991 | 0.470 | 0.131 |
| Clinical findings of TMD | |||||||
| Clicking on examination | 15/24 | 39 (21.2) | 20/11 | 31 (15.5) | 0.409 | 0.727 | 0.234 |
| Crepitation on examination | 0/2 | 2 (1.1) | 0/0 | 0 | – | – | – |
| TMJ tenderness | 18/15 | 33 (18) | 6/5 | 11 (5.5) | 0.041* | 0.932 | 0.001* |
| Muscular tenderness | 34/22 | 56 (30.4) | 6/2 | 8 (4) | 0.006* | 0.384 | 0.000* |
| Restricted mouth opening | 0/0 | 0 (0) | 0/0 | 0 (0) | – | – | – |
CLCPI, children living in child protection institutions; CLWP, children living with their parents; TMJ, temporomandibular joint.
Significant difference according to the chi-square test (P < 0.05).
The prevalence of all clinical signs of TMD was higher in the CLCPI group than in the CLWP group; however, statistically significant differences were found only for ‘TMJ tenderness’ and ‘muscular tenderness’ between the groups (Table 3). Additionally, the only statistically significant gender-based difference was found for both signs in the CLCPI group, which were higher in girls (P < 0.05) (Table 3). Dentition (mixed vs. permanent) did not affect the prevalence of clinical signs of TMD in either group. Restriction of mouth opening was not observed in any of the children examined.
With regard to the associations between symptoms and signs, positive associations were found between reported symptoms and clinical signs of TMD for both groups (P < 0.05) (Table 4). In the CLCPI group, ‘pain/tiredness in masticatory muscles’ was found to be associated with ‘TMJ tenderness’ and ‘muscular tenderness’. In addition, ‘joint sticking’ was found to be associated with ‘muscular tenderness’ in the same group. In the CLWP group, ‘pain or tiredness in masticatory muscles’ was associated with ‘TMJ tenderness’. Additionally, ‘reported joint noise’ and ‘joint sticking’ were associated with ‘clicking’ in the same group (Table 4).
Table 4.
Association between symptoms and signs of temporomandibular disorder (TMD)
| Variable | Signs of TMD | |||||||
|---|---|---|---|---|---|---|---|---|
| Clicking | Crepitation | TMJ tenderness | Muscle tenderness | |||||
| CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | |
| Symptoms of TMD | ||||||||
| Pain/tiredness in masticatory muscles | NS | NS | NS | – | 0.009* | 0.030* | 0.002* | NS |
| Joint noise | NS | 0.004* | NS | – | NS | NS | NS | NS |
| Joint sticking | NS | 0.005* | NS | – | NS | NS | 0.003* | NS |
CLCPI, children living in child protection institutions; CLWP, children living with their parents; NS, no significant association; TMJ, temporomandibular joint.
indicates significant positive association (P < 0.05, chi-square test).
Oral parafunctions
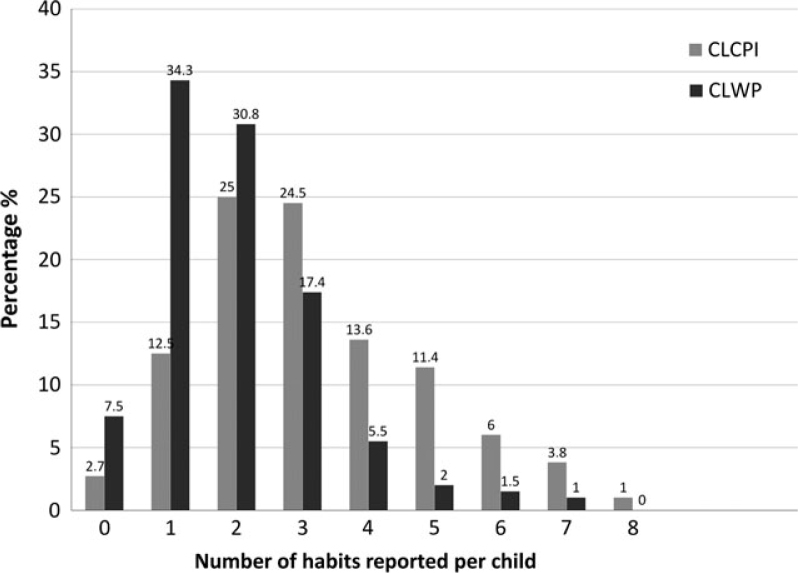
Overall, 97.3% of children in the CLCPI group and 92.5% in the CLWP group reported at least one oral parafunction (Figure 1). While 22.2% of the CLCPI group reported five or more oral parafunctions, only 4.5% of the CLWP group reported five or more oral parafunctions. The median value for the number of oral parafunctions reported was 3 in the CLCPI group and 2 in the CLWP group, and this is a statistically significant difference between groups (P < 0.05). While no significant difference was found between the number of oral parafunctions reported by boys and by girls in the CLCPI group (P < 0.05), a significant gender difference was found in the CLWP group (P < 0.05, chi-square test), with girls living at home more prone to oral parafunctions than boys.
Figure 1.
Distribution of the children according to the number of oral parafunctions.
Gum-chewing was the most common oral parafunction reported in both CLCPI and CLWP groups (Table 5). All oral parafunctions were reportedly more prevalent in the CLCPI group compared with the CLWP group, but differences between the groups were statistically significant only for ‘biting pencil’, ‘biting fingernails’, ‘chewing ice, hard candies’ and ‘opening bottles with teeth’ (P < 0.05). The only statistically significant gender-based difference was found for ‘chewing gum everyday’ and ‘jaw play’ in the CLWP group (Table 5).
Table 5.
Prevalence of oral parafunctions
| Variable | CLCPI | CLWP | P values | ||||
|---|---|---|---|---|---|---|---|
| Girls/Boys | Total (%) | Girls/Boys | Total (%) | CLCPI (Gender) | CLWP (Gender) | Difference between CLCPI and CLWP | |
| Biting pencil | 18/21 | 39 (21.2) | 12/11 | 23 (11.4) | 0.843 | 0.808 | 0.009* |
| Biting fingernails | 29/36 | 65 (35.3) | 28/20 | 48 (23.9) | 0.941 | 0.927 | 0.014* |
| Chewing ice, hard candies | 57/67 | 124 (67.4) | 60/37 | 97 (48.3) | 0.412 | 0.201 | 0.000* |
| Taking games apart with teeth | 14/18 | 32 (17.4) | 17/8 | 25 (12.4) | 1.00 | 0.317 | 0.172 |
| Opening bottles with teeth | 34/32 | 66 (35.9) | 21/16 | 37 (18.4) | 0.136 | 1.00 | 0.000* |
| Jaw play | 30/34 | 64 (34.8) | 40/16 | 56 (27.9) | 0.601 | 0.014* | 0.143 |
| Clenching/grinding teeth | 20/16 | 36 (19.6) | 14/12 | 26 (12.9) | 0.149 | 0.917 | 0.077 |
| Chewing gum everyday | 68/81 | 149 (81) | 96/63 | 159 (79.1) | 0.303 | 0.045* | 0.716 |
CLCPI, children living in child protection institutions; CLWP, children living with their parents.
Significant difference according to the chi-square test (P < 0.05).
With regard to the association between oral parafunctions and signs and symptoms of TMD, positive associations were found between findings in both the CLCPI and CLWP groups (P < 0.05). The mean number of oral parafunctions was higher in children with signs or symptoms of TMD than in children without signs and symptoms of TMD. A positive association was found between the number of oral parafunctions and ‘pain or tiredness in masticatory muscles’, ‘reported joint noises’ and ‘TMJ tenderness’ in the CLCPI group (P < 0.05). In the CLWP group, the number of oral parafunctions was associated with ‘reported joint noise’ and ‘muscular tenderness’ (P < 0.05, Mann–Whitney U-test).
Moreover, in the CLCPI group, ‘biting pencil’, ‘opening bottles with teeth’ and ‘clinching/grinding teeth’ were found to be associated with ‘pain or tiredness in masticatory muscles’ (Table 6). Also, ‘taking games apart with teeth’ and ‘jaw play’ were associated with ‘joint noises’. Additionally, ‘biting pencil’, ‘biting fingernails’, ‘opening bottles with teeth’ and ‘clenching/grinding teeth’ were associated with ‘TMJ tenderness’. Finally, ‘clenching/grinding teeth’ was associated with ‘muscle tenderness’ in the same group. In the CLWP group, ‘jaw play’ and ‘clenching/grinding teeth’ were found to be associated with ‘joint noise’. Additionally, ‘clenching/grinding teeth’ was found to be associated with ‘muscle tenderness’ (Table 6).
Table 6.
Associations between oral parafunctions and signs and symptoms of temporomandibular disorder (TMD) and stressful life events
| Signs | Symptoms | Stressful life events | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Pain/tiredness in masticatory muscles | Joint noise | Joint sticking | Clicking | Crepitation | TMJ tenderness | Muscle tenderness | Family-related problems | Lesson-related problems | Friend-related problems | ||||||||||
| CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | |
| Oral parafunctions | ||||||||||||||||||||
| Biting pencil | 0.018* | NS | NS | NS | NS | NS | NS | NS | NS | – | 0.010* | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Biting fingernails | NS | NS | NS | NS | NS | NS | NS | NS | NS | – | 0.001* | NS | NS | NS | NS | NS | NS | 0.005* | 0.041* | NS |
| Chewing ice, etc. | NS | NS | NS | NS | NS | NS | NS | NS | NS | – | NS | NS | NS | NS | NS | NS | NS | 0.043* | NS | NS |
| Taking games apart | NS | NS | 0.022* | NS | NS | NS | NS | NS | NS | – | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Opening bottle | 0.027* | NS | NS | NS | NS | NS | NS | NS | NS | – | 0.010* | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Jaw play | NS | NS | 0.008* | 0.047* | NS | NS | NS | NS | NS | – | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Clenching/grinding teeth | 0.007* | NS | NS | 0.007* | NS | NS | NS | NS | NS | – | 0.025* | NS | 0.011* | 0.001* | 0.004* | NS | NS | NS | 0.001* | NS |
| Chewing gum everyday | NS | NS | NS | NS | NS | NS | NS | NS | NS | – | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
CLCPI, children living in child protection institutions; CLWP, children living with their parents; NS, no significant association (P < 0.05); TMJ, temporomandibular joint.
indicates significant positive association (P < 0.05, chi-square test).
Stressful live events
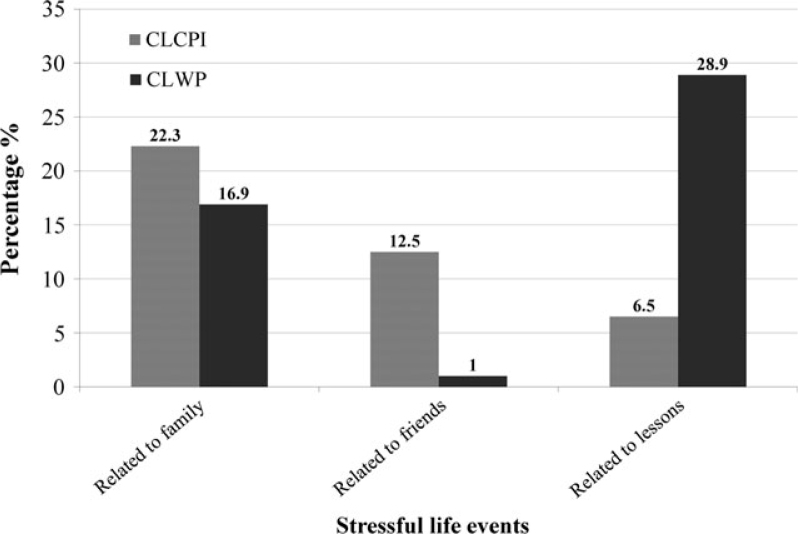
Significant differences (P < 0.05) were found between the CLCPI and CLWP groups in terms of stressful life events, with those in the CLCPI group reporting more problems associated with family and friends, and those in the CLWP group reporting more problems associated with school lessons (Figure 2). Significant gender differences were found only in problems with school lessons reported in the CLWP group, with more girls than boys in that group reporting problems relating to school lessons (P < 0.05, chi-square test).
Figure 2.
Distribution of the children according to the types of stressful life events.
With regard to the association between stressful life events and oral parafunctions, the mean number of oral parafunctions was higher in children who reported emotional problems than in children who did not report any problems. While familial problems were not statistically associated with the prevalence of oral habits in either group of children (P < 0.05), children in the CLCPI group who reported problems with friends had more oral parafunctions than did those who did not report problems with friends (P < 0.05), and children in the CLWP group who reported problems with school lessons had more oral parafunctions than those who did not report problems with school lessons (P < 0.05, Mann–Whitney U-test). Moreover, in the CLCPI group, ‘problems related to friends’ was found to be associated with ‘biting fingernails’ and ‘clenching/grinding teeth’ (P < 0.05) (Table 6). Also, ‘problems related to family’ was associated with ‘clenching/grinding teeth’ in the same group (P < 0.05). In the CLWP group, positive associations were found between ‘problems related to lessons’ and ‘biting fingernails’ and ‘chewing ice, etc.’ (P < 0.05) (Table 6).
Moreover, a significant, positive association was found between stressful life events and signs and symptoms of TMD (Table 7). In the CLCPI group, ‘problems related to friends’ was found to be associated with ‘pain/tiredness in masticatory muscles’, ‘joint sticking’, ‘TMJ tenderness’ and ‘muscular tenderness’ (P < 0.05). In the CLWP group, ‘problems related to lessons’ was associated with ‘joint noise’ and ‘joint sticking’ (P < 0.05) (Table 7).
Table 7.
Association between symptoms and signs of temporomandibular disorder (TMD) and stressful life events
| Symptoms | Signs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain/tiredness in masticatory muscles | Joint noise | Joint sticking | Clicking | Crepitation | TMJ tenderness | Muscle tenderness | ||||||||
| CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | CLCPI | CLWP | |
| Stressful life events | ||||||||||||||
| Family-related problems | NS | NS | NS | NS | NS | NS | NS | NS | NS | – | NS | NS | NS | NS |
| Lesson-related problems | NS | NS | NS | 0.001* | NS | 0.046* | NS | NS | NS | – | NS | NS | NS | NS |
| Friend-related problems | 0.005* | NS | NS | NS | 0.025* | NS | NS | NS | NS | – | 0.005* | NS | 0.012* | NS |
CLCPI, children living in child protection institutions; CLWP, children living with their parents; NS, no significant association (P < 0.05); TMJ, temporomandibular joint.
indicates significant positive association (P < 0.05, chi-square test).
Discussion
The present study found the prevalence of signs and symptoms of TMD and oral parafunctions to vary significantly between children living in protective institutions and children living with their families. To the best of our knowledge, no clinical study has made this comparison in either children or adults; thus, it is difficult to assess the findings of the current study in light of previous reports.
Previous studies have reported different incidence rates for signs and symptoms of TMD6., 22., 23.. While some studies have reported TMJ clicking to be the most frequent sign of TMD6., 24., other studies report a higher incidence of muscle or TMJ tenderness22. In the present study, the most frequent clinical sign of TMD was found to be clicking in the CLWP group and muscular tenderness in the CLCPI group. Previous studies of children have reported rates of clicking to range from 6.8% to 65%6., 9., 25.. This wide range can be attributed to differences in examination methods, sample size and age groups. In the present study, incidences of clicking were found to be lower compared with the results of previous studies conducted in Turkish children using a stethoscope6., 22.. Rather than a stethoscope, the present study used a manual technique to detect joint sounds because, in line with Okeson21, joint sounds may be adequately identified by palpation, whereas the use of more sensitive detection devices, such as a stethoscope, could increase the detection rate and lead to unnecessary and inappropriate treatment.
Children may not be aware of their TMD symptoms because they may have fewer or more moderate TMD symptoms than adults or because the disease may be at an earlier stage. Okeson found many children to be unaware of sounds in relation to the TMJ that were later observed during clinical examinations2. Similarly, Riolo et al.26 reported almost no relationship between self-reported symptoms and clinically observable signs. In contrast, Emodi-Perlman et al.11 reported a significant association between self-reported symptoms and clinically observable signs, and the authors attributed this to the fact that children were assisted by their parents when completing self-reporting questionnaires, thereby increasing the reliability of the findings. In the present study, positive correlations were found between self-reported symptoms and clinically observable signs in both CLCPI and CLWP groups. Given that children in the CLCPI group could not receive parental assistance in completing the questionnaires, and that caregivers dealing with multiple children in an institutional setting could not be expected to be as helpful as parents with regard to reporting on children's TMD symptoms, to ensure standardisation between the CLCPI and CLWP groups, the questionnaires were implemented through interviews with a dentist to eliminate the possibility of misunderstanding and increase the reliability of children's self-reporting.
Many reports have been published on the prevalence of oral parafunctions in children11., 27.. The incidence of oral parafunction in the present study was higher in both groups compared with previous studies11., 27.. The higher rate found in the present study could be a result of differences in ethnic characteristics or the age of the participants.
Although some studies indicate no associations between oral parafunctional habits, such as bruxism, and other signs and symptoms of TMD10., 28., in general, oral parafunctions are considered to be important factors in TMD aetiology2., 29.. Sari et al.30 reported an association between parafunctions and TMJ dysfunction among children with mixed dentition (the parafunctions were thumb/finger sucking and nail biting) as well as among children with permanent dentition (the parafunction was bruxism). Similarly, Emodi-Perlman et al.11 found an association between jaw play and joint noise and pain during mastication in children, and Widmalm et al.29 stated that most symptoms of pain in connection with TMJ dysfunction were associated with parafunctions, primarily bruxism. The present study demonstrated a high association between all oral parafunctions and signs and symptoms of TMD in both groups of children. Bruxism was especially associated with more than one TMD sign or symptom in both groups. In a study by Karibe et al.31 conducted with children 11–15 years of age, children's reports of habitual diurnal clenching correlated significantly with TMD symptoms, whereas reported nocturnal tooth grinding did not show any correlation. The authors attributed this finding to the fact that children might not be aware of nocturnal tooth grinding while asleep and concluded that behaviour modification to control habitual diurnal tooth clenching could play an important role in treating or preventing TMD. In the present study, to ensure standardisation, only the relationship between diurnal clenching and TMD was evaluated because it was thought that children in the CLCPI group might provide unreliable answers regarding night-time behaviour because they did not live with their families.
Psychological factors, such as increased stress levels and emotional challenges, have been reported in adolescents with TMD2., 11., 12., 13., 14., 15., 31.. Karibe et al.31 found that subjects with TMD had higher anxiety scores than did subjects without TMD, and Alamoudi et al.12 found a significant association between emotional status and multiple signs and symptoms of TMD. In the present study, the impact of emotional status on TMD was observed in both self-reported and clinically observable TMD signs and symptoms. In both groups, a higher incidence of TMD signs and symptoms was found among children who reported a problem with school, family or friends when compared with children who did not report problems. Moreover, the overall prevalence of TMD signs and symptoms was higher in the CLCPI group than in the CLWP group. This is most likely attributable to higher levels of stress among children in the CLCPI group compared with children in the CLWP group and is related to the fact that children in institutionalised care live away from their families, tend to have a lower SES than their non-institutionalised counterparts, spend most of their time with their peers and may have only one or no living parents. In a cross-sectional study of adolescents, the prevalence of emotional and behavioural problems among adolescents brought up in institutions was found to be two- to five times higher than among a national sample of adolescents in general19. In the present study, a significant association was also found between the numbers of oral parafunctions and children's emotional status in both CLWP and CLCPI groups. In addition, in line with the higher incidences of TMD signs and symptoms found among children in the CLCPI group, the incidences of oral parafunctions were higher in the CLCPI group than in the CLWP group. Furthermore, the rate of association between oral habits and multiple signs and symptoms of TMD was higher in the CLCPI group than in the CLWP group. This could be due to a greater frequency and intensity of parafunctions related to greater stress levels or to a lack of parental control that might prevent children from engaging in negative oral habits.
With regard to gender differences, some previous studies have reported TMD-related pain and other symptoms to be more common in girls than in boys29., 32., whereas other studies did not show differences between genders11., 24.. The present study reported a higher rate of TMD signs and symptoms in girls than in boys; however, this difference was only significant in signs for the CLWP group.
Conclusion
The results of this study demonstrate that institutionalised children present with a higher prevalence of TMD signs and symptoms and oral parafunctions than do children who live with their parents, and they also present with a more positive association between TMD-related symptoms and stressful life events and oral parafunctions. However, additional long-term comparative studies are needed to confirm the results reported here.
Acknowledgement
There is no funding for this study.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Manfredini D, Guarda-Nardini L, Winocur E, et al. Research diagnostic criteria for temporomandibular disorders: a systematic review of axis | epidemiologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:453–462. doi: 10.1016/j.tripleo.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Okeson JP. In: Management of Temporomandibular Disorders and Occlusion. 7th ed. Okeson JP, editor. Mosby Elsevier; St. Louis: 2013. Etiology of functional disturbances in the masticatory system; pp. 102–128. [Google Scholar]
- 3.Costen JB. Syndrome of ear and sinus symptoms dependent upon disturbed function of the temporomandibular joint. Ann Otol Rhinol Laryngol. 1997;106:805–819. doi: 10.1177/000348949710601002. [DOI] [PubMed] [Google Scholar]
- 4.Okeson JP. In: Management of Temporomandibular Disorders and Occlusion. 7th ed. Okeson JP, editor. Mosby Elsevier; St. Louis: 2013. Signs and symptoms of temporomandibular disorders; pp. 129–169. [Google Scholar]
- 5.Minghelli B, Cardoso I, Porfirio M, et al. Prevalence of temporpmandibular disorder in children and adolescents from public schools in southern portugal. N Am J Med Sci. 2014;6:126–132. doi: 10.4103/1947-2714.128474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sönmez H, Sari S, Oksak Oray G, et al. Prevalence of temporomandibular dysfunction in Turkish children with mixed and permanent dentition. J Oral Rehabil. 2001;28:280–285. [PubMed] [Google Scholar]
- 7.Okeson JP. Temporomandibular disorders in children. Pediatr Dent. 1989;11:325–329. [PubMed] [Google Scholar]
- 8.Sena MF, Mesquita KS, Santos FR, et al. Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paul Pediatr. 2013;31:538–545. doi: 10.1590/S0103-05822013000400018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kritsineli M, Shim YS. Malocclusion, body posture, and temporomandibular disorders in children with primary and mixed dentition. J Clin Pediatr Dent. 1992;16:86–93. [PubMed] [Google Scholar]
- 10.Castelo PM, Gaviao MB, Pereira LJ, et al. Relationship between oral parafunctional/nutritive sucking habits and temporomandibular joint dysfunction in primary dentition. Int J Paediatr Dent. 2005;15:29–36. doi: 10.1111/j.1365-263X.2005.00608.x. [DOI] [PubMed] [Google Scholar]
- 11.Emodi-Perlman A, Eli I, Friedman-Rubin P, et al. Bruxism, oral parafunctions, anamnestic and clinical findings of temporomandibular disorders in children. J Oral Rehabil. 2012;39:126–135. doi: 10.1111/j.1365-2842.2011.02254.x. [DOI] [PubMed] [Google Scholar]
- 12.Alamoudi N. Correlation between oral parafunction and temporomandibular disorders and emotional status among saudi children. J Clin Pediatr Dent. 2001;26:71–80. doi: 10.17796/jcpd.26.1.m24280163t5q65x6. [DOI] [PubMed] [Google Scholar]
- 13.Pizolato RA, Freitas-Fernandes FS, Gaviao MB. Anxiety/depression and orofacial myofacial disorders as factors associated with TMD in children. Braz Oral Res. 2013;27:156–162. doi: 10.1590/s1806-83242013000100021. [DOI] [PubMed] [Google Scholar]
- 14.van Selms MK, Lobbezoo F, Wicks DJ, et al. Craniomandibular pain, oral parafunctions, and psychological stress in a longitudinal case study. J Oral Rehabil. 2004;31:738. doi: 10.1111/j.1365-2842.2004.01313.x. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi FY, Gaviao MB, Montes AB, et al. Evaluation of oro-facial function in young subjects with temporomandibular disorders. J Oral Rehabil. 2014;41:496–506. doi: 10.1111/joor.12163. [DOI] [PubMed] [Google Scholar]
- 16.Al-Jobair AM, Al-Sadhan SA, Al-Faifi AA, et al. Medical and dental health status of orphan children in central Saudi Arabia. Saudi Med J. 2013;34:531–536. [PubMed] [Google Scholar]
- 17.Khare V, Koshy A, Rani P, et al. Prevalence of dental caries and treatment needs among the orphan children and adolescents of Udaipur district, Rajasthan, India. J Contemp Dent Pract. 2012;13:182–187. doi: 10.5005/jp-journals-10024-1118. [DOI] [PubMed] [Google Scholar]
- 18.Kanbur N, Tüzün Z, Derman O. Psychiatric symptoms of adolescents reared in an orphanage in Ankara. Turk J Pediatr. 2011;53:281–284. [PubMed] [Google Scholar]
- 19.Şimşek Z, Erol N, Oztop D, et al. Epidemiology of emotional and behavioral problems in children and adolescents reared in orphanages: a national comparative study. Turk Psikiyatri Derg. 2008;19:235–246. [PubMed] [Google Scholar]
- 20.Virk P, Jain RL, Pathak A, et al. Inter-relationship of intelligence-quotient and self-concept with dental caries amongst socially handicapped orphan children. J Indian Soc Pedod Prev Dent. 2012;30:127–132. doi: 10.4103/0970-4388.99986. [DOI] [PubMed] [Google Scholar]
- 21.Okeson JP. In: Management of Temporomandibular Disorders and Occlusion. 7th ed. Okeson JP, editor. Mosby Elsevier; St. Louis: 2013. History and examination for temporomandibuler disorders; pp. 170–221. [Google Scholar]
- 22.Muhtaroğulları M, Demirel F, Saygılı G. Temporomandibular disorders in Turkish children with mixed and primary dentition: prevalence of signs and symptoms. Turk J Pediatr. 2004;46:159–163. [PubMed] [Google Scholar]
- 23.Köhler AA, Helkimo AN, Magnusson T, et al. Prevalence of symptoms and signs indicative of temporomandibular disorders in children and adolescents. A cross-sectional epidemiological investigation covering two decades. Eur Arch Paediatr Dent. 2009;10:16–25. doi: 10.1007/BF03262695. [DOI] [PubMed] [Google Scholar]
- 24.Bonjardim LR, Gaviao MB, Pereira LJ, et al. Signs and symptoms of temporomandibular disorders in adolescents. Braz Oral Res. 2005;19:93–98. doi: 10.1590/s1806-83242005000200004. [DOI] [PubMed] [Google Scholar]
- 25.Nilner M. Prevalence of functional disturbances and diseases of the stomatognathic system in 15–18 year olds. Swed Dent J. 1981;5:189–197. [PubMed] [Google Scholar]
- 26.Riolo ML, TenHave TR, Brandt D. Clinical validity of the relationship between TMJ signs and symptoms in children and youth. ASDC J Dent Child. 1988;55:110–113. [PubMed] [Google Scholar]
- 27.Widmalm SE, Christiansen RL, Gunn SM, et al. Prevalence of signs and symptoms of craniomandibular disorders and orofacial parafunction in 4–6-year-old African-American and Caucasian children. J Oral Rehabil. 1995;22:87–93. doi: 10.1111/j.1365-2842.1995.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 28.Cheifetz AT, Osganian SK, Allred EN, et al. Prevalence of bruxism and associated correlates in children as reported by parents. J Dent Child. 2005;72:67–73. [PubMed] [Google Scholar]
- 29.Wildmalm SE, Gunn SM, Christiansen RL, et al. Association between CMD signs and symptoms, oral parafunctions, race and sex, in 4–6-year-old African-American and Caucasian children. J Oral Rehabil. 1995;22:95–100. doi: 10.1111/j.1365-2842.1995.tb00241.x. [DOI] [PubMed] [Google Scholar]
- 30.Sari S, Sonmez H. Investigation of the relationship between oral parafunctions and temporomandibular joint dysfunction in Turkish children with mixed and permanent dentition. J Oral Rehabil. 2002;29:108–112. doi: 10.1046/j.1365-2842.2002.00781.x. [DOI] [PubMed] [Google Scholar]
- 31.Karibe H, Shimazu K, Okamoto A, et al. Prevalence and association of self-reported anxiety, pain, and oral parafunctional habits with temporomandibular disorders in Japanese children and adolescents: a cross-sectional survey. BMC Oral Health. 2015;15:15–18. doi: 10.1186/1472-6831-15-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.List T, Wahlund K, Wenneberg B, et al. TMD in children and adolescents: prevalence of pain, gender differences, and perceived treatment need. J Orofac Pain. 1999;13:9–20. [PubMed] [Google Scholar]