Abstract
Objectives: Both temporomandibular disorder (TMD) and knee osteoarthritis (KOA) are prevalent joint diseases; however, an association between them has not been reported. Therefore, this study investigated the prevalence of the specific symptoms and signs of TMDs (SSTs) in patients with KOA. Methods: In total, 200 patients with KOA and 150 healthy individuals were recruited. The prevalence of specific SSTs in patients with mild or severe KOA was compared with the prevalence of specific SSTs in the control group and the results were analysed using a chi-square test. Logistic regression was used to adjust for potential confounders, such as gender and age. Results: The prevalence of ‘impaired range of jaw movement (IRM)’ was 63.6% (n = 77) in the mild KOA group and 62.4% (n = 117) in the severe KOA group; the values for both KOA groups were significantly higher than that for the non-OA control group (34.7%, n = 144; P < 0.017). In addition, 54.7% of the patients with severe KOA reported ‘impaired temporomandibular joint (TMJ) function’, a value significantly higher than that of the control group (39.6%, P < 0.017). No significant differences between groups were found for other SSTs. Conclusions: Patients with KOA might be more likely to experience SSTs, such as IRM and impaired TMJ function.
Key words: Joint diseases, temporomandibular disorders, osteoarthritis, geriatrics
Introduction
Temporomandibular disorder (TMD) is a common orofacial disorder featuring a spectrum of clinical signs and symptoms involving the masticatory muscles, the temporomandibular joint (TMJ) and the adjacent structures1., 2.. The typical symptoms and signs of TMD (SSTs) include pain, impaired range of jaw movement (IRM) and TMJ clicking, all of which can undermine a patient's quality of life3. TMD is generally classified into three subtypes: muscular disorders; disc displacement; and degenerative diseases. Distinct ages have been identified for specific TMD conditions: inflammatory-degenerative joint disorders most commonly occur in participants over 50 years old and disc displacements usually occur in subjects about 30 years old4. Nevertheless, the explicit relationship and aetiology of all the three subtypes remains unclear, and they actually have overlapping symptoms and signs5.
Osteoarthritis (OA) is a chronic and degenerative joint disease, primarily afflicting weight-bearing joints, such as the knee and the hip. The typical course of OA is characterised by the gradual and uneven loss of articular cartilage, osteophyte formation, subchondral sclerosis and degeneration of periarticular structures6. As a result of its high prevalence in the elderly population, OA has been considered as the leading cause of physical disability in the USA7. Osteoarthritis of the knee (KOA) is extremely common globally, being associated with certain occupational activities (repeated loading of the joint), injury and obesity. The symptoms of KOA include joint pain, stiffness and limitation of movement8.
Up to 25% of the population may experience symptoms of TMD. However, only a small percentage of the afflicted individuals seek treatment for pain and dysfunction2. Complicated etiology, ignored early symptoms and the lack of specific diagnostic criteria make early diagnosis of TMD a challenge.
A high incidence of TMD has recently been reported in patients with OA9. A radiographic study showed that more than half of patients with KOA have generalised OA (GOA)10. Moreover, another study found reduced joint space and osteophytes in TMJs in patients with OA, which is similar to the common form of TMJ osteoarthritis11. Nevertheless, no study has yet reported an association between the occurrence of SSTs and KOA. Therefore, in the present study, we investigated the prevalence of specific SSTs in patients with KOA and sought to identify whether patients with KOA are more susceptible to the development of TMD.
Materials and Methods
Participants and groups
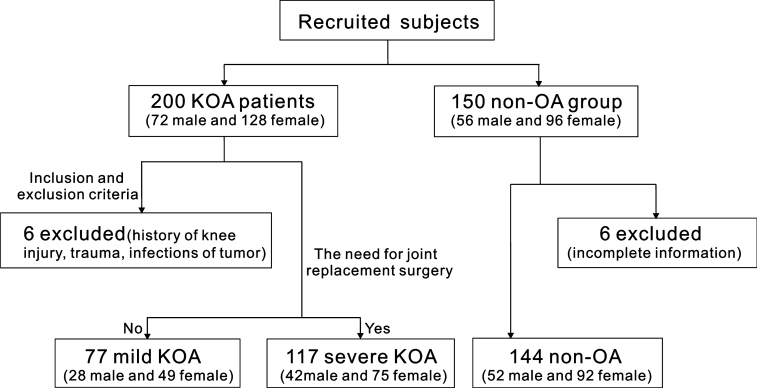
This cross-sectional case–control clinical trial began on 1 October 2013 and ended on 30 June 2014, and was conducted in full accordance with the World Medical Association's Declaration of Helsinki (2008). All participants received a written introduction to the experiment and consented verbally to the research. The West China Hospital of Stomatology Institutional Review Board approved the consent procedure and the study protocol. In total, 200 patients with KOA (128 female patients and 72 male patients) were recruited from the Department of Orthopaedics of a hospital in southwestern China. They were diagnosed as having KOA according to the criteria established by the Osteoarthritis Research Society International12. Based on the treatment guidelines issued by the German Society for Orthopedics and Orthopedic Surgery and the German Professional Association of Orthopedists and Trauma Surgeons, conservative physiotherapy, followed by orthopaedic aids and orthoses, pharmacotherapy and even joint replacement surgery were performed, depending on the severity of the KOA13. Because pain levels and radiological data were classified into several different grades after evaluation and the results were not always consistent with clinical symptoms, patients with KOA were divided into ‘mild KOA’ and ‘severe KOA’ subgroups, according to the requirements, or not, for joint replacement surgery14., 15.. Patients with an explicit history of knee injury, trauma, infections or tumours were excluded. Another group of 150 people were randomly selected from the physical examination database of the hospital, and were matched with the KOA patients for age and gender. These people had no symptoms or signs of OA and served as the non-OA control group. The specific inclusion process is presented in Figure 1.
Figure 1.
Flow chart of the study showing detailed inclusion and exclusion criteria, as well as distribution of subjects in the knee osteoarthritis (KOA) group and the non-osteoarthritis (non-OA) group.
Examination of SSTs
All participants were asked to complete a questionnaire, with the purpose being to collect personal information, such as gender and age. SSTs were recorded using the Helkimo index16, which has been used widely in similar epidemiological studies3., 17.. The specific SSTs investigated are listed in Table 1. ‘Range of movement’ refers to the interincisal distance and overbite at the maximal mouth opening position, and was measured using a millimeter ruler18. The participants were grouped into several levels based on the degree of their subjective [anamnestic indices (Ai)] and objective [dysfunction indices (Di)] SSTs: subjectively symptom-free (Ai0); subjective mild symptoms (AiI); subjective severe symptoms (AiII); objectively sign-free (Di0); objective mild signs (DiI); objective moderate signs (DiII); and objective severe signs (DiIII). The specific calculation methods were based on a modified version of the Helkimo index19.
Table 1.
Symptoms and signs of temporomandibular disorder (SSTs) questionnaire
| Items | SSTs | Grade |
|---|---|---|
| Symptoms | Symptom free | 0 |
| TMJ sounds or fatigue/stiffness in the jaws on awakening or on movement of the lower jaw | I | |
| Difficulties in opening the mouth wide, locking or luxation Pain on mandible movement Pain in the TMJ or masticatory muscles |
II | |
| Signs | ||
| IRM | Normal range of movement: ≥40 mm | 0 |
| Slightly impaired mobility: 30–39 mm | I | |
| Severely impaired mobility: <30 mm | II | |
| Impaired TMJ function | Smooth movement without TMJ sounds, or deviation of ≤2 mm in opening or closing movements | 0 |
| TMJ sounds in one or both joints, ≥2 mm deviation on opening or closing movements, or both | I | |
| Locking, luxation, or both, of the TMJ | II | |
| Muscle pain | No tenderness to palpation in the masticatory muscles | 0 |
| Tenderness to palpation at one to three sites | I | |
| Tenderness to palpation at four or more palpation sites | II | |
| TMJ pain | No tenderness to palpation | 0 |
| Tenderness to lateral palpation | I | |
| Tenderness to posterior palpation | II | |
| Pain on mandible movement | No pain on movement | 0 |
| Pain on one movement | I | |
| Pain on two or more movements | II |
IRM, impaired range of jaw movement; TMD, temporomandibular disorder; TMJ, temporomandibular joint.
Before the investigation, the examiners participated in a 2-week training course on clinical examination of participants using unified diagnostic criteria and detailed protocols. The reproducibility of the clinical recordings (interexaminer agreement) was tested via blind re-examination. The kappa value was used to judge the inter-examiner agreement, and this value ranged from 0.62 to 0.79, indicating ‘good’ agreement. Two examiners performed the evaluation together to minimise the bias associated with participant interviews.
Data analyses
The Kruskal–Wallis H test was used to compare the distribution of each grade of SST (Ai and Di) among the three study groups (i.e. the mild KOA group, the severe KOA group and the non-OA control group). Values of P <0.05 denoted that the distributions of each grade of SST differed among the three groups; thus, pairwise comparisons were performed. The chi-square test was employed to examine the differences among the three groups regarding specific SST prevalence. Logistic regression was used to examine the influence of potential confounders (e.g. gender and age) on the prevalence of SSTs, and the results are shown as odds ratios (ORs) with 95% confidence interval (95% CI). A two-tailed value of P <0.05 was considered significant. All data from the questionnaires and clinical examinations were processed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Of a total of 350 participants who were recruited for this study, 338 (96.6%) completed the questionnaires and TMD-related examination: 144 non-OA volunteers (61.57 ± 0.65 years of age; 52 male and 92 female); 77 patients with mild KOA (59.57 ± 1.01 years of age; 28 male and 49 female); and 117 patients with severe KOA (64.91 ± 0.82 years of age; 42 male and 75 female).
The percentage distribution of Ai and Di categories according to the Helkimo index, across the non-OA, mild KOA and severe KOA groups, is shown in Figure 2. Most participants indicated no subjective symptoms, and no significant differences were observed among the three groups (P = 0.871). Differences were observed for the Di categories (P < 0.001). Therefore, additional Wilcoxon tests were performed between each pair at an adjusted significance level (α’) of 0.017, using the formula . Significant differences were found between the non-OA and mild KOA groups (P = 0.006, <0.017), and between the non-OA and severe KOA groups (P < 0.001), but not between the mild KOA and severe KOA groups (P = 0.293, >0.017). Taken together, the patients with KOA showed a significantly higher risk of physical signs of TMD compared with the non-OA individuals in this study.
Figure 2.
Percentage distributions of the anamnestic indices (Ai) and dysfunction indices (Di), based on the Helkimo index, for the non-osteoarthritis (non-OA) group as well as for the mild and severe knee osteoarthritis (mild KOA and severe KOA, respectively) groups. Ai0, subjectively symptom-free; AiI, subjective mild symptoms; AiII, subjective severe symptoms; Di0, objectively sign-free; DiI, objective mild signs; DiII, objective moderate signs; DiIII, objective severe signs.
Pearson's chi-square test was used to examine the prevalence of specific SSTs among the three groups. Only two items showed significant differences (IRM, χ2 = 26.26, P < 0.001; and impaired TMJ function, χ2 = 6.14, P = 0.47). Additional pairwise comparisons were performed to determine where these differences existed (Tables 2 2). Significantly more patients with mild KOA (63.6%) or severe KOA (62.4%) had IRM compared with subjects of the non-OA group (34.7%; P < 0.017). Moreover, patients in the severe KOA group had a significantly higher prevalence (54.7%) of impaired TMJ function (i.e. sounds or deviation in opening and closing movements) compared with patients in the non-OA group (39.6%; P < 0.017). The prevalence of this SST in the mild KOA group was 49.4%, also higher than the non-OA group; however the difference was not statistically significant. No significant differences were found between the mild KOA and severe KOA groups for these two signs (P > 0.017, data not shown). No significant differences were found among the three groups for muscle pain or TMJ pain during movement.
Table 2.
Comparison of symptoms and signs of temporomandibular disorder (SST) rates between non-osteoarthritis (non-OA) and mild knee osteoarthritis (KOA) groups
| SSTs | Non-OA group | Mild KOA group | χ2 | P |
|---|---|---|---|---|
| Subjective symptoms | 21 (14.6) | 13 (16.9) | 0.20 | 0.65 |
| IRM | 50 (34.7) | 49 (63.6)* | 16.26 | <0.01 |
| Impaired TMJ function | 57 (39.6) | 38 (49.4) | 1.95 | 0.16 |
| Muscle pain | 15 (10.4) | 7 (9.1) | 0.09 | 0.75 |
| TMJ pain | 15 (10.4) | 9 (11.7) | 0.08 | 0.77 |
| Pain on mandible movement | 7 (4.9) | 6 (7.8) | 0.78 | 0.37 |
Values are given as n (%).
IRM, impaired range of jaw movement; TMJ, temporomandibular joint.
P < 0.01.
Patients with KOA seemed more likely to have moderate or severe SSTs compared with subjects in the non-OA group, although no significant differences were found (Figure 3).
Figure 3.
Prevalence of symptoms and signs of temporomandibular disorder (SST) for the mild and severe knee osteoarthritis (KOA) groups, as well as for the non-osteoarthritis (non-OA) group. (a) Prevalence of mild and severe SSTs. (b) Prevalence of severe SSTs. TMJ, temporomandibular disorder.
Logistic regression analyses were performed to adjust for confounders such as gender and age (Table 4). Compared with the non-OA participants, patients with severe KOA had higher rates of IRM (OR = 3.43, 95% CI: 2.02–5.84, P < 0.01) and impaired TMJ function (OR = 1.81, 95% CI: 1.09–2.99, P = 0.02), whereas the patients with mild KOA were more likely to have IRM (OR = 3.41, 95% CI: 1.88–6.19, P < 0.01) only.
Table 4.
Association between the rates of specific symptoms and signs of temporomandibular disorder (SSTs) and knee osteoarthritis (KOA) and other potential confounders
| Variable | IRM | Impaired joint function | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Severe KOA | 3.43 | 2.02–5.85 | <0.01 | 1.81 | 1.09–2.99 | 0.02 |
| Mild KOA | 3.41 | 1.88–6.19 | <0.01 | 1.52 | 0.86–2.66 | 0.15 |
| Age (years) | 0.99 | 0.96–1.02 | 0.366 | 1.01 | 0.98–1.03 | 0.58 |
| Sex (male) | 2.45 | 1.52–3.95 | <0.01 | 1.42 | 0.89–2.23 | 0.13 |
| Constant | 0.63 | 0.604 | 0.33 | 0.20 | ||
95% CI, 95% confidence interval; IRM, impaired range of jaw movement; OR, odds ratio; TMD, temporomandibular disorder; TMJ, temporomandibular joint.
Discussion
Impaired general health is related to TMD-type pain20. This study explored the relationship between KOA and specific SSTs. The results demonstrated that patients with KOA seem more likely to have IRM and impaired TMJ function compared with non-OA control subjects.
The Research Diagnostic Criteria for Temporomandibular Dysfunction (RDC/TMD) is recognized as the gold standard for diagnosis, in which axis I represents physical examinations and axis II concerns the evaluation of pain and mental-psychological status; however, several studies have used both the RDC/TMD and the Helkimo dysfunction index as diagnostic criteria21., 22.. As one of the diagnostic standards for TMD, the Helkimo index had been widely used, with proven reliability, in different studies to evaluate the clinical status and subjective symptoms of TMJ dysfunction23. Consistency between the clinical manifestation of TMD and radiographic changes has been poor in previous research24, while the Helkimo index focuses on clinical performance and functional status and has the advantages of simplicity, practicality and scale. It has also been translated into Chinese and successfully used in previous studies25., 26., 27.. Therefore, we chose the Helkimo index to determine the severity of TMD. Nevertheless, we propose that the RDC/TMD standards (axis I/II) and radiographic examinations should be used in future studies.
Range of movement is a generally accepted evaluation of TMJ function. The average range of painless maximal vertical opening is approximately 45.0 ± 1.1 mm in healthy, elderly participants28. As one of the most common signs of TMD, IRM has been the diagnostic basis for many TMD criteria, such as the RDC/TMD. Thus, the increased prevalence of IRM in patients with KOA indicates a tendency for TMD. In addition, compared with participants in the non-OA group, patients with severe KOA were more likely to have impaired TMJ function (Table 3). These findings may agree with the radiographic changes in the TMJs of patients with KOA24. On the other hand, pain, a universal subjective symptom of TMD and usually the chief complaint of people seeking treatment, is less prevalent among the elderly29.
Table 3.
Comparison of symptoms and signs of temporomandibular disorder (SST) rates between non-osteoarthritis (non-OA) and severe knee osteoarthritis (KOA) groups
| SSTs | Non-OA group | Severe KOA group | χ2 | P |
|---|---|---|---|---|
| Subjective symptoms | 21 (14.6) | 19 (16.2) | 0.14 | 0.71 |
| IRM | 50 (34.7) | 73 (62.4)* | 19.84 | <0.01 |
| Impaired TMJ function | 57 (39.6) | 64 (54.7)* | 5.93 | 0.01 |
| Muscle pain | 15 (10.4) | 11 (9.4) | 0.07 | 0.78 |
| TMJ pain | 15 (10.4) | 17 (14.5) | 1.01 | 0.31 |
| Pain on mandible movement | 7 (4.9) | 11 (9.4) | 2.07 | 0.15 |
Values are given as n (%).
IRM, impaired range of jaw movement; TMD, temporomandibular disorder; TMJ, temporomandibular joint.
P < 0.01.
There are at least two reasons why patients with KOA are more likely to be affected by IRM and impaired TMJ function. First, KOA and TMD might share some common hereditary susceptibility. Genetic studies have identified many risk alleles for OA, and mutations in these genes might directly cause general OA, including TMJ OA30., 31.. There may be specific genetic risk factors for mechanical joint damage. Second, as OA pain can lead to depression through its effects on fatigue and disability32., 33., subsequent psychological complaints, such as fatigue, anxiety and depression, can be translated into the sensation of pain and contribute to the development of TMD34. These are assumptions, and the true mechanism remains to be verified in future studies.
The prevalence of the subjective symptoms was much lower (Figure 3) than that of the objective signs. The great majority of all participants reported little or no TMD symptoms. This result is reasonable considering the advanced age of participants in the investigation. Previous studies have reported that fewer than 4% of 65- to 75-year-old participants consider their TMD symptoms to be great or severe29. As the structure and function of our organs and systems degenerate with age, patients may be less sensitive to pain. Geriatric patients more often display objective symptoms related to TMD, but they rarely report pain in the TMJ or masticatory muscles28.
In conclusion, the current cross-sectional study found that patients with KOA, especially those with severe KOA, were more likely to be affected by symptoms and signs of TMD, such as IRM and impaired TMJ function.
Acknowledgements
The present study was supported by Student's Platform for Innovation and Entrepreneurship Training Program; Orthodontic National Key Clinical Specialty Construction Program of China, West China Hospital of Stomatology, Sichuan University; and National Nature Science Foundation of China (no. 11372202). We declare no potential conflicts of interest that might have influenced this article.
References
- 1.Steven JS, David AK, Leonard BK. Temporomandibular disorders. N Engl J Med. 2008;359:2693–2705. doi: 10.1056/NEJMra0802472. [DOI] [PubMed] [Google Scholar]
- 2.Murphy MK, MacBarb RF, Wong ME, et al. Temporomandibular disorders: a review of etiology, clinical management, and tissue engineering strategies. Int J Oral Maxillofac Implants. 2013;28:E393–E414. doi: 10.11607/jomi.te20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yekkalam N, Wänman A. Prevalence of signs and symptoms indicative of temporomandibular disorders and headaches in 35-, 50-, 65- and 75-year-olds living in Vasterbotten, Sweden. Acta Odontol Scand. 2014;72:458–465. doi: 10.3109/00016357.2013.860620. [DOI] [PubMed] [Google Scholar]
- 4.Manfredini D, Guarda-Nardini L, Winocur E, et al. Research diagnostic criteria for temporomandibular disorders: a systematic review of axis I epidemiologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:453–462. doi: 10.1016/j.tripleo.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Roh H-S, Kim W, Kim Y-K, et al. Relationships between disk displacement, joint effusion, and degenerative changes of the TMJ in TMD patients based on MRI findings. J Craniomaxillofac Surg. 2012;40:283–286. doi: 10.1016/j.jcms.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Zhu S, Chen K, Lan Y, et al. Alendronate protects against articular cartilage erosion by inhibiting subchondral bone loss in ovariectomized rats. Bone. 2013;53:340–349. doi: 10.1016/j.bone.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Sampson ER, Hilton MJ, Tian Y, et al. Teriparatide as a chondroregenerative therapy for injury-induced osteoarthritis. Sci Transl Med. 2011;3:101ra93. doi: 10.1126/scitranslmed.3002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dulay GS, Cooper C, Dennison EM. Knee pain, knee injury, knee osteoarthritis & work. Best Pract Res Clin Rheumatol. 2015;29:454–461. doi: 10.1016/j.berh.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Aceves-Avila F, Chávez-López M, Chavira-González JR, et al. Temporomandibular joint dysfunction in various rheumatic diseases. Reumatismo. 2013;65:126–130. doi: 10.4081/reumatismo.2013.126. [DOI] [PubMed] [Google Scholar]
- 10.Forestier R, Francon A, Briole V, et al. Prevalence of generalized osteoarthritis in a population with knee osteoarthritis. Joint Bone Spine. 2011;78:275–278. doi: 10.1016/j.jbspin.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Gynther GW, Tronje G, Holmlund AB. Radiographic changes in the temporomandibular joint in patients with generalized osteoarthritis and rheumatoid arthritis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:613–618. doi: 10.1016/s1079-2104(96)80058-8. [DOI] [PubMed] [Google Scholar]
- 12.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):A1–A56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Michael JW, Schluter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152–162. doi: 10.3238/arztebl.2010.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elbaz A, Mor A, Segal G, et al. Novel classification of knee osteoarthritis severity based on spatiotemporal gait analysis. Osteoarthritis Cartilage. 2014;22:457–463. doi: 10.1016/j.joca.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Moreton BJ, Tew V, das Nair R, et al. Pain phenotype in patients with knee osteoarthritis: classification and measurement properties of pain detect and self-report leeds assessment of neuropathic symptoms and signs scale in a cross-sectional study. Arthrit Care Res. 2015;67:519–528. doi: 10.1002/acr.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helkimo M. Studies on function and dysfunction of the masticatory system. II. Index for anamnestic and clinical dysfunction and occlusal state. Sven Tandlak Tidskr. 1974;67:101–121. [PubMed] [Google Scholar]
- 17.Witulski S, Vogl TJ, Rehart S, et al. Evaluation of the TMJ by means of Clinical TMD Examination and MRI Diagnostics in Patients with Rheumatoid Arthritis. Biomed Res Int. 2014;2014:328–560. doi: 10.1155/2014/328560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti AC, Oltramari PV, Navarro Rde L, et al. Examination of temporomandibular disorders in the orthodontic patient: a clinical guide. J Appl Oral Sci. 2007;15:77–82. doi: 10.1590/S1678-77572007000100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Der Weele LT, Dibbets JMH. Helkimo's index: a scale or just a set of symptoms. J Oral Rehabil. 1987;14:229–237. doi: 10.1111/j.1365-2842.1987.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 20.Yekkalam N, Wänman A. Associations between craniomandibular disorders, sociodemographic factors and self-perceived general and oral health in an adult population. Acta Odontol Scand. 2014;72:1054–1065. doi: 10.3109/00016357.2014.949843. [DOI] [PubMed] [Google Scholar]
- 21.Sena MF, Mesquita KS, Santos FR, et al. Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paul Pediatr. 2013;31:538–545. doi: 10.1590/S0103-05822013000400018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Hashmi A, Al-Azri A, Al-Ismaily M, et al. Temporomandibular disorders in patients with mandibular fractures: a preliminary comparative case-control study between South Australia and Oman. Int J Oral Maxillofac Surg. 2011;40:1369–1372. doi: 10.1016/j.ijom.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Sirirunqrojying S, Kerdpon D. Relationship between oral tori and temporomandibular disorders. Int Dent J. 1999;49:101–104. doi: 10.1111/j.1875-595x.1999.tb00516.x. [DOI] [PubMed] [Google Scholar]
- 24.Rando C, Waldron T. TMJ osteoarthritis: a new approach to diagnosis. Am J Phys Anthropol. 2012;148:45–53. doi: 10.1002/ajpa.22039. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Long X, Deng M, et al. Osteoarthritic changes after superior and inferior joint space injection of hyaluronic acid for the treatment of temporomandibular joint osteoarthritis with anterior disc displacement without reduction: a cone-beam computed tomographic evaluation. J Oral Maxillofac Surg. 2015;73:232–244. doi: 10.1016/j.joms.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 26.He SS, Li F, Song F, et al. Spontaneous neural activity alterations in temporomandibular disorders: a cross-sectional and longitudinal resting-state functional magnetic resonance imaging study. Neuroscience. 2014;278:1–10. doi: 10.1016/j.neuroscience.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Chen Z, Xu B. Causes and treatment of mandibular and condylar fractures in children and adolescents: a review of 104 cases. JAMA Otolaryngol Head Neck Surg. 2014;140:203–207. doi: 10.1001/jamaoto.2013.6300. [DOI] [PubMed] [Google Scholar]
- 28.Schmitter M, Rammelsberg P, Hassel A. The prevalence of signs and symptoms of temporomandibular disorders in very old subjects. J Oral Rehabil. 2005;32:467–473. doi: 10.1111/j.1365-2842.2005.01449.x. [DOI] [PubMed] [Google Scholar]
- 29.Unell L, Johansson A, Ekback G, et al. Prevalence of troublesome symptoms related to temporomandibular disorders and awareness of bruxism in 65- and 75-year-old subjects. Gerodontology. 2012;29:1741–2358. doi: 10.1111/j.1741-2358.2011.00558.x. [DOI] [PubMed] [Google Scholar]
- 30.Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012;8:77–89. doi: 10.1038/nrrheum.2011.199. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Nakaoka H, Yamamoto K, et al. Genome-wide association study of degenerative bony changes of the temporomandibular joint. Oral Dis. 2014;20:409–415. doi: 10.1111/odi.12141. [DOI] [PubMed] [Google Scholar]
- 32.Ozcetin A, Ataoglu S, Kocer E, et al. Effects of depression and anxiety on quality of life of patients with rheumatoid arthritis, knee osteoarthritis and fibromyalgia syndrome. West Indian Med J. 2007;56:122–129. doi: 10.1590/s0043-31442007000200004. [DOI] [PubMed] [Google Scholar]
- 33.Hawker GA, Gignac MAM, Badley E, et al. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:1382–1390. doi: 10.1002/acr.20298. [DOI] [PubMed] [Google Scholar]
- 34.Gameiro GH, da Silva Andrade A, Nouer DF, et al. How may stressful experiences contribute to the development of temporomandibular disorders? Clin Oral Invest. 2006;10:261–268. doi: 10.1007/s00784-006-0064-1. [DOI] [PubMed] [Google Scholar]