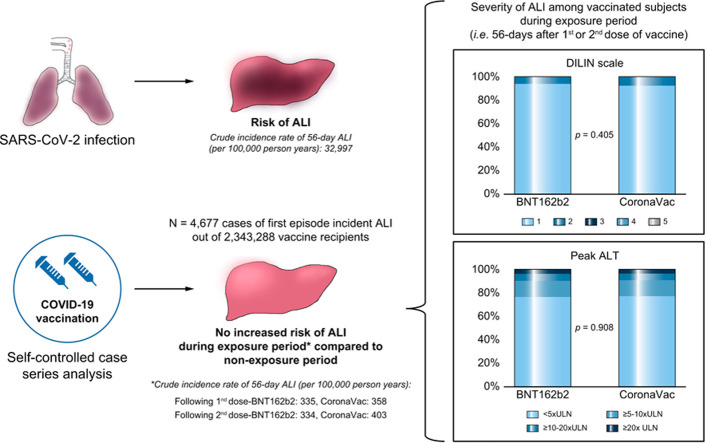
Abstract
Background & Aims
Case reports of severe acute liver injury (ALI) following COVID-19 vaccination have recently been published. We evaluated the risks of ALI following COVID-19 vaccination (BNT162b2 or CoronaVac).
Methods
We conducted a modified self-controlled case series analysis using the vaccination records in Hong Kong with data linkage to electronic medical records from a territory-wide healthcare database. Incidence rate ratios (IRRs) for ALI outcome in the 56-day period following first and second doses of COVID-19 vaccines in comparison to the non-exposure period were estimated and compared to the ALI risk in patients with SARS-CoV-2 infection.
Results
Among 2,343,288 COVID-19 vaccine recipients who were at risk, 4,677 patients developed ALI for the first time between 23rd February 2021 to 30th September 2021. The number of ALI cases within 56 days after the first and second dose of vaccination were 307 and 521 (335 and 334 per 100,000 person-years) for BNT162b2, and 304 and 474 (358 and 403 per 100,000 person-years) for CoronaVac, respectively, compared to 32,997 ALI cases per 100,000 person-years among patients within 56 days of SARS-CoV-2 infection. Compared to the non-exposure period, no increased risk was observed in the 56-day risk period for first (IRR 0.800; 95% CI 0.680–0.942) and second (IRR 0.944; 95% CI 0.816–1.091) dose of BNT162b2, or first (IRR 0.689; 95% CI 0.588–0.807) and second (IRR 0.905; 95% CI 0.781–1.048) dose of CoronaVac. There were no severe or fatal cases of ALI following COVID-19 vaccination.
Conclusion
There was no evidence of an increased risk of ALI associated with BNT162b2 or CoronaVac vaccination. Based on all current available evidence from previous studies and our study, the benefit of mass vaccination far outweighs the ALI risk from vaccination.
Lay summary
There have been some recent reports that COVID-19 vaccination could be associated with acute liver injury. In our study, we found no evidence that COVID-19 vaccination increased the risk of acute liver injury, which was much more common after SARS-CoV-2 infection than after vaccination. Hence, our study provides further data indicating that the benefits of mass COVID-19 vaccination outweigh the potential risks.
Keywords: Acute liver injury, COVID-19 vaccine, BNT162b2, CoronaVac, case series
Graphical abstract
Introduction
Since the global outbreak of COVID-19 in early 2020, tremendous efforts have been placed on preventive vaccination. Within a very short time, several types of vaccines were approved for emergency use for immunization against severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) infection and a population-based vaccination campaign was promoted across the globe following the World Health Organization’s declaration of the pandemic. Acute liver injury (ALI) was not reported in registrational clinical trials for COVID-19 as the sample sizes were insufficient to detect rare adverse events following vaccination. Since the widespread rollout of COVID-19 vaccination programs across the globe, cases of adverse events including rare complications affecting the liver in the form of ALI mimicking autoimmune hepatitis (AIH) have been described.[1], [2], [3], [4], [5], [6] The hepatotoxicity was evidenced by elevated liver enzyme levels (either hepatocellular or cholestatic pattern) as well as elevated bilirubin levels, which presented clinically as jaundice.[1], [2], [3], [4], [5] The seropositivity rate for anti-nuclear antibody and anti-smooth muscle antibody were variable among different reports. Individual liver biopsies revealed heterogenous patterns including histopathological features typical of AIH, such as interface hepatitis, portal inflammation or non-specific changes of centrilobular necrosis.2 There were also reports of severe venous thrombosis of unusual sites, such as the portal and splenic vein, and immune thrombocytopenic purpura associated with ALI.7 , 8 Incidents were reported from recipients of various vaccines including the adenovirus-based vaccine of Oxford-AstraZeneca and the mRNA vaccines of Pfizer-BioNTech (also known as BNT162b2 mRNA) and Moderna, suggesting that these adverse events may be independent of the vaccine mechanisms.2 , 4 The concern over vaccine-associated autoimmunity was raised, while the molecular mimicry and bystander activation of proinflammatory cascade are the possible mechanisms.1 , 2
Although new variants of SARS-CoV-2 are emerging, vaccination remains an effective and reliable way to reduce disease severity, hospitalization requirement and more importantly, mortality.9 BNT162b2 mRNA vaccine and CoronaVac inactivated vaccine are the only 2 vaccines currently available in Hong Kong. Both vaccines trigger a robust adaptive immune response and the production of neutralizing antibodies.10 , 11 In response to the safety signal of ALI generated from the published case reports, this pharmacovigilance study was conducted to investigate the risk and severity of ALI following COVID-19 vaccination.
Materials and methods
Data source
Anonymized, population-wide COVID-19 vaccination records in the Hong Kong Special Administrative Region, China were obtained from the Department of Health. These records included date of vaccination, and brand of vaccine. Electronic medical records were retrieved from the Hong Kong Hospital Authority (the statutory body managing public healthcare services in the region). These records included demographics, date of registered death, drug dispensing records, diagnoses, procedures and laboratory tests. Records were linked using a unique de-identified mapping key. These 2 linked sources of data have been extensively used for COVID-19 vaccine pharmacovigilance research[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25] and drug-induced liver injury research.26
Study design and study population
A self-controlled case series (SCCS) design was used to investigate the risk of ALI following BNT162b2 and CoronaVac. The SCCS was originally developed for vaccine safety,[27], [28], [29] and has recently been used to investigate COVID-19 vaccine-associated thromboembolic events30 , 31 and hematological disorders including leukopenia, neutropenia and thrombocytopenia.18 , 30 , 32 Such hematological disorders are diagnosed based on laboratory parameters. It is appropriate to apply the SCCS design on the ALI outcome, a laboratory-based diagnosis, using the electronic medical record database of the Hong Kong Hospital Authority. Laboratory results are automatically updated and transferred to the research database daily.
The population of interest was described as adults who had received at least 1 dose of COVID-19 vaccine between 23rd February 2021 and 30th September 2021. A schematic presentation of the SCCS design is shown in Fig. 1 . Based on the requirements for SCCS analysis, we only included those diagnosed with ALI for the first time between 23rd February 2021 and 30th September 2021 who had been identified by liver function test results extracted from the electronic medical records. ALI was defined by the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin according to the criteria listed in Asia Pacific Association of Study of Liver consensus guidelines33 and the drug-induced liver injury clinical practice guidelines of the European Association for the Study of Liver Disease.34 Briefly, ALI is diagnosed when one of the following thresholds is met: 1) ≥5x upper limit of normal (ULN) elevation in ALT, 2) ≥2x ULN elevation in AST, or 3) ≥3x ULN elevation in ALT and simultaneous elevation of total bilirubin ≥2x ULN, with the absence of other etiologies, where the ULN of ALT, AST, and total bilirubin are 40 U/L, 40 U/L and 19 μmol/L, respectively.
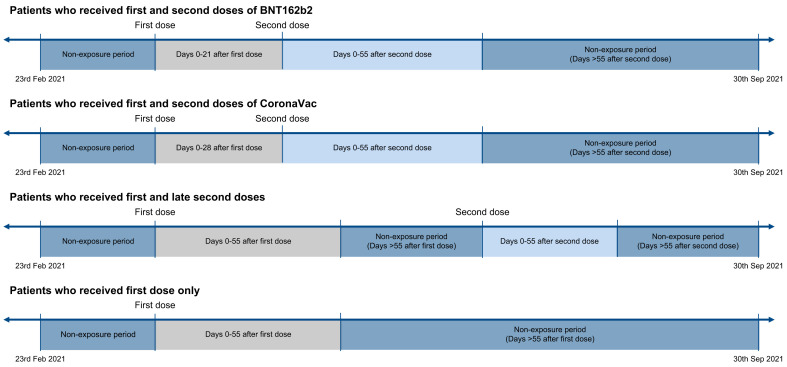
Fig. 1.
Observation timeline of patients in the modified self-controlled case series.
A review of published case reports showed ALI following COVID-19 vaccination could present from several days up to 46 days post-vaccination.35 , 36 Hence, a risk window of 56 days post-vaccination was chosen to define the exposure period. Two risk periods which covered 0-55 days after first and second doses were included. For individuals who received the second dose within 56 days of the first dose, the period after the second dose was considered as post-second dose exposure. For instance, when the second dose of vaccine was received on day 21, day 0-20 was regarded as post-first dose risk period, whereas day 21-77 was regarded as post-second dose risk period (Fig. 1). The remaining periods during the observation period were identified as non-exposure periods.
In addition, adult patients who had SARS-CoV-2 positivity on PCR between 1st September 2020 and 30th September 2021, with no prior history of ALI before the positive result, were selected to compare the risk of ALI following SARS-CoV-2 infection and following COVID-19 vaccination. Governmental statutory policy during the study period required every person with a confirmed diagnosis of SARS-CoV-2 infection to undergo mandatory hospitalization or quarantine in medical facilities, regardless of the severity of symptoms from the infection. Therefore, our study captured the data from all patients with SARS-CoV-2 infection during the specified period.
Statistical analyses
The crude incidence rate of ALI following COVID-19 vaccination (cases per 100,000 person-years) and that of ALI following SARS-CoV-2 infection (cases per 100,000 person-years) were estimated. A propensity score model in which the dependent variable was the receipt of the first dose of BNT162b2, the first dose of CoronaVac, or the diagnosis of SARS-CoV-2 infection was constructed by a logistic regression. Inverse probability of treatment weighting (IPTW) was used to equilibrate the baseline characteristics such as age, sex, pre-existing comorbidities and medication used across the 3 groups. Conditional Poisson regression models weighted by IPTW were fitted to estimate the incidence rate ratio (IRR) and 95% CI of ALI risks for BNT162b2 or CoronaVac recipients relative to patients with SARS-CoV-2 infection.
The severity of ALI following vaccination and SARS-CoV-2 infection was assessed by the following parameters: peak ALT level, peak AST level, Drug-Induced Liver Injury Network (DILIN) scale,37 the AST:ALT ratio (which has been associated with poor prognosis in SARS-CoV-2 infection),38 , 39 proportion of patients requiring hospitalization, and intensive care unit (ICU) admission. Follow-up information about the ALI, such as the proportion of patients who had a delayed second dose (>42 days after the first dose of BNT162b2 or >56 days after the first dose of CoronaVac),24 incident AIH, or new-onset liver disease was explored in those with a follow-up period of at least 28 days following ALI. Differences in ALI severity and follow-up information between the 2 vaccines were compared using ordered or binary logistic regressions adjusting for baseline characteristics.
The SCCS study design has been used in pharmacovigilance studies to investigate vaccine safety, including COVID-19 vaccines.[40], [41], [42] Three assumptions should be satisfied to ensure the appropriate use of SCCS(27). Firstly, the event should be independently recurrent such that each occurrence does not affect subsequent events. However, events could occur repeatedly and may increase the probability of future episodes. Therefore, only the first episode was treated as the outcome of interest in this study. Secondly, the event of interest should be independent of the exposure. Patients with events might be less likely to receive the vaccines and hence this assumption might not be supported when applying the standard SCCS model, especially for the second dose vaccination. Therefore, we applied a modified SCCS model, which was designed to investigate outcomes that are associated with exposure.43 Lastly, the event should not censor the observation period. The modified SCCS for event dependent exposure analysis was applied using the R function “eventdepenexp” in the R-package “SCCS”.28 IRRs and their corresponding 95% CIs were estimated using conditional Poisson regression, with non-exposure period as the reference period and length of risk period as offset variable. Unlike the classical SCCS, the modified SCCS requires unvaccinated patients with ALI during the observation period (e.g. no scheduled vaccination appointment, or cancellation of vaccination appointment if the ALI events occurred before receiving the vaccine) to guide the timing of events by enabling adjustment for the monthly seasonal effects. It is important to note that these unvaccinated patients did not act as controls. A comprehensive discussion on use of the modified SCCS for COVID-19 vaccine research can be found in a recent publication.44
To ensure the robustness of the main results, sensitivity analyses were conducted by 1) varying the duration of risk window from 14 to 42 days; 2) excluding the pre-vaccination period due to potential increased liver function abnormalities detected during the pre-vaccination interval when people might have had health check-up prior to vaccination; 3) adding pre-risk periods of days -56 to -1 prior to 2 doses as people who had events in the pre-risk period might decrease the likelihood of vaccination, 4) excluding patients with SARS-CoV-2 infection after the rollout of the vaccination program in Hong Kong as they would have had exposure to both the vaccination and natural infection which might trigger a greater immune response and increase the ALI risk; and 5) excluding patients who had no liver panel results during the observation period. Subgroup analyses of patients with and without chronic liver diseases were conducted, where chronic liver disease is defined as patients with cirrhosis, fatty liver disease, hepatitis, hepatocellular carcinoma, and use of viral hepatitis drugs.
All statistical analyses were performed with R version 4.1.1 and Stata MP v17.0 (StataCorp LLC). A 2-sided significance level of 5% was used in all statistical analyses. Two investigators (ICHA and FWTC) independently conducted the statistical analyses for quality assurance.
Ethical approval
Ethical approval for this study was granted by the Institutional Review Board of the University of HK/HA HK West Cluster (UW21-149 and UW21-138) and the Department of Health Ethics Committee (LM 21/2021).
Results
Baseline characteristics
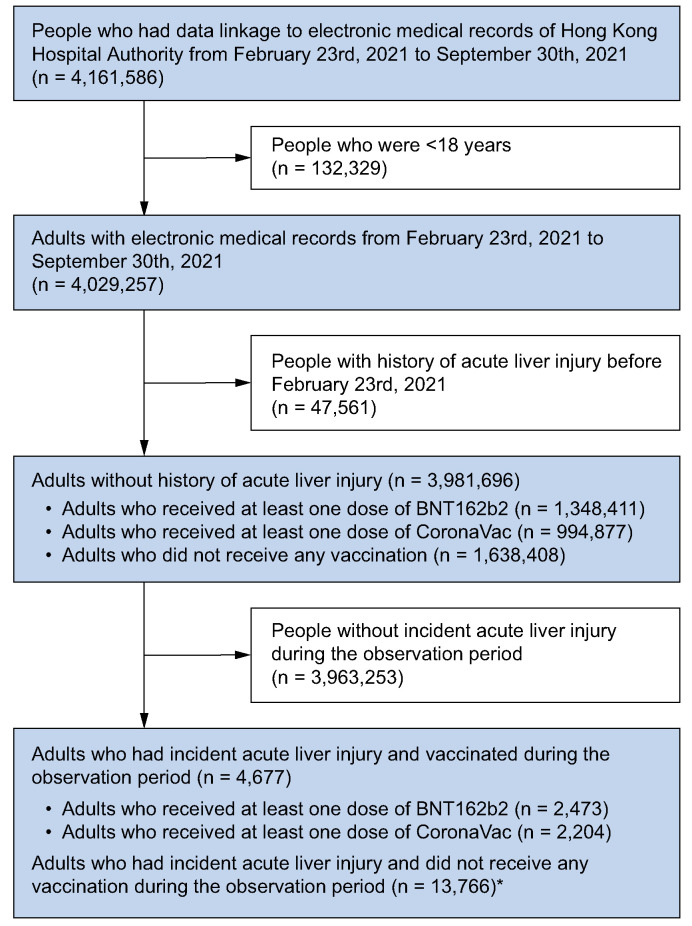
A study population of 4,029,257 people was identified from the database after excluding 132,329 people aged below 18 years. Of the 3,981,696 people without a history of ALI, 2,343,288 had been COVID-19 vaccine recipients (BNT162b2: 1,348,411; CoronaVac: 994,877) in the period between 23rd February 2021 and 30th September 2021. These include 1,267,318 (94.0%) and 915,690 (92.0%) people who had received the second dose of BNT162b2 or CoronaVac, respectively. Fig. 2 summarizes the patient deposition of the study. Table 1 shows the baseline characteristics of 4,677 patients who had been vaccinated between 23rd February 2021 to 30th September 2021 and who developed ALI for the first time during the same period. The median (interquartile age range [IQR]) of ALI patients who received BNT162b2 or CoronaVac was 52 (IQR 38-63) and 60 (IQR 49-68) years, respectively. Females accounted for 41.4% and 40.5% of ALI cases following BNT162b2 and CoronaVac vaccination, respectively.
Fig. 2.
Inclusion and exclusion criteria for modified self-controlled case series analysis of people from February 23rd, 2021 to September 30th, 2021 in Hong Kong SAR, China.
∗Unvaccinated patients with acute liver injury during the observation period (e.g. no scheduled vaccination appointment, or cancellation of vaccination appointment if the acute liver injury events occurred before the scheduled appointment) was included to inform the timing of events by adjusting for the monthly seasonal effects.44 These unvaccinated patients did not act as controls.
Table 1.
Baseline characteristics of people developing ALI for the first time between 23rd February 2021 to 30th September 2021 following BNT162b2 or CoronaVac vaccination in the self-controlled case series study.
| Baseline characteristics | Acute liver injury (n = 4,677) n (%) or median [IQR] |
|
|---|---|---|
| BNT162b2 (n = 2,473) | CoronaVac (n = 2,204) | |
| Age, yr | 52 [38-63] | 60 [49-68] |
| 18-40 | 699 (28.3%) | 233 (10.6%) |
| 41-65 | 1,281 (51.8%) | 1,266 (57.4%) |
| >65 | 493 (19.9%) | 705 (32.0%) |
| Sex | ||
| Male | 1,449 (58.6%) | 1,311 (59.5%) |
| Female | 1,024 (41.4%) | 893 (40.5%) |
| Pre-existing comorbidities | ||
| Charlson's index | 2 [0-3] | 3 [2-4] |
| 0-1 | 1,088 (44.0%) | 550 (25.0%) |
| 2-4 | 1,179 (47.7%) | 1,361 (61.8%) |
| ≥5 | 206 (8.3%) | 293 (13.3%) |
| Chronic Liver diseases | 77 (3.1%) | 93 (4.2%) |
| Myocardial infarction | 26 (1.1%) | 23 (1.0%) |
| Hypertension | 508 (20.5%) | 622 (28.2%) |
| Peripheral vascular disease | 13 (0.5%) | 8 (0.4%) |
| Cerebrovascular disease | 55 (2.2%) | 72 (3.3%) |
| Chronic obstructive pulmonary disease | 44 (1.8%) | 46 (2.1%) |
| Dementia | 4 (0.2%) | 6 (0.3%) |
| Paralysis | 3 (0.1%) | 4 (0.2%) |
| Diabetes without chronic complication | 223 (9.0%) | 315 (14.3%) |
| Diabetes with chronic complication | 15 (0.6%) | 18 (0.8%) |
| Malignancy | 112 (4.5%) | 122 (5.5%) |
| Metastatic solid tumor | 19 (0.8%) | 24 (1.1%) |
| Medications used | ||
| Renin-angiotensin-system agents | 394 (15.9%) | 427 (19.4%) |
| Beta blockers | 278 (11.2%) | 314 (14.2%) |
| Calcium channel blockers | 525 (21.2%) | 656 (29.8%) |
| Diuretics | 134 (5.4%) | 124 (5.6%) |
| Nitrates | 85 (3.4%) | 77 (3.5%) |
| Lipid lowering agents | 486 (19.7%) | 552 (25.0%) |
| Insulins | 76 (3.1%) | 70 (3.2%) |
| Antidiabetic drugs | 278 (11.2%) | 341 (15.5%) |
| NSAID | 269 (10.9%) | 201 (9.1%) |
| Antivirals | 148 (6.0%) | 183 (8.3%) |
| Antibiotics | 499 (20.2%) | 411 (18.6%) |
| Immunosuppressants | 32 (1.3%) | 18 (0.8%) |
| COVID-19 survivor | 15 (0.6%) | 4 (0.2%) |
| Received second dose | 2,158 (87.3%) | 1,840 (83.5%) |
ALI, acute liver injury; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drugs.
Clinical presentation and severity of ALI among individuals with ALI during risk periods
The median duration from vaccination to onset of ALI was 20 (IQR 11-33) days. There were 307 ALI events which occurred within 56 days after the first dose of BNT162b2, and 304 ALI events which occurred within 56 days after the first dose of CoronaVac vaccination. The crude incidence rate of 56-day ALI was 335 cases per 100,000 person-years (307 cases/91,722 person-years) for the first dose BNT162b2 recipients, and 358 cases per 100,000 person-years (304 cases/84,993 person-years) for first dose CoronaVac recipients within 56 days. A total of 521 and 474 ALI events occurred within 56 days after the second dose of BNT162b2 and CoronaVac vaccination, respectively. The crude incidence rate of 56-day ALI was 334 cases per 100,000 person-years (521 cases/156,161 person-years) for second dose BNT162b2 recipients, and 403 cases per 100,000 person-years (474 cases/117,607 person-years) for second dose CoronaVac recipients within 56 days.
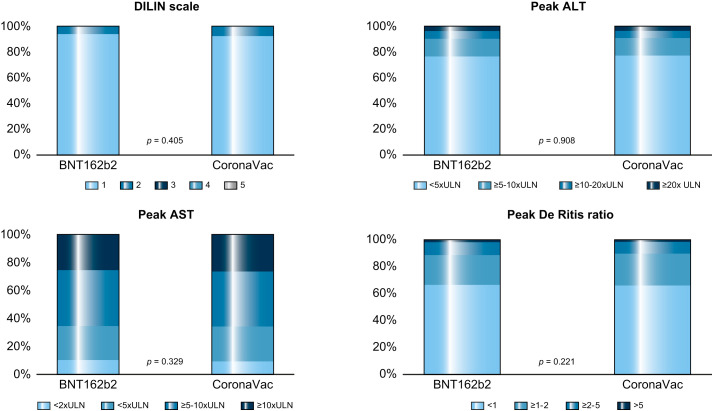
Of the 1,606 vaccinated individuals who developed ALI for the first time during the exposure periods (i.e. within 56 days after the first or second dose of COVID-19 vaccination), 676 (42.1%) patients required hospitalization (median length of stay: 4 days, IQR 2-7 days) and 19 (1.2%) stayed in the ICU during the hospitalization. In total, 2.9% of BNT162b2 recipients and 3.5% of CoronaVac recipients were hospitalized because of a primary diagnosis of ALI. The median duration of ALI was 4 days (IQR 2-7); most cases (99.2%) had resolved by day 42. No patients developed severe or fatal ALI (i.e., DILIN scale 4 or 5) in either vaccinated group. Most patients with ALI had a peak ALT <20x ULN or AST <10 x ULN, while a peak ALT >20x ULN and a peak AST >10x ULN were observed in 3.8% and 25.5% of BNT162b2 recipients, and 3.6% and 26.5% of CoronaVac recipients, respectively. There were no significant differences in the ALI severity defined by the DILIN scale (p = 0.405), peak ALT (p = 0.908), peak AST (p = 0.329), the AST:ALT ratio (p = 0.221), hospitalization rate (p = 0.237), and ICU admission (p = 0.193) between the 2 vaccine recipient groups (Fig. 3 ). Of those patients who were followed-up for at least 28 days after ALI onset, there were no significant differences in the proportion of recipients delaying the second dose of vaccine (p = 0.260), risks of incident AIH (0% for both groups) and new-onset liver diseases (p = 0.269). Chronic liver disease was the leading potential cause of post-vaccination ALI (BNT162b2: 8.0%; CoronaVac: 10.5%), followed by cholestasis/cholangitis/cholecystitis/cholelithiasis (BNT162b2: 3.3%; CoronaVac: 4.0%), and cancer (BNT162b2: 1.9%; CoronaVac: 3.7%) (Table S1).
Fig. 3.
Severity of ALI in people who received BNT162b2 and CoronaVac vaccination.
p value of differences in ALI severity between people who received BNT162b2 and CoronaVac vaccination using ordered logistic regression adjusting for baseline characteristics. ALI, acute liver injury; ALT, alanine aminotransferase, AST, aspartate aminotransferase; DILIN, Drug Induced Liver Injury Network; ULN, upper limit of normal.
Modified SCCS analysis
The total number of ALI events during the observation period in both vaccinated groups was 4,677 (2,473 from BNT162b2 and 2,204 from CoronaVac) (Fig. 2). The proportion of individuals who received both doses of vaccines were 87.3% and 83.5% for BNT162b2 and CoronaVac, respectively.
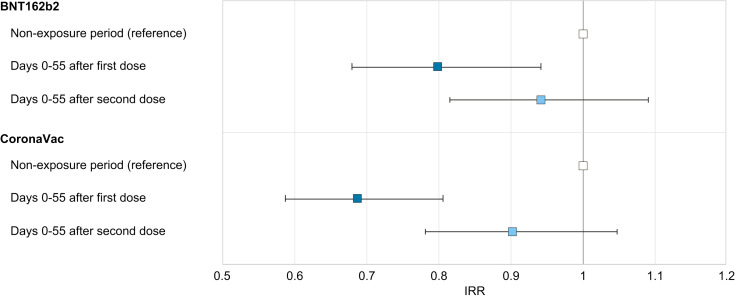
Fig. 4 and Table S2 show the IRRs of ALI following COVID-19 vaccination and a comparison by event-dependent exposure SCCS. Compared with the non-exposure period, no increased risk was observed in the 56-day risk period for the first dose of BNT162b2 (IRR 0.800; 95% CI 0.680–0.942), first dose of CoronaVac (IRR 0.689; 95% CI 0.588–0.807), the second dose of BNT162b2 (IRR 0.949; 95% CI 0.816–1.091), or the second dose of CoronaVac (IRR 0.905; 95% CI 0.781–1.048). We compared the peak liver enzymes of the non-exposure vs. the exposure period using linear regression. The peak ALT and peak AST for BNT162b2 recipients was 288 vs. 268 U/L (p = 0.204) and 216 vs. 224 U/L (p = 0.716), respectively. Similarly, the peak ALT and peak AST for CoronaVac recipients was 288 vs. 250 U/L (p = 0.030) and 245 vs. 215 U/L (p = 0.458), respectively.
Fig. 4.
Risks of acute liver injury among people who received BNT162b2 and CoronaVac vaccination in the modified self-controlled case series analysis.
Error bar is a 95% CI of the incidence rate ratio. IRR, incidence rate ratio.
Sensitivity analyses with various risk periods, adding pre-risk periods, excluding patients who died during the observation period, excluding the pre-vaccination period including patients who had liver panel results, and subgroup analyses of patients with and without chronic liver diseases, demonstrated consistent findings (Table S3) in both vaccines.
ALI risk in comparison with patients with SARS-CoV-2 infection
Of the 6,353 adult patients with SARS-CoV-2 infection without prior ALI (Table S4), the number of ALI cases within a 56-day period of SARS-CoV-2 infection was 309. Crude incidence rate was 32,997 cases per 100,000 person-years.
After propensity score weighting (Table S5), vaccinated individuals had lower 56-day ALI risks than patients with SARS-CoV-2 infection (BNT162b2: IRR 0.052, 95% CI 0.045-0.059, p <0.001; CoronaVac: IRR 0.051, 95% CI 0.045-0.058, p <0.001). The median time from SARS-CoV-2 infection to ALI onset was 9 days (IQR 4–14). The median length of hospitalization was 13 (IQR 6 to 21) days and 62 (19.1%) required ICU admission. The mean peak ALT and peak AST were 254 and 185 U/L, respectively. The proportion of patients with SARS-CoV-2 infection who developed moderate ALI (i.e., DILIN scale 2) was 3.02%, the proportion with peak ALT >20x ULN was 10.87%, and with peak AST >10x ULN was 1.63%.
Discussion
To the best of our knowledge, this is the first SCCS study using population-based data to examine the risk of ALI after COVID-19 vaccinations. The current study did not identify any increased risk of ALI after BNT162b2 or CoronaVac vaccination. Our findings suggested that the absolute risk of ALI is very low following COVID-19 vaccination and much lower than following SARS-CoV-2 infection. Over 90% of the ALI cases following COVID-19 vaccination were mild cases, while none of the ALI cases were severe or fatal.
ALI following COVID-19 vaccination was reported in cases which presented with a variety of clinical signs and laboratory results. The majority of liver injury cases in mRNA vaccine recipients showed hepatocellular injury along with high titers of autoantibodies, elevated IgG and response to corticosteroid therapy.35 , 36 Only association, but not causality, were suggested between COVID-19 vaccination and ALI due to the presence of other confounding factors in these case reports.4 , 5 Although most studies did not suggest direct vaccine-induced liver injury and AIH, vaccine-induced immune-mediated hepatitis was postulated as one of the possible mechanisms.35 , 36 , 45 Theoretically, mRNA vaccines are more immunogenic than inactivated whole-virus vaccines as observed from the superior seroconversion rates of anti-spike receptor binding domain IgG after vaccination.46 mRNA vaccines are known to be “self-adjuvanted” due to the capacity of the mRNA to stimulate innate responses via the endosomal toll-like receptors 3,7,8 and 9, as well as stimulation of type I interferon from RIG-I sensing. In comparison, inactivated whole-virus vaccines are not sufficiently immunogenic on their own and require the addition of adjuvants, which in the case of CoronaVac is aluminium hydroxide.47 Detailed analyses with head-to-head comparison of the T-cell response following mRNA vaccine or inactivated whole-virus vaccines are however lacking. One study reported a different spectrum of T-cell responses (in terms of epitopes and IFN-γ-positive T-cell response) between BNT162b2 and BBIBP-CorV (inactivated virus) but it was concluded that both vaccines were immunologically effective.48 A recent case report has shed some light on deciphering the potential immunopathogenesis of mRNA vaccine-associated AIH.49 In a 52-year-old man with clinically robust AIH which was steroid-responsive, SARS-CoV-2-specific CD8+ T cells were enriched and demonstrated an activated phenotype both peripherally and intrahepatically, in comparison with vaccinated individuals who did not develop hepatitis. In addition, CD38 expression level corresponding to T-cell activation mirrored transaminase levels and response to systemic corticosteroid therapy. These findings strongly support the hypothesis that the mRNA COVID-19 vaccine is capable of causing AIH. However, considering that 760 million doses of mRNA COVID-19 vaccine have been administrated worldwide,50 if there was a causal association, the risk is extremely small.
Taking a broader view, not all cases of ALI following COVID-19 vaccination can be accounted for by AIH. The exact mechanisms for the majority of ALI cases following COVID-19 vaccination remain unknown. Previous population-based studies have shown that drug-induced hepatoxicity occurred in 19 cases per 100,000 recipients, while hepatoxicity with autoimmune features was seen in fewer than 1 per million cases.35 , 51 In the reported cases of suspected vaccine-induced immune hepatitis, the affected individuals had good previous health with no history of autoimmune disease, or exposure to hepatotoxic agents or other AIH-related risk factors. The response to immunosuppressive treatment in those reports and the presence of autoimmune comorbidities (e.g. Hashimoto thyroiditis)52 , 53 would speak for immune-mediated events, yet a definite causality between COVID-19 vaccination and AIH could not be established.[1], [2], [3], [4] Although elevated IgG level, interface hepatitis and lymphoplasmacytic infiltrate are typical features of AIH, such features were not consistently observed in some cases associated with COVID-19 vaccination.4 , 5 Previous studies found 10% to 34% of these patients were asymptomatic and had significantly lower or normal aminotransferase and immunoglobulin levels but similar liver histopathological features as symptomatic patients; this suggests poor correlation between clinical features and liver histology.54 , 55 Therefore, we cannot exclude the possibility that the number of injurious cases has been underestimated in the literature to date, as well as in our population-based data. Indeed, access to care and patients’ hesitancy to comply with medical follow-ups during the pandemic likely masked some cases of ALI as shown in a single-center study in Germany.56 The same group reported no increased risk of AIH after COVID-19 vaccination and 25 patients with “newly diagnosed AIH” that was temporally related to the vaccine displayed features of definite pre-existing chronic liver disease.56 Therefore, the true incidence of ALI (and possibly AIH) following COVID-19 vaccination is confounded by many factors such as case identification, alternative causes of ALI, and pre-existing liver disease.
In addition, cases of immune thrombocytopenic purpura with or without coincidental liver injury were reported following COVID-19 vaccination, further supporting the possible mechanism of immune-mediated injury.5 , 7 , 57 However, this hypothesis was challenged by the lack of interaction between anti-PF-4 antibody and spike protein, and the absence of relapse.58 Because cases of AIH and immune thrombocytopenic purpura have only been reported rarely, despite the overwhelming number of COVID-19 vaccine doses administered daily and globally, direct causation between vaccination and liver injury is difficult to establish.35 Nevertheless, the vast majority of individuals with ALI following COVID-19 vaccination in our cohort presented with mild self-limiting disease, with low rates of hospitalization and ICU admission, thereby speaking against the phenomenon of vaccine-induced chronic AIH or immune-mediated DILI, which typically requires corticosteroid therapy to induce remission. In our cohort, the mean platelet count among vaccinated individuals with ALI was not significantly lower during the ALI period compared to pre-ALI onset (ALI onset: 241.8±121.3 109/L vs. pre-ALI onset 242.8±110.2 109/L, p = 0.617; data not shown), findings which are not supportive of immune phenomenon. Our analysis showed that the risk of ALI following COVID-19 vaccination was not higher than in the non-exposure period. Vaccinated individuals in Hong Kong might have had a health check-up prior to vaccination and therefore have a higher chance of ALI being detected in the pre-vaccination period. Nevertheless, the results remained the same after discounting the pre-vaccination period (Table S3). Overall, the risk of severe liver injury or liver failure was extremely low after COVID-19 vaccination. The incidence rate of ALI following COVID-19 vaccination was much less than that following SARS-CoV-2 infection. Since presentation of severe SARS-CoV-2 infection is associated with ALI,59 , 60 vaccination is recommended for protection against ALI associated with severe COVID-19.
Although vaccination should be considered a pharmaceutical product for the prevention of different diseases, there are no registries like DILIN to comprehensively track and investigate cases of ALI.61 Whether intrinsic or idiosyncratic, most cases of DILI occur within the first few weeks of exposure to a drug. Intrinsic DILI typically presents with a dose-related pattern and occurs in a large proportion of individuals exposed to the drug within a short time span (hours to days). In idiosyncratic DILI, cases are not dose-related and exhibit a variable latency to onset of days to weeks.34 Therefore, defining the exposure period as the first 55 days after vaccination in our cohort can reasonably account for the majority of ALI cases potentially associated with COVID-19 vaccination. Moreover, we performed an additional sensitivity analysis considering different lengths of exposure period, which yielded similar findings of no increase in the risk of ALI in the exposure period compared to the non-exposure period (Table S3).
There are several limitations in the current study. Firstly, our study reported a low rate of chronic liver disease among vaccinated individuals with incident ALI so it is possible that some underlying liver diseases may have been underdiagnosed. However, our previous publications have shown that vaccine recipients in Hong Kong were younger, healthier, and on fewer medications than unvaccinated people during the observation period.12 , 15 , 16 Hence, our findings may not be fully applicable to people in poor health. Nevertheless, a “healthy vaccine recipient effect” is unlikely to affect our results because of the within-person comparison nature of the SCCS. Furthermore, our study yielded negative results. This confirms that its study design does not suffer from detection bias, i.e. better surveillance of ALI post vaccination causing an apparent association. Secondly, results of liver biopsy and the levels of ductal enzymes like alkaline phosphatase were not available in our data source; only laboratory data on ALT, AST, and total bilirubin were used for outcome definition. Supportive data on autoantibodies and immunoglobulin pattern will be helpful in future studies to improve characterization of ALI and identify immune-mediated hepatitis following COVID-19 vaccination. However, severe autoimmune conditions following COVID-19 vaccination are rare.25 It is worth considering that the current literature is potentially influenced by publication bias, where cases with features of AIH following vaccination are preferentially reported. Currently, the relative number of COVID-19 vaccines administered significantly outweighs the number of SARS-CoV-2 infections on a global level.50 , 62 This could also contribute to the numerous case reports of post-vaccination ALI in the literature compared to ALI associated with natural infection, the latter being mediated by non-immunological mechanisms,63 The unmasking of pre-existing cases and the under-diagnosis of asymptomatic cases cannot be completely ruled out. However, severe cases which required emergency services and hospitalization are likely to have been captured in our study. Lastly, the assumption used in SCCS analysis meant that only new-onset ALI but not recurrent events were included in our analysis. Future studies are needed to explore the risk of ALI following COVID-19 vaccination in patients with underlying liver diseases or previous episodes of ALI.
Although case reports of severe ALI following COVID-19 vaccination have been recently described, our analytical study found no increased risk of ALI after BNT162b2 or CoronaVac COVID-19 vaccination. Our findings showed that the absolute risk of ALI is very low following COVID-19 vaccination in our cohort of over 2 million vaccine recipients. Based on all current available evidence from previous studies and our study, the potential benefit of mass vaccination far outweighs the potential ALI risk from vaccination and SARS-CoV-2 infection.
Abbreviations
AIH, autoimmune hepatitis; ALI, acute liver injury; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DILI, drug-induced liver injury; DILIN - Drug-Induced Liver Injury Network; ICU, intensive care unit; IRR, incidence rate ratio; IPTW, inverse probability of treatment weighting; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SCCS, self-controlled case series; ULN, upper limit of normal.
Financial support
Research Grant from the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (Ref. No. COVID19F01). The funders did not have any role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. FTTL and ICKW’s post were partly funded by D24H; hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission.
Authors’ contributions
CKHW and ICKW were responsible for the study concept. CKHW, LYM, ICHA reviewed the literature, designed statistical analysis, conducted analyses, wrote the manuscript; CKHW, LYM, ICHA reviewed the literature, contributed to the interpretation of the analysis, and wrote the manuscript. ICHA and FWTC conducted analyses. CKHW, LYM, FTTL, XL, EYFW, CSLC, EWYC, WYC, FWTC, MFY, ICKW contributed to the interpretation of the analysis, critically reviewed and revised the manuscript. All authors contributed to the interpretation of the analysis, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data availability statement
The data used in this study are not freely available. Approvals for the use of data were obtained from the Department of Health and the Hospital Authority specifically for this COVID-19 vaccine safety monitoring study. Authors are bound by ethical, legal and contractual conditions imposed by both Department of Health and the Hospital Authority, and are not allowed to use the data for any other purposes or divulge the data to any third parties. The vaccination record data are owned by the Department of Health. Clinical records are owned by Hospital Authority. Vaccination records were linked to clinical records on de-identified patients of the Hospital Authority. Following approvals from the Institutional Review Board, data requests were submitted and assessed by both Department of Health and Hospital Authority prior to data release for use by specified research delegates only. For further information regarding the data request and approval process, please see: (https://www3.ha.org.hk/data/Provision/Submission).
Conflicts of interest
Carlos King Ho Wong reports receipt of research funding from the EuroQoL Group Research Foundation, the Hong Kong Research Grants Council, and the Hong Kong Health and Medical Research Fund. Francisco Tsz Tsun Lai has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council. Xue Li has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, research and educational grants from Janssen and Pfizer; internal funding from University of Hong Kong; consultancy fee from Merck Sharp & Dohme, unrelated to this work. Eric Yuk Fai Wan has received research grants from the Food and Health Bureau of the Government of the Hong Kong SAR, and the Hong Kong Research Grants Council, outside the submitted work. Celine Sze Ling Chui has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, and Amgen; personal fee from Primevigilance Ltd.; outside the submitted work. Esther Wai Yin Chan reports honorarium from Hospital Authority, grants from Research Grants Council (RGC, Hong Kong), grants from Research Fund Secretariat of the Food and Health Bureau, grants from National Natural Science Fund of China, grants from Wellcome Trust, grants from Bayer, grants from Bristol-Myers Squibb, grants from Pfizer, grants from Janssen, grants from Amgen, grants from Takeda, grants from Narcotics Division of the Security Bureau of HKSAR, outside the submitted work. MF Yuen is an advisory board member and/or received research funding from AbbVie, Arbutus Biopharma, Assembly Biosciences, Bristol Myer Squibb, Dicerna Pharmaceuticals, GlaxoSmithKline, Gilead Sciences, Janssen, Merck Sharp and Dohme, Clear B Therapeutics, Springbank Pharmaceuticals; and received research funding from Arrowhead Pharmaceuticals, Fujirebio Incorporation and Sysmex Corporation, outside the submitted work. Ian Chi Kei Wong reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong RGC, and the Hong Kong Health and Medical Research Fund, National Institute for Health Research in England, European Commission, National Health and Medical Research Council in Australia.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
The authors thank the Hospital Authority and the Department of Health for the generous provision of data for this study. FTTL and ICKW’s post were partly funded by D24H; hence this work was partly supported by AIR@InnoHK administered by Innovation and Technology Commission, and Miss Jasmine Lok for English copyediting.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2022.06.032.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., Dayer E., Vergani D., et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avci E., Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: new-onset or flare-up? J Autoimmun. 2021;125 doi: 10.1016/j.jaut.2021.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodato F., Larocca A., D’Errico A., Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug-related liver injury. J Hepatol. 2021;75(5):1254–1256. doi: 10.1016/j.jhep.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clayton-Chubb D., Schneider D., Freeman E., Kemp W., Roberts S.K. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J Hepatol. 2021;75(5):1249–1250. doi: 10.1016/j.jhep.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann R., Sekhon S., Sekhon S. Drug-induced liver injury after COVID-19 vaccine. Cureus. 2021;13(7) doi: 10.7759/cureus.16491. e16491-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hines A., Shen J.G., Olazagasti C., Shams S. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccine. BMJ Case Rep. 2021;14(7) doi: 10.1136/bcr-2021-242678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. New Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . 2021. Monitoring COVID-19 vaccine effectiveness.https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/how-they-work.html Available from: [Google Scholar]
- 10.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. New Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X., Tong X., Yeung W.W.Y., Kuan P., Yum S.H.H., Chui C.S.L., et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2021;81(4):564–568. doi: 10.1136/annrheumdis-2021-221571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan E.Y.F., Chui C.S.L., Lai F.T.T., Chan E.W.Y., Li X., Yan V.K.C., et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi: 10.1016/S1473-3099(21)00451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua G.T., Kwan M.Y.W., Chui C.S.L., Smith R.D., Cheung E.C.-L., Tian T., et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai F.T.T., Huang L., Peng K., Li X., Chui C.S.L., Wan E.Y.F., et al. Post-Covid-19-vaccination adverse events and healthcare utilization among individuals with or without previous SARS-CoV-2 infection. J Intern Med. 2022;291(6):864–869. doi: 10.1111/joim.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai F.T.T., Huang L., Chui C.S.L., Wan E.Y.F., Li X., Wong C.K.H., et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun. 2022;13(1):411. doi: 10.1038/s41467-022-28068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai F.T.T., Li X., Peng K., Huang L., Ip P., Tong X., et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine. Ann Intern Med. 2022;175(3):362–370. doi: 10.7326/M21-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sing C.W., Tang C.T.L., Chui C.S.L., Fan M., Lai F.T.T., Li X., et al. COVID-19 vaccines and risks of hematological abnormalities: nested case-control and self-controlled case series study. Am J Hematol. 2022;97(4):470–480. doi: 10.1002/ajh.26478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan E.Y.F., Chui C.S.L., Wang Y., Ng V.W.S., Yan V.K.C., Lai F.T.T., et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. Lancet Reg Health – West Pac. 2022;21 doi: 10.1016/j.lanwpc.2022.100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Tong X., Wong I.C.K., Peng K., Chui C.S.L., Lai F.T.T., et al. Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study. Gut. 2022 doi: 10.1136/gutjnl-2021-326860. [DOI] [PubMed] [Google Scholar]
- 21.Xiong X., Wong C.K.H., Au I.C.H., Lai F.T.T., Li X., Wan E.Y.F., et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022;32(5):505–514. doi: 10.1089/thy.2021.0684. [DOI] [PubMed] [Google Scholar]
- 22.Li X., Lai F.T.T., Chua G.T., Kwan M.Y.W., Lau Y.L., Ip P., et al. Myocarditis following COVID-19 BNT162b2 vaccination among adolescents in Hong Kong. JAMA Pediatr. 2022;176(6):612–614. doi: 10.1001/jamapediatrics.2022.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai F.T.T., Chua G.T., Chan E.W.W., Huang L., Kwan M.Y.W., Ma T., et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population-based retrospective cohort study. Emerg Microbes Infect. 2022;11(1):885–893. doi: 10.1080/22221751.2022.2050952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong C.K.H., Xiong X., Lau K.T.K., Chui C.S.L., Lai F.T.T., Li X., et al. Impact of a delayed second dose of mRNA vaccine (BNT162b2) and inactivated SARS-CoV-2 vaccine (CoronaVac) on risks of all-cause mortality, emergency department visit, and unscheduled hospitalization. BMC Med. 2022;20(1):119. doi: 10.1186/s12916-022-02321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Gao L., Tong X., Chan V.K.Y., Chui C.S.L., Lai F.T.T., et al. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: a descriptive cohort study among 1.1 million vaccinated people in Hong Kong. J Autoimmun. 2022 doi: 10.1016/j.jaut.2022.102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J.B.J., Chui C.S.L., Suh I.H., Chen E.Y.H., Seto W.K., Mok M.T., et al. Association between nonvitamin K antagonist oral anticoagulants or warfarin and liver injury: a cohort study. Am J Gastroenterol. 2020;115(9):1513–1524. doi: 10.14309/ajg.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 27.Petersen I., Douglas I., Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 28.Weldeselassie Y.G., Whitaker H.J., Farrington C.P. Use of the self-controlled case-series method in vaccine safety studies: review and recommendations for best practice. Epidemiol Infect. 2011;139(12):1805–1817. doi: 10.1017/S0950268811001531. [DOI] [PubMed] [Google Scholar]
- 29.Whitaker H.J., Farrington C.P., Spiessens B., Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 30.Hippisley-Cox J., Patone M., Mei X.W., Saatci D., Dixon S., Khunti K., et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jabagi M.J., Botton J., Bertrand M., Weill A., Farrington P., Zureik M., et al. Myocardial infarction, stroke, and pulmonary embolism after BNT162b2 mRNA COVID-19 vaccine in people aged 75 years or older. JAMA. 2022;327(1):80–82. doi: 10.1001/jama.2021.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devarbhavi H., Aithal G., Treeprasertsuk S., Takikawa H., Mao Y., Shasthry S.M., et al. Drug-induced liver injury: Asia Pacific Association of Study of Liver consensus guidelines. Hepatol Int. 2021;15(2):258–282. doi: 10.1007/s12072-021-10144-3. [DOI] [PubMed] [Google Scholar]
- 34.EASL Clinical Practice Guidelines Drug-induced liver injury. J Hepatol. 2019;70(6):1222–1261. doi: 10.1016/j.jhep.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Bethesda (MD) 2012. Covid-19 vaccines. LiverTox: clinical and research information on drug-induced liver injury. [PubMed] [Google Scholar]
- 36.Shroff H., Satapathy S.K., Crawford J.M., Todd N.J., VanWagner L.B. Liver injury following SARS-CoV-2 vaccination: a multicenter case series. J Hepatol. 2022;76(1):211–214. doi: 10.1016/j.jhep.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute of Diabetes and Digestive and Kidney Diseases . 2012. Severity grading in drug induced liver injury Bethesda (MD)https://www.ncbi.nlm.nih.gov/books/NBK548241/ updated 2019 May 4. Available from: [PubMed] [Google Scholar]
- 38.Pranata R., Huang I., Lim M.A., Yonas E., Vania R., Lukito A.A., et al. Elevated de ritis ratio is associated with poor prognosis in COVID-19: a systematic review and meta-analysis. Front Med. 2021;8 doi: 10.3389/fmed.2021.676581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinellu A., Arru F., De Vito A., Sassu A., Valdes G., Scano V., et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest. 2021;51(1) doi: 10.1111/eci.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smeeth L., Thomas S.L., Hall A.J., Hubbard R., Farrington P., Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 41.Forbes H., Douglas I., Finn A., Breuer J., Bhaskaran K., Smeeth L., et al. Risk of herpes zoster after exposure to varicella to explore the exogenous boosting hypothesis: self controlled case series study using UK electronic healthcare data. BMJ. 2020;368:l6987. doi: 10.1136/bmj.l6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong A.Y., Wong I.C., Chui C.S., Lee E.H., Chang W.C., Chen E.Y., et al. Association between acute neuropsychiatric events and helicobacter pylori therapy containing clarithromycin. JAMA Intern Med. 2016;176(6):828–834. doi: 10.1001/jamainternmed.2016.1586. [DOI] [PubMed] [Google Scholar]
- 43.Farrington P., Whitaker H., Weldeselassie Y.G. 2018. Self-controlled case series studies. [Google Scholar]
- 44.Ghebremichael-Weldeselassie Y., Jabagi M.J., Botton J., Bertrand M., Baricault B., Drouin J., et al. A modified self-controlled case series method for event-dependent exposures and high event-related mortality, with application to COVID-19 vaccine safety. Stat Med. 2022 doi: 10.1002/sim.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 vaccination: true causality or mere association? J Hepatol. 2021;75(5):1250–1252. doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim W.W., Mak L., Leung G.M., Cowling B.J., Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2(9):e423. doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinz F.X., Stiasny K. Distinguishing features of current COVID-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccin. 2021;6(1):104. doi: 10.1038/s41541-021-00369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vályi-Nagy I., Matula Z., Gönczi M., Tasnády S., Bekő G., Réti M., et al. Comparison of antibody and T cell responses elicited by BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) vaccines against SARS-CoV-2 in healthy adult humans. Geroscience. 2021;43(5):2321–2331. doi: 10.1007/s11357-021-00471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boettler T., Csernalabics B., Salié H., Luxenburger H., Wischer L., Salimi Alizei E., et al. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022 doi: 10.1016/j.jhep.2022.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritchie H., Mathieu E., Rodés-Guirao L., Appel C., Giattino C., Ortiz-Ospina E., et al. 2020. Coronavirus pandemic (COVID-19): our world in data.https://ourworldindata.org/covid-vaccinations Available from: [Google Scholar]
- 51.Björnsson E.S., Bergmann O.M., Björnsson H.K., Kvaran R.B., Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–1425. doi: 10.1053/j.gastro.2013.02.006. 25.e1-3; quiz e19-20. [DOI] [PubMed] [Google Scholar]
- 52.Vuille-Lessard É, Montani M., Bosch J., Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021;123 doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J Hepatol. 2021;75(3):728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kogan J., Safadi R., Ashur Y., Shouval D., Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol. 2002;35(1):75–81. doi: 10.1097/00004836-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Hartl J., Miquel R., Zachou K., Wong G.-W., Asghar A., Pape S., et al. Features and outcome of AIH patients without elevation of IgG. JHEP Rep. 2020;2(3) doi: 10.1016/j.jhepr.2020.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rüther D.F., Weltzsch J.P., Schramm C., Sebode M., Lohse A.W. Autoimmune hepatitis and COVID-19: No increased risk for AIH after vaccination but reduced care. J Hepatol. 2022 doi: 10.1016/j.jhep.2022.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., et al. Thrombocytopenia following pfizer and Moderna SARS-CoV-2 vaccination. Am J Hematol. 2021;96(5):534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacy J., Pavord S., Brown K.E. VITT and second doses of Covid-19 vaccine. New Engl J Med. 2021;386(1):95. doi: 10.1056/NEJMc2118507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hundt M.A., Deng Y., Ciarleglio M.M., Nathanson M.H., Lim J.K. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. 2020;72(4):1169–1176. doi: 10.1002/hep.31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parohan M., Yaghoubi S., Seraji A. Liver injury is associated with severe coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of retrospective studies. Hepatol Res. 2020;50(8):924–935. doi: 10.1111/hepr.13510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fontana R.J., Watkins P.B., Bonkovsky H.L., Chalasani N., Davern T., Serrano J., et al. Drug-induced liver injury network (DILIN) prospective study. Drug Saf. 2009;32(1):55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.World Health Organization . 2022. WHO coronavirus (COVID-19) dashboard.https://covid19.who.int Available from: [Google Scholar]
- 63.Nardo A.D., Schneeweiss-Gleixner M., Bakail M., Dixon E.D., Lax S.F., Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are not freely available. Approvals for the use of data were obtained from the Department of Health and the Hospital Authority specifically for this COVID-19 vaccine safety monitoring study. Authors are bound by ethical, legal and contractual conditions imposed by both Department of Health and the Hospital Authority, and are not allowed to use the data for any other purposes or divulge the data to any third parties. The vaccination record data are owned by the Department of Health. Clinical records are owned by Hospital Authority. Vaccination records were linked to clinical records on de-identified patients of the Hospital Authority. Following approvals from the Institutional Review Board, data requests were submitted and assessed by both Department of Health and Hospital Authority prior to data release for use by specified research delegates only. For further information regarding the data request and approval process, please see: (https://www3.ha.org.hk/data/Provision/Submission).