Abstract
Upper aero-digestive tract (UADT) cancers are collectively cancers of various human body sites, such as the oral cavity, pharynx, oesophagus and larynx. Worldwide, they are the fourth most frequent cancer type and the fourth most common cause of mortality from cancer. Many studies have shown that several chronic diseases, such as cancer, which occur more commonly in later adulthood, are influenced by social and psychological circumstances during birth, childhood, adolescence and early adult life. It is suggested that the build up of problematic circumstances throughout life is the cause of disease, rather than circumstances that happen at one point in time. UADT cancer is a chronic disease of complex multifactorial origin and most of the underlying exposures/risks cannot be considered as individual factors or in isolation, as they act at different levels, which differ from time to time. Thus, life-course epidemiology, rather than drawing false dichotomies between different risk factors of the underlying disease, attempts to integrate biological and social risk processes that cause the chronic disease. It studies how socially patterned exposures during all stages of life – childhood, adolescence and early adult – influence disease risk in adulthood and socio-economic position and hence may account for social inequalities in adult health and mortality. Furthermore, varying health effects, according to the timing or duration of exposure to socio-economic circumstances, may indicate important traces to the causes of cancer. In this paper, we have attempted to draw a conceptual framework on the relationships between socio-economic inequalities, oral health risk factors along the life-course of an individual and incidence of UADT cancer.
Key words: Life-course, social determinants, cancer of upper aero-digestive tract
INTRODUCTION
Definition of upper aero-digestive tract cancer
Here we define squamous cell carcinomas of the upper aero-digestive tract (UADT) according to the following International Classification of Disease (ICD) cancer diagnostic groups: intra-oral sites (ICD-10 C00-C06); oro-pharynx (ICD-10 C09-C10), and other ill-defined sites of the lip, oral cavity and pharynx (ICD-10 C12-C14)1; larynx (ICD-10 C32); and oesophagus (ICD-10 C15)2. We exclude nasopharynx (C11) and salivary glands (C07-C08) as they have different risk factors and biology from the predominantly squamous cell carcinoma of the foregoing sites3. These cancer sites are often grouped together in epidemiological data4 with over 400,000 cases estimated annually5.
UADT squamous cell carcinoma is a devastating chronic disease of multifactorial origin and is an important global health problem6., 7.. Chronic diseases, such as UADT cancer, often involve diagnosis at the tail end of a long, accumulating pathological process and a stepwise accumulation of genetic alterations8., 9.. Globally, estimated age-standardised incidence rates per 100,000 for all ages and both sexes for malignant neoplasms of lip plus oral cavity are 3.8, for larynx are 2.2, for other pharynx are 2.0 and for oesophagus are 7.07. Two-thirds of the global burden of these cancer cases occurs in developing countries, with the Indian subcontinent accounting for one-third of the global burden for cancers of lip and oral cavity10. At the time of diagnosis, most UADT cancers are restricted to the primary site and the regional lymph nodes. At this stage, cancer may be cured with local treatment with surgery and/or radiotherapy11. However, when local control is not achieved within a few years of diagnosis, these cancers usually progress rapidly, resulting in mortality12., 13..
Cancer of the UADT, being a chronic disease, has roots years, decades or even generations before the date of diagnosis, or even of the earliest clinical symptoms14. Explanation for the social variations in general and oral health are incompletely explored and understood by the traditional aetiological study models that emphasise risk factors at the stage of life when studies are conducted14.
Therefore, applying the life-course approach (which is explained in the following sections) may further elucidate the relationship between socio-economic, behavioural, psychosocial and dental risk factors and the incidence and mortality of UADT cancer14., 15., 16., 17.. Furthermore, social inequalities in cause-specific adult incidence and mortality lie in socially patterned exposures in different stages of the life-course18.
RISK FACTORS FOR UADT CANCER
The wide variation in worldwide incidence and mortality from cancers of the UADT are mainly attributed to variations in exposure to the major environmental and behavioural risk factors, namely: tobacco and alcohol; low intake of fruits and vegetables; and infection with human papilloma virus (HPV)19., 20., 8., 6., 21., 22., 23., 24., 25.. Chronic trauma to the oral mucosa from poor restorations and prostheses, and poor oral hygiene with a consequent heavy bacterial load in the mouth, are re-emerging as significant risk factors26., 27., 28.. Some epidemiological studies29., 30., 31. show that employment in industries with occupational exposure to wood dust, asbestos, acid mists or solvents, and in textiles and leather manufacturing, are associated with an increased risk of UADT cancer16., 31., 30., 32.. Furthermore, a positive relationship between a family history of head and neck cancer and UADT cancer risk have been suggested by a few studies33., 34.. Low socio-economic status (SES) has been independently linked to an increased incidence of, and poorer survival for those diagnosed with, UADT cancer35., 36., 37..
Different behavioural/environmental or genetically present exposures/risk factors to health do not occur in solitude14. The sequence of episodes within the risk factors leading to an adverse health outcome includes both proximal and distal causes – proximal factors act directly or almost directly to cause disease, and distal causes are further back in the causal chain and act via a number of intermediary causes14. The risk factors that make an individual susceptible to diagnosis of a chronic disease, such as cancer, at early or later stages of life, are likely to have their roots in a complex chain of environmental events that may have begun years previously, which, in turn, were shaped by broader socio-economic determinants14. Most of the risks are interlinked and cannot be untwined from each other to be considered separately, as they act at different levels and vary over different periods of life14.
LIFE-COURSE PERSPECTIVE TO UADT CANCER
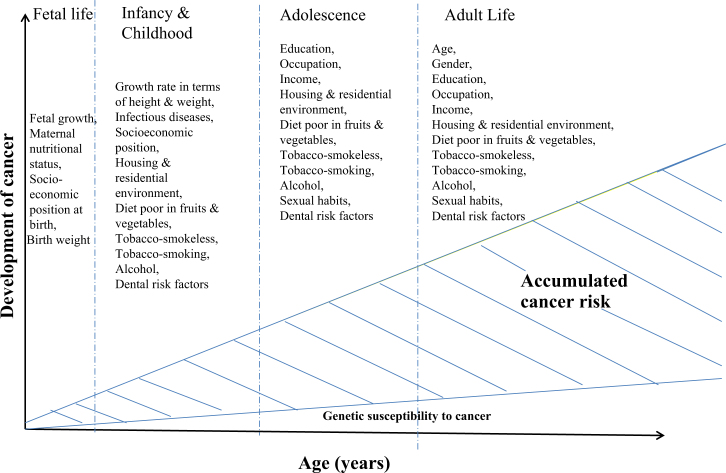
Socio-economic factors (education, occupation and family income), which are considered as determinants of health, are effective throughout life, across cause, place and time14., 31.. Their study involves two broad perspectives: one based upon the life-course; and the other emphasising contemporary life circumstances31., 38.. The life-course approach and health perspectives consider chronic disease epidemiology in terms of social and physical hazards, and the consequent biological, behavioural and psychosocial processes, that act across all stages of the lifespan – gestation, infancy, childhood, adolescence, young adulthood and midlife – to affect the disease risk or health in later adult life39.
The independent interactive and cumulative interconnections between spheres of various biological and psychological pathways and social determinants, such as education, occupation, income and family environment, and the fact that chronic disease development is influenced by participation of individuals in multiple spheres, can be studied under the life-course framework approach14., 39., 40., 41..
Figure 1 illustrates early-life factors, including biological and social factors, which may play a crucial role in causing many later-life conditions in addition to – or in synergy with – those of well-established adult exposures42., 43., 44., 45., 46., 47..
Figure 1.
A life-course perspective to upper aero-digestive tract cancer.
LIFE-COURSE EPIDEMIOLOGY – THEORETICAL MODELS (WITH RELEVANT EXAMPLES)
Life-course epidemiology is not solely a longitudinal study. It basically relies on two models: the first is a theoretical model, whereas the second is a study design14., 48.. The purpose of life-course epidemiology is to build and test theoretical models that postulate pathways linking exposures across the life-course to later-life health outcomes14., 48. (Table 1). Considering the wide range of exposures over the life-course and the potential importance of timing and duration, exposures may affect disease risk in a variety of ways. Based on this concept, life-course has been divided into four broad hypothetical models, which are listed here briefly and then are explained in detail in further sections14:
Table 1.
Structure of life-course epidemiology: concepts
| Causal pathway in relation to time |
| Accumulation |
| Chain of risk |
| Trajectory |
| Timing of casual actions (stages of life) |
| Birth cohort |
| Critical period |
| Sensitive period |
| Induction and latency period |
| Different types of mechanisms |
| Embodiment |
| Mediating |
| Modifying factors |
| Resilience |
| Susceptibility and vulnerability |
Source: Ben-Shlomo and Kuh14.
-
•
Critical period model14 – where an insult during a specific period of growth or development has a lasting, lifelong effect on physical functioning or structure that results in disease later on
-
•
Critical period influences with later modifiers of their effects14 – the later factors modify a risk incurred earlier
-
•
Accumulation of risk with correlated results14 – where risk factors cluster in socially or biologically patterned ways and hence raise the risk of disease through social and/or biological chains or pathways of risk where one adverse (or protective) experience will tend to lead to another adverse (or protective) experience in a cumulative way
-
•
Accumulation of risk with independent and uncorrelated results14 – where separate and independent risk factors at each stage of life combine to raise disease risk.
These models explicitly require the temporal ordering of exposures and their inter-relationships. The development of these models in life-course epidemiology provides a persuasive rationale for time-related study designs39., 49., 50.. The above-mentioned life-course models occur simultaneously and thereby cannot be segregated from each other. It is hard to distinguish these models empirically, or to develop standardised and acceptable methods of combining cumulative exposures48. Before we understand the four life-course models, we need to understand what the terms ‘critical periods’ and ‘sensitive periods’ mean. Therefore, a brief description of these terms is given in the following section.
CRITICAL PERIOD VERSUS SENSITIVE PERIODS
Qualitatively different exposure-time interactions throughout life describe the ‘critical’ and ‘sensitive’ periods. During both of these periods, exposure to disease risk factors are deterministic, or especially powerful, in predisposing to, or lessening the risk of, disease in later stages of life14., 39., 48.. Early life exposures that must occur in some specified window(s) of time, and often involve exposures that alter normal biological development which may have a long-term effect on body function or disease (protective or an adverse effect), are referred to as the ‘critical period’39., 48.. A broader class of influences that may have greater impact on later outcomes if they occur in certain periods outside the developmental time window are referred to as the ‘sensitive period’, which is more likely to be seen in behavioural development39., 48.. The accumulation of risk – chain of risk or pathway model – can have an additive or a trigger effect on the incidence of cancer in later stages of life.
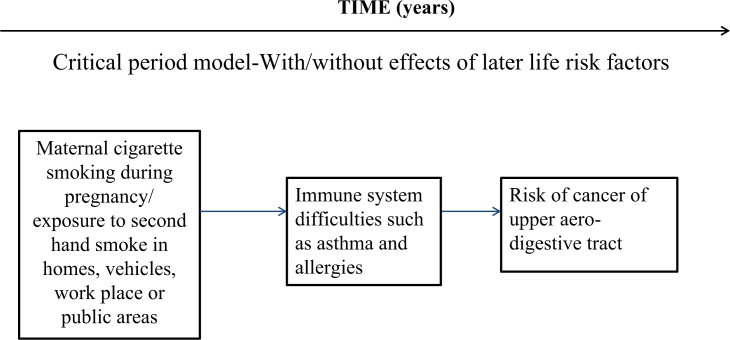
MODEL 1: CRITICAL PERIOD
The critical period model can be with or without effect of later-life risk factors or with later-life effect modifiers14. Critical period of development refers to a time window when intrinsic changes in the organisation of living systems or subsystems towards increasing complexity, greater adaptivity and more efficient functioning occurs rapidly and may be most easily modified in a favourable or unfavourable direction51. This is also known as ‘biological programming’ or as a ‘latency model’38, and is the basis of the ‘fetal origins of adult disease hypothesis’52.
The period of in utero development is one of the most critical windows during which adverse intrauterine conditions and exposures may influence the growth and development of the fetus as well as its future postnatal health and behaviour, for example: maternal cigarette smoking during pregnancy has been associated with altered DNA methylation and dysregulated expression of microRNA. Another example of ‘biological programming’ can be explained by the following example: the delay in tooth emergence at 12 months of age was associated with those who were shorter at birth and those who were classified as stunted53. Also, the prevalence of not having lower permanent molars at 6 years of age was higher among children who had a chronic malnutrition indicator at 6 months of life53.
During this important period, ‘critical windows’ are narrow and certain disturbances may alter fetal growth and development, leading to health and behavioural consequences that manifest, and possibly persist, across the life-course53 as illustrated in Figure 2, Model 154., 55..
Figure 2.
Life-course epidemiology: Model 1 – critical period/sensitive period, with/without effects of later-life risk factors.
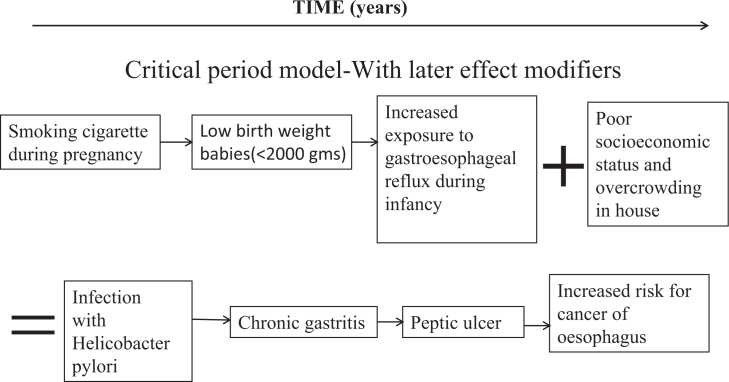
MODEL 2: CRITICAL PERIOD MODEL WITH LATER EFFECTS MODIFIERS
This model is an extension of the first model. Active risk factors in later stages of life may interact with exposures in early life either to increase the effects on cancer (‘synergism’) or to diminish them (‘antagonism’)14. This model includes the possibility that the effect of an exposure during the critical period of development on later disease risk may be suddenly altered by later active stressors – physiological or psychological14., 56., 57., 58. – as illustrated in Figure 3, Model 2. An example of this period can be taken from a study investigating the effect of breastfeeding and non-nutritive sucking habits on the occlusion patterns at 6 years of age, which highlighted that the presence of breastfeeding combined with the non-use of pacifiers ensured a protective effect of the presence of posterior cross-bite in the primary dentition59.
Figure 3.
Life-course epidemiology: Model 2 – critical period/sensitive period with later effect modifiers.
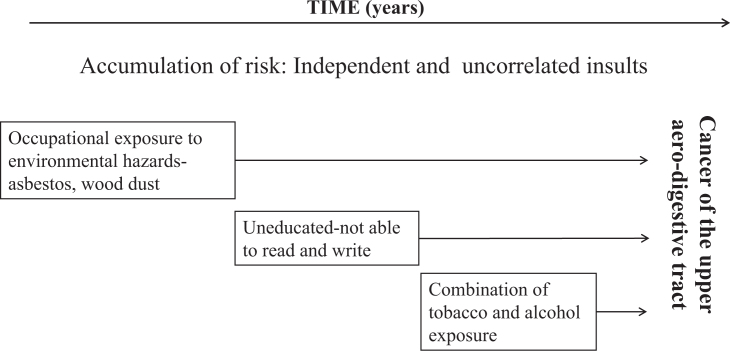
MODEL 3: ACCUMULATION OF RISK – INDEPENDENT AND UNCORRELATED INSULTS
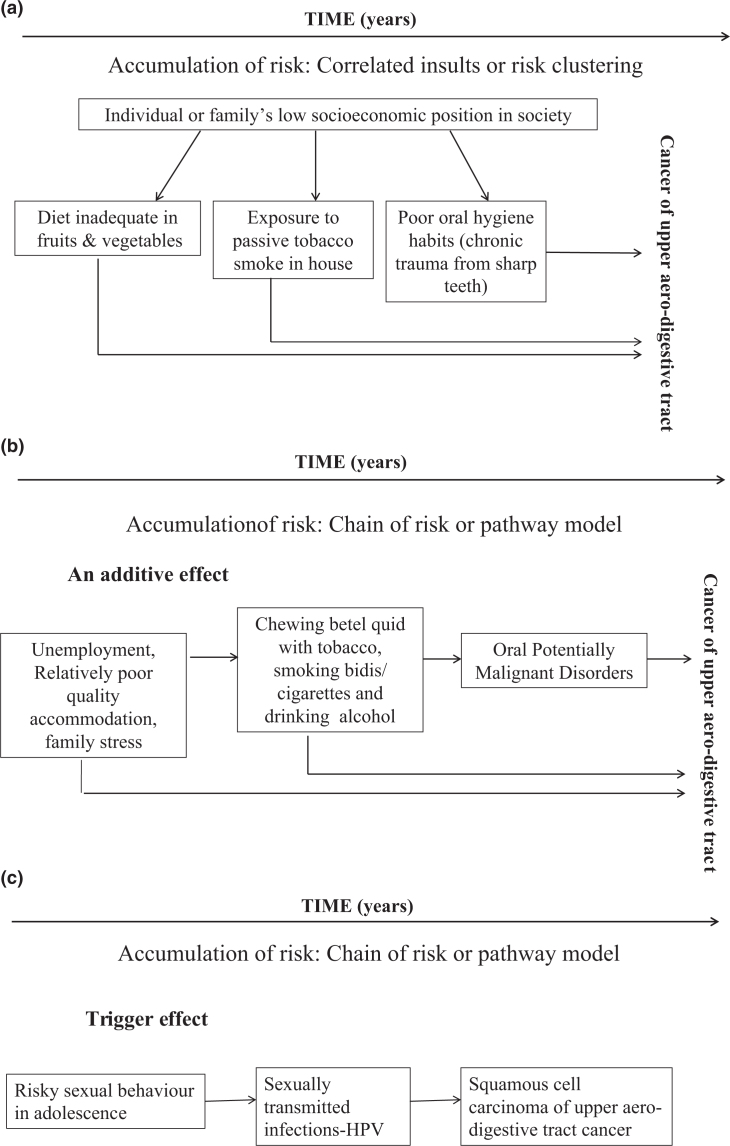
In contrast to the first two models55, the ‘accumulation of risk model’ assumes that risks to health or factors that promote good health amalgamate gradually over the life-course (through episodes of illness and injury, unfavourable environmental conditions and health-undesirable behaviours) without neglecting factors acting at sensitive developmental periods, which may have a greater impact on health in later life. This idea is complementary to the notion of allostatic load59 which means that as the number, duration and severity of exposures increases, there is increasing cumulative damage to biological systems59. Risk exposures may be independent and uncorrelated insults, as illustrated in Figure 4, Model 3, or clustered, as shown in Figure 5a, Model 455., 60., 61., 62.; the latter is known as an ‘accumulation model with risk clustering’14., 39..
Figure 4.
Life-course epidemiology: Model 3: accumulation of risk. Independent and uncorrelated results.
Figure 5.
Life-course epidemiology: Model 4: accumulation of risk. (a) Correlated insults or risk clustering. (b) Chain of risk or pathway model (additive effect). (c) Chain of risk or pathway model (trigger effect). HPV, human papillomavirus.
MODEL 4: ACCUMULATION OF RISK – CORRELATED INSULTS OR RISK CLUSTERING
A common reason for risk factors clustering is where all exposures relate to an individual’s or family’s socio-economic position in society, as illustrated in Figure 5a, Model 4.
MODEL 4: ACCUMULATION OF RISK – CHAIN OF RISK OR PATHWAY MODEL – AN ADDITIVE EFFECT
A ‘chain of risk or pathway model – an additive effect’ refers to a sequence of linked exposures that lead to impaired function and an increased likelihood of getting the disease, as one negative experience/exposure/risk leads to another, and so on (in a probabilistic manner)38; it may also have an independent ‘additive effect’ on later function or disease, as shown in Figure 5b, Model 460., 61., 62..
Alternatively, a ‘trigger effect’ describes a chain of risk where it is only the final association in the chain that has any considerable influence on the outcome, as shown in Figure 5c, Model 461., 62..
The life-course models as explained above are inseparable and may operate concurrently. It may not be easy to distinguish these models empirically or to develop standardised and acceptable methods of combining cumulative exposures14. Use of the life grid technique has been shown to improve recall and the reliability of past recalled information in life-course model-based studies63.
Few studies have explored the relationship between life-course risk factors and the incidence of UADT cancer. However, the results of a recent European study suggest that a downward life-course social trajectory is an unbiased discrete risk factor for head and neck cancer among men31. Studies in the field of breast, prostate and testicular cancers have obtained similar results50. Similarly, a couple of previous studies provide evidence that parental health and their health behaviours have direct and early impacts on their children, placing them at greater risk for subsequent detrimental health outcomes64., 65.. On the contrary, others have reported loss of effect of social factors when adjusted for smoking and alcohol66.
Nonetheless, there are large gaps in knowledge to confirm any definite linkage or influence of socio-economic determinants along the life-course of an individual on incidence of UADT cancer.
CHALLENGES WITH THE LIFE – COURSE MODEL
Understanding the life-course is about understanding lives through time. This requires the researchers to consider the dynamic process and multidimensional nature of health and well-being in adulthood associated with time and place. Another barrier for application of life-course approach to cancer is the lack of intermediate end-points, such as serum lipids and hypertension (which exists for cardiovascular diseases), inadequate knowledge of the biological mechanism related to the development of cancer and the relative rarity of specific cancers in populations14. Case–control studies are limited in types and in accuracy of early life information obtained through recall from adult participants. Missing data as a result of low response rates among participants or surrogates, and the inability to recall exposures in the distant past, presents with other major difficulties14.
Apart from the initial study design, implementation of life-course models present many methodological challenges. They include the analytical problems associated with modelling repeat observations, hierarchical data, latent exposures or multiple interactive or small effects14., 67.. When distal exposures operate through different levels of risk factors, their complete impact may not be recorded in traditional regression analysis methods in which both proximal and distal variables are included. More complex multilevel models and characterisation of causal webs of interactions among risk factors may lead to more appropriate estimates, as well as facilitating estimation of the effect of simultaneous changes in two or more risk factor distributions68.
POLICY IMPLICATIONS
Explicit recognition of life-course models can help improve the design and effectiveness of future intervention programmes to promote healthy aging69. Based on life-course theories and models, policies targeting health prevention at individual and at social levels should target the health care of infants, children and adults. This would require a major reconfiguration of existing health-delivery systems, with more focus on longitudinal integration of services over time49. Investment in child health care would be a key stage for preventing many risk factors that increase the incidence of chronic diseases in adulthood. For example, instructions given to children attending dental clinics, by a dental hygienist/dentist, regarding maintaining good oral hygiene at all stages of life during adolescence, have a positive impact in reduction of tooth loss70., 71.. Another example could be advocating a message about being a non-smoker, or, for a young adult who smokes, the importance of smoking cessation72. The reformed health policies should target the socio-behavioural development, rather than the health outcomes only.
CONCLUSIONS
Chronic diseases, such as cancer, are a major public health problem in both developed and developing countries and there is increasing evidence that life-course exposures during intrauterine growth, postnatal growth and developmental trajectories play a crucial role in the development of these diseases73. Poverty and socio-economic disadvantage are integral in the complex aetiopathogenesis of many chronic diseases74. Most longitudinal studies on the association of life-course exposure with development of cancer have been performed in developed countries and there is a great need to collect data from developing countries so that consistency between exposure and disease associations can be examined in different contexts38. Research has proved that a more holistic approach to cancer is more effective than the medical approach alone.
Acknowledgement
The authors have no acknowledgements.
Conflict of interests
The authors have no competing interests.
REFERENCES
- 1.Slootweg PJ, Eveson JW. In: World Health Organisation Classification of Tumours Pathology and Genetics of Head and Neck Tumours. Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Lyon; IARC: 2005. Introduction: tumours of the oral cavity and oropharynx; pp. 166–167. [Google Scholar]
- 2.Richiardi L, Corbin M, Marron M, et al. Occupation and risk of upper aerodigestive tract cancer: the ARCAGE study. Int J Cancer. 2012;130:2397–2406. doi: 10.1002/ijc.26237. [DOI] [PubMed] [Google Scholar]
- 3.Bell D, Hanna EY. Salivary gland cancers: biology and molecular targets for therapy. Curr Oncol Rep. 2012;14:166–174. doi: 10.1007/s11912-012-0220-5. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. International Classification of Diseases: Malignant neoplasms of lip, oral cavity and pharynx 2007. Available from: http://apps.who.int/classifications/apps/icd/icd10online/. Accessed 19 January 2015
- 5.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Pelucchi C, Gallus S, Garavello W, et al. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol Res Health. 2006;29:193–198. [PMC free article] [PubMed] [Google Scholar]
- 7.Ferlay J, Soerjomataram I, Ervik M, et al. International Agency for Research on Cancer; Lyon, France: 2012. GLOBOCAN v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Available from: http://globocan.iarc.fr. Accessed 19 August 2014. [Google Scholar]
- 8.Gupta B, Johnson NW. Emerging and established global life-style risk factors for cancer of upper aero-digestive tract. Asian Pac J Cancer Prev. 2014;15:5983–5991. doi: 10.7314/apjcp.2014.15.15.5983. [DOI] [PubMed] [Google Scholar]
- 9.Muir C, Weiland L. Upper aerodigestive tract cancers. Cancer. 1995;75:147–153. doi: 10.1002/1097-0142(19950101)75:1+<147::aid-cncr2820751304>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Subramanian S, Sankaranarayanan R, Bapat B, et al. Cost-effectiveness of oral cancer screening: results from a cluster randomized controlled trial in India. Bull World Health Organ. 2009;87:200–206. doi: 10.2471/BLT.08.053231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sloan D, Goepfert H. Conventional therapy of head and neck cancer. Hematol Oncol Clin North Am. 1991;5:601–625. [PubMed] [Google Scholar]
- 12.Boysen M, Natvig K, Winther FO, et al. Value of routine follow-up in patients treated for squamous cell carcinoma of the head and neck. J Otolaryngol. 1985;14:211–214. [PubMed] [Google Scholar]
- 13.Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Shlomo Y, Kuh D. A life-course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- 15.Nicolau B, Marcenes W, Bartley M, et al. A life-course approach to assessing causes of dental caries experience: the relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res. 2003;37:319–326. doi: 10.1159/000072162. [DOI] [PubMed] [Google Scholar]
- 16.Nicolau B, Thomson WM, Steele JG, et al. Life-course epidemiology: concepts and theoretical models and its relevance to chronic oral conditions. Community Dent Oral Epidemiol. 2007;35:241–249. doi: 10.1111/j.1600-0528.2007.00332.x. [DOI] [PubMed] [Google Scholar]
- 17.Marmot MG, Wilkinson RG. 2nd ed. Oxford University Press; Oxford: 2006. Social Determinants of Health. [Google Scholar]
- 18.Davey Smith G, Gunnell DJ, Ben-Shlomo Y. In: Poverty, Inequality and Health: An International Perspective. Leon D, Walt G, editors. Oxford University Press; Oxford: 2000. Life-course approaches to socio-economic differentials in cause specific adult mortality; pp. 88–124. [Google Scholar]
- 19.Ansary-Moghaddam A, Martiniuk A, Lam TH, et al. Smoking and the risk of upper aero digestive tract cancers for men and women in the Asia-Pacific region. Int J Environ Res Public Health. 2009;6:1358–1370. doi: 10.3390/ijerph6041358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polesel J, Talamini R, La Vecchia C, et al. Tobacco smoking and the risk of upper aero-digestive tract cancers: a reanalysis of case-control studies using spline models. Int J Cancer. 2008;122:2398–2402. doi: 10.1002/ijc.23385. [DOI] [PubMed] [Google Scholar]
- 21.Boeing H, Dietrich T, Hoffmann K, et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control. 2006;17:957–969. doi: 10.1007/s10552-006-0036-4. [DOI] [PubMed] [Google Scholar]
- 22.Dal Maso L, La Vecchia C, Polesel J, et al. Alcohol drinking outside meals and cancers of the upper aero-digestive tract. Int J Cancer. 2002;102:435–437. doi: 10.1002/ijc.10723. [DOI] [PubMed] [Google Scholar]
- 23.Franceschi S, Levi F, Conti E, et al. Energy intake and dietary pattern in cancer of the oral cavity and pharynx. Cancer Causes Control. 1999;10:439–444. doi: 10.1023/a:1008918104757. [DOI] [PubMed] [Google Scholar]
- 24.Anantharaman D, Gheit T, Waterboer T, et al. Human papillomavirus infections and upper aero-digestive tract cancers: the ARCAGE study. J Natl Cancer Inst. 2013;105:536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 25.Gupta B, Ariyawardana A, Johnson NW. Oral cancer in India continues in epidemic proportions: evidence base and policy initiatives. Int Dent J. 2013;63:12–25. doi: 10.1111/j.1875-595x.2012.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piemonte ED, Lazos JP, Brunotto M. Relationship between chronic trauma of the oral mucosa, oral potentially malignant disorders and oral cancer. J Oral Pathol Med. 2010;39:513–517. doi: 10.1111/j.1600-0714.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 27.Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34:284–291. [PubMed] [Google Scholar]
- 28.Rosenquist K, Wennerberg J, Schildt EB, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 29.Conway DI, McMahon AD, Smith K, et al. Socioeconomic factors influence selection and participation in a population-based case-control study of head and neck cancer in Scotland. J Clin Epidemiol. 2008;61:1187–1193. doi: 10.1016/j.jclinepi.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Jayaprakash V, Natarajan KK, Moysich KB, et al. Wood dust exposure and the risk of upper aero-digestive and respiratory cancers in males. Occup Environ Med. 2008;65:647–654. doi: 10.1136/oem.2007.036210. [DOI] [PubMed] [Google Scholar]
- 31.Schmeisser N, Conway DI, McKinney PA, et al. Life-course social mobility and risk of upper aerodigestive tract cancer in men. Eur J Epidemiol. 2010;25:173–182. doi: 10.1007/s10654-010-9429-5. [DOI] [PubMed] [Google Scholar]
- 32.Maier H, Tisch M, Enderle G, et al. Occupational exposure to paint, lacquer and solvents, and cancer risk in the area of the upper aero-digestive tract. HNO. 1997;45:905–908. doi: 10.1007/s001060050172. [DOI] [PubMed] [Google Scholar]
- 33.Negri E, Boffetta P, Berthiller J, et al. Family history of cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Int J Cancer. 2009;124:394–401. doi: 10.1002/ijc.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein AM, Blot WJ, Greenberg RS, et al. Familial risk in oral and pharyngeal cancer. Eur J Cancer B Oral Oncol. 1994;30B:319–322. doi: 10.1016/0964-1955(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 35.Conway DI, McKinney PA, McMahon AD, et al. Socioeconomic factors associated with risk of upper aerodigestive tract cancer in Europe. Eur J Cancer. 2010;46:588–598. doi: 10.1016/j.ejca.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Conway DI, McMahon AD, Smith K, et al. Components of socioeconomic risk associated with head and neck cancer: a population-based case-control study in Scotland. Br J Oral Maxillofac Surg. 2010;48:11–17. doi: 10.1016/j.bjoms.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Sharpe KH, McMahon AD, McClements P, et al. Socioeconomic inequalities in incidence of lung and upper aero-digestive tract cancer by age, tumour subtype and sex: a population-based study in Scotland (2000-2007) Cancer Epidemiol. 2012;36:e164–e170. doi: 10.1016/j.canep.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Hertzman C, Power C, Matthews S, et al. Using an interactive framework of society and lifecourse to explain self-rated health in early adulthood. Soc Sci Med. 2001;53:1575–1585. doi: 10.1016/s0277-9536(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 39.Kuh D, Ben-Shlomo Y, Lynch J, et al. Life-course epidemiology. J Epidemiol Community Health. 2003;57:778–783. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chittleborough CR, Baum FE, Taylor AW, et al. A life-course approach to measuring socioeconomic position in population health surveillance systems. J Epidemiol Community Health. 2006;60:981–992. doi: 10.1136/jech.2006.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. doi: 10.1007/s11095-008-9661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall AJ, Yee LJ, Thomas SL. Life-course epidemiology and infectious diseases. Int J Epidemiol. 2002;31:300–301. [PubMed] [Google Scholar]
- 43.McCormack VA, dos Santos Silva I, De Stavola BL, et al. Fetal growth and subsequent risk of breast cancer: results from long term follow up of Swedish cohort. BMJ. 2003;326:248. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore SE, Cole TJ, Collinson AC, et al. Prenatal or early postnatal events predict infectious deaths in young adulthood in rural Africa. Int J Epidemiol. 1999;28:1088–1095. doi: 10.1093/ije/28.6.1088. [DOI] [PubMed] [Google Scholar]
- 45.Power C, Manor O, Matthews S. The duration and timing of exposure: effects of socioeconomic environment on adult health. Am J Public Health. 1999;89:1059–1065. doi: 10.2105/ajph.89.7.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith GD, Hart C, Blane D, et al. Adverse socioeconomic conditions in childhood and cause specific adult mortality: prospective observational study. BMJ. 1998;316:1631–1635. doi: 10.1136/bmj.316.7145.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bircher J. Towards a Dynamic Definition of Health and Disease. Med Health Care Philos. 2005;8:335–341. doi: 10.1007/s11019-005-0538-y. [DOI] [PubMed] [Google Scholar]
- 48.Mishra G, Ben-Shlomo Y, Kuh D. In: Handbook of Behavioral Medicine. Steptoe A, editor. Springer; New York: 2010. A life course approach to health behaviors: theory and methods; pp. 525–539. [Google Scholar]
- 49.Halfon N, Hochstein M. Life-course health development: an integrated framework for developing health, policy, and research. Milbank Q. 2002;80:433–479. doi: 10.1111/1468-0009.00019. iii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch J, Smith GD. A life-course approach to chronic disease epidemiology. Annu Rev Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 51.Scott JP, Stewart JM, De Ghett VJ. Critical periods in the organization of systems. Dev Psychobiol. 1974;7:489–513. doi: 10.1002/dev.420070602. [DOI] [PubMed] [Google Scholar]
- 52.Barker DJP. Growth in utero and coronary heart disease. Nutr Rev. 1996;54:S1–S7. doi: 10.1111/j.1753-4887.1996.tb03864.x. [DOI] [PubMed] [Google Scholar]
- 53.Bastos JL, Peres MA, Peres KG, et al. Infant growth, development and tooth emergence patterns: a longitudinal study from birth to 6 years of age. Arch Oral Biol. 2007;52:598–606. doi: 10.1016/j.archoralbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Knopik VS, Maccani MA, Francazio S, et al. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol. 2012;24:1377–1390. doi: 10.1017/S0954579412000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra GD, Cooper R, Kuh D. A life-course approach to reproductive health: theory and methods. Maturitas. 2010;65:92–97. doi: 10.1016/j.maturitas.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lina F, Sven C, Matteo B, et al. Risk of oesophageal adenocarcinoma among individuals born preterm or small for gestational age. Eur J Cancer. 2013;49:2207–2213. doi: 10.1016/j.ejca.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 57.Forssell L, Cnattingius S, Bottai M, et al. Risk of esophagitis among individuals born preterm or small for gestational age. Clin Gastroenterol Hepatol. 2012;10:1369–1375. doi: 10.1016/j.cgh.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 58.Jansson C, Johansson AL, Nyren O, et al. Socioeconomic factors and risk of esophageal adenocarcinoma: a nationwide Swedish case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1754–1761. doi: 10.1158/1055-9965.EPI-05-0140. [DOI] [PubMed] [Google Scholar]
- 59.Peres KG, Barros AJ, Peres MA, et al. Effects of breastfeeding and sucking habits on malocclusion in a birth cohort study. Rev Saude Publica. 2007;41:343–350. doi: 10.1590/s0034-89102007000300004. [DOI] [PubMed] [Google Scholar]
- 60.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 61.Kraunz KS, McClean MD, Nelson HH, et al. Duration but not intensity of alcohol and tobacco exposure predicts p16INK4A homozygous deletion in head and neck squamous cell carcinoma. Cancer Res. 2006;66:4512–4515. doi: 10.1158/0008-5472.CAN-05-3748. [DOI] [PubMed] [Google Scholar]
- 62.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 63.Lu HX, Wong MC, Lo EC, et al. Trends in oral health from childhood to early adulthood: a life course approach. Community Dent Oral Epidemiol. 2011;39:352–360. doi: 10.1111/j.1600-0528.2011.00611.x. [DOI] [PubMed] [Google Scholar]
- 64.Blane D, Berney L, Smith GD, et al. Reconstructing the life-course: health during early old age in a follow-up study based on the Boyd Orr cohort. Public Health. 1999;113:117–124. doi: 10.1016/s0033-3506(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 65.Cowell AJ. The relationship between education and health behavior: some empirical evidence. Health Econ. 2006;15:125–146. doi: 10.1002/hec.1019. [DOI] [PubMed] [Google Scholar]
- 66.Gilman SE, Martin LT, Abrams DB, et al. Educational attainment and cigarette smoking: a causal association? Int J Epidemiol. 2008;37:615–624. doi: 10.1093/ije/dym250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blane D, Netuveli G, Stone J. The development of life course epidemiology. Rev Epidemiol Sante Publique. 2007;55:31–38. doi: 10.1016/j.respe.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 68.De Stavola BL, Nitsch D, dos Santos Silva I, et al. Statistical issues in life-course epidemiology. Am J Epidemiol. 2006;163:84–96. doi: 10.1093/aje/kwj003. [DOI] [PubMed] [Google Scholar]
- 69.Berkman LF. Social epidemiology: social determinants of health in the United States: are we losing ground? Annu Rev Public Health. 2009;30:27–41. doi: 10.1146/annurev.publhealth.031308.100310. [DOI] [PubMed] [Google Scholar]
- 70.Tu YK, Woolston A, Baxter PD, et al. Assessing the impact of body size in childhood and adolescence on blood pressure: an application of partial least squares regression. Epidemiology. 2010;21:440–448. doi: 10.1097/EDE.0b013e3181d62123. [DOI] [PubMed] [Google Scholar]
- 71.Watt R. Health. Education Authority; London: 1999. Oral Health Promotion: A Guide to Effective Working in Pre-School Settings. [Google Scholar]
- 72.Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003;18:1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu S, Jones RN, Glymour MM. Implications of lifecourse epidemiology for research on determinants of adult disease. Public Health Rev. 2010;32:489–511. doi: 10.1007/BF03391613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poulton R, Caspi A, Milne BJ, et al. Association between children’s experience of socioeconomic disadvantage and adult health: a life-course study. Lancet. 2002;360:1640–1645. doi: 10.1016/S0140-6736(02)11602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]