Abstract
Introduction:
Tobacco manufacturers design and marketed products with appealing sensory characteristics to drive product uptake and continued use. We assessed smokers’ and non-smokers’ cognitive, affective, and sensory responses to Camel Snus (CS) and Nicotine gum (NG) to gauge future intentions to use.
Method:
In a single laboratory session, 348 participants (including current smokers and nonsmokers in Buffalo, NY and Boston, MA) were exposed to CS and NG products in counterbalanced order. Exposure involved a cumulative set of 3 steps in which participants i) viewed an advertisement; ii) viewed the packaging, and iii) touched and smelled the product, without actual use. Current daily and non-daily smokers were invited to undertake a fourth exposure step by sampling the product. Following product exposure, participants completed perception measures and reported future intentions to use either product at the end of the survey. After each exposure, participants’ reported feelings of valence and arousal.
Results:
Smokers reported greater preference to try NG (63.8%) compared with CS (17.4%) or neither (18.8%), whereas majority of nonsmokers preferred neither product (64.3%) (p<0.01). Of those offered to sample the products, 78.3% daily smokers and 68.4% non-daily smokers opted to sample. When asked about intentions to try, a greater proportion of smokers stated a preference to try NG over CS, as did the small number of nonsmokers who expressed a preference.
Conclusion:
Intentions to try CS were low despite different levels of exposure to product, and this low product appeal and interest in use may translate to limited potential of CS to serve as a reduced harm product for smokers.
Keywords: tobacco, nicotine, smokeless, harm reduction, advertising, packaging
1. Introduction
Trial and adoption of new tobacco products is a function of two related areas of consumer response: 1) perceptions of messaging, including knowledge, attitudes, and cognitive responses to marketing and promotion; and 2) response to product use, including affective, sensory, and subjective responses (O’Connor et al., 2018; Rees et al., 2009). As cigarette use has declined in the United States, the smokeless tobacco (SLT) market has expanded. Cigarette manufacturers entered the U.S. SLT market in 2006 and promoted Swedish-style snus products as an alternative to cigarettes, often framed around circumvention of smoke-free laws and with implied health claims (Mejia et al., 2010). Both smoking and snus use have also been associated with low socio-economic status, which could be an important factor for consideration among cigarette smokers’ when planning to switch products for harm reduction (Tiora et al., 2020). Several Norwegian studies have demonstrated a link between a sharp rise in snus use with lowered smoking prevalence, providing a foundation for population-level tobacco harm reduction impact (Tiora et al.,2020; Foulds et al., 2003; Ramström, & Foulds, 2006; Rodu et al., 2002; Gilljam and Galanti, 2003). However, it remains unresolved as to whether increased use of SLT and nicotine gum (NG) are causally related to a decline in smoking rates in Sweden (Henningfield and Fagerstrom, 2001), and the potential risk of unintended consequences from risk of continued nicotine dependence (Henningfield and Fagerstrom, 2001; Bergsvik and Rogeberg, 2018)
A range of products are now available that provide an alternative to smokers seeking nicotine but with lower exposure to the carcinogens that are typical of combusted cigarette products. NG is a long-standing oral nicotine replacement therapy (NRT) used as a first-line smoking cessation medication which has also been suggested as a potential path toward harm reduction for smokers (Kozlowski & O’Connor, 2002; Abrams et al., 2018; Le Houezec et al., 2011). In 2013, the FDA authorized longer-term use of NRT to prevent smoking relapse. The product also became available in smaller-count boxes (e.g. 20 pieces) to reduce cost barriers. However, there is little evidence of substantial growth in nicotine gum use for harm reduction (Felberbaum, 2013). Meanwhile, tobacco manufacturers have begun to request modified risk tobacco product (MRTP) marketing authorizations for smokeless tobacco products, including General Snus (authorized in 2019), Camel Snus (CS) and Copenhagen (both pending approval). More recently, tobacco companies have introduced ‘modern oral’ products that appear to share many similarities to snus (nicotine pouches such as Zyn and On) and NG (hard and soft lozenges including Velo). The rapid growth of this market sector underscores the need for evidence-based methods to assess the relative appeal of new and novel tobacco products with the potential to lower health risks, and gauge smokers’ likely future use of those products, in the context of a dynamic tobacco marketplace.
Initiation and continued use of tobacco products have been associated with consumers’ receptivity to various marketing strategies. Consumers’ perceptions of advertisements and reduced risk claims may differ by smoking status, with lower skepticism and greater belief in claims among current smokers, compared with former smokers or non-smokers (Berman et al., 2018). Evaluations using behavioral economic measures have also suggested that CS has lower abuse liability and lower purchase likelihood compared with nicotine gum among smokers (Rousu et al., 2015). Yet despite being perceptions of lowered health risks, reduced risk claims in CS advertisements were viewed as less truthful and evoked greater skepticism (Fix et al., 2017). Even so, product advertising and marketing, while a longstanding strategy used by manufacturers to enhance appeal, comprise only part of an overall strategy (Smith et al., 2015; Bansal-Travers et al., 2011). Products themselves are designed by manufacturers to enhance appeal through manipulation of sensory experiences, and the interplay between consumers’ perceptions of marketing and response to product use may drive initial and future intentions to use those products (Hatsukami et al., 2013). Snus users have reported a less rewarding sensory experience compared to cigarettes or nicotine gum (Hatsukami et al., 2016; Nelson et al., 2019). Nevertheless, an experimental study with 80 smokers randomly assigned to product use indicated that smoking quit rates with use of CS (Robust & Winterchill) and NG (mint flavor) were comparable (Kotlyar et al., 2011), suggesting that CS has viability as a reduced risk product.
Because a consumer’s decision to adopt a product is a result of a complex interplay between product perception, product response, and behavioral intention, strategies to evaluate the influence of exposure to tobacco pack marketing and response to actual trial and use on future use intentions are needed. The present study aimed to understand the interest in trying and likelihood of purchase of CS and NG, integrating measures of sensory, affective, and cognitive assessment, in current smokers, former smokers, and non-smokers.
2.1. Materials and Methods
Participants were recruited in Buffalo NY and Boston MA from August 2012 to September 2018 via advertisements in community newspapers, Craigslist, Facebook, Google AdWords, for a 60 mins long single session in lab. Participants’ ages ranged from 18–65 years and included current daily, current non-daily, and former smokers, as well as non-smokers. Daily smokers were those who reported 100 or more lifetime cigarettes and currently smoked every day. Non-daily smokers included those who have smoked 100 lifetime cigarettes and currently smoked some days. Former smokers included those who reported 100 or more cigarettes smoked but currently do not smoke at all. Never smokers included participants who reported not smoking 100 or more cigarettes in their lifetime and do not currently smoke.
2.2. Procedures:
A within-subjects, cross sectional design was used in which participants were randomly assigned to CS and NG exposure in a counterbalanced order (i.e., CS first or NG first). Participants completed a core questionnaire using either a pen/pencil or a computer assisted self-interview. Participants were then exposed to CS and NG products in a set of three steps, which involved: i) viewing a product advertisement; ii) viewing the packaging, and iii) touching and smelling the product without actual use. Current daily and non-daily smokers were also given an option to sample the product, which means they were allowed to place the products, a single CS pouch or NG pellet in their mouth, as suggested by the respective manufacturers. In the first step, participants were shown an ad for 15 seconds and were asked to complete an ad rating with consumer perception measures. The ads for both CS and NG were colorful and contained their respective flavored products (Supplemental Fig.1). Next, participants received a sealed package of the same product to inspect for 20–30 seconds before completing ratings and consumer perceptions questions. Third, participants were given a freshly opened package of the same product and instructed to touch and smell the contents for 30 seconds. Participants then completed the assessment of sensory perceptions of the product (haptic and olfactory) as well as the perception measures. Current daily and non-daily smokers were given the option of a fourth step, in which they were invited to sample each product for 5 minutes before completing sensory and subjective evaluations. Participants who opted to sample the product were given guidance on oral administration, as per manufacturer’s instructions. Participants had an option to opt out of sampling the product with no effect on their compensation for the study. Finally, all participants completed a task specifying which products they were most interested in trying and how likely they were to try the product of interest in next month. The exposure protocol was then repeated with the other product, according to the counterbalanced order. A debriefing statement in the end of the session stressed that no tobacco products are safe to use, and assistance was offered to anyone interested in quitting tobacco. All participants received $50 as compensation. The study was approved by institutional review boards [IRB].
2.3. Measures:
The core questionnaire included demographic measures of age, gender, race and ethnicity, tobacco use history, annual household income, and education level. After viewing ad for each product, the participants were asked ‘how likely is it that the ad you just saw contained truthful information?’ The responses ranged on a five-point Likert scale from ‘not at all likely’ to ‘extremely likely’. Further, they were asked ‘how skeptical are you about the truthfulness of the ad?’, for which the responses ranged on a five-point scale from ‘not at all skeptical’ to ‘extremely skeptical’. Truthfulness and skepticism were recoded and computed to achieve a summed variable for analysis purposes (Felberbaum, 2013). After each exposure to an ad, the packaging and being able to smell and handle both products, the participants responded to a self-assessment “manikin”, which depicted a figure with varying degrees of expression of affective responses (O’Connor et al., 2017). The self-assessment manikin assessed affective responses in terms of valence (good-bad), and arousal (excited-calm) on five-point Likert scales. Finally, at the end of survey, after experiencing both CS and NG, the participants were asked, ‘Of all the ads you saw today, which of the products you would be most interested in trying?’ [CS, NG, Neither]. Regardless of their choice in the previous question, all participants were further asked, ‘How likely are you to purchase [product selected above] in the next month?’. Responses varied on a 11-point scale from ‘10= Certain, practically certain’ to ‘0= No chance, almost no chance’. This study included several sensory measures designed to evaluate participants’ subjective responses to the products. Daily and non-daily smokers were given an option to sample the products (CS and NG), which means, they could keep the product in their mouth for the time recommended by the manufacturer and complete a product evaluation scale and drug effects/liking questionnaire (O’Connor et al., 2018; Hatsukami et al., 2013) for each product. [Supplemental fig.1]
2.4. Data analysis:
Our primary outcome of interest was interest in use measured at the end of the survey, assessed by three measures: Product preference (CS vs NG vs Neither); likelihood of purchase (recoded as low [0–4] vs high [5–10]); and Uptake of the offer to sample the products (among current and former smokers, Yes vs No). Key independent variables used in analysis included smoking status, truthfulness and skepticism towards ad of CS and NG, and stage of exposure to the product (advertising, packaging, smell and handling). Multivariable models were adjusted for sex, age, study location (Buffalo vs Boston), and product sequence (CS first vs NG first). As an intermediate step to understand affective product response, valence and arousal within participants across different exposures of CS and NG, were assessed using repeated measures ANOVA (adjusted for product and ad truthfulness). Here, a higher estimated marginal mean indicated feeling ‘good’ (valence) and ‘calm’ (arousal). Binomial regressions modeled product preference, intent to purchase, and decision to sample the products. All analyses were performed using SPSS version 25 (IBM, Armonk, NY).
3. Results
3.1. Participant characteristics:
A description of the study sample is shown in Table 1. Of the initial participants (n=384), n=3 were subsequently determined to not meet eligibility criteria; 20 were missing information related to demographics, smoking status, and primary outcomes; and 13 answered sensory questions despite not sampling products (indicating noncompliance with procedures). This yielded a final sample of n=348. Of these, approximately 55.8% were accrued in Buffalo. The mean (M[SD]) age was 42.0 (14.3). Most participants were non-Hispanic whites (56.4%), daily-smokers (52.2%) and below an annual income of $20,000 (42.5%). There was an equal number of males and females in the sample. There were significant differences between Buffalo and Boston’s’ participants in terms of age, gender, ethnicity, education and smoking status
Table 1:
Distribution of study population in two locations, based on Demographics and Behavioral factors.
| Variable names | Categories | Boston n (%) |
Buffalo n (%) |
Total n (%) |
Chi-square (p-value) |
|---|---|---|---|---|---|
| Age (yrs.) (n=348) |
≤30 | 70 (52.2) | 64 (47.8) | 134 (38.7) |
26.5 (<0.01*) |
| 31–50 | 54 (56.2) | 42 (43.8) | 96 (27.7) | ||
| >50 | 29 (25.0) | 87 (75.0) | 116 (33.6) | ||
| Sex (n=346) |
Male | 100 (58.1) | 72 (41.9) | 172 (50.0) |
28.3 (<0.01*) |
| Female | 51 (29.7) | 121 (70.3) | 172 (50.0) | ||
| Ethnicity (n=346) |
Hispanics | 2 (22.2) | 7 (77.8) | 9 (2.6) |
9.9 (0.02*) |
| Non-Hispanic White | 80 (41.2) | 114 (58.8) | 194 (56.4) | ||
| Non-Hispanic Black | 45 (42.9) | 60 (57.1) | 105 (30.5) | ||
| Others | 24 (66.7) | 12 (33.3) | 36 (10.5) | ||
| Annual Income (in US Dollars) (n=346) |
<20,000 | 71 (48.3) | 76 (51.7) | 147 (42.7) |
2.1 (0.35) |
| 20,000–49,999 | 51 (41.5) | 72 (58.5) | 123 (35.8) | ||
| ≥50,000 | 29 (39.2) | 45 (60.8) | 74 (21.5) | ||
| Educational qualification (n=346) |
Upto high school/equivalent | 62 (52.5) | 56 (47.5) | 118 (34.3) |
6.4 (0.04*) |
| Some college or 2-year degree | 52 (42.3) | 71 (57.7) | 123 (35.8) | ||
| College graduate or professional | 37 (35.9) | 66 (64.1) | 103 (29.9) | ||
| Smoking status (n=345) |
Daily Smoker | 98 (54.7) | 81 (45.3) | 179 (52.2) |
71.5 (<0.01*) |
| Non-daily smoker | 32 (84.2) | 6 (15.8) | 38 (11.1) | ||
| Former smoker | 7 (16.3) | 36 (83.7) | 43 (12.5) | ||
| Never-smoker | 14 (16.9) | 69 (83.1) | 83 (24.2) |
3.2. Interest in trying CS and/or NG in the future:
The analysis shows response to the question ‘Of all the ads you saw today, which product would you be most interested in trying?’ In a multivariate logistic regression model, we observed that daily smokers were approximately 12 times more likely (OR=11.55; 95% CI: 5.38–24.77, p<0.01) and non-daily smokers were 10 times more likely (OR=10.0; p<0.01, 95% CI:3.21–31.16) to report an interest in trying NG compared to never-smokers [Table 2]. Similarly, daily smokers were approximately 10 times more likely to show an interest in trying CS compared to non-smokers (OR=10.08; p<0.01, 95% CI: 2.91–34.90) [Table 3]. Per unit increase in truthfulness and decrease in skepticism for NG ad resulted in a 36% (OR=1.36; p<0.01; 95% CI: 1.13–1.64,) greater interest in trying NG and a 47% (OR=1.47; p=0.01;95% CI: 1.12–1.94,) greater interest in trying CS. [Table 2&3].
Table 2.
Multinomial regression with purchase intention for NG as outcome, perceptions towards CS and NG advertisements as predictors and location, age, gender, order of products sampled and smoking status as covariates. Response option ‘Neither of the two’ is used as reference category.
| Variable | aOR Purchase NG |
95% CI | p | |
|---|---|---|---|---|
| NG ad truthfulness | 1.36 | 1.13 | 1.64 | 0.00* |
| CS ad truthfulness | 0.93 | 0.81 | 1.08 | 0.35 |
| Buffalo | 1.64 | 0.81 | 3.35 | 0.17 |
| Boston | Ref | - | - | - |
| Age <30 | 1.81 | 0.90 | 3.65 | 0.10 |
| Age 30–50 | 0.84 | 0.40 | 1.76 | 0.64 |
| Age >50 | Ref | - | - | - |
| Male | 1.10 | 0.61 | 2.00 | 0.10 |
| Female | Ref | - | - | - |
| Daily smoker | 11.55 | 5.38 | 24.77 | 0.00* |
| Nondaily smoker | 10.00 | 3.21 | 31.16 | 0.00* |
| Former smoker | 1.56 | 0.65 | 3.75 | 0.32 |
| Never smoker | Ref | - | - | - |
| CS First | 0.95 | 0.54 | 1.66 | 0.85 |
| NG First | Ref | - | - | - |
OR= Odds ratio, CI= Confidence interval, P= p-value,
Differences are significant at α=0.05. Reference category for outcome is ‘Neither of the two’.
Table 3.
Multinomial regression with purchase intention for CS as outcome, perceptions towards CS and NG advertisements as predictors and location, age, gender, order of products sampled and smoking status as covariates. Response option ‘Neither of the two’ is used as reference category.
| Variable | aOR Purchase CS |
95% CI | p | |
|---|---|---|---|---|
| NG ad truthfulness | 1.47 | 1.12 | 1.94 | 0.01* |
| CS ad truthfulness | 1.09 | 0.88 | 1.36 | 0.43 |
| Buffalo | 0.44 | 0.17 | 1.15 | 0.09 |
| Boston | Ref | - | - | - |
| Age <30 | 1.06 | 0.38 | 2.94 | 0.91 |
| Age 30–50 | 0.79 | 0.26 | 2.25 | 0.66 |
| Age >50 | Ref | - | - | - |
| Male | 1.22 | 0.52 | 2.84 | 0.65 |
| Female | Ref | - | - | - |
| Daily smoker | 10.08 | 2.91 | 34.90 | 0.00* |
| Nondaily smoker | 3.20 | 0.56 | 18.46 | 0.19 |
| Former smoker | 1.42 | 0.28 | 7.18 | 0.68 |
| Never smoker | Ref | - | - | - |
| CS First | 0.87 | 0.39 | 1.93 | 0.73 |
| NG First | Ref | - | - | - |
OR= Odds ratio, CI= Confidence interval, P= p-value,
Differences are significant at α=0.05. Reference category for outcome is ‘Neither of the two’.
3.3. Product preference:
A Pearson Chi-square test of product preference based on smoking status showed that 63.8% of smokers (daily and non-daily) preferred NG whereas only 17.4% smokers preferred CS with 64.3% never smokers preferred neither of the two products. The difference between groups was statistically significant (Chi-square =70.15, p<0.001).
3.4. Likelihood of purchase in next month:
This analysis shows the likelihood of purchasing the product when asked ‘How likely you are to purchase [product selected above] in the next month?’ The analysis was restricted to those who chose CS or NG when asked ‘Of all the ads you saw today, which product would you be most interested in trying?’, and excluded those who chose neither, Chi-square tests showed a significant association between daily smokers and higher likelihood of purchasing a product in next month (Chi-square= 23.2, p<0.01), compared to never-smokers and former smokers, who reported a lower likelihood of purchasing a given product. Similarly, the current and former smokers, who chose to sample any one or both products were more likely to report an intention to purchase the product of interest in the next month compared to those who declined to sample any product (Chi-square=17.5, p<0.01). We did not find any significant associations in the binomial regression model based on demographics or smoking status. (Supplemental Table 1).
3.5. Sampling of product and interest in trying product in future:
This analysis shows how the choice to sample the product during lab session was associated with response to choice of product for trying when asked ‘Of all the ads you saw today, which product would you be most interested in trying?’. Sample was restricted to daily and non-daily smokers (n=218), who were offered the option of trying the products during the lab session. Of these, 78.3% of daily smokers and 68.4% of non-daily smokers sampled at least one product. Of those who sampled at least one product, 59.9% were males vs 40.1% females. Majority of the participants who sampled both or any one of the two products showed an interest in trying NG in near future when compared to CS or neither product. However, the difference was not statistically significant (Chi-square= 6.38, p=0.38).
A binomial regression model (Table 4) with sampling of the product (sampled any vs sampled none) as the main outcome and product ad perceptions as covariate showed that those who perceived the NG ad as more truthful and less skeptical were 1.7 times more likely to sample any product (OR=1.65, p=0.05; 95% CI: 1.01–2.70). Buffalo participants were approximately 8 times more likely to sample any product compared to Boston participants (OR=8.38, p<0.01, 95% CI: 2.52–27.93). Those older than 50 years showed greater likelihood of sampling one or both the products (Chi-square= 11.68 p>0.01*) when offered. Additional analyses including valence showed, those reporting higher valence after smell and handling of CS (Chi-square=6.37, p=0.04) and NG (Chi-square=8.74, p=0.01) were more likely to sample any one or both products (Supplemental Table 2).
Table 4.
Binomial regression model with sampling of product (any vs none) as outcome and perceptions towards CS and NG advertisements as predictors and location, age, gender, order of products sampled and smoking status as covariates.
| Variable | aOR Opted to Sample |
95% CI | p | |
|---|---|---|---|---|
| NG ad truthfulness | 1.65 | 1.01 | 2.70 | 0.05* |
| CS ad truthfulness | 1.58 | 0.90 | 1.30 | 0.06 |
| Buffalo | 8.38 | 2.52 | 27.93 | 0.001* |
| Boston | Ref | - | - | - |
| Age <30 | Ref | - | - | - |
| Age 30–50 | 0.39 | 0.11 | 1.37 | 0.14 |
| Age >50 | 0.60 | 0.17 | 2.17 | 0.44 |
| Male | 1.68 | 0.73 | 3.90 | 0.23 |
| Female | Ref | - | - | - |
| Daily smoker | 1.49 | 0.59 | 3.73 | 0.40 |
| Nondaily smoker | Ref | - | - | - |
| CS First | 1.04 | 0.47 | 2.30 | 0.92 |
| NG First | Ref | - | - | - |
OR= Odds ratio, CI= Confidence interval, P= p-value,
Differences are significant at α=0.05.
3.6. Ad truthfulness:
The mean [M(±SD)] values for perceived truthfulness of NG and CS ads were 2.45 (±0.79) and 2.58 (±0.69) respectively and showed a significant but weak non-linear correlation (rs=0.1, p=0.05). Truthfulness ratings did not differ by location, age, sex, or smoking status.
3.7. Valence and arousal measures:
Supplemental Figure 2 illustrates the adjusted marginal mean changes in valence and arousal as a function of exposure condition (ad, packaging, smell and handle) using repeated measures ANOVA. Valence increased across exposure stages for CS (F (df1,df2) =10.72 (2.00,300.00), p<0.01) with higher valence in those who perceived the CS ad as more truthful and expressed a preference for CS (F(df1,df2)=19.09 (2.00,298.00), p<0.01). In contrast, valence did not increase across NG exposure conditions. Significant differences in valence were observed between different levels of exposure for NG (F(df)=7.49(2), p<0.01) and CS (F(df)=17.59, p<0.01), while arousal was significantly different only for NG exposure (F(df)=4.89(2), p<0.01). Supplemental Tables 3 and 4 present results from a multivariate logistic regression model assessing valence and arousal, with product choice and ad perceptions as independent factors. Results from this model were comparable to the repeated measures ANOVA.
4. Discussion
The present study evaluated the cognitive, affective, and sensory responses of participants to CS and NG. A greater overall preference was observed for NG in comparison to CS, which is consistent with findings reported in an experimental study by Rousu and colleagues that showed greater inclination to purchase NG compared with CS in smokers participating in an experimental auction (Rousu et al., 2015). However, these findings contradict those from an open-label crossover study where heavy smokers preferred snus over NG for smoking cessation, citing gastro-intestinal side effects from short-term use of NG (Caldwell et al., 2010). Most non-smokers in our study preferred neither of the two products (64.3 %). Of those non-smokers who chose one of the two products, most of them (29.5%) preferred NG over CS. Current smokers were more likely to choose either CS or NG with an increased preference for NG, compared to non-smokers. This shows an increased likelihood of current smokers’ willingness to try alternative tobacco products, perhaps as a long-term alternative to smoking. Non-smokers, on the other hand, might have perceived both products as a source of nicotine and hence, as an addictive product. Additionally, the participants who sampled any one or both products, majority of them showed an increased interest in trying NG in near future. These results support findings by O’Connor et al., where a majority of smokers who sampled four different types of nicotine-containing products including snus and nicotine gum, preferred to try NG to reduce smoking (O’Connor et al., 2011).
According to a survey determining the reasons for trial and quitting of snus among smokers, a greater number of smokers used snus as a means of reducing cigarette smoking rather than completely quitting (Biener et al., 2016). This is indicative of a greater inclination towards dual usage. This could be due to the marketing of NG as a pharmaceutical product, contrasting with a perception of CS as a tobacco product, which may elicit an association with the health risks of smoking (Lund, 2012; O’Connor, 2012). These differences in perceived health risks could explain NG’s lower popularity among smokers not intending to quit. Additionally, studies reporting a tendency among smokers to use snus and cigarettes dually, rather than completely switching to snus, may also point towards a comparatively lower rate of acceptance of snus products for smoking cessation in United States compared with Swedish snus’ acceptance in Norway and Sweden. This observation further underscores a pressing need for evidence-based methods to understand the factors that shape product choice and predict future use of alternative tobacco products.
A positive association was observed between increasing truthfulness and decreasing skepticism for the NG ad and an increased purchase intention for both NG and CS. These observations might be due to greater familiarity with NG as a pharmacological product in both smokers and non-smokers. A greater trust in truthfulness of a pharmacologic agent translated well into trying either CS or NG respectively. In contrast, a study by O’Connor et al. (O’Connor et al., 2014) reported no significant effects on purchase intention from the SLT or control ads shown to participants. In this study, there were no control ads, but participants displayed greater influence from the NG ad on their purchase intentions. The presence of health warning label on the ad shown for CS and its absence on NG ad could be one of the factors influencing smokers’ perception of safety of the product.
We did not find a significant association between gender and choice of product. Among smokers who were offered a choice to sample the product, equal proportions of men and women opted to try either product. However, both men and women were more likely to opt to try NG over CS. These results are in contrast with findings from Allen et al. who found that men used more snus and women used more gum (Allen et al., 2016). While traditional ST products have low rates of use among women, snus and emerging ‘modern oral’ products may hold greater appeal in not requiring spitting and having an overall more positive image and marketing. Thus, greater attention to the appeal of novel oral nicotine products among women smokers and nonsmokers is warranted.
Studies have associated emotional responses to tobacco packaging and advertisements with intentions to smoke or quit smoking (Wu et al., 2015; Cho et al., 2018). In accordance with the existing literature, we observed an association between “feeling good” and “more excited” with a greater perception of truthfulness for the ads. Emotional arousal could be a critical predictor of smoking related decisions – valence in particular seemed to differ by product choice and perceived truthfulness, suggesting this could be an important marker of product response. This also served as a manipulation check, as these ratings did vary as a function of different exposures to the product. Uptake of the offer to try can be viewed as a behavioral marker of intention to use and appeared to vary primarily as a function of current smoking status.
5. Conclusions
The results from this study add to the evidence base on methods to assess consumer responses, including sensory perceptions and future use intentions, to novel tobacco products. We found limited appeal for CS relative to nicotine gum, even when participants were given an opportunity to handle the package, smell and handle the product.
Participants who reported a preference for CS showed increased valence with greater exposure to CS, and interest in trying was associated with higher perceptions of message truthfulness and lower skepticism. Nonetheless, NG was the product of choice among smokers as well as a small number of non-smokers who indicated a choice when asked about intentions to try in near future. The low preference for CS among current smokers suggests that this product may have limited utility if marketed as a harm reduction alternative to combustible tobacco products in the United States. The methods used here may find broader application in the assessment of new and novel tobacco products, including non-combustible products that have the potential to reduce exposure among smokers, and could inform future regulatory approaches.
Supplementary Material
Fig 1.
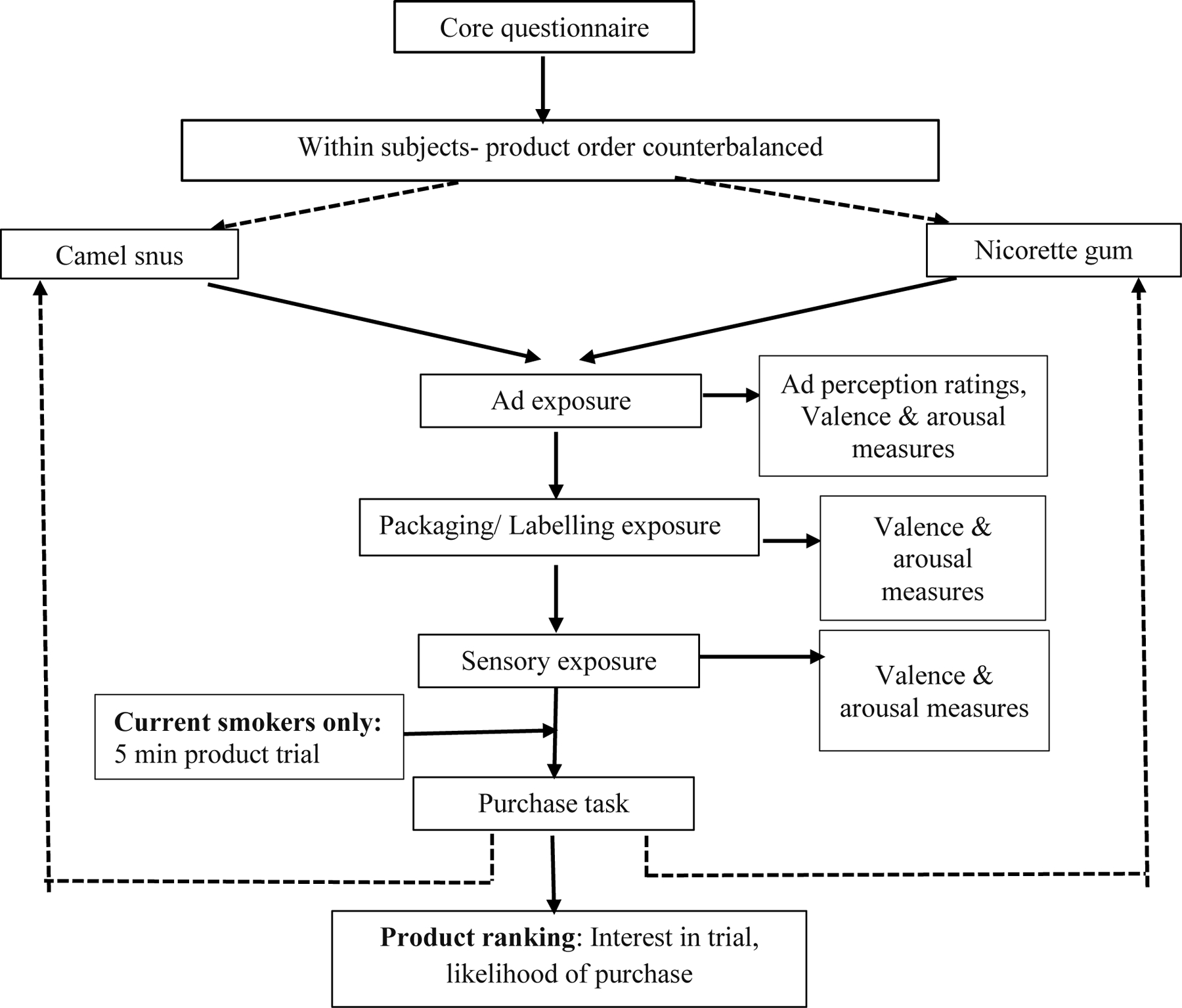
Flowchart representing the schematic of the study session.
Acknowledgments:
Thanks to Afsaneh Mollaie for assistance with data cleaning, Ayesha Javed for critical reading, and Rosalie Marlsberger, Justin Rodgers & Emeka Agudile for research assistance at the Boston site.
Abbreviations
- CS
Camel Snus
- NG
Nicotine Gum
- SLT
smokeless tobacco
- NRT
nicotine replacement therapy
- MRTP
modified risk tobacco product
References
- Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, & Niaura RS (2018). Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annual review of public health, 39, 193–213. 10.1146/annurev-publhealth-040617-013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A, Vogel RI, Meier E, Anderson A, Jensen J, Severson HH, & Hatsukami D (2016). Gender differences in snus versus nicotine gum for cigarette avoidance among a sample of US smokers. Drug and alcohol dependence, 168, 8–12. 10.1016/j.drugalcdep.2016.08.624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal-Travers M, O’Connor R, Fix BV, & Cummings KM (2011). What do cigarette pack colors communicate to smokers in the U.S.? American journal of preventive medicine,40(6),683–689. 10.1016/j.amepre.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsvik D, & Rogeberg O (2018). Assessing the effect of public health information by incentivised risk estimation: An example on Swedish snus. Int J Drug Policy, 54, 51–57. doi: 10.1016/j.drugpo.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Berman ML, Bickel WK, Harris AC, LeSage MG, O’Connor RJ, Stepanov I, Shields PG, & Hatsukami DK (2018). Consortium on Methods Evaluating Tobacco: Research Tools to Inform US Food and Drug Administration Regulation of Snus. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 20(11),1292–1300. 10.1093/ntr/ntx228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Roman AM, Mc Inerney SA, Bolcic-Jankovic D, Hatsukami DK, Loukas A, O’Connor RJ, & Romito L (2016). Snus use and rejection in the USA. Tobacco control, 25(4),386–392. 10.1136/tobaccocontrol-2013-051342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell B, Burgess C, & Crane J (2010). Randomized crossover trial of the acceptability of snus, nicotine gum, and Zonnic therapy for smoking reduction in heavy smokers. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 12(2), 179–183. 10.1093/ntr/ntp189 [DOI] [PubMed] [Google Scholar]
- Cho YJ, Thrasher JF, Yong HH, Szklo AS, O’Connor RJ, Bansal-Travers M, Hammond D, Fong GT, Hardin J, & Borland R (2018). Path analysis of warning label effects on negative emotions and quit attempts: A longitudinal study of smokers in Australia, Canada, Mexico, and the US. Social science & medicine (1982), 197, 226–234. 10.1016/j.socscimed.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felberbaum M (2013). FDA: Longer use of nicotine gum is ok USA Today. Published April 1, 2013.Accessed November 11, 2020. https://www.usatoday.com/story/news/nation/2013/04/01/fda-nicotine-gum/2042175/ [Google Scholar]
- Fix BV, Adkison SE, O’Connor RJ, Bansal-Travers M, Cummings KM, Rees VW, & Hatsukami DK (2017). Evaluation of modified risk claim advertising formats for Camel Snus. Health Education Journal, 76(8), 971–985. 10.1177/0017896917729723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Ramstrom L, Burke M, & Fagerström K (2003). Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco control, 12(4), 349–359. 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilljam H, & Galanti MR (2003). Role of snus (oral moist snuff) in smoking cessation and smoking reduction in Sweden. Addiction (Abingdon, England), 98(9), 1183–1189. 10.1046/j.1360-0443.2003.00379.x. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Severson H, Anderson A, Vogel RI, Jensen J, Broadbent B, Murphy SE, Carmella S, & Hecht SS (2016). Randomised clinical trial of snus versus medicinal nicotine among smokers interested in product switching, Tobacco Control, 25, 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Zhang Y, O’Connor RJ, & Severson HH (2013). Subjective responses to oral tobacco products: scale validation. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 15(7), 1259–1264. 10.1093/ntr/nts265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, & Fagerstrom KO (2001). Swedish Match Company, Swedish snus and public health: a harm reduction experiment in progress? Tobacco Control, 10(3), 253–257. DOI: 10.1136/tc.10.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar M, Hertsgaard LA, Lindgren BR, Jensen JA, Carmella SG, Stepanov I, Murphy SE, Hecht SS, & Hatsukami DK (2011). Effect of oral snus and medicinal nicotine in smokers on toxicant exposure and withdrawal symptoms: a feasibility study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 20(1), 91–100. 10.1158/1055-9965.EPI-10-0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, & O’Connor RJ (2002). Cigarette filter ventilation is a defective design because of misleading taste, bigger puffs, and blocked vents. Tobacco control, 11 Suppl 1(Suppl 1), I40–I50. 10.1136/tc.11.suppl_1.i40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Houezec J, McNeill A, & Britton J (2011). Tobacco, nicotine and harm reduction. Drug and alcohol review, 30(2), 119–123. 10.1111/j.1465-3362.2010.00264.x. [DOI] [PubMed] [Google Scholar]
- Lund KE (2012). Association between willingness to use snus to quit smoking and perception of relative risk between snus and cigarettes. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 14(10), 1221–1228. 10.1093/ntr/nts077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia AB, Ling PM, & Glantz SA (2010). Quantifying the effects of promoting smokeless tobacco as a harm reduction strategy in the USA. Tobacco control, 19(4), 297–305. 10.1136/tc.2009.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PR, Chen P, Battista DR, Pillitteri JL, & Shiffman S (2019). Randomized Trial to Compare Smoking Cessation Rates of Snus, With and Without Smokeless Tobacco Health-Related Information, and a Nicotine Lozenge. Nicotine Tob Res, 21(1), 88–94. Doi: 10.1093/ntr/nty011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Lewis MJ, Adkison SE, Bansal-Travers M, & Cummings KM (2017). Perceptions of “Natural” and “Additive-Free” Cigarettes and Intentions to Purchase. Health education & behavior: the official publication of the Society for Public Health Education, 44(2), 222–226. 10.1177/1090198116653935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ (2012). Non-cigarette tobacco products: what have we learnt and where are we headed? Tobacco control, 21(2),181–190. 10.1136/tobaccocontrol-2011-050281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, June KM, Bansal-Travers M, Rousu MC, Thrasher JF, Hyland A, & Cummings KM (2014). Estimating demand for alternatives to cigarettes with online purchase tasks. American journal of health behavior, 38(1), 103–113. 10.5993/AJHB.38.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor RJ, Lindgren BR, Schneller LM, Shields PG, & Hatsukami DK (2018). Evaluating the utility of subjective effects measures for predicting product sampling, enrollment, and retention in a clinical trial of a smokeless tobacco product. Addictive behaviors, 76, 95–99. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Norton KJ, Bansal-Travers M, Mahoney MC, Cummings KM, & Borland R (2011). US smokers’ reactions to a brief trial of oral nicotine products. Harm Reduct J, 8, 1 10.1186/1477-7517-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramström LM, & Foulds J (2006). Role of snus in initiation and cessation of tobacco smoking in Sweden. Tobacco control, 15(3),210–214. 10.1136/tc.2005.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees VW, Kreslake JM, Cummings KM, O’Connor RJ, Hatsukami DK, Parascandola M, Shields PG, & Connolly GN (2009). Assessing consumer responses to potential reduced-exposure tobacco products: a review of tobacco industry and independent research methods. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 18(12), 3225–3240. 10.1158/1055-9965.EPI-09-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodu B, Stegmayr B, Nasic S, & Asplund K (2002). Impact of smokeless tobacco use on smoking in northern Sweden. Journal of internal medicine, 252(5), 398–404. 10.1046/j.1365-2796.2002.01057.x [DOI] [PubMed] [Google Scholar]
- Rousu MC, O’Connor RJ, Bansal-Travers M, Pitcavage JM, & Thrasher JF (2015). The impact of free trial acceptance on demand for alternative nicotine products: evidence from experimental auctions. Harm reduction journal, 12, 18. 10.1186/s12954-015-0052-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Bansal-Travers M, O’Connor RJ, Goniewicz ML, & Hyland A (2015). Associations between perceptions of e-cigarette advertising and interest in product trial amongst US adult smokers and non-smokers: results from an internet-based pilot survey. Tobacco induced diseases, 13(1), 14. 10.1186/s12971-015-0039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjora Tore, Skogen Jens, & Sivertsen Børge. (2020). Increasing similarities between young adults’ smoking and snus use in Norway: a study of the trends and stages of smoking and snus epidemic from 2010 to 2018. BMC Public Health 20, 10.1186/s12889-020-09604-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Winkler MH, Wieser MJ, Andreatta M, Li Y, & Pauli P (2015). Emotion regulation in heavy smokers: experiential, expressive and physiological consequences of cognitive reappraisal. Frontiers in psychology, 6, 1555. 10.3389/fpsyg.2015.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


