Abstract
Radiation therapy exerts a tumoricidal local effect as well as both local and systemic immunomodulation. Immune checkpoint blockade has become a widely utilized treatment modality across cancer types with a rapidly growing list of agents and FDA-approved indications. Moreover, there may be synergy between radiation therapy and immune checkpoint blockade. A variety of strategies have been employed but the optimal sequencing of these therapies is unclear. In this review, we discuss the major mechanisms of available immune checkpoint inhibitors and explore the available preclinical and clinical evidence regarding treatment sequencing. We also review safety considerations and conclude with possible future directions.”
Keywords: radiation therapy, immunotherapy, immune checkpoint blockade, cancer therapy sequencing, radiosensitization
Precis:
Combined immune checkpoint blockade and radiation therapy have important roles in the treatment of an increasing number of malignancies, but the optimal sequencing is unknown. In this review we summarize the available preclinical and clinical evidence regarding the timing of therapy.
Introduction:
Both radiation therapy (RT) and immunotherapy are well established components of therapy across a wide variety of cancers and clinical presentations. Radiation therapy acts predominantly by causing overwhelming DNA damage to localized targets, although its cytotoxic effect also results in the release of tumor antigens and changes in the tumor microenvironment which may be recognized by the host immune system.
Given the rapid acceleration of utilization and its major impact on the therapeutic paradigm for several malignancies, immunotherapy is often considered a recent advance. However, its use dates as far back as 1868 when the German physicians W. Busch and F. Fehleisen noted the regression of tumors in cancer patients when infected with erysipelas. 1,2 In 1891, William Coley reported on the intratumoral injection of mixtures of Streptococcus pyogenes and Serratia marcescens. 3
The normal immune system must operate within a safe window between being too permissive to foreign pathogens and being overly aggressive toward the normal self. Remarkably, it typically successfully functions within these confines with guidance from crucial regulatory mechanisms including regulatory T cells (Tregs) and T-cell anergy. Unfortunately, in the setting of cancer, the immune system applies selective pressure and immune tumor editing which can result in deleterious immune suppression and tolerance and the subsequent escape of tumor cells from immune recognition. 4
There are several therapeutic mechanisms exploited by modern immunotherapy. The most widely utilized at present is immune checkpoint blockade (ICB), which removes inhibitory signals of T cell activation, thereby allowing tumor-reactive T cells to mount an antitumoral response. 5,6
Over the past several years there has been rapid development and increasing administration of immunotherapy to treat cancer, with the CTLA-4 inhibitor ipilimumab first approved for use in metastatic melanoma in 2011 and now also approved for use in renal cell carcinoma, colorectal cancer, and hepatocellular carcinoma.7 Pembrolizumab, a monoclonal PD-1 blocking antibody, received its first FDA approval for use in the United States in 2014. It is now approved for at least 15 separate cancer types8 and has been joined by nivolumab and cemiplimab, along with three FDA-approved PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab). Several other agents are on the horizon9.
There is emerging evidence for synergy between RT and ICB due to both local and systemic RT-induced immunomodulation, 10–12 which has prompted many ongoing and upcoming clinical trials exploring combination therapy, the extensive cataloging of which is beyond the scope of this review. Figure 1 shows an overview of the interplay between RT and ICB. Radiation’s local cytotoxic effect causes cell death, releases tumor antigens, and can activate CD8+ T cells, which can promote the migration of tumor-infiltrating lymphocytes (TILs)13. Moreover, RT can also lead to an increased immune response via changes in the tumor microenvironment and nearby stromal tissue.14 Conversely, there is evidence that ICB can enhance the anti-tumor effect of RT15; taken together, this supports the concept of a combined approach.
Figure 1:
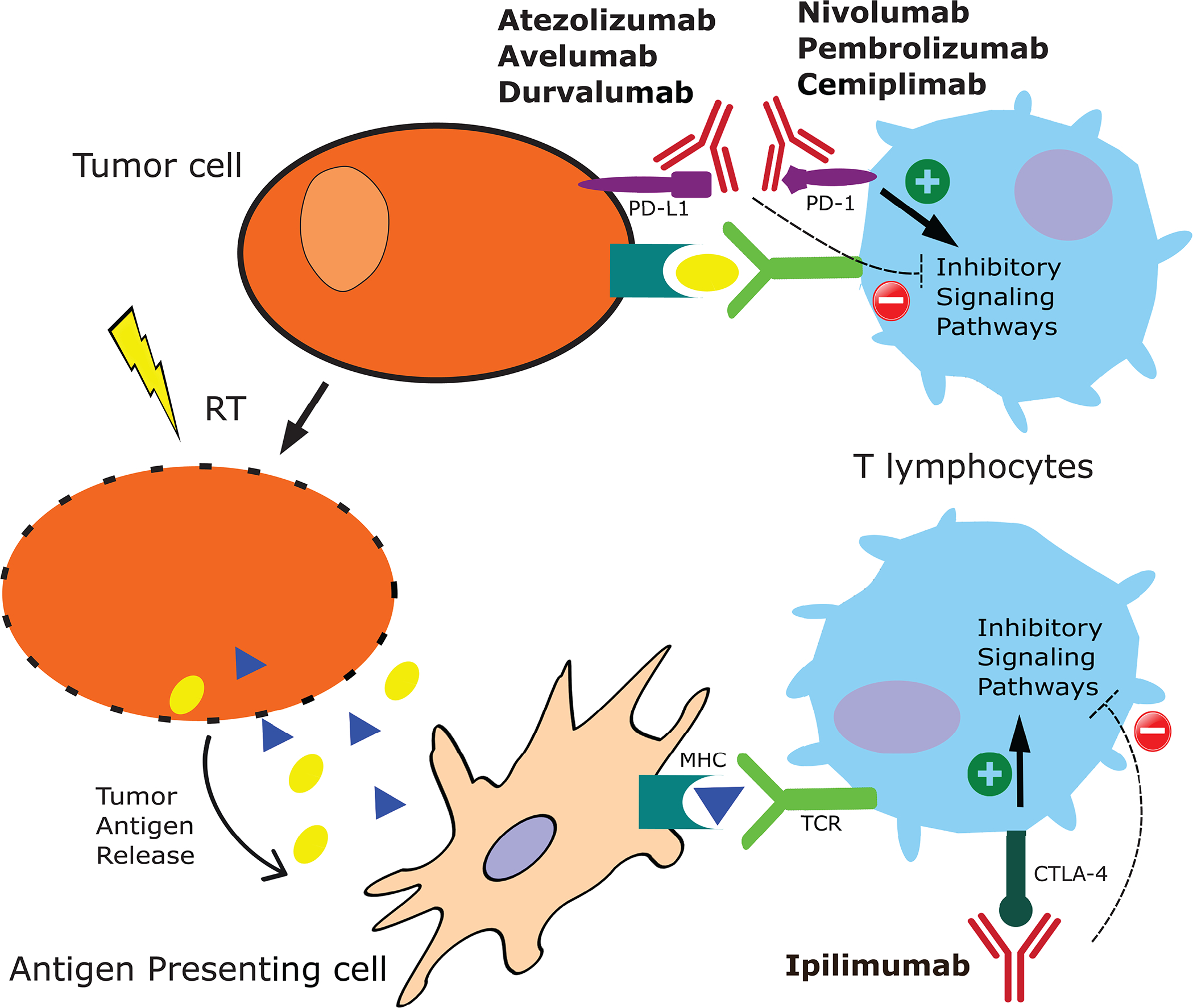
Basic schematic of interactions between RT and ICB on tumor cells and immune modulation. MHC = major histocompatibility complex, TCR = T-cell receptor.
Although interest and utilization of ICB is clearly accelerating, the optimal sequencing with radiation therapy is still an unresolved question, both in terms of efficacy and toxicity. There are considerations that could favor ICB as a priming agent prior to RT, ICB given concurrently with RT, and consolidative ICB following RT (Figure 2). In this review we explore the available preclinical and clinical data surrounding the timing of immunotherapy and RT and will highlight key upcoming clinical trials that will shed more light on this critical issue.
Figure 2:
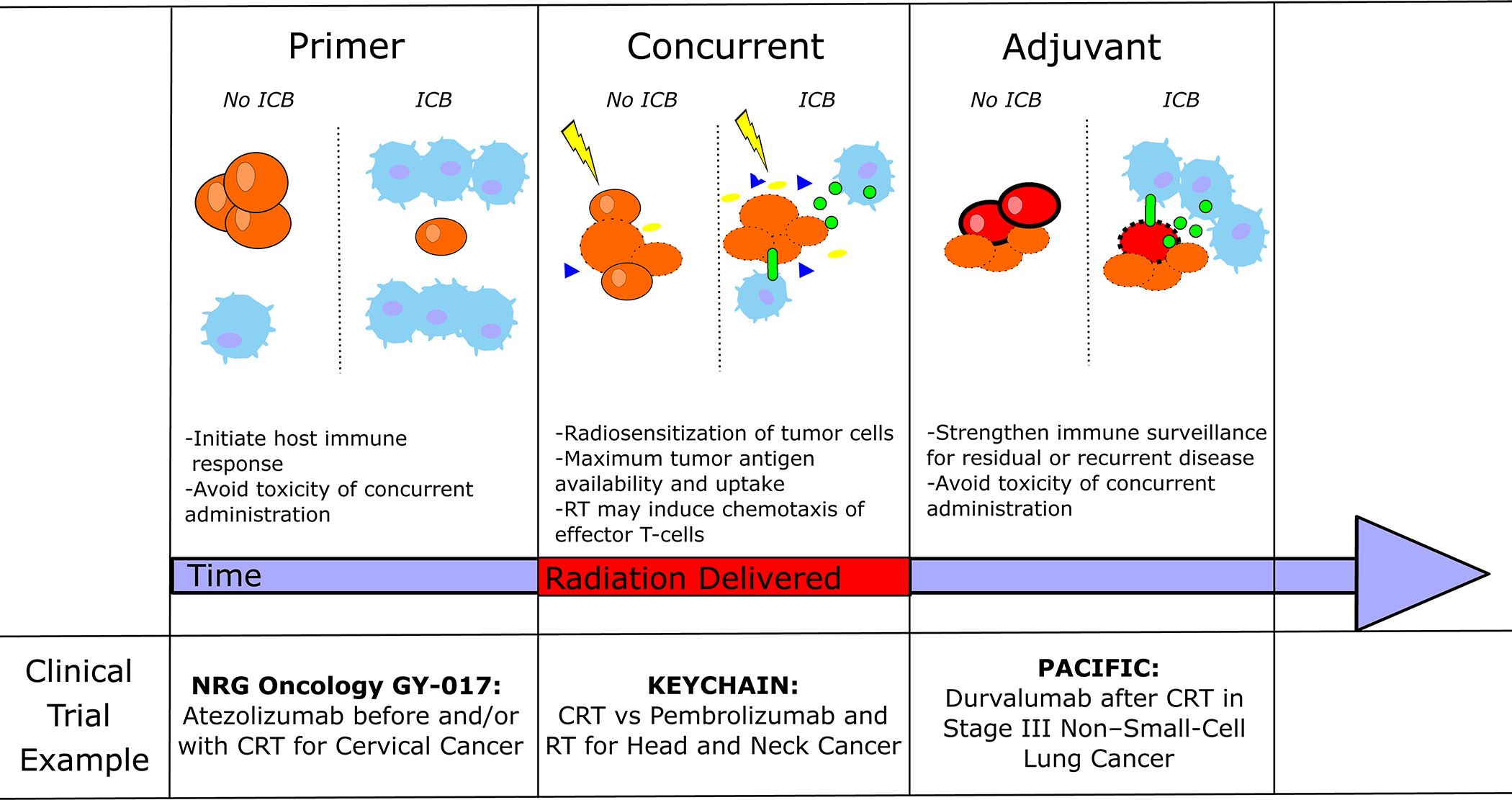
Framework for considerations regarding immune checkpoint blockade (ICB) and RT sequencing, including priming (ICB prior to RT), concurrent administration, and adjuvant/consolidative use. CRT = chemoradiotherapy.
CTLA-4 Blockade and RT:
The protein receptor CTLA4 on T cells is upregulated following T-cell receptor (TCR) engagement and serves to dampen TCR signaling by competing with the costimulatory agent CD28,5 which results in downregulation of TCR signal amplitude, T-cell activity, and therefore tumor surveillance. There is also a cell-extrinsic suppressive capacity of CTLA-4 which is mediated through Tregs. 16,17 Ipilimumab is currently the only FDA-approved CTLA-4 inhibitor7.
Preclinical Data:
Several preclinical studies have demonstrated promising synergy between RT and CTLA4 blockade 18–20 Young et al. performed a study in tumor-bearing mice treated with 20 Gy of radiation to the tumor with anti-CTLA4 antibody, anti-OX40 antibody (which targets recently-activated T-cells), or both. RT alone led to transient tumor control but all tumors in this group eventually regrew. Ultimately, the best results were achieved when anti-CTLA4 was given on day 7 prior to RT followed shortly by OX40, suggesting that anti CTLA-4 could produce a priming immunomodulatory effect when given prior to RT.21 Table 1 highlights preclinical data exploring sequencing questions for ICB and RT.
Table 1:
Relevant preclinical studies investigating sequencing between ICB and RT
| Title | ICB Mechanism and timing with RT | Disease Site/Model | Key Results |
|---|---|---|---|
| Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer18 | CTLA-4 blockade given at 1, 4, and 7 days after RT | Murine breast model | RT alone inhibited tumor growth initially but survival was ultimately similar to control mice; however, mice treated with RT followed by anti-CTLA4 had improvement in development of lung metastases as well as survival |
| Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model19 | CTLA-4 blockade given at 1, 4, and 7 days after RT | Murine syngeneic EL4 lymphoma cells and Lewis lung carcinoma cells | CTLA-4 blockade significantly increased the anti-tumor activity of RT when given in combination in terms of tumor growth delay |
| Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer20 | RT given before or concurrently with anti-CTLA-4 | Murine melanoma model | Combination therapy was more effective than single agent therapy, with similar results when given concurrently or with RT before |
| Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy21 | RT and CTLA-4 blockade along with anti-OX40 agonist antibody | Murine colorectal carcinoma model | Anti-CTLA-4 therapy was most effective when given prior to RT |
| Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies37 | Anti-PD-1 (as well as anti-CD137, anti-CD40, and/or MAC4 antibodies); anti-PD-1 was given on days 0, 4, 8, and 12 relative to RT | Subcutaneous and orthotopically implanted triple negative mammary tumors | Combination of immunostimulatory and inhibitory checkpoints enhanced the curative potential of RT |
| Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen38 | RT and PD-1 blockade | Murine melanoma and breast models | Immune-stimulating effects of RT were significantly increased when combined with PD-1 blockade or regulatory T-cell depletion |
| Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma39 | Concurrent RT and PD-1 blockade (RT on days 14, 16 and 18) and anti-PD-1 on days 7, 14, and 21 | Orthotopic murine model of hepatocellular carcinoma | Tumor response to stereotactic radiation was augmented with concurrent PD-1 blockade although the efficacy of the combination was transient |
| The role of PD-L1 in the radiation response and clinical outcome for bladder cancer15 | RT and PD-L1 blockade | Murine bladder model | PD-L1 blockade induced a longer tumor growth delay following RT. PD-L1 expression peaked 72 hours after RT delivery and significantly declined 7 days after RT |
| Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade57 | RT and PD-1 or PD-L1 blockade | Multiple murine models | Fractionated RT with PD-1 or PD-L1 blockade led to improved local control, long-term survival, and protection against tumor rechallenge. Concurrent PD-L1 blockade (rather than sequential)_was required for improved survival |
| PD ‐L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy58 | PD-L1 blockade with RT | Murine pancreatic carcinoma models | In vivo PD-L1 blockade with high dose RT improved tumor response; mathematical modeling suggesting that radiosensitization was abolished if anti-PD-L1 was delayed to 7 days after RT |
Clinical Data:
There are several ongoing or recently completed early-phase clinical trials testing various combinations of anti-CTLA4 therapy and RT. A comprehensive listing is beyond the scope of this review and this topic is rapidly evolving but Table 2 summarizes a sampling of relevant trials in a variety of disease sites.
Table 2:
Relevant Prospective Trials Involving CTLA-4 Blockade and RT. MTD= maximum tolerable dose, DLT = dose-limiting toxicity, WBRT = whole brain RT, SRS = stereotactic radiosurgery
| Trial | ClinicalTrials.gov ID | Setting | Phase | Treatment | Primary Endpoint | Toxicity | Status |
|---|---|---|---|---|---|---|---|
| Immune Activation in Patients with Locally Advanced Cervical Cancer Treated with Ipilimumab Following Definitive Chemoradiation (GOG-9929)87,88 | NCT01711515 | Cervical cancer (node positive) | 1 | Extended field RT with concurrent cisplatin followed by ipilimumab | MTD, DLT | 2/21 patients with self-limited grade 3 toxicity (lipase increase and dermatitis) | Published |
| Phase II Trial of Ipilimumab with Stereotactic Radiation Therapy for Metastatic Disease: Outcomes, Toxicities, and Low-Dose Radiation-Related Abscopal Responses82 | NCT02239900 | Metastatic solid tumors | 2 | SBRT concurrently (1 day after first dose) of ipilimumab or sequentially (1 week after second dose) | MTD, DLT | Several grade 3 events, most commonly diarrhea (6%), liver enzyme elevation, and skin rash (5% each); no treatment-related grade 4 or 5 toxicities | Published |
| Phase 1 Study of Ipilimumab Combined With Whole Brain Radiation Therapy or Radiosurgery for Melanoma Patients With Brain Metastases83 | NCT01703507 | Metastatic melanoma (brain) | 1 | WBRT or SRS with ipilimumab starting on day 3 of WBRT or 2 days after SRS | MTD | 16 patients enrolled, total of 21 grade 1–2 neurotoxic effects, 1 grade 3 neurotoxicity prior to ipilumumab, 10 additional grade 3 toxicities, mostly commonly GI (31%), no grade 4 or 5 toxicity | Published |
| Ipilimumab and radiation in patients with unresectable melanoma brain metastases: A multicenter, open label, phase-2, Spanish Melanoma Group (GEM) study89 | NCT02115139 | Metastatic melanoma (brain) | 2 | Ipilimumab every 3 weeks with whole brain radiation therapy between cycles 1 and 2 | 1-year survival rate | 11/58 patients with treatment-related significant events including liver enzyme elevation (n=4), diarrhea (n=4), and intestinal perforation (n=1) | Abstract Presented |
| Trial of SBRT With Concurrent Ipilimumab in Metastatic Melanoma | NCT02406183 | Metastatic Melanoma | 1 | Ipilimumab every 3 weeks for 4 cycles with concurrent RT | MTD | Pending report | Completed |
| Study of Combined Ionizing Radiation and Ipilimumab in Metastatic Non-small Cell Lung Cancer (NSCLC) | NCT02221739 | Metastatic NSCLC | 2 | Ipilimumab within 24 hours of starting RT | Best tumor response (excluding treated lesion) | Pending report | Completed |
| Neoadjuvant Durvalumab and Tremelimumab Plus Radiation for High Risk Soft-Tissue Sarcoma (NEXIS) | NCT02221739 | Soft-tissue sarcoma | 1/2 | Three doses of tremelimumab (CTLA-4) and durvalumab (PD-L1) prior to RT followed by surgical resection followed by additional durvalumab | High-grade toxicity and histopathologic response | Pending report | Open |
| Ipilimumab, Cetuximab, and Intensity-Modulated Radiation Therapy in Treating Patients With Previously Untreated Stage III-IVB Head and Neck Cancer | NCT01935921 | Head and Neck | 1 | Concurrent cetuximab and ipilimumab with RT in locally advanced treatment-naïve head and neck cancer | Toxicity | Pending report | Completed |
| Combination of Chemoradiation With Immunotherapy in Inoperable œsophageal Cancer (CRUCIAL) | NCT03437200 | Esophagus | 2 | Ipilumumab plus nivolumab with concurrent chemoradiation for inoperable early or locally advanced esophageal cancer | 1 year PFS | Pending report | Open |
The clinical data are limited with respect to the specific question of the optimal sequencing of anti-CTLA4 and RT. Kiess et al reported outcomes from 46 patients with melanoma who received ipilimumab and single fraction stereotactic radiosurgery (SRS) for brain metastases. Patients who received SRS during or before ipilimumab appeared to have better OS and less regional recurrence than those treated with ipilimumab first and there was a trend toward less local failure with concurrent treatment22. Similarly, Schoenfeld et al reported a case review of 16 patients were treated with whole brain radiation therapy (WBRT) plus SRS with ipilimumab and found that SRS before ipilimumab appeared to be associated with improved survival compared to ipilimumab first. However, another group reported outcomes from 77 melanoma patients who received SRS for brain metastases and did not see a difference in survival based on the sequencing of the two modalities23. Perhaps treatment within close temporal proximity is most important for maximum efficacy irrespective of the exact sequencing. For example, Jiang et al reported that outcomes were best for patients with brain metastases treated with SRS and ipilimumab when the SRS was given within 5.5 months of the last ipilimumab treatment. 24
In the setting of advanced cervical cancer, patients with lymph-node positive cervical cancer were treated on a recent phase I study of ipilimumab sequentially following standard of care chemoradiation therapy and demonstrated at 1-year disease-free survival of 74%, showing possible activity of a consolidative strategy in a population with a poor prognosis. This study also illustrated an increase in PD-1 expression during chemoradiation therapy that was sustained with administration of ipilimumab.
Programmed Death-1 Receptor (PD-1) Blockade and RT:
The PD-1 pathway has been one of the key targets for therapeutic intervention over recent years. PD-1 is predominantly expressed on mature cytotoxic T lymphocytes both within peripheral tissues and the tumor microenvironment 25,26 and serves as a “brake” on the activation of T-cells, which can thereby mediate tumor cell escape from immune surveillance. PD-1 binds PD-L1 and PD-L2 which are expressed by tumor cells as well as antigen presenting cells (APCs). 27 Once bound, T cell activity is inhibited and apoptosis signaling is increased. ICB can reactivate immunity in some patients but predicting benefit is difficult and may depend on factors such as age and sex.28
PD-1 expression may serve as a marker of T cell activation as it is expressed on activated but not resting T-cells in vitro 29 and its expression is associated with antigen-specific T cells in cancer patients. 30–32 However, PD-1 expression may also be correlated with an “exhausted” T-cell phenotype in which cytokine production is impaired.30,33,34 Nivolumab35, pembrolizumab8, and most recently cemiplimab36 are PD-1 inhibitors currently on the market.
Preclinical Data:
Several studies have investigated the combination of RT and ICB. Verbrugge et al demonstrated that concurrent RT and PD1 blockade can enhance the curative capacity of RT in a murine breast model. 37 Sharabi et. al showed that stereotactic body radiation therapy (SBRT), which allows for the precisely targeted delivery of high-dose radiation, given one day prior to PD-1 blockade, augments the PD-1 antitumor response and induces formation of memory T-cells via cross-presentation of tumor antigens. 38 Similarly, another group showed that anti-PD-1 therapy increased the tumor response to SBRT in a preclinical hepatocellular carcinoma model.39
Clinical Data:
The KEYNOTE-001 study investigated the use of pembrolizumab monotherapy for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) and reported durable activity and encouraging 5-year overall survival (OS) rates, particularly for patients with a PD-L1 tumor proportion score of at least 50%. 40 A pooled analysis of the phase 2 PEMBRO-RT and the phase 1/2 MDACC trials for patients with metastatic NSCLC randomizing to ICB with or without RT showed significantly improved PFS and OS along with increased response to therapy in the RT arms, adding more evidence supportive of combination therapy.41 The ETOP NICOLAS trial, also in the setting of lung cancer, has reported encouraging early safety data for 83 patients treated with upfront chemoradiation therapy with concurrent nivolumab, 42 with pending report of 1-year PFS data. A secondary analysis of KEYNOTE-001 showed that patients who had received RT prior to pembrolizumab appeared to have longer progression-free survival (PFS) and OS with pembrolizumab treatment than those who had not previously received RT, 43 although the design of this trial did not include a comparison to the converse sequencing option.
Multiple studies, predominantly phase 1/2 trials, testing a variety of sequencing approaches have been published or are underway. In the setting of head and neck cancer, for example, there are early reports from trials adding nivolumab to concurrent chemoradiation with cetuximab44 or testing cetuximab head-to-head with prembrolizumab, also concurrently.45
Duska et al. reported on their randomized Phase II study of chemoradiation and concurrent pembrolizumab for locally advanced cervical cancer, which showed safety and tolerability with the addition a PD-1 inhibitor with chemoradiation.46 Treatment-related grade ≥2 toxicity was experienced by 88%, 11 with at least one grade 4 AE and another 23 with at least one grade 3 AE. Grade ≥1 diarrhea was reported in 34 (65%; 50% grade 1) patients with no difference between arms (63 vs 68%, respectively). Two patients experienced 3 DLT’s. Most patients completed cisplatin (100% vs 82%), and 83% in both arms completed all pembrolizumab. The preliminary report from this study show that the combination of immune checkpoint inhibitors and pelvic chemo-radiotherapy in locally advanced cervical cancer appears to be safe and effective.
For additional examples and to highlight future directions, Table 3 provides a non-exhaustive sampling of reported or ongoing prospective trials testing the combination of RT with PD-1 blockade, including lung, head and neck, breast, gastrointestinal, genitourinary, central nervous system, hematologic, and gynecologic malignancies.
Table 3:
Relevant prospective trials involving PD-1 Blockade and RT. NSCLC = non-small cell lung cancer, PFS= progression-free survival, AE = adverse event, SCC= squamous cell carcinoma, DFS = disease-free survival
| Trial | ClinicalTrials.gov ID | Setting | Phase/Type | Treatment | Endpoint | Toxicity | Status |
|---|---|---|---|---|---|---|---|
| Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials 41 |
NCT02492568
NCT02444741 |
Metastatic NSCLC | 1/2 | Immunotherapy with or without RT | Response rate | High grade events uncommon, no new safety signals | Published |
| Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer-The ETOP NICOLAS trial42 | NCT02434081 | Stage III NCSCC | 2 | Chemoradiation with concurrent nivolumab | Safety | Anemia (47.5%), fatigue (45%), pneumonitis (42.5%); 55% mild, 30.3% moderate, 10.9% severe | Published |
| Phase II Randomized Trial of Radiotherapy With Concurrent and Adjuvant Pembrolizumab (Keytruda) Versus Concurrent Chemotherapy in Patients With Advanced/Intermediate-Risk p16+ Head and Neck Squamous Cell Carcinoma (KEYCHAIN) | NCT03383094 | Head and neck (intermediate/high risk p16+, locoregionally advanced) | 2 | RT and concurrent cisplatin versus RT and concurrent and adjuvant pembrolizumab | PFS | Lead-in results reported for 8 patients; included one DLT (grade 4 adrenal insufficiency), one grade 3 weight loss, one grade 3 infusion reaction | Recruiting |
| Safety evaluation of nivolumab (Nivo) concomitant with cetuximab-radiotherapy for intermediate (IR) and high-risk (HR) local-regionally advanced head and neck squamous cell carcinoma (HNSCC): RTOG 3504 44 | NCT02764593 | Head and neck (Intermediate and high risk) | 1 | Chemoradiation with cetuximab with addition of concurrent nivolumab (various schedules) | DLT | 1 DLT (mucositis) out of 8 evaluable patients along with one other grade 3 AE (lipase increase) | Initial safety report presented |
| Targeting PD-1 Therapy Resistance With Focused High or High and Low Dose Radiation in SCCHN | NCT03085719 | Metastatic head and neck | 2 | High and/or low dose RT with pembrolizumab on day one | Overall response rate | Pending Report | Recruiting |
| A phase II randomized trial of pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced (LA) squamous cell carcinoma of the head and neck (SCCHN): First results of the GORTEC 2015–01 “PembroRad” trial45 | NCT02707588 | Head and neck; patients unfit for high dose cisplatin with nonoperable disease | 2 | RT with cetuxumab or pembrolizumab | Locoregional control | Less grade ≥3 mucositis and dermatitis in pembrolizumab arm; similar dysphagia | Early results presented |
| A Study of Chemoradiation Plus Pembrolizumab for Locally Advanced Laryngeal Squamous Cell Carcinoma | NCT02759575 | Locally advanced head and neck (larynx) | 1/2 | RT with concurrent cisplatin and concurrent pembrolizumab | Rate of grade 3 or 4 AEs | Pending Report | Active |
| Study of Nivolumab Alone or in Combination With Ipilimumab as Immunotherapy vs Standard Follow-up in Surgical Resectable HNSCC After Adjuvant Therapy (IMSTAR-HN) | NCT03700905 | Postoperative head and neck SCC | 3 | Nivolumab or nivolumab plus ipilimumab following surgical resection and adjuvant RT (with or without chemo) | DFS | Pending report | Open |
| Anti PD-1 Antibody With Radiation Therapy in Patients With HER2-negative Metastatic Breast Cancer | NCT03430479 | Metastatic Her2-negative breast | I/2 | RT + nivolumab + hormonal therapy | DLT | Pending report | Active |
| Study of PD1 Blockade by Pembrolizumab With Stereotactic Body Radiotherapy in Advanced Solid Tumors | NCT02608385 | Limited metastatic solid tumors | 1 | 3 or 5 doses of SBRT to chosen metastatic sites followed by pembrolizumab | Recommended SBRT dose | Pending report | Active |
| Safety and Feasibility of PD-1 Blockade in the Treatment of Rectal Cancer | NCT04357587 | Rectal cancer | 1 | RT with concurrent capecitabine with pembrolizumab given on days 1, 22, and 43 | Rate of AEs, completion of therapy, feasibility | Pending report | Recruiting |
| A Phase II Randomized Trial of Immunotherapy Plus Radiotherapy in Metastatic Genitourinary Cancers | NCT03115801 | Metastatic genitourinary cancers | 2 | Immunotherapy (nivolumab, atezolizumab, or pembrolizumab) with or without RT | Response rate | Pending report | Recruiting |
| Combination of Nivolumab Immunotherapy With Radiation Therapy and Androgen Deprivation Therapy | NCT03543189 | Grade group 5 prostate cancer | 1–2 | ADT followed by nivolumab and RT | Safety, replase-free survival | Pending report | Recruiting |
| Nivolumab With Radiation Therapy and Bevacizumab for Recurrent MGMT Methylated Glioblastoma | NCT03743662 | Recurrent MGMT Methylated Glioblastoma | 2 | Hypofractionated reirradiation with concurrent nivolumab followed by adjuvant nivolumab | Overall survival | Pending report | Recruiting |
| Pembrolizumab for Newly Diagnosed Glioblastoma (PERGOLA) | NCT03899857 | Newly diagnosed glioblastoma | 2 | RT with concurrent temozolomide with addition of concurrent Pembrolizumab | OS | Pending report | Not yet recruiting |
| A Multicenter, Phase 3, Randomized Trial of Sequencial Chemoradiotherapy With or Without Toripalimab (PD-1 Antibody) in Newly Diagnosed Early-Stage Extranodal Natural Killer/T Cell Lymphoma, Nasal Type (ENKTL) | NCT04365036 | Early stage NK/T Cell lymphoma | 3 | Toripalimab and induction chemotherapy followed by RT with concurrent toripalimab versus induction followed by RT | PFS | Pending report | Recruiting |
| Study of Chemoradiotherapy With or Without Pembrolizumab (MK-3475) For The Treatment of Locally Advanced Cervical Cancer (MK-3475-A18/KEYNOTE-A18/ENGOT-cx11) | NCT04221945 | Locally advanced cervix | 3 | Chemoradiation with or without concurrent pembrolizumab | PFS, OS | Pending report | Recruiting |
Programmed Death-Ligand 1 (PD-L1) Blockade and RT:
The transmembrane protein PD-L1 is generally not expressed in cell lines in vitro but can become induced on tumors and in the tumor microenvironment 47,48 by IFN-γ which is in turn produced by effector T-cells as well as by Toll-like receptor ligands through several signaling pathways including JAK/STAT/IRF-1, MEK/ERK, MyD88/TRAF6, and p38 MAPK. 49–53 When bound to its partner PD-1, downstream signaling results in immune inhibition. In addition to PD-1, PD-L1 has emerged as a pharmaceutical target with a similar goal of preventing immunologic downregulation by removing the “brake”. Atezolizumab54, avelumab55, and durvalumab56 are monoclonal anti-PD-L1 agents with current indications.
Preclinical Data:
Wu et al. showed in a human bladder cancer cell line and a murine model of bladder cancer that RT induces expression of PDL1 in a dose-dependent manner. PD-L1 expression peaked 72 hours after RT delivery and significantly declined when assessed 7 days after RT. Furthermore, it appeared that the anti-PD-L1 therapy sensitized the tumor to RT in a preclinical bladder cancer model. 15 Similarly, Dovedi et al showed in a colorectal model that concurrent administration of anti-PD-L1 with RT appeared more efficacious than sequential administration. 57 Moreover, a separate study found that in vivo PD-L1 inhibition added to high dose RT significantly improved tumor response in two murine models of pancreatic ductal adenocarcinoma and reported mathematical modeling suggesting that radiosensitization was completely abolished if anti-PD-L1 was delayed to 7 days after RT. 58
Clinical Data:
The PACIFIC trial randomly assigned patients with stage III NSCLC to receive durvalumab or placebo following standard chemoradiation therapy. Median PFS was significantly longer for patients treated with durvalumab compared to placebo (16.8 months versus 5.6 months, respectively), and there was also improvement in secondary endpoints of response rate and duration of response. Toxicity endpoints were similar between the groups 59 and this landmark trial rapidly changed the standard of care for stage III NSCLC. On subgroup analysis, patients who were randomized within 14 days of completion of RT had improved survival compared to patients who were randomized after a longer interval. 60
Table 4 shows examples across multiple disease sites of published and ongoing studies exploring the combination of RT and PD-L1 blockade.
Table 4:
relevant prospective trials involving PD-L1 Blockade and RT. NSCLC= non-small cell lung cancer, PFS = progression-free survival, OS = overall survival, DLT = dose limiting toxicity
| Trial | ClinicalTrials.gov ID | Setting | Phase/Type | Treatment | Endpoint | Toxicity | Status |
|---|---|---|---|---|---|---|---|
| Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer59 | NCT02125461 | Stage III NSCLC | 3 | Chemoradiation with or without consolidative durvalumab | PFS and OS | Grade 3/4 events in 29.9% of patients in durvalumab arm and 26.1% of placebo arm; most common was pneumonia | Published |
| Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial90 | NCT01693562 | Advanced head and neck (palliative intent) | 1/2 | Palliative RT plus durvalumab | Safety and efficacy | 5 patients reported grade 1–2 AEs; no grade ≥3; most common was transient mucositis | Published |
| Durvalumab And Radiation Therapy Followed by Adjuvant Durvalumab in Patients With Urothelial Cancer (T2–4 N0–2 M0) of the Bladder (DUART) | NCT02891161 | Urothelial cancer | 1/2 | RT with concurrent durvalumab followed by adjuvant durvalumab | Safety/PFS | Pending report | Active |
| Prostate Cancer With OligometaSTatic Relapse: Combining Stereotactic Ablative Radiotherapy and Durvalumab (MEDI4736) (POSTCARD) | NCT03795207 | Oligometastatic prostate cancer | 2 | SBRT with or without durvalumab (to be started 1 month prior to SBRT and then given for 12 months total) | PFS | Pending Report | Active |
| Avelumab With Chemoradiation in Locally Advanced Rectal Cancer | NCT03299660 | Locally advanced rectal cancer | 2 | Standard long-course chemoradiation followed by 4 cycles of avelumab followed by resection | Pathologic response rate | Pending report | Open |
| A Study Evaluating the Association of Hypofractionated Stereotactic Radiation Therapy and Durvalumab for Patients With Recurrent Glioblastoma (STERIMGLI) | NCT02866747 | Recurrent glioblastoma | 1/2 | Hypofractionated RT with durvalumab starting on the last day of RT | DLT/OS | Pending report | Open |
| CALLA: Efficacy and safety of durvalumab with and following concurrent chemoradiotherapy (CCRT) versus CCRT alone in women with locally advanced cervical cancer: A phase III, randomized, double-blind, multicenter study. | NCT03830866 | Cervix (FIGO IB2-IIB with positive nodes or IIIA-IVA with any node) | 3 | Durvalumab + chemoradiation or placebo + chemoradiation followed by durvalumab or placebo maintenance for 24 months | PFS | Pending report | Open |
Toxicity and Tolerability of ICB and RT:
As ICB is increasingly utilized and combined with other treatment modalities including RT and chemotherapy, more refined knowledge pertaining to toxicity is paramount. Untoward activation of the immune system can lead to a host of immune-related overactivation toxicity affecting systems throughout the body.
The incidence of radionecrosis after SRS to the brain varies based on diagnostic criteria and between series but is typically on the order of 13–34% within two years following treatment. 61–64 Fang et al. conducted a retrospective review of 137 patients treated with CNS-directed RT in combination with either PD-1 or CTLA-4 and found evidence of radionecrosis in 27% of patients. OS at 1 year did not differ between those that developed radionecrosis and those that did not.65 Ahmed et al reported no undue toxicity for combination nivolumab and SRS for brain metastases from melanoma and the toxicity identified was consistent with what would be expected with ICB alone. 66
Another series of 133 patients with metastatic cancer (small cell lung cancer, melanoma, or renal cell carcinoma) received RT and a variety of ICB sequences and agents. Overall, therapy was well-tolerated with mostly mild toxicity and there were no associations between the site treated and specific immune-related adverse events (ir-AEs), although patients who received both CTLA4 and PD-1 blockade experienced more any-grade ir-AEs compared to either individually (71% vs 29%, p<0.001) and there was a trend toward more any-grade ir-AEs in patients who received RT within 14 days of immunotherapy (39% vs 23%, p= 0.01).67
A large retrospective review of 750 patients suggests a benefit to OS with concurrent ICB and RT, especially pronounced with at least 1 month of induction ICB. 68 Qian et al analyzed outcomes from 75 patients with 566 brain metastases treated with SRS and found a greater reduction in tumor volume persistent at 1.5, 3, and 6 months when SRS was given concurrently with ICB (defined as within 4 weeks) versus more than 4 weeks apart; this effect was most pronounced for PD-1 compared to CTLA-4 inhibition. 69
A phase I dose-finding study of SBRT and durvalumab or the CTLA-4 inhibitor tremelimumab administered as second-line therapy for metastatic pancreatic adenocarcinoma patients did not report any dose-limiting toxicities thus far. 70 Notably, the PACIFIC trial showed similar toxicity between durvalumab and placebo in the consolidation setting. 59
Radiation recall, an acute inflammatory reaction limited to previously irradiated areas has been reported with a variety of systemic agents.71 The data are limited with respect to RT and ICB, although nivolumab-induced RT recall pneumonitis was reported in a case even two years after the delivery of RT. 72
Upcoming Clinical Trials and Future Directions:
There are many ongoing and upcoming trials that will further elucidate the sequencing of ICB and RT. Furthermore, the combination and sequencing of other systemic therapy as well as RT is a critical area of interest, especially as ICB moves more into the upfront and definitive setting alongside traditional cytotoxic chemotherapy and/or targeted therapy.
For locally advanced cervical cancer, NRG-GY017 is testing atezulizumab as an immune primer and plus concurrent to chemoradiation versus concurrent only for the definitive treatment of lymph-node positive locally advanced cervical cancer. 73 The CALLA study, also in the setting of advanced cervical cancer, is a global phase III randomized placebo controlled trial investigating concurrent and consolidative durvalumab plus standard of care platinum-based chemoradiation therapy versus standard chemoradiation alone. 74 Additionally, the NRG-GY020 trial is examining adjuvant RT with or without pembrolizumab for resected high-intermediate risk microsatellite instability-high endometrial cancer patients (NCT04214067).
In the lung cancer arena, ICB is under active investigation in a variety of sequences. Table 5 highlights several trials directly exploring sequencing questions. The SABRseq trial is currently recruiting patients and will examine SBRT followed by pembrolizumab versus pembrolizumab followed by SBRT for patients with metastatic NSCLC (NCT 03307759). In the definitive setting, patients with stage I-II NSCLC eligible for SBRT monotherapy are eligible for the PACIFIC-4 trial which will randomize to SBRT plus concurrent durvalumab versus placebo. 75 Additionally, the I-SABR trial will test the use of nivolumab within 36 hours of SBRT (NCT 01463423). Finally, the phase III SWOG S1914 trial is randomizing patients with high risk early stage NSCLC to induction/consolidation atezolizumab plus SBRT versus SBRT alone (NCT 04214262).
Table 5:
Prospective trials directly comparing sequencing of ICB and RT
| Trial | ClinicalTrials.gov ID | Setting | Phase/Type | Treatment | Endpoint | Toxicity | Status |
|---|---|---|---|---|---|---|---|
| Results of an early safety analysis of a study of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer 46 | NCT02635360 | Locally advanced cervical cancer | 2 | Pembrolizumab given after or during chemoradiation | Safety | 88% of patients with grade 2 or higher toxicity; no difference between arms | Early report published |
| Anti PD-L1 (Atezolizumab) as an Immune Primer and Concurrently with Extended Field Chemoradiotherapy for Node Positive Locally Advanced Cervical Cancer73 | NCT03738228 | Node positive locally advanced cervix | 1 | Loading atezolizumab followed by chemoRT (extended field) versus chemoRT (extended field) with concurrent atezolizumab | Anti-tumor immune response | Pending Report | Active |
| Sequencing of Stereotactic Ablative Body Radiotherapy in Combination With PD-1 Blockade Using Pembrolizumab in Metastatic Non-Small Cell Lung Carcinoma (SABRseq) | NCT03307759 | Metastatic non-smell lung cancer | 1 | Pembrolizumab given before or after SBRT | Adverse Events | Pending report | Recruiting |
The phase II KEYCHAIN trial is randomizing patients with intermediate/high risk p16-positive locoregionally advanced head and neck squamous cell carcinoma to concurrent and adjuvant pembrolizumab with RT versus standard of care therapy (RT with concurrent cisplatin). 76
Ultimately, ICB will continue to incorporate into the standard of care paradigms for many malignancies. Understanding and confirming how to best incorporate ICB, either in the neoadjuvant, concurrent, or adjuvant settings will require well designed clinical trials and will likely differ between cancer types. Along these lines, strategic use of orthotopic pre-clinical models, which more accurately reflect tissue specific tumor microenvironments, may help to guide rational design of these trials.77 Additionally, precise analysis of patient samples from ongoing trials will be critical to understanding the effects of sequencing on the tumor microenvironment and mechanisms of tumor cell death or resistance. For example, the ongoing NRG-GY017 study is testing loading and concurrent atezolizumab plus chemoradiation vs concurrent atezolizumab plus chemoradiation and is collecting tissue and blood samples; the primary endpoint of the study is differential immune activation assessed by clonal expansion of T cell receptor beta repertoires in peripheral blood.73
Most groups have focused solely on the effects of ICB on T-cells, but it is important to recognize that many other immune cells are regulated by the PD-1 pathway including NK-cells, B-cells, macrophages, and dendritic cells. For example, it was recently shown that B-cells play a key role in anti-tumor immune responses after ICB and that B-cells can be activated by radiation and PD-1 blockade.78–80 Understanding how these multiple cell types work together to effectuate tumor immune surveillance and will be key to unlocking anti-tumor immunity and increasing the response rates to ICB.
Sequencing Summary and Recommendations:
The combination of ICB and RT is already a viable treatment strategy for patients with solid malignancies across a large range of histology and stage, although there remain several unresolved questions, including the sequencing and timing of this combination. Although there are preclinical and clinical studies that have addressed this topic, further prospective clinical work is needed to better clarify and directly compare sequencing options, and conclusions are present are limited. Here we reviewed the available evidence and identified emerging lines of evidence.
With respect to anti-CTLA4 therapy, the data are insufficient to make a firm recommendation for sequencing, as most reports retrospectively detail a variety of treatment regimens with relatively small numbers of patients. However, preclinical data supports treatment with RT and anti-CTLA4 within close proximity and early clinical trial data shows a signal for efficacy when given in the concurrend and consolidative setting. 21,81–83
Regarding PD1/PD-L1 blockade, the preponderance of available clinical data most firmly supports use after RT or in the adjuvant setting, such as in the PACIFIC trial, which rapidly changed the standard of care for a subset of patients with NSCLC by adding consolidative ICB after definitive chemoradiation60. Subset analysis supports the notion of starting durvalumab as soon as feasibly possible following chemoradiation, although this may be confounded to some extent by patients with more favorable disease or overall health status recovering faster from chemoradiation and perhaps moving sooner to the next phase of therapy.
However, a large number of studies are underway exploring either concurrent or neoadjuvant therapy. Preclinical work shows a radiosensitization effect of ICB and a modeling study has suggested a limited window of opportunity for optimizing this synergy. 58 Concurrent administration may also maximize the release and availability of tumor-related cytokines for better recognition by the host immune system.
Outside of a clinical trial, most ICB is given in the setting of advanced and often metastatic disease. Although durable responses are possible, many patients today unfortunately will not be cured, and therefore toxicity of treatment and effects on quality of life are of great importance. Both RT and ICB have distinct side effect profiles, with RT-related adverse events generally related to normal tissue damage to structures surrounding the target, while ICB can result in autoimmune damage essentially anywhere in the body. The expected toxicity profiles of each must be carefully considered and there are reports of interrelated toxicity, e.g. radiation recall dermatitis or pneumonitis. For the most part, however, the available evidence suggests that toxicity is not usually synergistic and that ICB given before, during, or after RT is typically well-tolerated. Von Reibnitz et al published a retrospective series of 79 patients treated with RT and several ICB agents and did not find a significant correlation between adverse events when comparing concurrent versus sequential treatment. 84
As ICB moves more into the upfront setting, it will be increasingly employed either concurrently or in close proximity with other forms of systemic therapy. Some trials, such as KEYCHAIN76, are exploring the replacement of standard of care chemotherapy in a definitive head and neck setting, whereas the NRG GY-017 trial is adding atezulizumab as an immune primer as well as concurrently with standard chemotherapy and RT. 73 Efficacy and toxicity reports from these and other trials will allow for future determinations about whether ICB can replace traditional systemic therapy or if not, if it can be safely administered alongside it. Future studies of the addition of immunotherapy to traditional chemo-radiation are planned to determine the best way to deliver the treatment and if any improvement is seen with adding immunotherapy to traditional therapy.
Moreover, there are several other related areas of investigation of importance and interest, including the optimal dose, fractionation, and target volumes of RT when combined with ICB. Indeed, preclinical work has shown that elective nodal irradiation can actually attenuate the effectiveness of combined SRS and immunotherapy. 85 Indeed, focal irradiation of a tumor may stimulate CD8+ and stem-like CD8+ T cells in regional lymph nodes and irradiation of these nodes in preclinical modeling has been shown to reduce distant control86, which may have implications for size of radiation fields in the future.
There are also an increasing number of other types of immunotherapy platforms either already in use or on the horizon, including other T cell targets, NK cell targets, dendritic cell vaccines, and intralesional injections.
Conclusions:
Taken together, ICB is under active investigation in conjunction with RT in several disease sites and is moving into the upfront definitive setting, including increasing emphasis on concomitant administration. For curative treatment, there is evidence supporting the use of induction, concurrent, and consolidative usage, and the combination of RT and ICB is frequently employed in the management of metastatic disease. There are potential merits to each approach. An induction strategy may allow for maximal immune priming and may allow for earlier systemic control. Concurrent therapy may maximize synergy between modalities by taking advantage of RT-induced tumor antigen release as well as RT-mediated immunologic changes. Consolidation may allow for long-term duration of immune activation and surveillance after definitive local treatment. There is at least a theoretical risk of synergistic toxicity with concomitant administration, although early data has not shown disproportionate adverse events associated with concurrent therapy and at present there is not strong evidence suggesting differences in toxicity. The overall weight of evidence supports the framework that the efficacy of the combination of therapies is likely optimized when given in relatively close proximity.
Overall, there is insufficient evidence to declare one sequencing strategy universally better than others, and response vary based on tumor type and stage, but ongoing clinical trials will provide valuable additional insight and should be well-supported by the oncology community.
Footnotes
Conflict of Interest: Dr. Sharabi reports consultant fees from Jounce Therapeutics, AstraZeneca, and Merck; reports receiving research grants from Varian Medical Systems and Pfizer; holds ownership interest in Toragen outside the submitted work. Dr. Zamarin reports personal fees from Merck, grants from Merck, grants, personal fees and other from Genentech, personal fees from Agenus, grants and personal fees from Hookipa Biotech, personal fees from Western Oncolytics, other from Calidi Biotherapeutics, grants from Astra Zeneca, outside the submitted work. Dr. Mayadev has disclosed that she serves as the Co-Chair of the Cervix/Vulva Cancer Subcommittee at NRG Oncology, is a consultant for AstraZeneca, and serves on the GOG Foundation Advisory Board for ad-hoc clinical trial meetings. LKM reports conflict of interest: AstraZeneca, Merck. The remaining authors report that they have no potential conflicts of interest.
References:
- 1.Busch W Aus der Sitzung der medicinischen Section vom 13. November 1867. Berliner Klin Wochenschrift (1868). [Google Scholar]
- 2.Fehleisen. Ueber die Züchtung der Erysipelkokken auf künstlichem Nåhrboden und ihre Uebertragbarkeit auf den Menschen. Dtsch. Medizinische Wochenschrift (1882) doi: 10.1055/s-0029-1196806. [DOI] [Google Scholar]
- 3.COLEY WB TREATMENT OF INOPERABLE MALIGNANT TUMORS WITH THE TOXINES OF ERYSIPELAS AND THE BACILLUS PRODIGIOSUS. Am. J. Med. Sci. (1894) doi: 10.1097/00000441-189407000-00006. [DOI] [Google Scholar]
- 4.Dunn GP, Old LJ & Schreiber RD The three Es of cancer immunoediting. Annual Review of Immunology (2004) doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 5.Wei SC, Duffy CR & Allison JP Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discovery (2018) doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer (2012) doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.FDA Fact Sheet, Ipilimumab. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s108lbl.pdf (2020).
- 8.FDA Fact Sheet, Pembrolizumab. (2020).
- 9.Galon J & Bruni D Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nature Reviews Drug Discovery (2019) doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 10.Frey B et al. Immunomodulation by ionizing radiation—impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunological Reviews (2017) doi: 10.1111/imr.12572. [DOI] [PubMed] [Google Scholar]
- 11.Son CH, Fleming GF & Moroney JW Potential role of radiation therapy in augmenting the activity of immunotherapy for gynecologic cancers. Cancer Management and Research (2017) doi: 10.2147/CMAR.S116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y et al. Combining immunotherapy and radiotherapy for cancer treatment: Current challenges and future directions. Frontiers in Pharmacology (2018) doi: 10.3389/fphar.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y et al. Therapeutic effects of ablative radiation on local tumor require CD8 + T cells: Changing strategies for cancer treatment. Blood (2009) doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang W, Chan CK, Weissman IL, Kim BYS & Hahn SM Immune Priming of the Tumor Microenvironment by Radiation. Trends in Cancer (2016) doi: 10.1016/j.trecan.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Wu C. Te, Chen WC, Chang YH, Lin WY & Chen MF The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. (2016) doi: 10.1038/srep19740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedline RH et al. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. (2009) doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read S et al. Blockade of CTLA-4 on CD4 + CD25 + Regulatory T Cells Abrogates Their Function In Vivo. J. Immunol. (2006) doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demaria S et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. (2005). [PubMed] [Google Scholar]
- 19.Yoshimoto Y et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS One (2014) doi: 10.1371/journal.pone.0092572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twyman-Saint Victor C et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature (2015) doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young KH et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One (2016) doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiess AP et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: Safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. (2015) doi: 10.1016/j.ijrobp.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knisely JPS et al. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival: Clinical article. J. Neurosurg. (2012) doi: 10.3171/2012.5.JNS111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang W et al. Temporally-Dependent Intracranial Control of Melanoma Brain Metastasis by Stereotactic Radiation Therapy in Patients Treated With Immune Checkpoint Blockade. Int. J. Radiat. Oncol. (2015) doi: 10.1016/j.ijrobp.2015.07.137. [DOI] [Google Scholar]
- 25.Vinay DS et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Seminars in Cancer Biology (2015) doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P & Allison JP Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell (2015) doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latchman Y et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. (2001) doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 28.Castro A et al. Strength of immune selection in tumors varies with sex and age. Nat. Commun. (2020) doi: 10.1038/s41467-020-17981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu-Monette ZY, Zhang M, Li J & Young KH PD-1/PD-L1 blockade: Have we found the key to unleash the antitumor immune response? Frontiers in Immunology (2017) doi: 10.3389/fimmu.2017.01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmadzadeh M et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood (2009) doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mognol GP et al. Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl. Acad. Sci. U. S. A. (2017) doi: 10.1073/pnas.1620498114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez-Poma SM et al. Expansion of tumor-infiltrating CD8+ T cells expressing PD-1 improves the efficacy of adoptive T-cell therapy. Cancer Res. (2017) doi: 10.1158/0008-5472.CAN-17-0236. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q et al. Blockade of Programmed Death-1 Pathway Rescues the Effector Function of Tumor-Infiltrating T Cells and Enhances the Antitumor Efficacy of Lentivector Immunization. J. Immunol. (2010) doi: 10.4049/jimmunol.1001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngiow SF et al. A threshold level of intratumor CD8+ T-cell PD1 expression dictates therapeutic response to anti-PD1. Cancer Res. (2015) doi: 10.1158/0008-5472.CAN-15-1082. [DOI] [PubMed] [Google Scholar]
- 35.FDA Fact Sheet: Nivolumab.
- 36.FDA Fact Sheet: Cemiplimab.
- 37.Verbrugge I et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. (2012) doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 38.Sharabi AB et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. (2015) doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman D et al. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol. Res. (2017) doi: 10.1111/hepr.12789. [DOI] [PubMed] [Google Scholar]
- 40.Garon EB et al. Five-year long-term overall survival for patients with advanced NSCLC treated with pembrolizumab: Results from KEYNOTE-001. J. Clin. Oncol. (2019) doi: 10.1200/jco.2019.37.18_suppl.lba9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theelen WSME et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir. Med. (2020). [DOI] [PubMed] [Google Scholar]
- 42.Peters S et al. Safety evaluation of nivolumab added concurrently to radiotherapy in a standard first line chemo-radiotherapy regimen in stage III non-small cell lung cancer—The ETOP NICOLAS trial. Lung Cancer (2019) doi: 10.1016/j.lungcan.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Shaverdian N et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. (2017) doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferris RL et al. Safety evaluation of nivolumab (Nivo) concomitant with cetuximab-radiotherapy for intermediate (IR) and high-risk (HR) local-regionally advanced head and neck squamous cell carcinoma (HNSCC): RTOG 3504. J. Clin. Oncol. (2018) doi: 10.1200/jco.2018.36.15_suppl.6010. [DOI] [Google Scholar]
- 45.Sun XS et al. A phase II randomized trial of pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced (LA) squamous cell carcinoma of the head and neck (SCCHN): First results of the GORTEC 2015–01 “PembroRad” trial. J. Clin. Oncol. (2018) doi: 10.1200/jco.2018.36.15_suppl.6018. [DOI] [Google Scholar]
- 46.Duska LR et al. Results of an early safety analysis of a study of the combination of pembrolizumab and pelvic chemoradiation in locally advanced cervical cancer. Cancer (2020) doi: 10.1002/cncr.33136. [DOI] [PubMed] [Google Scholar]
- 47.Iwai Y, Terawaki S & Honjo T PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. (2005) doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 48.Andorsky DJ et al. Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin. Cancer Res. (2011) doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 49.Lee SJ et al. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-γ-induced upregulation of B7-H1 (CD274). FEBS Lett. (2006) doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- 50.Liu J et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood (2007) doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 51.Hao Y et al. Selective JAK2 inhibition specifically decreases Hodgkin lymphoma and Mediastinal large B-cell lymphoma growth in vitro and in vivo. Clin. Cancer Res. (2014) doi: 10.1158/1078-0432.CCR-13-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia-Diaz A et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. (2017) doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto R et al. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. (2009) doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.FDA Fact Sheet: Atezolizumab.
- 55.FDA Fact Sheet: Avelumab.
- 56.FDA Fact Sheet: Durvalumab.
- 57.Dovedi SJ et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. (2014) doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 58.Azad A et al. PD -L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol. Med. (2017) doi: 10.15252/emmm.201606674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antonia SJ et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N. Engl. J. Med. (2017) doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 60.Antonia SJ et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. (2018) doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 61.Sneed PK et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J. Neurosurg. (2015) doi: 10.3171/2014.10.JNS141610. [DOI] [PubMed] [Google Scholar]
- 62.Giglio P & Gilbert MR Cerebral radiation necrosis. Neurologist (2003) doi: 10.1097/01.nrl.0000080951.78533.c4. [DOI] [PubMed] [Google Scholar]
- 63.Kohutek ZA et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J. Neurooncol. (2015) doi: 10.1007/s11060-015-1881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Le Rhun E, Dhermain F, Vogin G, Reyns N & Metellus P Radionecrosis after stereotactic radiotherapy for brain metastases. Expert Review of Neurotherapeutics (2016) doi: 10.1080/14737175.2016.1184572. [DOI] [PubMed] [Google Scholar]
- 65.Fang P et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J. Neurooncol. (2017) doi: 10.1007/s11060-017-2470-4. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed KA et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J. Neurooncol. (2017) doi: 10.1007/s11060-017-2437-5. [DOI] [PubMed] [Google Scholar]
- 67.Bang A et al. Multicenter Evaluation of the Tolerability of Combined Treatment With PD-1 and CTLA-4 Immune Checkpoint Inhibitors and Palliative Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. (2017) doi: 10.1016/j.ijrobp.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Samstein R, Rimner A, Barker CA & Yamada Y Combined Immune Checkpoint Blockade and Radiation Therapy: Timing and Dose Fractionation Associated with Greatest Survival Duration Among Over 750 Treated Patients. Int. J. Radiat. Oncol. (2017) doi: 10.1016/j.ijrobp.2017.06.303. [DOI] [Google Scholar]
- 69.Qian JM, Yu JB, Kluger HM & Chiang VLS Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer (2016) doi: 10.1002/cncr.30138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duffy AG et al. A pilot study of immune checkpoint inhibition in combination with radiation therapy in patients with metastatic pancreatic cancer. J. Clin. Oncol. (2017) doi: 10.1200/jco.2017.35.15_suppl.e15786. [DOI] [Google Scholar]
- 71.Burris HA & Hurtig J Radiation Recall with Anticancer Agents. Oncologist (2010) doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shibaki R, Akamatsu M, Fujimoto H, Koh Y & Yamamoto N Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Annals of Oncology (2017) doi: 10.1093/annonc/mdx115. [DOI] [PubMed] [Google Scholar]
- 73.Mayadev J et al. Anti-PD-L1 (atezolizumab) as an immune primer and concurrently with extended-field chemoradiotherapy for node-positive locally advanced cervical cancer. Int. J. Gynecol. Cancer (2019) doi: 10.1136/ijgc-2019-001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Monk BJ et al. CALLA: Efficacy and safety of durvalumab with and following concurrent chemoradiotherapy (CCRT) versus CCRT alone in women with locally advanced cervical cancer: A phase III, randomized, double-blind, multicenter study. J. Clin. Oncol. 37, TPS5597–TPS5597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robinson C et al. P1.18–12 PACIFIC-4/RTOG 3515: Phase III Study of Durvalumab Following SBRT for Unresected Stage I/II, Lymph-Node Negative NSCLC. J. Thorac. Oncol. (2019) doi: 10.1016/j.jtho.2019.08.1328. [DOI] [Google Scholar]
- 76.Sacco AG et al. Radiotherapy with Concurrent and Adjuvant Pembrolizumab in Patients with P16-Positive Locoregionally Advanced Head and Neck Cancer: KEYCHAIN Trial Lead-In Results. Int. J. Radiat. Oncol. (2019) doi: 10.1016/j.ijrobp.2019.06.1701. [DOI] [Google Scholar]
- 77.Olson B, Li Y, Lin Y, Liu ET & Patnaik A Mouse models for cancer immunotherapy research. Cancer Discovery (2018) doi: 10.1158/2159-8290.CD-18-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Helmink BA et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature (2020) doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petitprez F et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature (2020) doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 80.Kim SS et al. B Cells Improve Overall Survival in HPV-Associated Squamous Cell Carcinomas and Are Activated by Radiation and PD-1 Blockade. Clin. Cancer Res. (2020) doi: 10.1158/1078-0432.CCR-19-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayadev J et al. A phase I study of sequential ipilimumab in the definitive treatment of node positive cervical cancer: GOG 9929. J. Clin. Oncol. (2017) doi: 10.1200/jco.2017.35.15_suppl.5526. [DOI] [Google Scholar]
- 82.Welsh JW et al. Phase II trial of ipilimumab with stereotactic radiation therapy for metastatic disease: Outcomes, toxicities, and low-dose radiation-related abscopal responses. Cancer Immunol. Res. (2019) doi: 10.1158/2326-6066.CIR-18-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams NL et al. Phase 1 Study of Ipilimumab Combined With Whole Brain Radiation Therapy or Radiosurgery for Melanoma Patients With Brain Metastases. in International Journal of Radiation Oncology Biology Physics (2017). doi: 10.1016/j.ijrobp.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 84.von Reibnitz D et al. Safety of combining thoracic radiation therapy with concurrent versus sequential immune checkpoint inhibition. Adv. Radiat. Oncol. (2018) doi: 10.1016/j.adro.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marciscano AE et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin. Cancer Res. (2018) doi: 10.1158/1078-0432.CCR-17-3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buchwald ZS et al. Tumor-draining lymph node is important for a robust abscopal effect stimulated by radiotherapy. J. Immunother. Cancer (2020) doi: 10.1136/jitc-2020-000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Da Silva DM et al. Immune Activation in Patients with Locally Advanced Cervical Cancer Treated with Ipilimumab Following Definitive Chemoradiation (GOG-9929). Clin. Cancer Res. (2020) doi: 10.1158/1078-0432.ccr-20-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayadev JS et al. Sequential Ipilimumab after Chemoradiotherapy in Curative-Intent Treatment of Patients with Node-Positive Cervical Cancer. JAMA Oncol. (2020) doi: 10.1001/jamaoncol.2019.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lopez-Martin JA et al. Ipilimumab and radiation in patients with unresectable melanoma brain metastases: A multicenter, open label, phase-2, Spanish Melanoma Group (GEM) study (NCT-2013-001132-22). J. Clin. Oncol. (2018) doi: 10.1200/jco.2018.36.15_suppl.9546. [DOI] [Google Scholar]
- 90.Levy A, Massard C, Soria JC & Deutsch E Concurrent irradiation with the anti-programmed cell death ligand-1 immune checkpoint blocker durvalumab: Single centre subset analysis from a phase 1/2 trial. Eur. J. Cancer (2016) doi: 10.1016/j.ejca.2016.09.013. [DOI] [PubMed] [Google Scholar]


