Abstract
The CCAAT enhancer binding protein (C/EBP) family of transcription factors are important transcriptional mediators of a wide range of physiologic processes. C/EBP-γ is the shortest C/EBP protein and lacks a canonical activation domain for the recruitment of transcriptional machinery. Despite its ubiquitous expression and ability to dimerize with other C/EBP proteins, C/EBP-γ has been studied far less than other C/EBP proteins, and, to our knowledge, no review of its functions has been written. This review seeks to integrate the current knowledge about C/EBP-γ and its physiologic roles, especially in cell proliferation, the integrated stress response, oncogenesis, hematopoietic and nervous system development, and metabolism, as well as to identify areas for future research.
Keywords: Transcription factor, CCAAT-enhancer binding protein, integrated stress response, cancer, cell proliferation, neuronal development
1. Introduction
The CCAAT enhancer binding (C/EBP) family is a group of leucine zipper (bZIP) transcription factors consisting of C/EBP-α, β, δ, γ, and ε. These transcription factors are expressed in a wide range of tissues in a time and context-specific manner and are involved in regulating a diverse set of physiologic processes, including adipogenesis, hepatocyte differentiation, hematopoiesis, the acute phase response, cell cycle regulation, and the response to oxidative stress (Ramji and Folka, 2002; Tsukada, et al., 2011; Huggins, et al., 2013, 2015). bZIP proteins contain a C terminal DNA binding domain, a leucine zipper dimerization domain, and typically an N terminal activation domain. C/EBP-γ, previously known as Ig/EBP, lacks an N terminal activation domain which makes it the shortest of the C/EBP family members. C/EBP-γ is also the only family member expressed ubiquitously in humans (Roman, et al., 1990; Ramji and Folka, 2002).
Due to C/EBP-γ’s ubiquitous expression and lack of an activation domain, it has historically been described as a dominant negative repressor of gene expression that acts as a generalized buffer against other C/EBP proteins (Cooper, et al., 1995; Ramji and Folka, 2002; Tsukada, et al., 2011). However, this description of C/EBP-γ’s physiologic roles is not entirely accurate. C/EBP-γ activates or represses gene transcription depending on its heterodimeric partner, the specific gene promoter, and the temporospatial and cellular context.
Despite C/EBP-γ dimerizing with several other transcription factors, C/EBP-γ alone has been relatively understudied compared to other C/EBP proteins. Recently, mounting research into C/EBP-γ demonstrates its involvement in major physiologic processes like control of cellular proliferation, induction of the integrated stress response, pulmonary and hematopoietic development and function, and regulation of metabolism. In addition, C/EBP-γ is increasingly viewed as an oncogene for several cancers (Alberich-Jordà, et al., 2012; Blomquist, et al., 2013; Gao, et al., 2002; Huggins, et al., 2015; Kaisho, et al., 1999; Mullins, et al., 2005; Xu, et al., 2015; Yin, et al., 2020). This review seeks to examine the current research on C/EBP-γ and to identify areas of future investigation. As will be explored throughout this review, C/EBP-γ has enormous involvement in the regulation of a wide range of physiologic processes.
2. Structure, dimerization, DNA binding, and regulation
C/EBP-γ is encoded by the CEBPG gene at chromosomal position 19q13.11 in humans. The gene encodes two transcripts, both of which code for the same 16.4 kDa protein that consists of 150 amino acids. Like other bZIP proteins, C/EBP-γ has an alpha-helical structure that consists of a C-terminal basic DNA binding domain, followed by a leucine zipper dimerization domain. C/EBP-γ is the smallest C/EBP family member and is the only member that lacks a trans-activation domain (Ramji and Folka, 2002) (Figure 1).
Figure 1. Structure of the C/EBP transcription factor family.
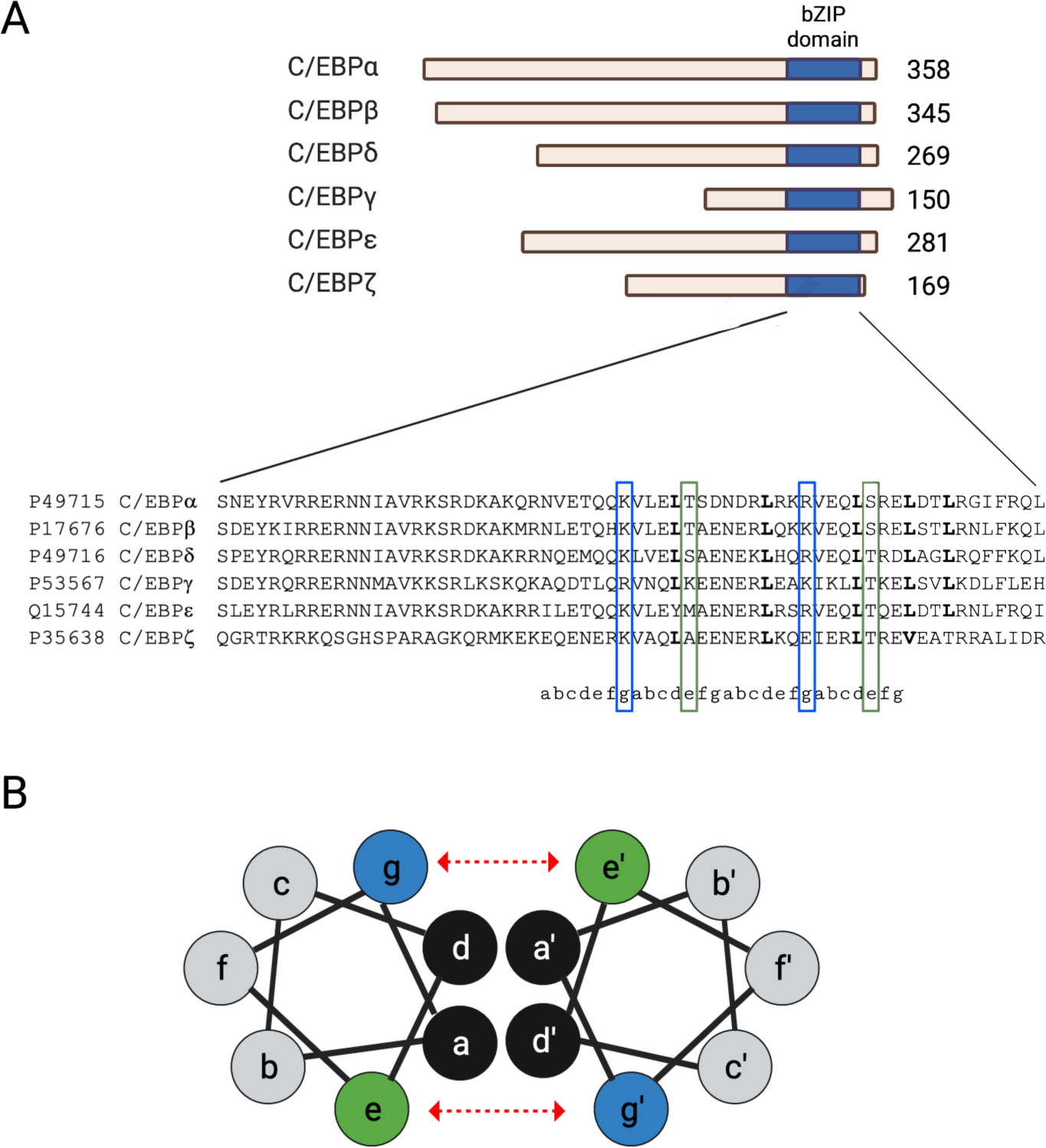
A) Scaled schematic representation of the 6 isoforms of the C/EBP transcription factor family (top). Protein sizes are provided to the right of each isoform. C/EBPγ does not contain the N-terminal activation and regulatory sequences that regulate the activity of other isoforms. The c-terminus of each isoform has a highly conserved bZIP domain (blue) which contains a basic region and a leucine zipper motif. The leucine zipper motif determines the dimerization partner for a specific C/EBP isoform. Multiple amino acid sequence alignment of bZIP the C/EBP isoforms retrieved from the Clustal Omega database (bottom). B) Cross-sectional view of the α-helix within bZIP dimer showing the amino acid positions (a through g) of the the heptad repeat of the leucine zipper. Leucine, designated by the letter “d”, interacts with other hydrophobic residues (i.e., “a”) which constitutes a low affinity interaction of the proteins (black). Electrostatic interactions between e (blue) and g (green) amino acids determine the dimerization specificity and serve to strength the interaction between C/EBP proteins (show by dashed, red arrows).
C/EBP-γ′s ubiquitous expression, ability to dimerize with multiple transcription factors, and flexibility in binding DNA sequences, confers an enormous regulatory capacity which is largely dependent on its cellular environment, heterodimeric partner, and the specific promoters or enhancers on which it is acting. C/EBP proteins must dimerize before they can bind to DNA (Parkin, et al., 2002; Ramji and Folka, 2002). Electrostatic interactions within the leucine zipper domain determine their dimerization specificity (Tsukada, et al., 2011). Post-translational modifications on specific amino acids can alter dimerization specificity. For example, γ phosphorylation of Ser273 of C/EBP-β promotes β:β homodimers, while dephosphorylation at this site promotes C/EBP-β heterodimerization with C/EBP-γ (Lee, et al., 2010). Unlike the other C/EBP family members, C/EBP-γ does not form homodimers (Hattori, et al., 2003). C/EBP-γ forms heterodimers with members of several bZIP transcription factors (e.g., activating transcription factor (ATF), Jun families) (Davydov, et al., 1995; Huggins, et al., 2015; Tsukada, et al., 2011; Vinson, et al., 1993). The most favorable C/EBP binding sequence is a dyad symmetrical repeat of RTTGC•GYAAY, although significant divergence is tolerated. In addition, C/EBP-γ can recognize noncanonical C/EBP binding sites depending on the identity of its heterodimeric partner (Ramji and Folka, 2002; Tsukada, et al., 2011). For example, ATF4/CEPB-γ heterodimers bind to a consensus CARE region as opposed to an ATF or C/EBP specific region (Huggins, et al., 2015).
The regulation of C/EBP-γ is poorly understood compared to other members of the C/EBP family. C/EBP-γ monomers are constitutively ubiquitinated and degraded in a proteasome-dependent manner which tightly regulates their intracellular concentration (Hattori, et al., 2003). C/EBP-γ likely undergoes post-translational modifications, although no evidence has been published to date. MLE12 lung epithelial cells treated with IL-1β increased C/EBP-γ DNA binding despite no measurable increase in transcription of the CEBPG which suggests that post-translational modifications and/or increased translation of CEBPG mRNA were responsible for the increase in C/EBP-γ activity (Yan, et al., 2020). In addition, sumoylation of C/EBP-α and C/EBP-β downregulates their activity; however, despite C/EBP-γ containing these same target residues, there is no evidence of sumoylation on C/EBP-γ (Tsukada, et al., 2011). Further, C/EBP-γ is the only member of the C/EBP family that is not known to be a target for phosphorylation (Cloutier, et al., 2009). Our lack of knowledge about potential post-translational modifications for C/EBP-γ hinders a comprehensive understanding of this transcription factor and the processes in which it is involved and should be a target for future research.
3. Cell cycle progression
C/EBP-γ promotes progression through the cell cycle and inhibits the differentiation of cells and C/EBP-γ is generally viewed as a pro-proliferative transcription factor. Several studies suggest that C/EBP-γ has a role in the progression of the cell cycle. Mouse embryonic fibroblasts from C/EBP-γ−/− mice experience a delayed and prolonged S phase of the cell cycle which suggests that C/EBP-γ is involved in progression through the G1/S phase (Huggins, et al., 2015). This finding is supported by prior work showing that C/EBP-γ mRNA transcript levels are highest during the G1/S transition and decrease shortly thereafter (Blomquist, et al., 2013).
C/EBP-γ expression is generally highest early in cell development and decrease as cells differentiate and exit the cell cycle, although C/EBP-γ retains a near-ubiquitous expression in most mature, differentiated cells. For example, in mouse vomerosensory neurons, C/EBP-γ is widely expressed on the day of birth and decreases significantly by postnatal day 14 (Nakano, et al., 2019). Similarly, C/EBP-γ expression is highest early in B cell development and decreases as cells age (Cooper, et al., 1992, 1994, 1995; Omori, et al., 1997). This temporal titration of C/EBP-γ expression underlies its potent regulation of cell differentiation programs. In most cell types analyzed, C/EBP-γ containing heterodimers predominate early on as cells proliferate, and over time non- C/EBP-γ heterodimers begin to predominate as cells begin to differentiate (Cooper, et al., 1992, 1995; Huggins et al., 2013, 2015; Nakano, et al., 2019; Parkin, et al., 2002).
Signaling pathways such as phosphatidylinositols (PIs) signaling have major roles in the regulation of the cell cycle from check points, proliferation, cell differentiation, gene expression and even membrane trafficking (Ratti, 2018). However, the mechanism by which C/EBP-γ promotes cell division remains unclear. C/EBP-γ may promote transcription of genes necessary for cell cycle continuation, or it may heterodimerize with transcription factors that typically prevent cell cycle continuation. Alternatively, C/EBP-γ may interact with other proteins involved in cell cycle regulation and modulate their function without DNA binding. For example, C/EBP-α can facilitate the cell cycle through protein interactions independent of DNA binding (Johnson, 2005).
The cell-type specificity of C/EBP-γ regulatory behavior facilitates normal and cancer cell functions. For example, C/EBP-γ positively regulates wound repair, a process reliant on increased cellular proliferation, both in vitro and in vivo (Melchionna, et al., 2012). Alternatively, C/EBP-γ upregulation in acute lymphocytic leukemia blocks granulocytic differentiation, demonstrating its role in the inhibition of differentiation into mature cells (Alberich-Jordà, et al., 2012).
4. Integrated stress response
C/EBP-γ is essential for the induction of the integrated stress response (Figure 2). The integrated stress response (ISR) is a coordinated cellular response to intra or extracellular stress (e.g., amino acid deprivation, oxidative redox imbalance, hypoxia, protein misfolding). The ISR is highly conserved among eukaryotes and is mediated primarily through transcriptional changes. Cell stress triggers phosphorylation of eukaryotic initiation factor 2α which initiates preferential translation of ATF4. ATF4 heterodimerizes with one of several bZIP transcription factors and promotes the transcription of genes necessary to respond to cellular stress. The specificity in the transcriptional response to cellular stress is provided by the heterodimeric partner for ATF4 (Harding, et al., 2003; Lu, et al., 2004; Pakos-Zebrucka, et al., 2016; Sikalidis, et al., 2011).
Figure 2. Role of C/EBPγ in the integrated stress response.
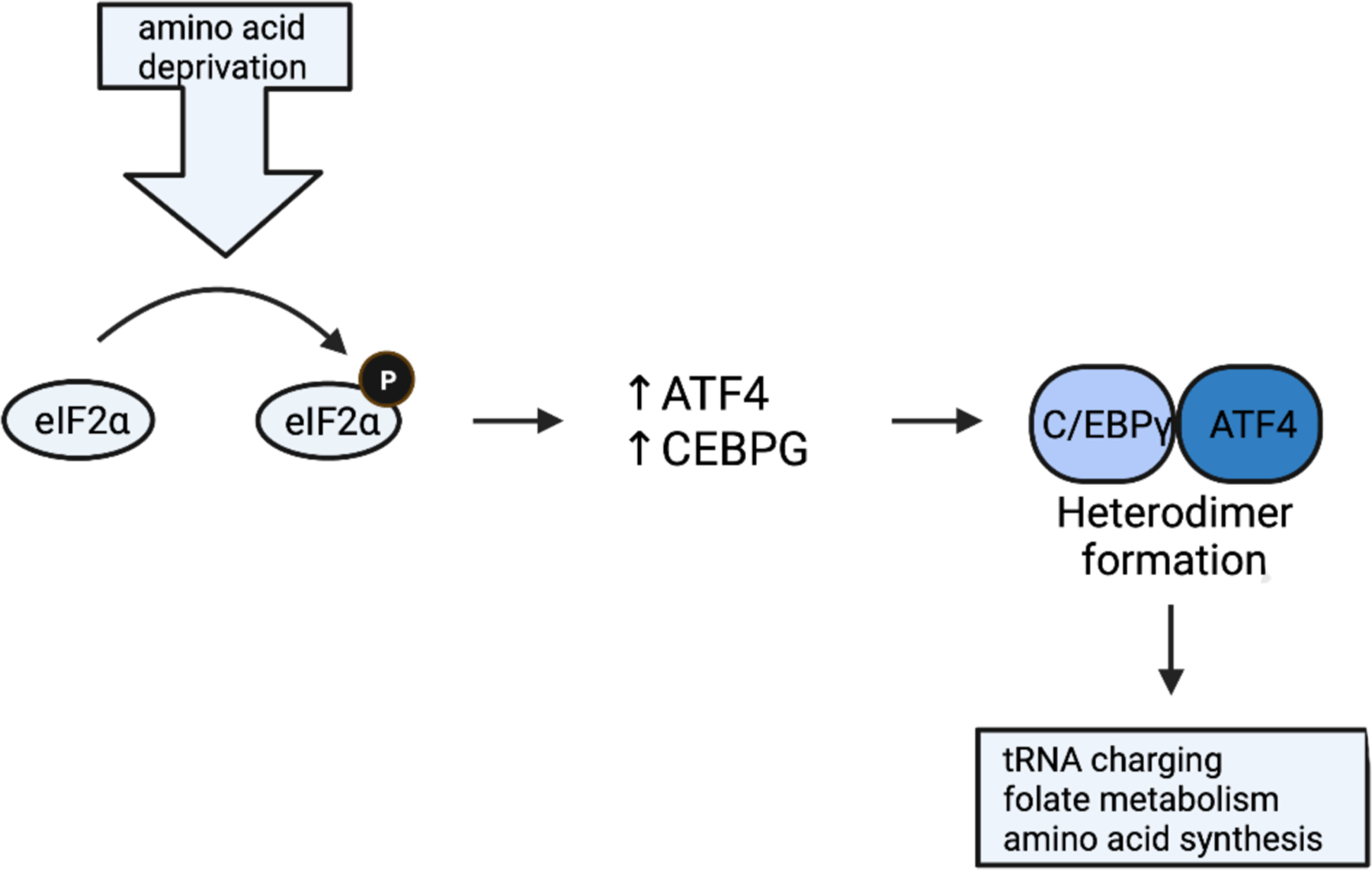
Cellular stress promotes eukaryotic initiation factor 2α (EIF2) phosphorylation which increases CEBPG transcription and translation of ATF4. Under conditions of amino acid deprivation or oxidative stress, ATF4 heterodimerizes with C/EBPγ to mediate transcription of genes necessary to mount the cellular response (i.e., tRNA charging, folate metabolism, amino acid synthesis).
Although ATF4 partners with C/EBP-γ or C/EBP-β, C/EBP-γ is a key heterodimeric partner of ATF4 in the regulation of cellular responses to amino acid deprivation and oxidative stress, while C/EBP-β is largely dispensable (Huggins, et al., 2015). Both amino acid deprivation and eukaryotic initiation factor 2α phosphorylation increases CEBPG transcription, and ATF4−/− cells have reduced CEBPG transcription (Harding, et al., 2003; Huggins, 2015; Lee, et al., 2008; Lu, et al, 2004; Sikalidis, et al., 2011), which suggests that C/EBP-γ is involved in the downstream mediation of the ISR. Mouse embryonic fibroblasts cultured in amino acid-deficient media contained C/EBP-γ and ATF4 but not C/EBP-β (Huggins, et al., 2015). This C/EBP-γ/ATF4 complex was entirely absent in C/EBP-γ−/− fibroblasts. Genes cobound by ATF4 and C/EBP-γ were associated with tRNA charging, folate metabolism, and amino acid synthesis. Further, mouse embryonic fibroblasts from C/EBP-γ−/− C57L6 mice experience significant oxidative stress, the induction of multiple oxidative stress response genes, and severe proliferative inhibition (Huggins, et al., 2015). C/EBP-γ/ATF4 appears to regulate the transcription of genes necessary for glutathione synthesis (e.g., Cth, Slc7a1, Gpx7, Mthfd2, Slc1a5, Gpt2). Importantly, C/EBP-γ was determined to be a key regulator of glutathione synthesis in both normal and lung adenocarcinoma cells (Huggins, et al., 2015). This study remains the only investigation of C/EBP-γ’s role in the ISR to date. Given the fundamental importance of the ISR to cell biology and its relevance to human pathology, the role of C/EBP-γ in the ISR is an area that deserves more research.
5. Oncogenesis and cancer
Cancer is characterized by increased proliferative activity and the loss of well-differentiated cells. Due to their abnormal growth rates, cancers frequently outgrow the available supply of oxygen and nutrients and result in a stressful microenvironment. Activation of the ISR ameliorates this stress and allows for greater survival of cancer cells (Huggins, et al., 2015). As the primary transcriptional mediator of the ISR, C/EBP-γ may be involved in the development of drug-resistant cancers (Williams, et al., 2020).
Given that C/EBP-γ is a pro-proliferative transcription factor and a fundamental mediator of the ISR, it is unsurprising that C/EBP-γ has roles in tumorigenesis and oncogenesis. C/EBP-γ is involved in hippo signaling-related tumorigenesis in a D. melanogaster tumor model (Atkins, et al., 2016). RNAi knockdown of CEBPG mRNA significantly impaired tumor size and invasiveness while non-tumor cells were unaffected (Atkins, et al. 2016). C/EBP-γ has also been implicated in several human cancers (e.g., lung cancer, acute myeloid leukemia, esophageal squamous cell carcinoma) (Alberich-Jordà, et al., 2012; Crawford, et al., 2007; De Kouchokovsky and Abdul-Hay, 2016; Huang, et al., 2020; Lou, 2013; Mullins, et al., 2005).
C/EBP-γ appears to be necessary for proper lung development. C/EBP-γ−/− mice display serious defects in pulmonary development, have atelectasis, and die within 96 hours (Kaisho, et al., 1999). However, relatively little is known about its role in pulmonary development and function. The large interindividual variation in lung cancer incidence cannot be attributed to environmental factors alone (Crawford, et al., 2007). Part of this variation can be attributed to individual differences in transcription and translation of key DNA repair genes in bronchial epithelial cells. A study of 49 human participants found a large interindividual variation in CEBPG mRNA expression levels, which was significantly correlated with the mRNA expression levels of four key antioxidant and DNA repair genes (i.e., XRCC1, ERCC5, GSTP1, SOD1) in individuals without bronchogenic carcinoma but was not correlated in individuals with bronchogenic carcinoma (Mullins, et al., 2005). The authors conclude that C/EBP-γ is responsible for optimal regulation of these four genes and that interindividual variation in the regulation of each of these genes by C/EBP-γ contributes to susceptibility to bronchogenic carcinoma (Mullins, et al., 2005). They further hypothesized that polymorphisms in the regulatory regions of these four genes might lead to suboptimal regulation by C/EBP-γ and therefore increase cancer risk. A follow-up study confirmed the positive regulation of the ERCC5 by C/EBP-γ transcription (Crawford, et al., 2007). In addition, C/EBP-γ may facilitate lung cancer metastasis through increased expression of ceramide synthase 6, an enzyme necessary for cancer cell migration (Shi et al., 2021).
Increased C/EBP-γ expression has recently been identified as a negative prognostic factor for survival for patients with esophageal squamous cell carcinoma (Huang et al., 2020). C/EBP-γ promotes the growth of esophageal squamous cell carcinoma tumors, both in vivo and in vitro, through activation of the PI3K/AKT signaling pathway. Interestingly, this increased C/EBP-γ expression is caused by copy number variations of CEBPG. In addition, the PI3K-AKT pathway is induced by the Epidermal Growth Factor Receptor (EGFR) family members, which together with C/EBP-β have been reported to drive themesenchymal transcriptional signature in glialblastoma (Steelman, et al., 2016) (Selagea, et al., 2016).
Acute myeloid leukemia (AML) is the most common acute leukemia in adults and is characterized by inhibition of myeloid cell differentiation and subsequent accumulation of myeloid precursors in the bone marrow (De Kouchokovsky and Abdul-Hay, 2016; Lou, 2013). C/EBP-α is a potent inducer of differentiation in myeloid cells, and approximately 13% of patients with AML have a mutation in CEBPA, which results in the inhibition of myeloid differentiation (Marchwicka and Marcinkowska, 2018). The mechanism involves hypermethylation of CEBPA in AML cells (Alberich-Jorda, et al., 2012). Because C/EBP-α acts as a repressor for CEBPG transcription, the increased CEBPA methylation reduces the repression of CEBPG and increased CEBPG transcription and translation, which then blocks granulopoietic differentiation. (Alberich-Jordà, et al., 2012). The anti-differentiation activity of C/EBP-γ may be a result of its inhibition of CEPBE and CSF3R transcription, both of which are involved in proper myeloid development (Alberich-Jordà, et al., 2012).
The most common cause of treatment failure in AML is the resistance of leukemia cells to chemotherapy drugs. Resistance to daunorubicin, a common AML treatment, is characterized by an ISR-like transcriptional program that is centered around ATF4. C/EBP-γ is strongly upregulated in daunorubicin-resistant K562 leukemia cells but not in daunorubicin-susceptible K562 cells (Williams, et al., 2020), which suggests that C/EBP-γ could be a potential drug target for patients with this particular type of AML. In addition, given the importance of an ISR-like transcriptional program focused on ATF4 in mediating the acquisition of resistance to common treatments, future research may investigate the role of C/EBP-γ in AML’s acquisition of drug resistance.
Similar to the estrogen receptor signaling pathway, C/EBP-γ may be involved in the development of other cancers (Akula, et al., 2020). The evidence surrounding the role of C/EBP-γ in breast cancer is conflicting. Higher expression of C/EBP-γ is associated with poorer outcomes in triple-negative breast cancer (Li, et al., 2019). However, MDA-MB-231 cells, a common model of triple-negative breast cancer, treated with docosahexaenoic acid significantly upregulated transcription of C/EBP-γ which suggests that C/EBP-γ may cooperate with the FOS protein to stimulate Akt dependent apoptosis (Chénais, et al., 2020). C/EBP-γ upregulation and an associated poor prognosis have also been shown in bladder tumor cells treated with an inhibitor of survivin, a mitotic protein frequently overexpressed in cancer (Tao, et al., 2012).
Given that C/EBP-γ has only recently been identified as an oncogene, there are many questions about its function which remain to be investigated. C/EBP-γ is clearly important for proper pulmonary function early in life, as evidenced by mortality in C/EBP-γ−/− mice (Kaisho, et al., 1999). However, the physiologic role that it has in pulmonary development is entirely unknown. Of the four genes regulated by C/EBP-γ investigated by Mullins et al. (i.e., XRCC1, ERCC5, GSTP1, SOP1), only ERCC5 has been further studied (Mullins et al., 2005). Follow-up studies could be performed to confirm the role of C/EBP-γ in the regulation of XRCC1, GSTP1, and SOP1 in pulmonary cells. Whether C/EBP-γ is overexpressed prior to oncogenesis and drives the process or is primarily induced as a component of the ISR after cells have become cancerous is not well understood and would be an interesting area for future research with implications beyond C/EBP-γ itself. This would be particularly important in the context of bronchogenic carcinoma and AML. Given the large individual and public health impacts of bronchogenic carcinoma, exploring C/EBP-γ’s role in pulmonary development and oncogenesis is an important task. The PI3K/AKT signaling pathway, regulated in part by nuclear inositide signaling, is a commonly dysregulated in cancer cells. Disruptions in nuclear inositide regulation of PI3K/AKT are especially important in the development of myeloid disruption syndromes like AML (Cappellini, et al., 2020; Blalock, et al., 2009). Given that increased C/EBP-γ expression is associated with disruptions in the PI3K/AKT pathway in esophageal squamous cell carcinoma and that C/EBP-γ is involved in AML leukemogenesis, it would be interesting to investigate whether C/EBP-γ impacts the PI3K/AKT pathway in the context of AML and if nuclear inositide signaling plays a role in this context (Follo, et al., 2010). Significant research is needed to clarify whether C/EBP-γ has a pro- or anti-cancer effect in breast cancer. Finally, given the dearth of research on the topic, a broader effort should be made to include C/EBP-γ in research on transcription factor biology and cancer.
6. Hematopoiesis and immune cell development and function
C/EBP-γ was first identified for its binding activity to two immunoglobulin Vh promoters in B cells (Cooper, et al., 1992; Roman, et al., 1990). C/EBP-γ has since been found to have roles in the development of several hematopoietic cell lineages and retains multiple functions in mature immune cells, primarily regulating the expression of cytokines, INF-γ, and cellular architectural proteins (Gao, et al., 2002; Kanai, et al, 2011; Omori, et al., 1997). C/EBP-γ’s immunologic role is highly conserved. C. elegans treated with RNAi-mediated CEBP-2, a CEBPG ortholog knockdown, show a significant decrease in survival upon challenge with P. aeruginosa, highlighting the role CEBP-γ plays in surveillance immunity in this model organism (Reddy, et al., 2016; Tjahjono and Kirienko, 2017).
C/EBP-γ is expressed throughout the lifespan of T and B cells (Omori, et al., 1997; Kaisho, et al., 1999). C/EBP-γ displays a reciprocal expression pattern with C/EBP-β in B cells, with C/EBP-γ levels being highest early in B cell development and decreasing as C/EBP-β levels increase (Cooper, et al., 1992; 1994, 1995). C/EBP-γ does not appear to be crucial for proper B or T cell development; C/EBP-γ−/− T and B cells develop normally and do not have decreased population nor abnormal surface markers or impaired proliferative response to mitogens or antibodies (Kaisho, et al., 1999).
In B cells, γ:β heterodimers bind to the promoters of IL6 and IL8 to prevent transcription in the absence of lipopolysaccharide (LPS). After exposure to LPS, γ:β dimers significantly augment the transcription of IL6 and IL8. The mechanism by which γ:β dimers switch from an inhibitory to an activating function on the IL6 and IL8 promoters in the presence of LPS in B cells is unknown and an area where more research is needed. It would be interesting to see if C/EBP-γ containing dimers can switch between inhibitory and activating roles in other cell or promoter contexts. C/EBP-γ has been identified as a possible mediator of downstream IL-4 signaling in the Ramos B cell line (Kanai, etal., 2011). C/EBP-γ binds to the PRE-1 enhancer element of IL4 in Jurkat T cells; however, the functional relevance remains unknown (Davydov, et al., 1995).
Although C/EBP-γ is widely expressed early in natural killer (NK) cell development, it is not necessary for proper NK cell development. NK cell numbers do not significantly decrease in mouse C/EBP-γ−/− bone marrow chimeras. However, splenic NK cell cytotoxic killing and IFNG transcription and translation in response to the proinflammatory cytokines IL-2, 1L-12, and IL-18 are decreased significantly in C/EBP-γ−/− bone marrow chimeras (Kaisho, et al., 1999). NK cell response to IL-15 signaling, which stimulates NK cell proliferation and is essential for proper NK cell survival and function, is also impaired in C/EBP-γ−/− mice (Kaisho, et al., 1999). Despite strong evidence that C/EBP-γ mediates mature NK cell cytotoxic killing and INFG transcription, the mechanism by which C/EBP-γ regulates these functions and its heterodimeric partners in this context remains unexplored.
CEBPG mRNA and protein levels are highest during the early myeloblast and promyelocyte phases of granulopoiesis and decrease to barely undetectable levels in mature neutrophils (Alberich-Jordà, et al., 2012; Bjerregaard, et al., 2003; Cloutier, et al., 2009). Silencing of CEBPG does not appreciably alter steady-state or emergency granulopoiesis in conditional knockout mice (Kardosova, et al., 2018). One study has confirmed that C/EBP-γ is expressed throughout Langerhans dendritic cell development but did not investigate any functional role that C/EBP-γ may have (Iwama, et al., 2002). Similarly, C/EBP-γ is expressed in macrophages and binds to the promoter element for granulocyte colony-stimulating factor in response to LPS, but the functional significance remains poorly characterized (Nishizawa, et al., 1991).
There are conflicting reports of the involvement of C/EBP-γ in globin switching, an important developmental milestone in erythropoiesis where erythrocytes switch from producing embryonic, fetal γ globin to adult β globin production. The human β globin locus contains five genes that are expressed at different times in development and are regulated by a single locus control region 5’ upstream of the genes. The locus control region interacts with and activates a single gene at a time through a looping mechanism, and these genes are thought to be regulated by competing for interactions with the locus control region. Altered expression of β globin leads to diseases such as sickle cell disease and β-thalassemia, which can be partially compensated by the reactivation of the fetal γ globin gene (Wall, et al., 1996; Iarovaia, et al., 2018).
C/EBP-γ activates transcription of the human β globin gene in murine erythroleukemia cells, but not in K562 human leukemia cells (Wall, et al., 1996). Further, C/EBP-γ alone was insufficient to induce expression of the β globin gene, but that wildtype expression was achieved when C/EBP-γ was present with another transcription factor, CF-1 (also known as NF-Y), which suggests that these C/EBP-γ and CF-1 bind to the same promoter region and interact to increase transcription of β globin; however, the CF-1 and C/EBP-γ binding sites in the β globin promoter overlap. Therefore, concurrent binding of the two transcription factors should not be possible (Wall, et al.,1996).
Two studies have refuted the conclusions of Wall et al. Zafarana et al. found that C/EBP-γ overexpression in murine erythroleukemia cells did not alter the expression of human β globin. Furthermore, transgenic mice expressing the human β globin locus with low-level CEBPG overexpression increased expression of fetal γ globin, while high levels of C/EBP-γ overexpression completed ablated fetal erythropoiesis and was fatal. In contrast to Wall, the authors conclude that CEBP-γ preferentially binds to and activates transcription of the γ globin gene. These authors did not suggest a dimeric partner for C/EBP-γ in the context of globin switching (Zafarana et al., 2000).
Gordon et al. agree with Zafarana et al. that C/EBP-γ does not promote β globin transcription and instead promotes expression of γ globin and that the model proposed by Wall is unlikely to occur in vivo (Gordon et al., 2005; Zafarana et al., 2000); however, these authors disagree as to the mechanism of how C/EBP-γ promotes γ globin transcription. Gordon et al. identified that C/EBP-δ binds to the β globin promoter and promotes transcription in coordination with the transcription factor EKLF, while C/EBP-γ suppresses transcription of β globin in coordination with EKLF. Instead of C/EBP-γ binding to and promoting transcription of γ globin directly, they instead suggest that C/EBP-γ negatively regulates β globin expression and allows for continued γ globin expression until C/EBP-δ begins to predominate through an unknown mechanism. The authors suggest that C/EBP-γ and C/EBP-δ could have a reciprocal relationship like that of C/EBP-γ and C/EBP-β in B cells, wherein C/EBP-γ levels are highest early in cell development and decrease over time as other C/EBP isoforms begin to predominate (Gordon et al., 2005).
Future research questions could focus on clarifying if C/EBP-γ binds to promoter regions for the β globin or γ globin genes, its heterodimeric partners, and resolving if its erythropoietic role is primarily activating or repressing transcription of select genes, or both depending on its dimeric partner. Investigation of relative CEBPG and CEBPD expression over time in erythrocytes could confirm or refute the model of β globin regulation proposed by Gordon et al. There is a significant interest in seeing if reactivation of γ globin in mature erythrocytes could be used clinically to ameliorate symptoms of hemoglobinopathies such as sickle cell disease and β thalassemia (Gordon et al., 2005; Iarovia, et al., 2018). As research in this area progresses, and the role of C/EBP-γ in globin switching is resolved, it would be interesting to see if C/EBP-γ could have a role in clinical γ globin reactivation.
HIV pathogenesis.
HIV gene transcription is driven by the interaction of human and viral transcription factors on the long terminal repeat region of the HIV genome (Schwartz, et al., 2000). C/EBP-γ is one of these transcription factors and mediates transcriptional changes responsible for several prominent neurologic consequences of HIV infection. HIV-1 strains can be classified by their preferred site of replication, with T tropic viruses replicating in CD4+ T cells and M tropic viruses replicating in macrophages. In the nervous system, M tropic HIV predominates, where it infects perivascular macrophages, microglia, and astrocytes (Schwartz, et al., 2000). The neurologic effects of HIV are mediated through two primary mechanisms: the direct cytotoxic effect of HIV proteins (e.g., Tat, Nef, and gp120) on neurons, and the increased production of proinflammatory cytokines (e.g., IL-1β, TNF-α, IL-6, IL-8, INF-γ, nitric oxide, quinolinic acid, TGF-β, IP-10, CCL5), which creates a neuroinflammatory milieu detrimental to neuronal health (Churchill, et al., 2015; Hong and Banks, 2005; Jones, et al., 2005; Nookala, et al., 2013; Schutz and Robinson-Papp, 2013).
In HIV-infected astrocytes, C/EBP-β binds to the HIV long terminal repeat region and activates transcription of several genes, whereas C/EBP-γ can heterodimerize with C/EBP-β and inhibit transcription of these genes (Schwartz, et al., 2000). C/EBP-γ overexpression does not abolish basal HIV gene transcription but does abolish C/EBP-β induced gene expression (Schwartz, et al., 2000). CC-chemokine ligand 5 (CCL5, also known as RANTES) is an inflammatory chemokine produced by astrocytes that recruits lymphocytes and monocytes to the site of inflammation (Nookala, et al., 2013). Several HIV proteins induce CCL5 production in astrocytes, including Nef, Tat, gp120, and Vpr (Liu and Kumar, 2015; Nookala, et al., 2013). Astrocytes transfected with CEBPG siRNA 48 hours before transfection with a Tat protein-encoding plasmid had reduced C/EBP-γ mRNA levels by 50% and protein levels by over 30% compared to controls (Nookala, et al., 2013). A similar experiment done with a Nef encoding plasmid showed that silencing C/EBP-γ with siRNA decreased CCL5 mRNA and protein levels by over 30% (Liu, et al., 2014). Therefore, C/EBP-γ is involved in mediating CCL5 production in response to HIV proteins and contributes to the neuroinflammatory environment detrimental to neurons characteristic of neuro HIV infection.
No study to date has investigated whether C/EBP-γ is involved in the upregulation of CCL5 production in response to gp120 or Vpr. However, upregulation of CCL5 in astrocytes in response to gp120 and Vpr is like that in response to Nef and Tat, and C/EBP-γ may be involved in this process (Gangwani, et al, 2013; Shah, et al., 2011). The C/EBP family can be activated by the p38-MAPK pathway. Silencing of p38 MAPK by siRNA significantly decreases CCL5 expression at the mRNA and protein levels in astrocytes (Gangwani, et al., 2013). These results suggest that C/EBP-γ may act downstream of p38-MAPK signaling and regulate CCL5 production in response to gp120 and Vpr. More research is needed to clarify the role C/EBP-γ has in mediating these effects.
Although astrocytes produce IL-6 and IL-8 in response to HIV proteins, C/EBP-γ is not involved in the induction of these cytokines in this context (Liu and Kumar, 2015). Despite macrophages serving both as important reservoirs of HIV infection in the brain and contributing to neuroinflammation, we could not identify any studies that investigate the role of C/EBP-γ in these cells in HIV. Although a role for C/EBP-γ in transcriptionally mediating HIV-related pathogenesis has been established, much remains to be investigated. Research is needed to confirm the hypothesized role that C/EBP-γ has in mediating CCL5 production in astrocytes in response to gp120 and Vpr. It is also unclear if C/EBP-γ upregulates astrocyte production of other inflammatory cytokines in HIV infection or if it has proinflammatory roles in other cells in the nervous system.
7. Other functions of C/EBP-γ
Roles in the nervous system.
C/EBP proteins, particularly C/EBP-β and C/EBP-δ, have major roles in the development of the nervous system (Pulido-Salgado, et al., 2015). Even though C/EBP-γ is expressed throughout the nervous system and heterodimerizes with both C/EBP-β and C/EBP-δ, less research has been conducted into its role in neural development and function in mature nervous system cells. Sensory neurons are a major component of the vomeronasal organ in mice, which is responsible for detecting pheromones and predator odors (Nakano, et al., 2019). CEPBG is widely expressed early in vomeronasal sensory neuron development, but within two weeks of birth, expression significantly decreases as neurons differentiate. C/EBP-γ and ATF5 are required for transcription of Vmn2r66, the gene that encodes vomeronasal 2, receptor 66.
Cebpg transcription is upregulated in mice four hours and one day post sciatic nerve crush injury and returns to baseline expression levels 3 days post-injury (Lopez de Heredia and Magoulas, 2013). Development of chronic pain after nerve injury is thought to be mediated in part through short- and long-term changes in transcription, and this transient increase in Cebpg expression may have a role in the development of chronic pain. Our group recently identified changes in chromatin structure at putative enhancer regions after chronic constriction injury to the sciatic nerve and confirmed C/EBP-γ binding to this region. Rats treated with AYX1, a DNA decoy drug targeting EGR1, prior to spared nerve injury surgery had significantly reduced mechanical hypersensitivity. EGR1 regulates the early transcriptional response to neural injury and facilitates the development of chronic pain. Of the 18 genes investigated, Cebpg was the most significantly downregulated in AYX1 treated rats. Cebpg mRNA was also decreased in the dorsal root ganglion and spinal cords of AYX1 treated dogs (Mamet, et al., 2014). These results suggest that C/EBP-γ is transiently induced as part of the neural response to injury and could have a role in mediating the development of chronic pain.
Metabolism and Energy Homeostasis.
Most research into how C/EBP proteins regulate metabolism has focused on C/EBP-𝛼, which is important for proper adipocyte and hepatocyte differentiation (Tsukada, et al., 2011). However, several studies have shown a role for C/EBP-γ in energy homeostasis as well. In C. elegans, CEBP-2 regulates the expression of ech-1.1 and fat-5, which encode proteins involved in mitochondrial beta-oxidation and fatty acid metabolism, respectively, and have human homologs (Xu, et al., 2015). In response to fasting, C/EBP-γ dimerizes with ATF5 and binds to the pgc-1α promoter of the genes encoding phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, two proteins important for gluconeogenesis, in human HepG2 cells (Shimizu, et al., 2009).
Recent work has described how the ATF3-C/EBP-γ axis directs cardiac and hematopoietic development in fish and mammalian cells through the regulation of glucose levels. C/EBP-γ binds to the promoter of Slc2a1a/Glut1, the primary fetal glucose transporter isoform, and activates its transcription while ATF3 binds to the CEBPG promoter and downregulates its expression, thereby reducing glucose levels. Alterations in glucose metabolism resulted in a change in cellular redox state which influences cell progenitor fate, and aberrant C/EBP-γ expression results in cardiac abnormalities (Yin, et la., 2020). This is the first study to implicate C/EBP-γ in the development of cardiac tissue.
8. Conclusion
C/EBP-γ remains the least characterized C/EBP transcription factor. Despite this, it is possible to draw conclusions about its important functions. C/EBP-γ is a crucial transcription factor for early life development, as evidenced by its role in directing cardiac and hematopoietic development through glucose regulation and the perinatal lethality of C/EBP-γ−/− mice (Kaisho, et al., 1999; Yin, et al., 2020). C/EBP-γ is a pro-proliferative transcription factor in multiple cell lineages and contexts, and its expression decreases as other C/EBP transcription factor expression increases, inducing differentiation. However, C/EBP-γ retains functional relevance in nearly every mature cell type. As a fundamental transcriptional mediator of the integrated stress response, it is crucial for cells’ ability to respond to cellular stressors like redox imbalance and amino acid deprivation (Huggins, et al., 2015). Because of its role in promoting cell proliferation and mediating the integrated stress response, CEBPG has been identified as a probable oncogene in multiple carcinomas, namely bronchogenic carcinoma and acute myeloid leukemia, with emerging evidence suggesting it may be involved in others (Alberich-Jordà, et al., 2012; Blomquist, et al., 2014, Huggins, et al., 2015). C/EBP-γ is also likely involved in other physiologic processes such as neural development, the development of chronic pain in response to injury, and regulation of metabolism (Mamet, et al., 2014; Nakano, et al., 2019; Xu, et al., 2015; Yin, et al., 2020). Given its ubiquitous expression and ability to heterodimerize with multiple transcription factors, as well as its displayed involvement in the processes above, C/EBP-γ is clearly of interest to cell biologists. Because of the paucity of research surrounding C/EBP-γ, there is a wide range of interesting research questions surrounding its physiologic roles that remain to be investigated.
Acknowledgements
This work was supported by, in part, by the Arkansas Children’s Research Institute, the Arkansas Biosciences Institute, the UAMS Winthrop P. Rockefeller Cancer Institute, and the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR003108). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Eric Rathman for his editing services through the UAMS Scientific Communication Group.
Abbreviations
- AML
acute myeloid leukemia
- ATF
activating transcription factor
- bZIP
leucine zipper
- CCL5
CC-chemokine ligand 5
- C/EBP
CCAAT enhancer binding
- ISR
integrated stress response
- LPS
lipopolysaccharide
- NK
natural killer
Footnotes
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Kimberly Stephens reports financial support was provided by Arkansas Biosciences Institute. Kimberly Stephens reports financial support was provided by National Institute of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akula SM, Candido S, Abrams SL, Steelman LS, Lertpiriyapong K, Cocco L, Ramazzotti G, Ratti S, Follo MY, Martelli AM, Murata RM, Rosalen PL, Bueno-Silva B, Matias de Alencar S, Falasca M, Montalto G, Cervello M, Notarbartolo M, Gizak A, Rakus D, Libra M, McCubrey JA, 2020. Abilities of β-Estradiol to interact with chemotherapeutic drugs, signal transduction inhibitors and nutraceuticals and alter the proliferation of pancreatic cancer cells. Adv Biol Regul 75, 100672. [DOI] [PubMed] [Google Scholar]
- Alberich-Jorda M, Wouters B, Balastik M, Shapiro-Koss C, Zhang H, Di Ruscio A, Radomska HS, Ebralidze AK, Amabile G, Ye M, Zhang J, Lowers I, Avellino R, Melnick A, Figueroa ME, Valk PJ, Delwel R, Tenen DG, 2012. C/EBPgamma deregulation results in differentiation arrest in acute myeloid leukemia. J Clin Invest 122(12), 4490–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins M, Potier D, Romanelli L, Jacobs J, Mach J, Hamaratoglu F, Aerts S, Halder G, 2016. An Ectopic Network of Transcription Factors Regulated by Hippo Signaling Drives Growth and Invasion of a Malignant Tumor Model. Curr Biol 26(16), 2101–2113. [DOI] [PubMed] [Google Scholar]
- Bjerregaard MD, Jurlander J, Klausen P, Borregaard N, Cowland JB, 2003. The in vivo profile of transcription factors during neutrophil differentiation in human bone marrow. Blood 101(11), 4322–4332. [DOI] [PubMed] [Google Scholar]
- Blalock WL, Grimaldi C, Fala F, Follo M, Horn S, Basecke J, Martinelli G, Cocco L, Martelli AM, 2009. PKR activity is required for acute leukemic cell maintenance and growth: a role for PKR-mediated phosphatase activity to regulate GSK-3 phosphorylation. J Cell Physiol 221(1), 232–41. [DOI] [PubMed] [Google Scholar]
- Blomquist TM, Brown RD, Crawford EL, de la Serna I, Williams K, Yoon Y, Hernandez DA, Willey JC, 2013. CEBPG Exhibits Allele-Specific Expression in Human Bronchial Epithelial Cells. Gene Regul Syst Bio 7, 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini A, Mongiorgi S, Finelli C, Fazio A, Ratti S, Marvi MV, Curti A, Salvestrini V, Pellagatti A, Billi AM, Suh PG, McCubrey JA, Boultwood J, Manzoli L, Cocco L, Follo MY, 2020. Phospholipase C beta1 (PI-PLCbeta1)/Cyclin D3/protein kinase C (PKC) alpha signaling modulation during iron-induced oxidative stress in myelodysplastic syndromes (MDS). FASEB J 34(11), 15400–15416. [DOI] [PubMed] [Google Scholar]
- Chenais B, Cornec M, Dumont S, Marchand J, Blanckaert V, 2020. Transcriptomic Response of Breast Cancer Cells MDA-MB-231 to Docosahexaenoic Acid: Downregulation of Lipid and Cholesterol Metabolism Genes and Upregulation of Genes of the Pro-Apoptotic ER-Stress Pathway. Int J Environ Res Public Health 17(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Cowley DJ, Wesselingh SL, Gorry PR, Gray LR, 2015. HIV-1 transcriptional regulation in the central nervous system and implications for HIV cure research. J Neurovirol 21(3), 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier A, Guindi C, Larivee P, Dubois CM, Amrani A, McDonald PP, 2009. Inflammatory cytokine production by human neutrophils involves C/EBP transcription factors. J Immunol 182(1), 563–571. [DOI] [PubMed] [Google Scholar]
- Cooper C, Henderson A, Artandi S, Avitahl N, Calame K, 1995. Ig/EBP (C/EBP gamma) is a transdominant negative inhibitor of C/EBP family transcriptional activators. Nucleic Acids Res 23(21), 4371–4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C, Johnson D, Roman C, Avitahl N, Tucker P, Calame K. The C/EBP family of transcriptional activators is functionally important for Ig VH promoter activity in vivo and in vitro. J Immunol. 1992;149(10):3225–31. [PubMed] [Google Scholar]
- Cooper CL, Berrier AL, Roman C, Calame KL, 1994. Limited expression of C/EBP family proteins during B lymphocyte development. Negative regulator Ig/EBP predominates early and activator NF-IL-6 is induced later. J Immunol 153(11), 5049–5058. [PubMed] [Google Scholar]
- Crawford EL, Blomquist T, Mullins DN, Yoon Y, Hernandez DR, Al-Bagdhadi M, Ruiz J, Hammersley J, Willey JC, 2007. CEBPG regulates ERCC5/XPG expression in human bronchial epithelial cells and this regulation is modified by E2F1/YY1 interactions. Carcinogenesis 28(12), 2552–2559. [DOI] [PubMed] [Google Scholar]
- Davydov IV, Bohmann D, Krammer PH, Li-Weber M, 1995. Cloning of the cDNA encoding human C/EBP gamma, a protein binding to the PRE-I enhancer element of the human interleukin-4 promoter. Gene 161(2), 271–275. [DOI] [PubMed] [Google Scholar]
- De Kouchkovsky I, Abdul-Hay M, 2016. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J 6(7), e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follo MY, Mongiorgi S, Finelli C, Clissa C, Ramazzotti G, Fiume R, Faenza I, Manzoli L, Martelli AM, Cocco L, 2010. Nuclear inositide signaling in myelodysplastic syndromes. J Cell Biochem 109(6),1065–71. [DOI] [PubMed] [Google Scholar]
- Gangwani MR, Noel RJ Jr., Shah A, Rivera-Amill V, Kumar A, 2013. Human immunodeficiency virus type 1 viral protein R (Vpr) induces CCL5 expression in astrocytes via PI3K and MAPK signaling pathways. J Neuroinflammation 10, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Parkin S, Johnson PF, Schwartz RC, 2002. C/EBP gamma has a stimulatory role on the IL-6 and IL-8 promoters. J Biol Chem 277(41), 38827–38837. [DOI] [PubMed] [Google Scholar]
- Gordon CT, Fox VJ, Najdovska S, Perkins AC, 2005. C/EBPdelta and C/EBPgamma bind the CCAAT-box in the human beta-globin promoter and modulate the activity of the CACC-box binding protein, EKLF. Biochim Biophys Acta 1729(1), 74–80. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D, 2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11(3), 619–633. [DOI] [PubMed] [Google Scholar]
- Hattori T, Ohoka N, Inoue Y, Hayashi H, Onozaki K, 2003. C/EBP family transcription factors are degraded by the proteasome but stabilized by forming dimer. Oncogene 22(9), 1273–1280. [DOI] [PubMed] [Google Scholar]
- Hong S, Banks WA, 2015. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 45, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Lin L, Shen Z, Li Y, Cao H, Peng L, Qiu Y, Cheng X, Meng M, Lu D, Yin D, 2020. CEBPG promotes esophageal squamous cell carcinoma progression by enhancing PI3K-AKT signaling. Amer J Cancer Res 10(10), 3328–3344. [PMC free article] [PubMed] [Google Scholar]
- Huggins CJ, Malik R, Lee S, Salotti J, Thomas S, Martin N, Quinones OA, Alvord WG, Olanich ME, Keller JR, Johnson PF, 2013. C/EBPgamma suppresses senescence and inflammatory gene expression by heterodimerizing with C/EBPbeta. Mol Cell Biol 33(16), 3242–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins CJ, Mayekar MK, Martin N, Saylor KL, Gonit M, Jailwala P, Kasoji M, Haines DC, Quinones OA, Johnson PF, 2015. C/EBPgamma Is a Critical Regulator of Cellular Stress Response Networks through Heterodimerization with ATF4. Mol Cell Biol 36(5), 693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarovaia OV, Kovina AP, Petrova NV, Razin SV, Ioudinkova ES, Vassetzky YS, Ulianov SV, 2018. Genetic and Epigenetic Mechanisms of beta-Globin Gene Switching. Biochemistry (Mosc) 83(4), 381–392. [DOI] [PubMed] [Google Scholar]
- Iwama A, Osawa M, Hirasawa R, Uchiyama N, Kaneko S, Onodera M, Shibuya K, Shibuya A, Vinson C, Tenen DG, Nakauchi H, 2002. Reciprocal roles for CCAAT/enhancer binding protein (C/EBP) and PU.1 transcription factors in Langerhans cell commitment. J Exp Med 195(5), 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wu S-Y, Chen Y-L, Zhang Z-M, Tao Y-F, Xie Y, Liao X-M, Li X-L, Li G, Wu D, Wang H-R, Zuo R, Cao H-B, Pan J-J, Yu J-J, Jia S-Q, Zhang Z, Chu X-R, Zhang Y-P, Chen C-X, Wang J-W, Hu S-Y, Li Z-H, Pan J, Fang F, Lu J, 2021. CEBPG promotes acute myeloid leukemia progression by enhancing EIF4EBP1. Cancer Cell Int, 21(1), 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PF, 2005. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci 118(Pt 12), 2545–2555. [DOI] [PubMed] [Google Scholar]
- Jones G, Zhu Y, Silva C, Tsutsui S, Pardo CA, Keppler OT, McArthur JC, Power C, 2005. Peripheral nerve-derived HIV-1 is predominantly CCR5-dependent and causes neuronal degeneration and neuroinflammation. Virology 334(2), 178–193. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Tsutsui H, Tanaka T, Tsujimura T, Takeda K, Kawai T, Yoshida N, Nakanishi K, Akira S, 1999. Impairment of natural killer cytotoxic activity and interferon gamma production in CCAAT/enhancer binding protein gamma-deficient mice. J Exp Med 190(11), 1573–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai A, Suzuki K, Tanimoto K, Mizushima-Sugano J, Suzuki Y, Sugano S, 2011. Characterization of STAT6 target genes in human B cells and lung epithelial cells. DNA Res 18(5), 379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardosova M, Zjablovskaja P, Danek P, Angelisova P, de Figueiredo-Pontes LL, Welner RS, Brdicka T, Lee S, Tenen DG, Alberich-Jorda M, 2018. C/EBPgamma is dispensable for steady-state and emergency granulopoiesis. Haematologica 103(8), e331–e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JI, Dominy JE Jr., Sikalidis AK, Hirschberger LL, Wang W, Stipanuk MH, 2008. HepG2/C3A cells respond to cysteine deprivation by induction of the amino acid deprivation/integrated stress response pathway. Physiol Genomics 33(2), 218–229. [DOI] [PubMed] [Google Scholar]
- Lee S, Shuman JD, Guszczynski T, Sakchaisri K, Sebastian T, Copeland TD, Miller M, Cohen MS, Taunton J, Smart RC, Xiao Z, Yu LR, Veenstra TD, Johnson PF, 2010. RSK-mediated phosphorylation in the C/EBP{beta} leucine zipper regulates DNA binding, dimerization, and growth arrest activity. Mol Cell Biol 30(11), 2621–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Xu C, Du Y, Junaid M, Kaushik AC, Wei DQ, 2019. Comprehensive epigenetic analyses reveal master regulators driving lung metastasis of breast cancer. J Cell Mol Med 23(8), 5415–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kumar A, 2015. Differential signaling mechanism for HIV-1 Nef-mediated production of IL-6 and IL-8 in human astrocytes. Sci Rep 5, 9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shah A, Gangwani MR, Silverstein PS, Fu M, Kumar A, 2014. HIV-1 Nef induces CCL5 production in astrocytes through p38-MAPK and PI3K/Akt pathway and utilizes NF-kB, CEBP and AP-1 transcription factors. Sci Rep 4, 4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez de Heredia L, Magoulas C, 2013. Lack of the transcription factor C/EBPdelta impairs the intrinsic capacity of peripheral neurons for regeneration. Exp Neurol 239, 148–157. [DOI] [PubMed] [Google Scholar]
- Lou YJ, 2013. CEBPA-CEBPG axis as a novel promising therapeutic target in acute myeloid leukemia. Acta Pharmacol Sin 34(2), 185–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP, 2004. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J 23(1), 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J, Klukinov M, Yaksh TL, Malkmus SA, Williams S, Harris S, Manning DC, Taylor BK, Donahue RR, Porreca F, Xie JY, Oyarzo J, Brennan TJ, Subieta A, Schmidt WK, Yeomans DC, 2014. Single intrathecal administration of the transcription factor decoy AYX1 prevents acute and chronic pain after incisional, inflammatory, or neuropathic injury. Pain 155(2), 322–333. [DOI] [PubMed] [Google Scholar]
- Marchwicka A, Marcinkowska E, 2018. Regulation of Expression of CEBP Genes by Variably Expressed Vitamin D Receptor and Retinoic Acid Receptor alpha in Human Acute Myeloid Leukemia Cell Lines. Int J Mol Sci 19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionna R, Bellavia G, Romani M, Straino S, Germani A, Di Carlo A, Capogrossi MC, Napolitano M, 2012. C/EBPgamma regulates wound repair and EGF receptor signaling. J Invest Dermatol 132(7), 1908–1917. [DOI] [PubMed] [Google Scholar]
- Mullins DN, Crawford EL, Khuder SA, Hernandez DA, Yoon Y, Willey JC, 2005. CEBPG transcription factor correlates with antioxidant and DNA repair genes in normal bronchial epithelial cells but not in individuals with bronchogenic carcinoma. BMC Cancer 5, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Iida Y, Murase T, Oyama N, Umemura M, Takahashi S, Takahashi Y, 2019. Co-expression of C/EBPgamma and ATF5 in mouse vomeronasal sensory neurons during early postnatal development. Cell Tissue Res 378(3), 427–440. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Wakabayashi-Ito N, Nagata S, 1991. Molecular cloning of cDNA and a chromosomal gene encoding GPE1-BP, a nuclear protein which binds to granulocyte colony-stimulating factor promoter element 1. FEBS Lett 282(1), 95–97. [DOI] [PubMed] [Google Scholar]
- Nookala AR, Shah A, Noel RJ, Kumar A, 2013. HIV-1 Tat-mediated induction of CCL5 in astrocytes involves NF-kappaB, AP-1, C/EBPalpha and C/EBPgamma transcription factors and JAK, PI3K/Akt and p38 MAPK signaling pathways. PLoS One 8(11), e78855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori SA, Smale S, O’Shea-Greenfield A, Wall R, 1997. Differential interaction of nuclear factors with the leukocyte-specific pp52 promoter in B and T cells. J Immunol 159(4), 1800–1808. [PubMed] [Google Scholar]
- Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM, 2016. The integrated stress response. EMBO Rep 17(10), 1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin SE, Baer M, Copeland TD, Schwartz RC, Johnson PF, 2002. Regulation of CCAAT/enhancer-binding protein (C/EBP) activator proteins by heterodimerization with C/EBPgamma (Ig/EBP). J Biol Chem 277(26), 23563–23572. [DOI] [PubMed] [Google Scholar]
- Pulido-Salgado M, Vidal-Taboada JM, Saura J, 2015. C/EBPbeta and C/EBPdelta transcription factors: Basic biology and roles in the CNS. Prog Neurobiol 132, 1–33. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P, 2002. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365(Pt 3), 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti S, Ramazzotti G, Faenza I, Fiume R, Mongiorgi S, Billi AM, McCubrey JA, Suh PG, Manzoli L, Cocco L, Follo MY, 2018. Nuclear inositide signaling and cell cycle. Adv Biol Regul 67, 1–6. [DOI] [PubMed] [Google Scholar]
- Reddy KC, Dunbar TL, Nargund AM, Haynes CM, Troemel ER, 2016. The C. elegans CCAAT-Enhancer-Binding Protein Gamma Is Required for Surveillance Immunity. Cell Rep 14(7), 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C, Platero JS, Shuman J, Calame K, 1990. Ig/EBP-1: a ubiquitously expressed immunoglobulin enhancer binding protein that is similar to C/EBP and heterodimerizes with C/EBP. Genes Dev 4(8), 1404–1415. [DOI] [PubMed] [Google Scholar]
- Schutz SG, Robinson-Papp J, 2013. HIV-related neuropathy: current perspectives. HIV AIDS (Auckl) 5, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Catez P, Rohr O, Lecestre D, Aunis D, Schaeffer E, 2000. Functional interactions between C/EBP, Sp1, and COUP-TF regulate human immunodeficiency virus type 1 gene transcription in human brain cells. J Virol 74(1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A, Singh DP, Buch S, Kumar A, 2011. HIV-1 envelope protein gp120 up regulates CCL5 production in astrocytes which can be circumvented by inhibitors of NF-kappaB pathway. Biochem Biophys Res Commun 414(1), 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Niimi A, Takeuchi T, Shiogama K, Mizutani Y, Kajino T, Inada K, Hase T, Hatta T, Shibata H, Fukui T, Chen- Yoshikawa TF, Nagano K, Murate T, Kawamoto Y, Tomida S, Takahashi T, Suzuki M, 2021. CEBPγ facilitates lamellipodia formation and cancer cell migration through cers6 upregulation. Cancer Sci 112(7), 2770–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu YI, Morita M, Ohmi A, Aoyagi S, Ebihara H, Tonaki D, Horino Y, Iijima M, Hirose H, Takahashi S, Takahashi Y, 2009. Fasting induced up-regulation of activating transcription factor 5 in mouse liver. Life Sci 84(25–26), 894–902. [DOI] [PubMed] [Google Scholar]
- Sikalidis AK, Lee JI, Stipanuk MH, 2011. Gene expression and integrated stress response in HepG2/C3A cells cultured in amino acid deficient medium. Amino Acids 41(1), 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman LS, Fitzgerald T, Lertpiriyapong K, Cocco L, Follo MY, Martelli AM, Neri LM, Marmiroli S, Libra M, Candido S, Nicoletti F, Scalisi A, Fenga C, Drobot L, Rakus D, Gizak A, Laidler P, Dulinska-Litewka J, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Montalto G, Cervello M, Milella M, Tafuri A, Demidenko Z, Abrams SL, McCubrey JA, 2016. Critical Roles of EGFR Family Members in Breast Cancer and Breast Cancer Stem Cells: Targets for Therapy. Curr Pharm Des 22(16), 2358–88. [DOI] [PubMed] [Google Scholar]
- Tao YF, Lu J, Du XJ, Sun LC, Zhao X, Peng L, Cao L, Xiao PF, Pang L, Wu D, Wang N, Feng X, Li YH, Ni J, Wang J, Pan J, 2012. Survivin selective inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor cells. BMC Cancer 12, 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjahjono E, Kirienko NV, 2017. A conserved mitochondrial surveillance pathway is required for defense against Pseudomonas aeruginosa. PLoS Genet 13(6), e1006876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada J, Yoshida Y, Kominato Y, Auron PE, 2011. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 54(1), 6–19. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Hai T, Boyd SM, 1993. Dimerization specificity of the leucine zipper-containing bZIP motif on DNA binding: prediction and rational design. Genes Dev 7(6), 1047–1058. [DOI] [PubMed] [Google Scholar]
- Wall L, Destroismaisons N, Delvoye N, Guy LG, 1996. CAAT/enhancer-binding proteins are involved in beta-globin gene expression and are differentially expressed in murine erythroleukemia and K562 cells. J Biol Chem 271(28), 16477–16484. [DOI] [PubMed] [Google Scholar]
- Williams MS, Amaral FM, Simeoni F, Somervaille TC, 2020. A stress-responsive enhancer induces dynamic drug resistance in acute myeloid leukemia. J Clin Invest 130(3), 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XY, Hu JP, Wu MM, Wang LS, Fang NY, 2015. CCAAT/enhancer-binding protein CEBP-2 controls fat consumption and fatty acid desaturation in Caenorhabditis elegans. Biochem Biophys Res Commun 468(1–2), 312–318. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhang L, Yang L, Zhang Q, Wang X, 2020. C/EBPgamma is a critical negative regulator of LPS-/IgG immune complex-induced acute lung injury through the downregulation of C/EBPbeta-/C/EBPdelta-dependent C/EBP transcription activation. FASEB J 34(10), 13696–13710. [DOI] [PubMed] [Google Scholar]
- Yin HM, Yan LF, Liu Q, Peng Z, Zhang CY, Xia Y, Su D, Gu AH, Zhou Y, 2020. Activating transcription factor 3 coordinates differentiation of cardiac and hematopoietic progenitors by regulating glucose metabolism. Sci Adv 6(19), eaay9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafarana G, Rottier R, Grosveld F, Philipsen S, 2000. Erythroid overexpression of C/EBPgamma in transgenic mice affects gamma-globin expression and fetal liver erythropoiesis. EMBO J 19(21), 5856–5863. [DOI] [PMC free article] [PubMed] [Google Scholar]


