Abstract
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by progressive joint destruction associated with increased pro-inflammatory mediators. In inflammatory microenvironments, exogenous ATP (eATP) is hydrolyzed to adenosine, which exerts immunosuppressive effects, by the consecutive action of the ectonucleotidases CD39 and CD73. Mature B cells constitutively express both ectonucleotidases, converting these cells to potential suppressors. Here, we assessed CD39 and CD73 expression on B cells from treated or untreated patients with RA. Neither the frequency of CD73+CD39+ and CD73−CD39+ B cell subsets nor the levels of CD73 and CD39 expression on B cells from untreated or treated RA patients showed significant changes in comparison to healthy controls (HC). CpG+IL-2-stimulated B cells from HC or untreated RA patients increased their CD39 expression, and suppressed CD4+ and CD8+ T cell proliferation and intracellular TNF-production. A CD39 inhibitor significantly restored proliferation and TNF-producing capacity in CD4+ T cells, but not in CD8+ T cells, from HC and untreated RA patients, indicating that B cells from untreated RA patients conserved CD39-mediated regulatory function. Good responder patients to therapy (R-RA) exhibited an increased CD39 but not CD73 expression on B cells after treatment, while most of the non-responder (NR) patients showed a reduction in ectoenzyme expression. The positive changes of CD39 expression on B cells exhibited a negative correlation with disease activity and rheumatoid factor levels. Our results suggest modulating the ectoenzymes/ADO pathway as a potential therapy target for improving the course of RA.
Keywords: Rheumatoid Arthritis, regulatory B cells, CD39+ B cells, ADO pathway, T cell suppression, eATP
Graphical Abstract
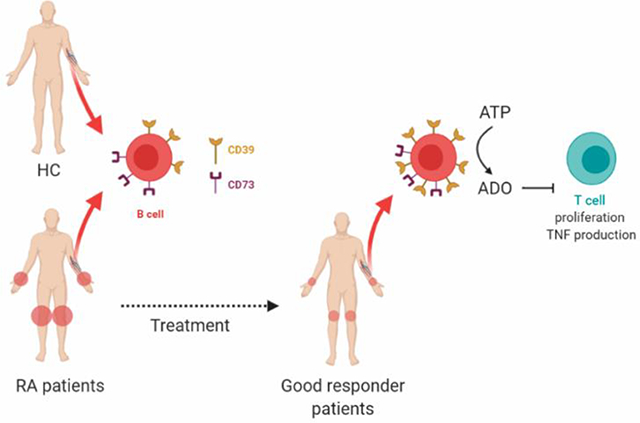
B cells from patients with Rheumatoid Arthritis (RA) have similar CD39 and CD73 expression to B cells from healthy controls (HC). After a positive response to therapy, B cells exhibit an increase in CD39, but not in CD73, expression and consequently higher capacity to hydrolyze eATP and to suppress CD4+ T cell proliferation and TNF production.
Introduction
During inflammation, adenosine triphosphate (ATP) is released from inflammatory and parenchymal cells. Extracellular ATP (eATP) acts as a “danger” signal stimulating immunity, i.e. by inflammasome activation. In order to regulate the immune response, eATP is hydrolyzed by the concerted action of two enzymes expressed in the plasma membrane, ectonucleoside triphosphate di-phosphohydrolase (NTPDase1/CD39) and ecto-5ǹucleotidase (CD73), into adenosine (ADO) [1]. By interacting with its specific receptors, ADO is able to downregulate the functions of different cell populations of the immune system. ADO attenuates the effector function of T cells and enhances the suppressive functions of regulatory T cells [2, 3]. Also, ADO is able to shape the humoral immune response. It has been reported that ADO plays an important role in immunoglobulin class switch recombination [4, 5].
Agreed with the sequential action of CD39 and CD73 degrades ATP, ADP, and AMP into ADO, the ectoenzymes CD39 and CD73 could be considered immunological mediators that contribute to dampens pro-inflammatory immune cell activity [6, 7]. Thus, human mature B cells that constitutively express CD39 and CD73 could potentially act as regulatory B cells. Activated, but not resting, B cells upregulate CD39 and downregulate CD73 expression, and therefore produce AMP but little ADO in the presence of eATP [8]. These B cells act as regulatory cells since they inhibit T cell proliferation and cytokine production by a mechanism presumably driven by AMP signaling [8]. Unlike human B cells, only 30% of murine B cells express CD39, but CD39 expression in murine B cells has also been associated to regulatory function [9].
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by progressive joint destruction associated with synovial proliferation and secretion of high levels of pro-inflammatory mediators, which include cytokines and growth factors among others [10, 11]. The huge tissue damage produced in the joints of RA patients gives rise to a considerable increase in ATP levels in the synovial fluid [12]. In addition, peripheral blood mononuclear cells (PBMC) from RA patients show higher expression levels of the ATP-gated P2X7 receptor (P2X7R) than those from normal individuals [13], suggesting a connection between ATP and the pathogenic behavior of T cells in RA.
CD39 and CD73 expression and activity undergo dynamic changes in response to different pathophysiological conditions [14–16]. It has been demonstrated that changes in the expression of these ectoenzymes may influence the outcome of various pathologies [17]. It has been demonstrated that Tregs from RA patients exhibit lower expression of CD39 than Tregs from healthy donors [18], and that patients with oligoarticular juvenile idiopathic arthritis exhibit low CD73 expression on T and B cells in the inflamed joints that correlate with clinical disease severity [19]. This shows the complexity of RA and highlights the importance of studying the different protagonists of the purinergic pathways involved in the development and progression of RA.
Given that B cells express high levels of CD39 and CD73, we assessed the CD39/CD73 ectonucleotidases involved in the ADO pathway in B cells from a cohort of patients with RA. In this study, we examined the frequency of CD73+CD39+ cells among B cell subsets, the NTPDase activity of CD39+ B cells before and after successful treatment, and the impact of this on T cell response in RA patients under different treatments.
Results
Similar frequencies of B cell subsets expressing CD39 and/or CD73 cells in RA patients and healthy controls
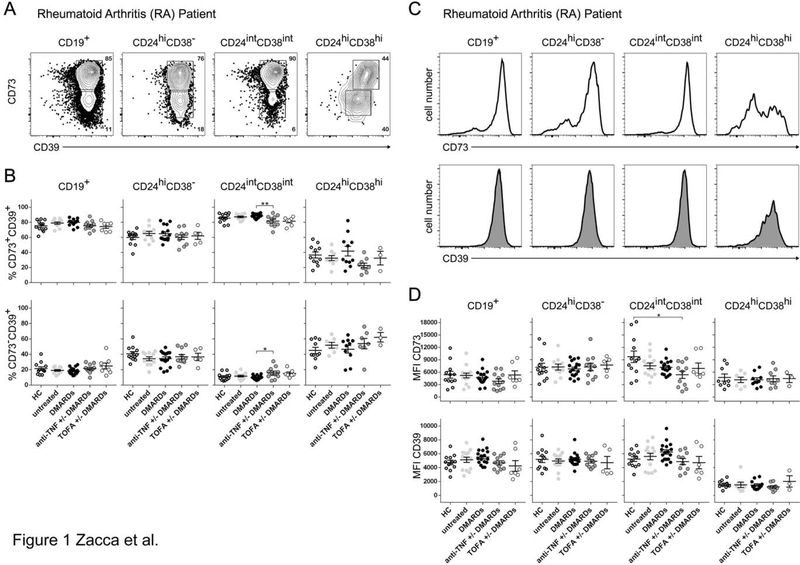
As a first step to analyzing the CD39/CD73 ectonucleotidases involved in the ADO pathway in B cells from RA patients, we determined by flow cytometry the percentages of CD39+ and CD73+ cells within the major circulating B cell populations in RA patients in comparison to healthy individuals. Peripheral blood was obtained from: healthy controls (HC), RA patients without specific treatment (untreated), and RA patients treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs, mainly MTX), anti-TNF +/− csDMARDs (any TNF-blocking biological treatment plus mainly MTX) and TOFA +/− csDMARDs (JAK inhibitor Tofacitinib plus mainly MTX). We evaluated CD73 and CD39 expression within different B cell subsets defined as total (CD19+), memory (CD19+CD24hiCD38−), mature (CD19+CD24intCD38int) and immature (CD19+CD24hiCD38hi) B cells. Fig. 1A illustrates a representative staining of CD73 and CD39 on B cell subsets from an untreated RA patient. As previously reported, most circulating B cells co-expressed CD39 and CD73 on the cell surface [8, 20].
Figure 1. CD39 and CD73 expression on B cell subsets from RA patients and healthy controls.
A) Representative dot plot of CD73 versus CD39 expression on total B cells (gate on lymphocytes CD19+) and different B cell subsets: memory B cells (CD19+CD24hiCD38− cells), mature B cells (CD19+CD24intCD38int cells) and immature B cells (CD19+CD24hiCD38hi cells) from untreated RA patients. B) Frequencies of CD73+CD39+ and CD73−CD30+ cells in total B cells and memory, mature and immature B cells in HC (n = 12), untreated (n = 13) or treated RA patients (csDMARDs, n = 17; anti-TNF+/− csDMARDS, n = 11; TOFA+/− csDMARDS, n = 7). Data are expressed as mean ±SEM. C) Representative histogram of CD73 or CD39 expression on total B cells (gate on lymphocytes CD19+) and the different B cell subsets from untreated RA patients D) Mean fluorescence intensity (MFI) of CD73 or CD39 on total, memory, mature and immature B cells (gated as mentioned before) from HC (n = 12), untreated (n = 13) or treated RA patients (csDMARDs, n = 17; anti-TNF+/− csDMARDS, n = 14; TOFA+/− csDMARDS, n = 7. MFI for immature B cells was determined on CD73+ or CD39+ cells.
p<0.05, **p<0.01, ***p<0.001. Kruskal-Wallis test with Dunn’s correction was used. csDMARDS is indicated as DMARDS.
Comparison of the frequencies of CD73+CD39+ B cells (Fig. 1B, upper panels), and CD73−CD39+ B cells (Fig. 1B, lower panels) among B cell subsets from our cohort of patients showed practically no significant differences between the groups of RA patients and HC (Fig. 1B). Within the immature B cell population, we observed higher frequencies of CD73−CD39+ B cells in comparison with mature B cells for all the samples analyzed (Fig 1B); therefore, the frequency of CD73+CD39+ cells was significantly lower than within mature B cells (p<0.01). Only a small but statistically significant difference was observed within CD73+CD39+ and CD73−CD39+ mature B cells when comparing RA patients treated with csDMARDs versus RA patients treated with anti-TNF± csDMARDs. However, despite this data, we can conclude that treatments did not modify the frequency of CD73+CD39+ and CD73−CD39+ B cell subsets. In addition, no differences were observed in the frequencies of single positive CD73 or single positive CD39-expressing cells within the different B cell subsets among all the groups analyzed (Supplementary Fig. 1).
The expression as well as the activity of CD39 and CD73 undergo dynamic changes depending on the pathophysiological context in which they are embedded [21]. Although we observed similar frequencies of CD73+CD39+ and of single positive CD73 or CD39 B cells in RA patients in comparison to HC, we next compared the mean fluorescence intensity (MFI) of CD73 and CD39 staining, as a measure of the levels of expression of these molecules, among the different B cell subsets from RA patients in comparison to HC. Fig. 1C displays representative histograms showing CD73 and CD39 expression on total, memory, mature and immature B cells from an untreated RA patient. After the analysis of CD73 and CD39 expression, we determined that there were no statistical differences in the MFI of CD39 and CD73 staining on peripheral blood B cell subsets from all the patients of our cohort in comparison to HC (Fig. 1D). Interestingly, immature B cells exhibited a bimodal expression of the ectoenzymes. CD39+ or CD73+ immature B cells had a significantly lower CD39 and CD73 expression in comparison to that observed on mature and memory B cells in all the groups analyzed (Supplementary Fig. 2), suggesting that B cells acquire CD73 and CD39 during maturation.
Altogether, these data indicate that circulating B cells expressing CD73 or CD39 or both ectoenzymes show no significant changes in their frequency or in the level of expression of these regulatory molecules in untreated or treated RA patients in comparison to HC.
Comparable CD39-mediated regulatory function in B cells from untreated RA patients and healthy controls
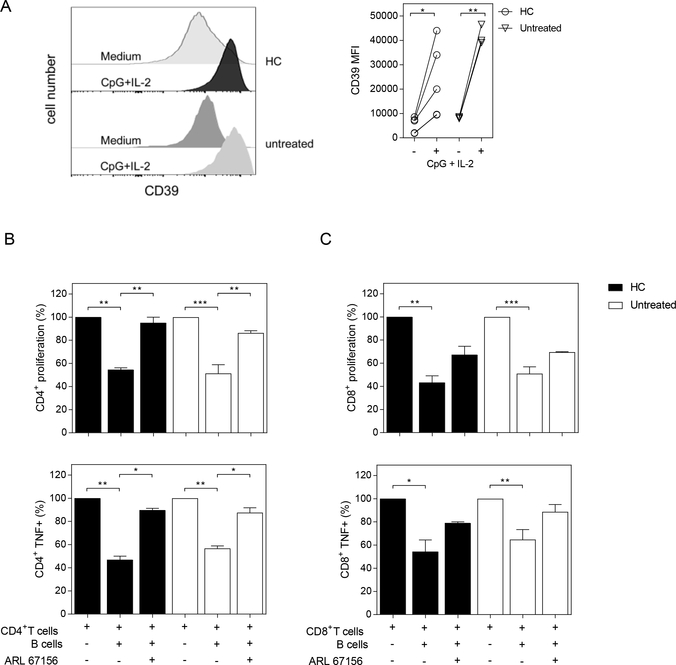
We next attempted to determine whether B cells from RA patients show dysfunctional regulatory features via CD39. To this end, we evaluated the capacity of B cells from HC and untreated RA patients to suppress the proliferation and the frequency of TNF-producing CD4+ and CD8+ T cells in a CD39-mediated fashion. As a first step, CD19+ B cells were purified from PBMC of HC and untreated RA patients and incubated during 72h with medium alone or with CpG+IL-2. We used CpG, a T-independent ligand to activate B cells [22], in the presence of IL-2, to provide signals that enable B cells to survive several days of culture. Later, activated B cells were incubated with autologous T cells previously stimulated with anti-CD3/anti-CD28.
As described for activated B cells [23], the MFI of CD39 expression increased significantly on B cells from HC and untreated RA patients upon stimulation with CpG plus IL-2 (Fig. 2A), while CD39 expression was not modified when B cells were incubated in the presence of IL-2 (data not shown). As expected, given our previous report [24], unstimulated B cells were not able to suppress T cell proliferation or cytokine production (data not shown). In contrast, when CD19+ B cells from HC, activated with CpG+IL-2, were added to the T cell culture, the proliferation of CD4+ or CD8+ T cells and the frequency of TNF+ CD4+ or CD8+ T cells significantly decreased (Fig. 2B and C). Remarkably, CD19+ B cells from untreated RA patients were also able to reduce CD4+ and CD8+ T cell proliferation and intracellular TNF production. To explore the role of CD39 in our co-culture systems, we added the ectoATPase CD39 inhibitor ARL67156 to the cultures [25], which significantly restored proliferation as well as TNF-producing capacity in CD4+ T cells from both HC and untreated RA patients (Fig. 2B). In contrast, the addition of ARL67156 did not significantly revert CD8+ T cell suppression or the frequency of TNF+CD8+ T cells elicited by CpG-stimulated B cells (Fig. 2C). Further, we observed that the addition of activated B cells to activated CD4+ T cells reduced the concentration of TNF, but not of IL-17A, in the supernatants of the co-cultures. An increase in TNF concentration was observed when ARL67156 was added to the culture of CD4+ T cells with activated B cells (Supplementary Fig. 3A and B). The addition of activated B cells to activated CD8+ T cells did not modify the levels of TNF in the culture supernants. IL-17A was not detected in the supernatants of activated B cells co-cultured with activated CD8+ T cells (data not shown).
Figure 2. CD39-dependent inhibition of T cell proliferation and cytokine production by activated B cells from HC and untreated RA patients.
A) Histograms and graph show CD39 expression (determined as MFI) on purified CD19+ B cells from HC or untreated RA patients incubated with medium alone (−) or with CpG+IL-2 (+) for 72 h. B-C) Purified CD19+ B cells, CD4+ and CD8+ T cells were obtained from PBMC of HC and untreated RA patients. CD19+ B cells were stimulated with CpG+IL-2 and then washed and incubated 2:1 with autologous CFSE-labeled CD4+ or CD8+ T cells cultured with anti-CD3/anti-CD28 mAb (plate bound) in absence or in presence of CD39 inhibitor (ARL 67156). T cell proliferation and TNF expression were analyzed by flow cytometry on CD3+CD19− live cells, after 72 h of culture. Percentage of proliferation and TNF production on (B) CD4+ T cells and (C) CD8+ T cells cultured in medium or in presence of CpG-activated CD19+ B cells with or without ARL 67156. Mean ± SEM of five independent experiments. *p<0.05, **p<0.01, ***p<0.001. One-way ANOVA followed by a Bonferroni’s post-test were used.
These results indicate that the CD39-mediated regulatory capacity of B cells on TNF− producing CD4+ T cells was not affected in RA patients.
RA patients showing good response to treatment exhibit increased CD39 expression on B cells
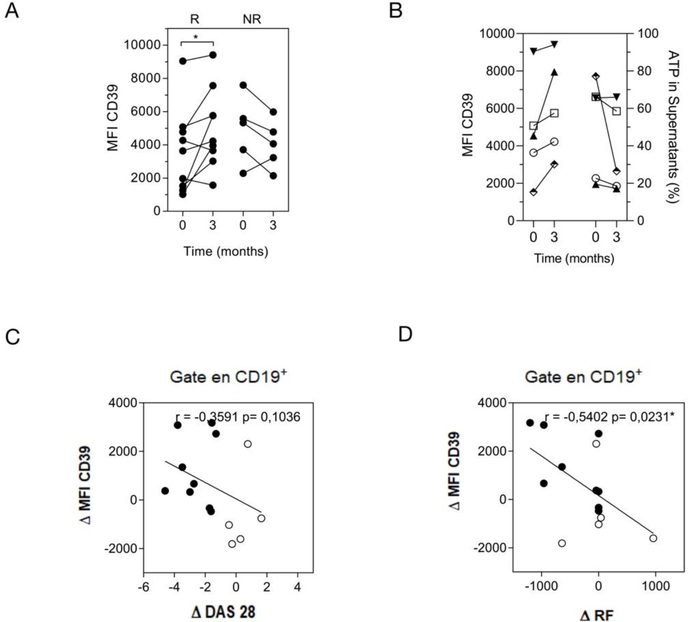
According to EULAR criteria [26, 27], the response of RA patients to drug therapy is classified as good, moderate or none, depending on the DAS28 variation. Considering this, we aimed at identifying whether changes in CD39 expression on B cells showed any correlation with the response to treatment in RA patients. To this end, we compared the level of CD39 expression on total B cells at baseline (before starting the treatment) and after 3 months of treatment, in RA patients who were classified as good responders (R-) and non-responders (NR-) (as indicated in Materials and Methods) after the follow-up evaluation of the clinical response. As evidenced by the changes in the MFI of CD39 expression, some R-RA patients exhibited a significant increase after treatment, while most of the NR patients exhibited a decrease in CD39 expression on B cells (Fig. 3A). We also evaluated whether CD39 expression increased in any particular B cell subset from R- and NR-RA patients. After treatment, R-RA patients showed a significant increase of CD39 expression on immature B cell subsets and a tendency, although without statistical significance, of CD39 increase on mature and memory B cells. As expected, NR patients did not exhibit any significant differences in CD39 expression on any B cell subset evaluated (Supplementary Fig. 4A). The results suggest that the CD39 increase detected in the total B cell population could be a consequence of small contributions of each subset.
Figure 3. B cells from RA patients increased CD39 expression after good response to treatment.
A) Mean fluorescence intensity (MFI) of CD39 on total B cells (CD19+CD4−CD8−) at baseline (time 0) and after 3 months (time 3) of treatment in good responder (R) and non-responder (NR) patients. (R n=9 and NR n=5). B) MFI of CD39 on total B cells from the same R-RA patient before (0) and after treatment (3) is shown together with ATP hydrolysis (ATP % in supernatant). C-D) Correlation plots show the relationship between the delta of mean fluorescence intensity (MFI) of CD39 on B cells, determined as the difference between the value of MFI after and before treatment (0–3 months) versus (C) delta of DAS 28 (0–3 months) and (D) delta of the titer of RF (0–3 months). Full and empty circles represent R and NR patients, respectively. Correlation analysis was performed with Pearson’s correlation test. p<0.05 was considered statistically significant.
We also evaluated the expression of CD73 in the different B cell subsets in R-RA and NR-RA patients. No change was observed in the expression of the CD73 ectoenzyme as a consequence of treatment, with the MFI of CD73 being similar before and after treatment in R- and NR-RA patients (Supplementary Fig. 4B). Finally, we evaluated the frequency of CD73+CD39+ cells in the different B cell subsets and observed no statistical differences before or after treatment in R- and NR-RA patients (Supplementary Fig. 4C).
To evaluate the activity of CD39 expressed on B cells as a functional ectoenzyme able to hydrolyze ATP, B cells purified from PBMCs of five R-RA patients at baseline and after treatment were cultured in the presence of exogenous ATP. The increase in the expression of CD39 on total B cells at treated versus baseline time points was confirmed in this group of R-RA patients (Fig. 3B). Culture supernatants from B cells obtained from R-RA patients after treatment showed lower ATP levels, although without statistical significance, than culture supernatants from B cells from the same patient obtained at baseline (Fig. 3B). Altogether, these results indicate that B cells from RA patients maintained their ability to hydrolyze ATP.
To understand the relevance of our findings, we evaluated different parameters associated with the disease (i.e. DAS28 and the levels of rheumatoid factor (RF) in RA patients with respect to changes in CD39 expression after therapy. Figure 3C depicts the delta of CD39 MFI, obtained from the values of CD39 expression on total CD19+ B cells before and after treatment vs the changes in disease activity determined as the delta of DAS28. We observed that high delta values of CD39 expression on CD19+B cells correlated with a better status of the disease (negative delta values of DAS28 correspond to R-RA patients; Fig. 3C indicated with fill circles). NR-RA patients have delta values of DAS28 near cero or higher than cero (Fig. 3 C, indicated with empty circles) and low delta values of CD39 expression. Also, high delta values of CD39 expression on total CD19+ B cells correlated with decreased levels of RF (negative delta values of RF titres). Considering that in the cohort we have evaluated, some patients were RF negative, delta values of RF were 0. Details of R-RA patients’ response can be observed in Supplementary Fig. 5 which shows the changes in CD39 expression as consequence of treatment and the changes in different parameters involved in progression of RA as DAS28, RF and anti-CCP antibodies.
Discussion
Despite the importance of B cells in the pathogenesis of human autoimmunity, they may also play a role in immunomodulation through IL-10-dependent [28] and -independent mechanisms. The independent mechanisms include participation of the inhibitory molecule PD-L1 [24, 29] or the expression of the ectoenzymes CD39/CD73 [8, 23]. Information about regulatory CD39+ CD73+B cells in patients with RA is limited, and therefore it is interesting to examine whether these cells are affected in RA and if therapy can modify their function.
In this work, we demonstrate that RA patients exhibit similar frequencies of B cell subsets that express CD73 and/or CD39 as HC. All subsets of circulating B cells expressing CD73 or CD39 or both ectoenzymes show no significant changes in their frequency or in the level of expression of these regulatory molecules in untreated or treated RA patients in comparison to HC, indicating that these B cell populations are not affected in RA.
The CD39/CD73 pathway has been previously evaluated in AR patients, but most of the studies were limited to T cells, both in patients and experimental models. Using an experimental model of collagen-induced arthritis, it has been reported that the IL-6 produced by pro-inflammatory cells reduced the frequency of CD39+ Tregs in lymph nodes and spleen [30]. Also, low CD73 expression on CD8+ and CD19+ cells in the inflamed site has been reported in patients with juvenile idiopathic arthritis [19]. On the other hand, peripheral blood from RA patients has been shown to have a higher frequency of Tregs with increased CD39 activity and decreased activity of adenosine deaminase, an enzyme involved in the metabolism of ADO [31], probably as a compensatory mechanism to maintain the levels of the anti-inflammatory ADO.
When we evaluated CD39 expression of B cells in the patients enrolled in our study, we observed that, as in HC, B cells from untreated RA patients increased the expression of CD39 after in vitro stimulation with an unspecific stimulus such as CpG. The activated B cell, from untreated RA patients or HC, suppressed CD4+ and CD8+ T cell proliferation and TNF production. The inhibition on CD4+ T cells by activated B cells was mediated by CD39, since the addition of CD39-activity inhibitor reverted the suppression. However, CD8+ T cell suppression was not modified by the CD39 inhibitor, indicating that the inhibition does not involve CD39. Probably, the inhibitory molecule PD-L1, also highly expressed on activated B cells from HC and RA patients, is responsible for the inhibition of CD8+ T cell proliferation and TNF production, as we previously reported [24]. This differential susceptibility among CD8+ and CD4+ T cells may depend on the differential expression of PD-1, the PD-L1 receptor, or ADO receptors on T cell populations [32] or differential responses to 5′-AMP signaling [33]. Further studies are needed to elucidate the causes of differential T cell responses to CD39-mediated inhibition.
Our data confirm the report that, after CpG stimulation, CD73 expression was downregulated on B cells [8]. Although it could be considered that CD73 downregulation affects the ability of B cells to generate ADO, it has been described that CD73 can be provided by neighboring cells. Indeed, CD39+CD73− Tr1 cells have been shown to be able to produce ADO with the collaboration of CD73+ cells [7, 34]. It is thus possible that, in the co-culture assays, activated T cells participate in ADO production providing CD73.
Remarkably, B cells from patients with good response to treatment upregulated CD39, but not CD73 expression. Although without statistical significance, we found B cells from R-RA patients showed a tendency toward increased ATP consumption. It is likely that enhanced CD39 expression favors the hydrolysis of exogenous ATP in AMP and ADO. Thus, B cells from R-RA patients may collaborate in the control of the inflammatory state. It has also been demonstrated that unresponsive RA patients exhibit lower CD39 expression on Tregs than good responder patients and HC. Additionally, Gupta et al [35] found that single nucleotide polymorphism (SNP) in the ENTPD1 (CD39) gene was associated with poor response to MTX and this could explain the patient’s differential response. The relevance of CD39 in RA was also evaluated in a murine model, finding that CD39 blockade reduced the antiarthritic effect of MTX, which suggests that CD39 and ADO production are involved in the mechanism of action of MTX and are required for responsiveness to the treatment [18]. In this context, it has been shown that two ADO receptors, A2A and A3ARs, are upregulated in lymphocytes from early RA patients or those treated with methotrexate (MTX) [36]. The authors suggested a correlation between A2A and A3AR expression and the inflammatory and clinical responses in RA, and that ADO receptor upregulation could represent a compensatory mechanism to reduce the inflammatory status.
Considering CD39 expression differs in the different subsets of B cells and that a different response to therapy in RA may result in a different composition of B cells [24, 37–39], we analyzed CD39 expression within the same subset of B cells before and after treatment and found a significant increase of CD39 expression in immature B cell subsets. Taking into account that R-RA patients from our cohort show decreased frequency of immature B cells [24], it is unlikely that this subset is responsible for the increase in CD39+ detected in the total B cell population. The increase of CD39 expression in the total CD19+CD4negCD8neg B cells appears to be due to a slight increase in each B cell subset.
The increase of CD39 in B cells correlated with a reduction in disease activity, determined as DAS28, and in the level of RF, suggesting an association between better patient status and high CD39 expression. These data indicate that the improvement observed in patients in response to the therapy could be due to an enhancement of the CD39/ADO pathway. It has thus been proposed that the frequency of CD39+ Tregs could be used as a biomarker of treatment response to MTX in RA [35].
Macrophages are a prevalent population in the inflamed joint and are one of the most important contributors of pro-inflammatory cytokines in RA. Under inflammatory conditions, they acquire a hyper-metabolism state in which the consumption of glucose and the production of ATP are increased [15, 40, 41]. Therefore, although this study focused on the role of regulatory B cells on T cell response, it would be interesting to know if CD73+CD39+B cells can condition macrophage function, considering that ADO downregulates classical macrophage activation [42]. Since this is a pilot study it would be interesting to go deeper in the study and include larger number of non-responder RA patients and evaluate patients with little or moderate response to the treatment. The incorporation of these patients would allow us to have a general vision of the role of CD39+ B cell in RA. Additionally, could be interesting to determine whether CD39+ B cells retain their regulatory function in the synovium, the primary site of the inflammatory process in RA.
Altogether our results position CD39 expression on B cells as an interesting pathway deserving deeper study in the context of RA. Considering that the immunoregulatory signal mediated by CD39-expressing B cells seems to be conserved in RA patients and enhanced in the context of good response to conventional treatments, therapies aimed at favoring the CD39/ADO pathway in B cells, and in other immunoregulatory populations, may help to improve RA treatments.
Materials and methods
Patients and healthy controls
Sex- and age-matched RA patients and healthy controls (HC) with an age range from 22–83 years were enrolled in this study after written informed consent. The RA patients were recruited from the Rheumatology Service (Hospital Nacional de Clínicas, HNC) under a variety of treatment conditions or before receiving treatment (baseline, untreated).
RA patients were diagnosed according to the American College of Rheumatology and the European League Against Rheumatism (EULAR) classification criteria [43]. The exclusion criteria include known or suspected ongoing infections or metabolic diseases for RA patients and any history of autoimmune disease, or treatment with glucocorticoid, or immunosupressive therapy for HD as reported in our previous study [24]. Some RA patients treated with less than 7.5 mg/day of glucocorticoid were included. Table 1 describes the clinical characteristics of the RA patients.
Table 1.
Demographic and clinical features of RA patients and controls.
| Characteristic | RA patients | Healthy Controls | |||
|
|
|
|
|||
| Age: range years | 22 – 83 | 31 – 74 | |||
| Sex: female/male | 53/10 | 15/3 | |||
| Treatment: | untreated | MTX | anti-TNF | TOFA | |
|
|
|||||
| CRP: median ± QD, mg/l | 12 ± 9.2 | 6.0 ± 2.8 | 5.0 ± 3.2 | 6.0 ± 4.5 | |
| ESR: median ± QD, mm/h | 19 ± 15 | 11 ± 7 | 9 ± 9 | 21 ± 7 | |
| DAS28 ESR: mean ± SEM | 4.9 ± 0.3 | 3.4 ± 0.3 | 3.9 ± 0.3 | 3.4 ± 0.4 | |
| Anti-CCP: positive/negative | 15/5 | 13/5 | 10/5 | 7/3 | |
| RF: positive/negative | 15/5 | 11/7 | 10/5 | 7/3 | |
RA, Rheumatoid arthritis; MTX, methotrexate; TOFA, JAK inhibitor Tofacitinib; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; DAS28, disease activity score 28; Anti-CCP, Anti-cyclic citrullinated peptide antibodies; RF, rheumatoid factor.
At the time of blood collection, the RA disease activity score (DAS28) was assessed as described [44]. Response to treatment was defined according to EULAR criteria. The DAS28 value reached after treatment was used to classify good responder (R) and non-responder (NR) patients [26, 27].
Peripheral blood was collected from HC, untreated RA patients (without specific therapy) and RA patients treated with csDMARDs (conventional synthetic disease-modifying antirheumatic drugs, mainly MTX), anti-TNF +/− csDMARDs (any TNF blocking biological treatment plus mainly MTX) and TOFA +/− csDMARDs (JAK inhibitor Tofacitinib plus mainly MTX) [24, 45]. In some patients, samples were taken at baseline and after 3 months of treatment. The Hospital Nacional de Clínicas ethics committee (CIEIS) approved the study, which was conducted according to the Declaration of Helsinki on studies with human subjects.
RF and anti-CCP antibodies determination
Levels of autoantibodies were determined in serum samples. A latex agglutination test was used to evaluate RF, according to the manufacturer’s instructions, with a cut-off value of 1/20 titer (Artritest, Wiener Laboratories). The anti-CCP antibodies (anti-cyclic citrullinated peptide) were quantified by ELISA, according to the manufacturer’s instructions, in which values ≥ 20U/ml were considered positive (Orgentec Diagnostika Gmbh).
Flow cytometry
Single cell suspensions from peripheral blood samples were stained with monoclonal Antibodies following the procedure previously described by Zacca et al. [24]. We used different combinations of the following antibodies: FITC anti-CD24 (ML5, BD, diluted 1/8), PE anti-CD39 (A1, Biolegend, diluted 1/20), FITC anti-human CD4 (13B8.2, Beckman Coulter, Brea, CA, diluted 1/20), PerCP-Cy5.5 anti-CD19 (HIB19, BD, diluted 1/10), APC/Cy7 anti-CD19 (HIB19, Biolegend, diluted 1/15), APC/Cy7 anti-CD38 (HIT2, Biolegend, diluted 1/20), PE/Cy7 anti-CD38 (HIT2, BD, diluted 1/25), APC anti-CD73 (AD2, Biolegend, diluted 1/20), PerCP anti-CD8 (diluted 1/20) and APC anti-CD8 (RPA-T8, eBioscience, diluted 1/50).
For intracellular cytokine staining, cells were stimulated with 50ng/ml PMA (Sigma-Aldrich) plus 1ug/ml ionomycin (Sigma-Aldrich) in the presence of Brefeldin A (GolgiPlug, BD) as we previously described [24]. PMA+ionomycin were used to activate the cells and induce cytokine secretion, and Brefeldin A to keep the cytokines inside the cells because this inhibits protein transport.
After stimulus, cells were washed and stained with APC/Cyanine7 anti-human CD19 (HIB19, BioLegend) and APC anti-human CD3 (UCHT-1, BD, diluted 1/20). Subsequently, cells were washed, fixed and permeabilized using Cytofix/Cytoperm (BD). Cells were washed with Perm/Wash (BD) and stained with PE/Cyanine7 anti-human TNF (MAb11, BD, diluted 1/100). Cell samples were acquired on a BD FACSCanto II Flow Cytometer and analyzed with FlowJo software (version 10). For the analysis of CD73+ and CD39+ cells on total B cell population, we performed a gate in CD19+ lymphocytes excluding CD4+ and CD8+ T cells (Supplementary Fig. 6). To define B cell subsets, we evaluated CD24 versus CD38 markers within a CD19+CD4−CD8− lymphocyte gate [24].
Cell purification procedures and cell cultures
Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll-Hypaque, as we described in Zacca et al. [24]. CD19+ B cells, CD4+ and CD8+ T cells from HC and RA patients were isolated from PBMCs by positive selection using magnetic beads following the manufacturer’s instructions (EasySep, Stemcell Technologies, Inc). Freshly isolated B cells were stimulated with CpG-ODN 2006 (1ug/ml; Invivogen), as we previously reported [24]. After stimulation, B cells were washed and stained for 30 min at 4°C with PE anti-CD39 (A1, Biolegend), APC/Cy7 anti-CD19 (HIB19, Biolegend, diluted 1/15) and APC anti-CD73 (AD2, Biolegend, diluted 1/20).
Autologous CFSE-labelled CD4+ or CD8+ T cells (0.1×106) stimulated with anti-CD3 (0.5 μg/ml, OKT3, Biolegend) and anti-CD28 (0.25 μg/ml, CD28.2, Biolegend) were co-cultured in presence or in absence of CpG-activated CD19+ B cells and T cell. The anti-CD3/CD28 is an antigen-independent stimulation that expands most T cells since they activate the TCR complex and co-stimulation, respectively [46]. After 72 h of culture, proliferation was evaluated as CFSE dilution by flow cytometry. In some experiments, 100uM ectoATPase inhibitor ARL67156 (Tocris) was added to the culture. Dead cells were excluded using Fixable Viability Stain 780 (eBioscience, diluted 1/1000), a viability dye that can be used to irreversibly label dead cells prior to fixation and permeabilization procedures.
Proliferation was calculated considering as 100% the values obtained from T cells stimulated with anti-CD3/CD28 alone, and the proliferation in the presence of activated B cells with or without ARL67156 (Tocris) was calculated relative to that 100%.
Cytokine Quantification
Cytokine concentrations in the supernatants of the co-cultures of activated- B and T cells were assessed by ELISA using paired antibodies for human TNF and IL-17A (Biolegend, USA) according to the manufacturer’s instructions.
ATP hydrolysis assay
PBMC from RA patients before and after treatment were incubated with recombinant human IL-2 (40ng/ml; Gibco) for 3 hours in 12-well plates at 5 × 105 cells/well. Then, B cells were purified as described above. To test whether CD39 on B cells is a functional E-NTPDase, purified B cells from RA patients, obtained before or after 3 months of treatment, were seeded into a 96-well plate at a density of 50000 cells per well in DMEM without phenol red (Gibco #21063). The hydrolysis of ATP in the culture medium by CD39 was assessed by a bioluminescent assay. Cells were incubated with 10μM ATP (Sigma-Aldrich A6419) in culture medium without serum, and the changes in eATP concentrations, after 40 minutes at 37°C and 5% CO2, were measured with an ATP bioluminescent assay kit (Invitrogen A22066) following manufacturer’s instructions. Cell-free medium with ATP alone was used as control. Briefly, 10 ul of culture supernatant was incubated with 90 ul of the reaction buffer in a 96-well plate at 28°C. After 15 minutes, the bioluminescence was measured at 560nm using the BIO TEK microplate spectrophotometer (Bio Tek instruments, Inc.). The values obtained of each experimental condition were referred to the value from medium culture alone with ATP, which is considered 100% of ATP.
Statistics
GraphPad Prism version 7 software was used for all the statistical analyses (GraphPad Software). P values <0.05 were considered significant. To define the distribution of the datasets we initially performed the D’Agostino-Pearson normality test. The specific test used in each experiment is described in each legend of Figures.
Supplementary Material
Supplementary Figure 1. Frequencies of CD39+ and CD73+ on peripheral blood B cell subsets from RA patients following different treatments. Graphs show frequencies of CD73+ (upper) and CD39+ B cells (lower) from HC, untreated or treated RA patients. Data are expressed as mean ± SEM. One-way ANOVA followed by a Bonferroni’s post-test were used.
Supplementary Figure 2. Comparison of CD39 and CD73 expression between primarily memory, immature and mature B cells from RA patients following different treatments. Graphs show MFI of CD39 (upper) and CD73 (lower) B cell subsets from HC, untreated or treated RA patients. Data are expressed as mean ± SEM. Patients treated with csDMARDs are indicated DMARDs. *p<0.05, **p<0.01, ***p<0.001. p values were determined using the Student’s t-test for unpaired simples.
Supplementary Figure 3: Levels of cytokines in the supernatant of B cells incubated with CD4+ T cells. Concentrations of A) TNF and B) IL-17 determined by ELISA in the supernatants of co-cultures of B cell and CD4+ T cells from untreated RA patients described in Figure 2. *p<0.05 **p< 0.005. p values were determined using the Student’s t-test for unpaired samples. ns=non-significant.
Supplementary Figure 4. CD39 and CD73 expression on total B cells and B cell subsets from R- and NR-RA patients. A) Graphs show MFI of CD39, B) MFI of CD73 and C) Frequency of CD73+CD39+ cells in the total B cell population and B cell subsets (memory, mature and immature B cells) from responder and non-responder RA patients at baseline (0) and after 3 months of treatment (3). Data are expressed as mean ± SEM. *p<0.05. P value was determined using the Student’s t-test for paired samples.
Supplementary Figure 5: Relation between different parameters involved in progression of RA and intensity of CD39 in total B cells. Intensity of CD39, determined as MFI, on total B cells at baseline (time 0) and after 3 months (time 3) of treatment in good responder (R) patients is shown together with A) DAS28, B) levels of serum RF and C) anti-CCP antibodies. *p<0.05, *** p<0.001. p values were determined using the Student’s t-test for paired samples. ns=non-significant
Supplementary Figure 6: Flow cytometry gating strategy for the analysis of total B cells A) Selection of the lymphocyte population from FSC-A vs SSC-A contour plot. B) Exclusion of doublets by gating in the diagonal between FSC-H vs FSC-A. C) Contour plot showing the expression of CD4 and CD8 after gating A and B. Negative population for CD4 and CD8 expression was selected and D) a contour plot of CD19 vs CD4 shows the gate for total B cells as CD19+ CD4neg CD8neg. Dot plots were performed with cells from HC as an example.
Highlights.
When compared cross-sectionally, rheumatoid arthritis patients under different treatments do not differ from each other or from healthy individuals in terms of the frequency of B cell subsets expressing the ectoenzymes CD39 and/or CD73.
The capacity of B cells to regulate the proliferation and TNF-production of CD4+ T cells mediated by CD39 is not affected in patients with rheumatoid arthritis.
In a longitudinal analysis, rheumatoid arthritis patients with good response to therapy exhibited an increase in CD39 expression on B cells after treatment while most of the non-responders exhibited a reduction in ectoenzyme expression.
B cells from some good responders increased their CD39 expression after treatment, which correlated with less DAS28, rheumatoid factor and anti-cyclic citrullinated peptide antibodies.
Acknowledgments:
We express our sincere gratitude to all the patients who participated in this study. We also thank MP Abadie and MP Crespo for their excellent technical assistance in flow cytometry and cell sorting, and A Constantino for help in auto-antibody determinations.
Funding: Research reported in this publication was supported by the Agencia Nacional de Promoción Científica y Técnica (PID 2012-0068) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grants Number R01AI116432A and R01AI110340). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Abbreviations
- RA
Rheumatoid arthritis
- eATP
exogenous ATP
- ADO
adenosine
- MTX
methotrexate
- HC
healthy controls
- csDMARDs
(conventional synthetic disease-modifying antirheumatic drugs)
- TOFA
Tofacitinib, JAK inhibitor
- MFI
means of fluorescence intensity
- PBMC
peripheral blood mononuclear cell
- R
good responders
- NR
non-responders
- RF
Rheumatoid factor
- anti-CCP antibodies
anti-cyclic citrullinated peptide antibodies
- DAS28
disease activity score 28
- ELISA
Enzyme-linked immunosorbent assays
Footnotes
Competing financial interests: The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Linden J and Cekic C, Regulation of lymphocyte function by adenosine. Arterioscler Thromb Vasc Biol, 2012. 32(9): p. 2097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bopp T, et al. , Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med, 2007. 204(6): p. 1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sitkovsky MV, T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol, 2009. 30(3): p. 102–8. [DOI] [PubMed] [Google Scholar]
- 4.Przybyla T, Sakowicz-Burkiewicz M, and Pawelczyk T, Purinergic signaling in B cells. Acta Biochim Pol, 2018. 65(1): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 5.Schena F, et al. , Dependence of immunoglobulin class switch recombination in B cells on vesicular release of ATP and CD73 ectonucleotidase activity. Cell Rep, 2013. 3(6): p. 1824–31. [DOI] [PubMed] [Google Scholar]
- 6.Beavis PA, et al. , CD73: a potent suppressor of antitumor immune responses. Trends Immunol, 2012. 33(5): p. 231–7. [DOI] [PubMed] [Google Scholar]
- 7.Mascanfroni ID, et al. , IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol, 2013. 14(10): p. 1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saze Z, et al. , Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood, 2013. 122(1): p. 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaku H, et al. , A novel mechanism of B cell-mediated immune suppression through CD73 expression and adenosine production. J Immunol, 2014. 193(12): p. 5904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joseph A, et al. , Immunologic rheumatic disorders. J Allergy Clin Immunol, 2010. 125(2 Suppl 2): p. S204–15. [DOI] [PubMed] [Google Scholar]
- 11.Weissmann G, The pathogenesis of rheumatoid arthritis. Bull NYU Hosp Jt Dis, 2006. 64(1–2): p. 12–5. [PubMed] [Google Scholar]
- 12.Ryan LM, Rachow JW, and McCarty DJ, Synovial fluid ATP: a potential substrate for the production of inorganic pyrophosphate. J Rheumatol, 1991. 18(5): p. 716–20. [PubMed] [Google Scholar]
- 13.Fan ZD, et al. , Involvement of P2X7 receptor signaling on regulating the differentiation of Th17 cells and type II collagen-induced arthritis in mice. Sci Rep, 2016. 6: p. 35804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allard B, et al. , The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev, 2017. 276(1): p. 121–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva JLG, et al. , ATP and adenosine: Role in the immunopathogenesis of rheumatoid arthritis. Immunol Lett, 2019. 214: p. 55–64. [DOI] [PubMed] [Google Scholar]
- 16.Takenaka MC, Robson S, and Quintana FJ, Regulation of the T Cell Response by CD39. Trends Immunol, 2016. 37(7): p. 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonioli L, et al. , CD39 and CD73 in immunity and inflammation. Trends Mol Med, 2013. 19(6): p. 355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peres RS, et al. , Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc Natl Acad Sci U S A, 2015. 112(8): p. 2509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Botta Gordon-Smith S, et al. , Correlation of low CD73 expression on synovial lymphocytes with reduced adenosine generation and higher disease severity in juvenile idiopathic arthritis. Arthritis Rheumatol, 2015. 67(2): p. 545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim ES, et al. , Down-regulation of CD73 on B cells of patients with viremic HIV correlates with B cell activation and disease progression. J Leukoc Biol, 2017. 101(5): p. 1263–1271. [DOI] [PubMed] [Google Scholar]
- 21.Schetinger MR, et al. , NTPDase and 5’-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors, 2007. 31(2): p. 77–98. [DOI] [PubMed] [Google Scholar]
- 22.Marasco E, et al. , B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients. Eur J Immunol, 2017. 47(1): p. 131–143. [DOI] [PubMed] [Google Scholar]
- 23.Figueiro F, et al. , Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg). Oncoimmunology, 2016. 5(2): p. e1082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacca ER, et al. , PD-L1(+) Regulatory B Cells Are Significantly Decreased in Rheumatoid Arthritis Patients and Increase After Successful Treatment. Front Immunol, 2018. 9: p. 2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canale FP, et al. , CD39 Expression Defines Cell Exhaustion in Tumor-Infiltrating CD8(+) T Cells-Response. Cancer Res, 2018. 78(17): p. 5175. [DOI] [PubMed] [Google Scholar]
- 26.Fransen J and van Riel PL, The Disease Activity Score and the EULAR response criteria. Rheum Dis Clin North Am, 2009. 35(4): p. 745–57, vii-viii. [DOI] [PubMed] [Google Scholar]
- 27.Smolen JS, et al. , Head-to-head comparison of certolizumab pegol versus adalimumab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet, 2016. 388(10061): p. 2763–2774. [DOI] [PubMed] [Google Scholar]
- 28.Mauri C and Menon M, The expanding family of regulatory B cells. Int Immunol, 2015. 27(10): p. 479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan AR, et al. , PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun, 2015. 6: p. 5997. [DOI] [PubMed] [Google Scholar]
- 30.Thiolat A, et al. , Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol, 2014. 66(2): p. 273–83. [DOI] [PubMed] [Google Scholar]
- 31.Dos Santos Jaques JA, et al. , Activities of enzymes that hydrolyze adenine nucleotides in lymphocytes from patients with rheumatoid arthritis. Cell Biochem Funct, 2013. 31(5): p. 395–9. [DOI] [PubMed] [Google Scholar]
- 32.Cockerham LR, et al. , Programmed death-1 expression on CD4(+) and CD8(+) T cells in treated and untreated HIV disease. AIDS, 2014. 28(12): p. 1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronstein BN and Sitkovsky M, Adenosine and adenosine receptors in the pathogenesis and treatment of rheumatic diseases. Nat Rev Rheumatol, 2017. 13(1): p. 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mascanfroni ID, et al. , Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med, 2015. 21(6): p. 638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta V, et al. , CD39 positive regulatory T cell frequency as a biomarker of treatment response to methotrexate in rheumatoid arthritis. Int J Rheum Dis, 2018. 21(8): p. 1548–1556. [DOI] [PubMed] [Google Scholar]
- 36.Varani K, et al. , A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res Ther, 2011. 13(6): p. R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glaesener S, et al. , Distinct effects of methotrexate and etanercept on the B cell compartment in patients with juvenile idiopathic arthritis. Arthritis Rheumatol, 2014. 66(9): p. 2590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McComish J, et al. , Changes in peripheral blood B cell subsets at diagnosis and after treatment with disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: correlation with clinical and laboratory parameters. Int J Rheum Dis, 2015. 18(4): p. 421–32. [DOI] [PubMed] [Google Scholar]
- 39.Souto-Carneiro MM, et al. , Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res Ther, 2009. 11(3): p. R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weyand CM, Zeisbrich M, and Goronzy JJ, Metabolic signatures of T-cells and macrophages in rheumatoid arthritis. Curr Opin Immunol, 2017. 46: p. 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeisbrich M, et al. , Hypermetabolic macrophages in rheumatoid arthritis and coronary artery disease due to glycogen synthase kinase 3b inactivation. Ann Rheum Dis, 2018. 77(7): p. 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasko G and Pacher P, Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol, 2012. 32(4): p. 865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aletaha D, et al. , 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum, 2010. 62(9): p. 2569–81. [DOI] [PubMed] [Google Scholar]
- 44.Prevoo ML, et al. , Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum, 1995. 38(1): p. 44–8. [DOI] [PubMed] [Google Scholar]
- 45.Onofrio LI, et al. , Inhibitory Receptor Expression on T Cells as a Marker of Disease Activity and Target to Regulate Effector Cellular Responses in Rheumatoid Arthritis. Arthritis Rheumatol, 2018. 70(9): p. 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trickett A and Kwan YL, T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods, 2003. 275(1–2): p. 251–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Frequencies of CD39+ and CD73+ on peripheral blood B cell subsets from RA patients following different treatments. Graphs show frequencies of CD73+ (upper) and CD39+ B cells (lower) from HC, untreated or treated RA patients. Data are expressed as mean ± SEM. One-way ANOVA followed by a Bonferroni’s post-test were used.
Supplementary Figure 2. Comparison of CD39 and CD73 expression between primarily memory, immature and mature B cells from RA patients following different treatments. Graphs show MFI of CD39 (upper) and CD73 (lower) B cell subsets from HC, untreated or treated RA patients. Data are expressed as mean ± SEM. Patients treated with csDMARDs are indicated DMARDs. *p<0.05, **p<0.01, ***p<0.001. p values were determined using the Student’s t-test for unpaired simples.
Supplementary Figure 3: Levels of cytokines in the supernatant of B cells incubated with CD4+ T cells. Concentrations of A) TNF and B) IL-17 determined by ELISA in the supernatants of co-cultures of B cell and CD4+ T cells from untreated RA patients described in Figure 2. *p<0.05 **p< 0.005. p values were determined using the Student’s t-test for unpaired samples. ns=non-significant.
Supplementary Figure 4. CD39 and CD73 expression on total B cells and B cell subsets from R- and NR-RA patients. A) Graphs show MFI of CD39, B) MFI of CD73 and C) Frequency of CD73+CD39+ cells in the total B cell population and B cell subsets (memory, mature and immature B cells) from responder and non-responder RA patients at baseline (0) and after 3 months of treatment (3). Data are expressed as mean ± SEM. *p<0.05. P value was determined using the Student’s t-test for paired samples.
Supplementary Figure 5: Relation between different parameters involved in progression of RA and intensity of CD39 in total B cells. Intensity of CD39, determined as MFI, on total B cells at baseline (time 0) and after 3 months (time 3) of treatment in good responder (R) patients is shown together with A) DAS28, B) levels of serum RF and C) anti-CCP antibodies. *p<0.05, *** p<0.001. p values were determined using the Student’s t-test for paired samples. ns=non-significant
Supplementary Figure 6: Flow cytometry gating strategy for the analysis of total B cells A) Selection of the lymphocyte population from FSC-A vs SSC-A contour plot. B) Exclusion of doublets by gating in the diagonal between FSC-H vs FSC-A. C) Contour plot showing the expression of CD4 and CD8 after gating A and B. Negative population for CD4 and CD8 expression was selected and D) a contour plot of CD19 vs CD4 shows the gate for total B cells as CD19+ CD4neg CD8neg. Dot plots were performed with cells from HC as an example.