Abstract
The COVID-19 pandemic highlighted a wide range of public health system challenges for infectious disease surveillance. The discovery that the SARS-CoV-2 virus was shed in feces and can be characterized using PCR-based testing of sewage samples offers new possibilities and challenges for wastewater surveillance (WWS). However, WWS standardization of practices is needed to provide actionable data for a public health response. A workshop was convened consisting of academic, federal government, and industry stakeholders. The objective was to review WWS sampling protocols, testing methods, analyses, and data interpretation approaches for WWS employed nationally and identify opportunities for standardizing practices, including the development of documentary standards or reference materials in the case of SARS-CoV-2 surveillance. Other WWS potential future threats to public health were also discussed. Several aspects of WWS were considered and each offers the opportunity for standards development. These areas included sampling strategies, analytical methods, and data reporting practices. Each of these areas converged on a common theme, the challenge of results comparability across facilities and jurisdictions. For sampling, the consensus solution was the development of documentary standards to guide appropriate sampling practices. In contrast, the predominant opportunity for analytical methods was reference material development, such as PCR-based standards and surrogate recovery controls. For data reporting practices, the need for establishing the minimal required metadata, a metadata vocabulary, and standardizing data units of measure including measurement threshold definitions was discussed. Beyond SARS-CoV-2 testing, there was general agreement that the WWS platform will continue to be a valuable tool for a wide range of public health threats and that future cross-sector engagements are needed to guide an enduring WWS capability.
Keywords: Wastewater surveillance, COVID-19, SARS-CoV-2, Standards, Public health
1. Introduction
The COVID-19 pandemic brought unprecedented challenges and exposed gaps in public health surveillance across the globe. The spread of the SARS-CoV-2 virus placed strains on public health agencies, commercial laboratories, and hospitals, and researchers around the world sought innovative ways to track, treat, and prevent the disease. The need for a reliable, scalable system for detecting pathogens early and continuously set the stage for massive experimentation. The discovery that many of those infected with SARS-CoV-2 also shed the virus in feces [1] which could be recovered from sanitary sewer systems reminded the public health community that wastewater monitoring could be a valuable tool for infectious disease surveillance. This practice, commonly referred to as wastewater surveillance (WWS), is not a new field; however, the benefits of WWS in infectious disease surveillance became more widely appreciated during the COVID-19 pandemic. This is demonstrated by the fact that a search for publications with titles that included the terms “wastewater surveillance” or “wastewater based epidemiology” resulted in 906 publications from 2020 to the present (search on Dimensions.ai July 8, 2022). In comparison, there were only 188 publications with those terms in the entire previous decade (2010–2019). Prior to the COVID-19 pandemic, one of the most notable use cases for WWS for infectious disease applications is in tracking poliovirus [[2], [3], [4]]. WWS has also been used to track a handful of pathogens, primarily those that are transmitted via the fecal-oral route [[5], [6], [2], [7], [3], [8], [4]]. WWS is not limited to infectious disease, another notable use case is to survey community pharmaceutical and opioid usage in support of the public health response to the opioid crisis [9,10]. In comparison to other surveillance efforts, WWS does not depend on individuals to take a clinical test and report results nor show symptoms. As a pooled community sample, wastewater testing is non-invasive, unbiased, anonymous, and less expensive than testing many individuals. Moreover, detection of SARS-CoV-2 in wastewater can be an early indicator of disease spread within a community, with increases in wastewater detected prior to clinical increases in infections [11,12]. However, there are no standardized practices for WWS, leading to a lack of comparability within and among programs. In June of 2020, the National Institute of Standards and Technology (NIST) hosted a workshop entitled: “A NIST-Hosted Webinar on Measuring SARS-CoV-2 in Wastewater and Fecal Material: A Call for Standards,” which framed some of the issues and challenges government and industry would need to consider as WWS and related sewage system constituent analysis programs were getting started [13]. Since then, many comprehensive reviews [[14], [15], [16]] of the methodologies and their results remind us that without convergence on standardized practices, we are limited in the ability to compare findings from one location to another or even at different points in time at the same location, which limits the extent to which the data can be used to support the public health response. WWS data can support public health in several ways including guiding public health investigation or intervention, measuring the burden of disease to monitor trends and identify high-risk populations, supporting early detection and identification of outbreaks and/or emerging health concern, and guiding programs to prevent and/or control disease ([17]). Regardless of the objective, the success of public health surveillance efforts rely on obtaining quality data that are complete, accurate, and timely. Even with the rapid increase in public reporting SARS-CoV-2 levels in wastewater, data are accessible but easily not comparable.
Standardized practices for WWS create an infrastructure to assure high confidence measurements and consists of many components, including standards and measurement services. Standards can be delivered in a variety of forms. For instance, documentary standards are written documents typically developed by consensus among a defined group. Documentary standards can be highly prescriptive with clearly stated methodologies to follow and performance criteria to meet. However, documentary standards can also include general guidance and best practice information. The type of documentary standards appropriate for a defined application will depend on the readiness level of the field. For instance, SARS-CoV-2 WWS is an evolving field and may likely benefit more from documents such as a standards development roadmap and standard guidance versus performance standards or validated standard test methods. Reference materials are another type of standard consisting of physical preparations certified to be homogeneous, stable, and fit for a defined purpose. They are often used as calibrants and controls. Other types of standards include reference datasets, reference instruments, and reference methods. Measurement services also play an important role in achieving high quality and scientifically defensible results. Common services include instrument and device calibrations, material measurement services, and Quality Assurance Programs (QAPs). A set of complementary standards and measurement services is typically needed to create a robust infrastructure for a given application space.
To address the need for standardized practices in WWS, a workshop was held to convene stakeholders from federal government agencies, academic, and industry. The agenda included reviewing the wide range of approaches implemented in WWS, discussing measurement challenges, and considering opportunities for development of standards, with a focus on documentary standards and reference materials to support WWS for SARS-CoV-2 and future targets of interest. The genesis for this workshop came through the convergence of several factors: 1) WWS efforts coordinated through the Centers for Disease Control and Prevention (CDC) and other organizations demonstrated the utility of WWS for tracking the prevalence of the SARS-CoV-2 virus in wastewater; 2) WWS can be used to track community prevalence of other pathogens or chemicals of concern, as well as biomarkers of human health; 3) the WWS infrastructure and capability for tracking SARS-CoV-2 provides momentum for transitioning to other public health threats; and 4) standardized practices could help underpin and sustain viable WWS capability that could rapidly be focused to address future concerns.
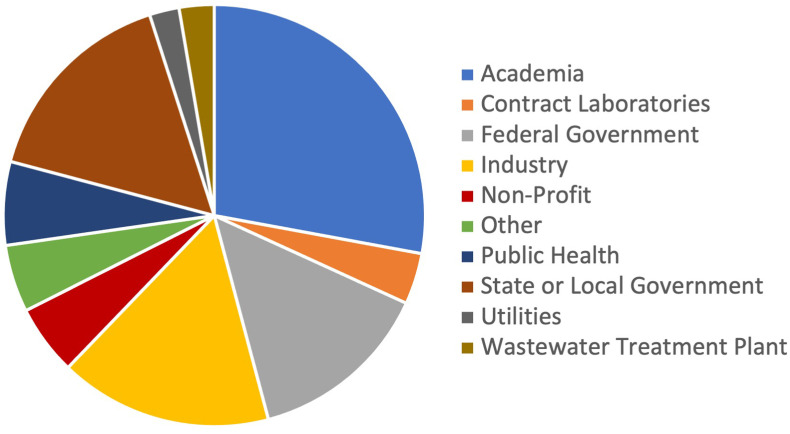
The virtual workshop entitled “Standards to Support an Enduring Capability in Wastewater Surveillance for Public Health” was hosted by the U.S. Department of Homeland Security Science and Technology Directorate and NIST in June 2021 with over 500 attendees (Fig. 1 ).
Fig. 1.
Breakdown of organizations represented by workshop registrants (n = 607).
Workshop documentation and recordings are available online [18], and a full report of the proceedings was published in July 2022 [19]. The following review will summarize key information discussed during the workshop focusing on wastewater sampling and testing methods; challenges of SARS-CoV-2 detection in wastewater; and WWS data reporting, analytics, and use as it pertains to standards development. While much of the workshop discussion was focused on detection of the SARS-CoV-2 pathogen, the conference delegates recognized that a need exists for an enduring capability that extends to other identifiers of population health risk and status (e.g., other pathogens, illicit and prescribed drugs, toxins, antimicrobial resistance genes, and indicators of human physiologic dysfunction). Thus, some standardized practice recommendations are offered towards building an enduring WWS capability.
2. Discussion
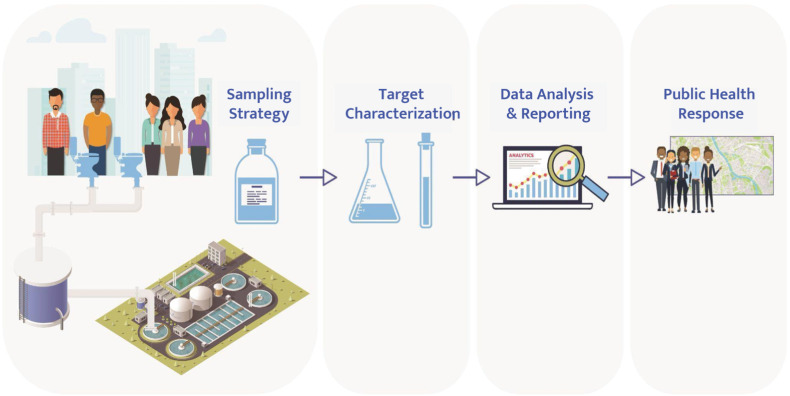
Each step of the WWS workflow from sample collection to the public health response can introduce variability that hinders comparable, reproducible, and quantitative data (Fig. 2 ).
Fig. 2.
Wastewater surveillance (WWS) workflow illustrating sampling strategy, target characterization (included testing methods), data analysis and reporting, and public health response steps.
A workshop poll showed that 93% of the 56 poll respondents see a need for the development of standards to support an enduring capability in WWS. To address these issues and identify appropriate standards, the workshop was organized into three broad topics: wastewater sampling strategies; analytical methods; and data reporting, analytics, and use. Using this framework, the main challenges in each topic area were identified and the proposed standards to support a WWS capability that persists beyond the COVID-19 pandemic are summarized below.
2.1. Wastewater sampling strategies
A first step in WWS is sampling strategy which includes site selection and sampling type (Fig. 2). Site selection must address the relationship between the population of interest, sampling site location [20], and sample type [[21], [22], [23]]. As a result, wastewater sampling practices have become an active and important research area. While sample collection may appear to be straightforward, in practice this seemingly simple step has many potential variations that could be improved with the application of documentary standards and reference materials. To learn more about these challenges, this workshop session included: two presentations, one case study, two rapid-fire presentations, and an expert panel discussion. Wastewater sampling parameters that were discussed included sample location (e.g., wastewater treatment plant, manhole), sample type (e.g., solids, liquid), sampling method (e.g., grab, composite), and sample handling (e.g., storage temperature, preservative addition). Workshop participants pointed out that a major challenge to the harmonization of sample collection is the diversity of wastewater infrastructure. This diversity is evident across wastewater treatment facilities that can vary in terms of size, design, and sampling access points. The source (e.g., residential, commercial, industrial, etc.) of waste streams deposited into collection systems of wastewater treatment facilities can also pose challenges. For example, some facilities have nearby hospitals that may contribute to increased WWS target concentrations. Additionally, inflow and infiltration can increase wastewater flows and contribute to decreased WWS target concentrations. The diversity of wastewater treatment facilities and corresponding waste stream sources make it very unlikely that a one-size-fits-all approach will be appropriate. In addition, approaches may be necessary to include dwellings that are not connected to centralized sewer systems, which the United States Environmental Protection Agency estimates to be one in five United States households[24].
The expert discussion panel pointed out that, given the breadth of sampling methods required to meet the diverse needs of all sewer systems, identifying appropriate standards and control materials becomes especially important for facilitating comparison of data. For wastewater sampling, the workshop consensus solution was the development of a documentary standard to guide appropriate sampling practices based on site type and sample characteristics. This document would encourage standardization for specific steps in the process such as safety protocols, storage time and conditions (holding times and storage temperatures), sample type (wastewater or settled solids), sampling frequency, sampling location, as well as packaging and transportation. The document should also provide guidance on metadata collection (e.g., storage time and temperature, flow rate, sample type, frequency, location, percent solids, pH, etc.) and how to handle any potential ethical considerations about what data are collected and how identifiable any data are (or could be in the future) to the population served. While workshop participants identified documentary standards as a primary need, the development of a mock wastewater control sample, such as a 24-h composite untreated wastewater matrix or a surrogate spike reference material to add to wastewater samples also received support. A summary of the challenges and recommendations is presented in Table 1 .
Table 1.
Summary of challenges and recommendations identified at the workshop.
| Workflow Step | Challenges | Recommendations |
|---|---|---|
| Wastewater Sampling Strategies |
|
|
| Analytical Methods |
|
|
| Data Reporting, Analytics and Use |
|
|
2.2. Analytical methods
This session focused on implementation experiences, research, and technologies for SARS-CoV-2 testing methods in wastewater. Since the onset of the COVID-19 pandemic, numerous laboratory testing methods have been developed and adopted for WWS. At least 36 standard operating procedures are in use across the world for wastewater and settled solids [25]. The rapid implementation of these methods combined with consumables’ supply challenges has limited opportunities to optimize test performance and standardize protocols. This lack of standardization has led to considerable variation among laboratories [26] and contributes to an emphasis on local data trend interpretations (single laboratory) rather than comparisons across laboratories. Three presentations, an expert panel discussion, workshop participant polling questions, and a rapid-fire session to showcase new technologies were included in this session to address the need for standards in analytical methods.
Method availability, consistency, and comparability are critical attributes of a successful WWS program, and the current lack of standardization confounds the ability to directly compare results generated from different protocols and laboratories. As a result, it was not surprising that in a workshop poll, 57% of 56 respondents identified testing methods as the step in the WWS workflow (Fig. 2) in most need of standards development. However, it was agreed that a single protocol may not be feasible for SARS-CoV-2 WWS and will likely not be useful across all testing laboratories due to variability in wastewater composition, supply chain constraints, and different sampling practices. Instead, workshop participants recommended the development of strategies and tools allowing for the comparison of different analytical methods. Possible steps to achieve comparability included the use of universal definitions for limit of detection and quantification as well as establishing a core set of laboratory testing quality control elements. Others suggested the development of a guidance framework similar to the Minimum Information for Publication of Quantitative Real-Time PCR Experiments for real-time quantitative PCR (qPCR) [27,28] and digital PCR (dPCR) [[29], [30]] The need for WWS laboratory accreditation requirements was also discussed to ensure laboratories have suitable QAPs and can consistently generate reliable results.
Multiple suggestions were also offered for the development of reference materials to serve as common controls used across locations to enable comparisons and improve SARS-CoV-2 testing methodologies. The need to measure the amount of human fecal waste in a wastewater sample for normalization purposes was discussed, with human-associated microbial source tracking methodologies suggested as a potential solution. Workshop polling (n = 44) identified the pepper mild mottle virus (43%) [[31], [32], [33]] and then bacteriophage (16%) as the recommended genetic marker associated with human fecal material; thus, a reference material for one of these targets may find immediate use. Additionally, 60% of respondents indicated a wastewater matrix reference material would be valuable, while another 21% responded “maybe” (n = 53). Sixty-five percent of respondents preferred real wastewater, whereas 24% preferred a synthetic wastewater with defined components (n = 46). In a follow-up poll focused on reference materials, respondents (n = 18) ranked matrix-based reference materials based on which would be most useful to develop; 24-h composite untreated wastewater was top ranked (average rank: 5 out of 6), followed by synthetic wastewater (3.3), 24-h composite treated wastewater (3.0), and pooled fecal material (2.9). The expert panel also pointed out that nucleic acid recovery and PCR-based amplification controls are needed to identify potential wastewater sample matrix interferences that can bias WWS target measurements. Finally, the need for improved statistical data analyses approaches was also discussed to estimate more accurately the uncertainty in WWS measurements. A summary of the challenges and recommendations is presented in Table 1.
This session also included a rapid-fire activity for industry participants to showcase new technologies and services to support SARS-CoV-2 WWS. Industry can play an important role in the implementation of testing practices, as commercialization through the development of test kits and service laboratories promotes protocol and data reporting standardization. Several technologies were described including GeneCount® SARS-CoV-2 Wastewater Test Kit (LuminUltra Technologies Ltd; New Brunswick, Canada), the Water SARS-CoV-2 RT-qPCR Test (IDEXX Laboratories, Inc; Maine, USA), the QIAcuity Digital PCR System (QIAGEN; Germantown, USA), the BioFire® COVID-19 Test Kit (BioFire® Defense; Salt Lake City, USA), Nanotrap (Ceres Nano; Manassas, USA), and SARS-CoV-2 Variant Profiling Service (Pangolin Health).
2.3. Data reporting, analytics, and use
WWS data is only useful to end users if there is confidence in the results and the ability to interpret findings improves the public health response. The large-scale surveillance efforts necessitated by the COVID-19 pandemic led to the generation of massive amounts of population-level data that needed to be analyzed and communicated in an effective manner [34]. This rapid implementation of SARS-CoV-2 tracking revealed several challenges for the WWS community, such as dissemination of data in a timely manner. This section summarizes two sessions, with the first including presentations on several use-cases and platforms from industry, academia, and government sectors [[35], [36], [37]]. Notably, the first session included a CDC presentation on the National Wastewater Surveillance System Data Collation and Integration for Public Health Event Responses platform. The second session included a keynote presentation on findings from an expert panel convened by the Communicating Sewage Surveillance for COVID-19 project [[38], [39]]. Presentations in both sessions were followed by panel discussions and participant polling during breakout session groups.
Similar to other sessions, the first challenge articulated was data comparability. There was a general lack of interoperability between different WWS reports due primarily to the use of variable units of measure. For example, some groups report normalized SARS-CoV-2 concentrations using wastewater flow or human fecal waste concentration estimates, while others report unadjusted values. Another key challenge identified by workshop participants was data interpretation. Public health officials were faced with unfamiliar data from advanced molecular methodologies with limited information on how to integrate this data with other sources of information such as local case and clinical testing results. Data interpretation and communication are often further complicated by inconsistent data and uncertainty in reporting practices of WWS measurements (Wade et al., 2022). During panel discussions, challenges in database flexibility to accommodate new data types were also identified. Using SARS-CoV-2 as an example, the early focus was on viral concentration (quantitative data), but as the pandemic continued, a desire to include variant tracking (qualitative data) emerged. The ability for a database system to handle both qualitative and quantitative data types will be critical for building an enduring WWS capability.
Several recommendations were made by workshop participants to address these data reporting practices challenges including standardizing data reporting practices rather than developing a single data system platform (e.g., one dashboard), and setting standards for each data type as well as guidance for documenting metadata. While exact specifications were not discussed, a general path forward was outlined to include minimal required metadata, standardized metadata vocabulary, and a standardized data input with unit of measure with threshold definitions. There was also support for the incorporation of standards and metadata to facilitate comparability (e.g., normalization controls, matrix spike recovery controls, detection protocols used). This response was reiterated in a poll question that asked for “suggestions on a universal data output for comparing data.” Here, the most common responses included reporting the WWS target concentration normalized to a human-associated genetic marker (9/16) and the need for the inclusion of metadata to help interpret results (4/16). It was also agreed that database dashboards do not need to be standardized. Instead, guidance outlining best practices including use of colors, downloadable data, video explanations, and options for further improvements of technical communication would be helpful. A summary of the challenges and recommendations is presented in Table 1.
2.4. Promising areas for standards/guidance to build an enduring capability
WWS is now routinely used around the world to assess the state of the current COVID-19 pandemic. Much has been learned and should be documented and leveraged to strengthen a WWS capability, both in continued response to COVID-19 and for future surveillance needs. The compelling need for standards to support this capability building was reiterated at the workshop by speakers, panelists, and attendees. They clearly indicated that reference materials and guidance documents are an appropriate starting point for standards development activities.
Since WWS can be used to track community prevalence of many public health targets, the adoption of practices that could be universally implemented for a variety of applications could help facilitate standardization and rapid deployment. As every WWS program must consider site selection and sample collection, documentary standards that organize best practices for these universal activities into guidance documents can serve to harmonize WWS workflows, as well as help practitioners avoid common pitfalls. Additionally, most attendees supported estimating the amount of human fecal material in a wastewater sample to normalize WWS target measurements. Standardized analytical methods to determine human fecal concentrations in wastewater using one or more human-associated fecal markers are needed. Since quantification of human-associated fecal markers often rely on PCR-based protocols, reference material for qPCR standard curves and dPCR positive controls could help reduce variability in concentration estimates and promote standardization of human fecal concentration estimations. Attendees were also supportive of a reference wastewater matrix to serve as a control sample when developing, comparing, and validating sample processing and WWS target detection protocols. While this reference material would not represent all wastewaters, it could serve as a benchmark to allow comparability studies and demonstration of detection capabilities, promote consistency among laboratories, and improve confidence in data. Standardized analytical methods to recover and quantify specific public health targets in wastewater are also needed. These methods would include appropriate and representative surrogates that can be used to assess recovery efficiency of sample processing methods as well as specific control material to use for standard curve generation (qPCR) or positive controls (dPCR).
3. Conclusions and outlook
Workshop presentations and panel discussions identified multiple factors to consider for the future development of standards for WWS applications. First, many standards can take at least one to two years to manufacture, certify, and distribute. It will thus be necessary to understand and anticipate the needs of the WWS community to ensure standards are useful and relevant when available to practitioners. It may be necessary to establish working groups that meet on a routine basis to assure the groups developing standards continue to meet practitioner needs. Second, the wide range of factors considered at this workshop were primarily focused on the detection and quantification of SARS-CoV-2. An enduring approach to WWS will need to consider a broader range of public health targets (e.g., viruses, bacteria, protozoans, and toxins), human waste types (e.g., feces and urine), and detection methodologies (e.g., genomic analysis). Standards development for chemical targets such as pharmaceuticals, toxins, and health biomarkers should also be considered. Also, education on the benefits of standards for researchers, practitioners, and the public will be important. For example, practitioners can be deterred from documentary standards because they believe prescribed practices will be too restrictive and prevent the generation of useful data. However, in practice, documentary standards elevate the quality of data and provide a framework for communicating findings resulting in increased confidence. In addition, a shared knowledge of the standards development process and a better understanding of how the type of standard corresponds to the technology readiness level is needed. For example, biomolecular WWS practices may not yet be ready for method standardization, but they could benefit from documentary standards focused on guidance and best practices allowing for improved data quality and reporting ultimately accelerating the method development process. Finally, given the interdisciplinary nature of the WWS field, the path forward will benefit from a “community” approach that engages cross-sector expertise, including government, academia, and industry.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclaimers
Information has been subjected to U.S. EPA peer and administrative review and has been approved for external publication. Any opinions expressed in this paper are those of the authors and do not necessarily reflect the official positions and policies of the U.S. EPA. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use. These opinions, recommendations, findings, and conclusions do not necessarily reflect the views or policies of NIST or the United States Government. Certain commercial equipment, instruments, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identification is not intended to imply recommendation or endorsement by NIST, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose. The research in this presentation was conducted with the U.S. Department of Homeland Security (DHS) Science and Technology Directorate (S&T) under contract FTST-21-FT005A. Any opinions contained herein are those of the author and do not necessarily reflect those of DHS S&T. While specific manufacturers of commercial products are presented in this document, this is not an official endorsement of any of these brands or models but serves as examples of the types of models considered appropriate for this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to acknowledge the additional members of the Workshop Organizing Committee: Michael Focazio, Sally Gutierrez, Scott Jackson, Amy Kirby, Katrice Lippa, Mia Mattioli, Yonas Nebiyeloul-Kifle, Kent Prinn, Renee Stevens, Paul Storella, and Sarah Wright.
References
- 1.Wang X., Zheng J., Guo L., Yao H., Wang L., Xia X., Zhang W. Fecal viral shedding in COVID-19 patients: clinical significance, viral load dynamics and survival analysis. Virus Res. 2020;289 doi: 10.1016/j.virusres.2020.198147. 198147-198147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ivanova O.E., Yarmolskaya M.S., Eremeeva T.P., Babkina G.M., Baykova O.Y., Akhmadishina L.V., Krasota A.Y., Kozlovskaya L.I., Lukashev A.N. Environmental surveillance for poliovirus and other enteroviruses: long-term experience in Moscow, Russian Federation, 2004-2017. Viruses. 2019;11(5):424. doi: 10.3390/v11050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Más Lago P., Gary H.E., Jr., Pérez L.S., Cáceres V., Olivera J.B., Puentes R.P., Corredor M.B., Jímenez P., Pallansch M.A., Cruz R.G. Poliovirus detection in wastewater and stools following an immunization campaign in Havana, Cuba. Int. J. Epidemiol. 2003;32(5):772–777. doi: 10.1093/ije/dyg185. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Vaccines and Biologicals; 2003. Guidelines for Environmental Surveillance of Poliovirus Circulation. [Google Scholar]
- 5.Brinkman N.E., Fout G.S., Keely S.P. Retrospective surveillance of wastewater to examine seasonal dynamics of enterovirus infections. mSphere. 2017;2(3) doi: 10.1128/mSphere.00099-17. e00099-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmér M., Paxéus N., Magnius L., Enache L., Arnholm B., Johansson A., Bergström T., Norder H., Schaffner D.W. Detection of pathogenic viruses in sewage provided early warnings of Hepatitis A virus and Norovirus outbreaks. Appl. Environ. Microbiol. 2014;80(21):6771–6781. doi: 10.1128/AEM.01981-14. https://pubmed.ncbi.nlm.nih.gov/25172863/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lugo D., Krogstad P. Enteroviruses in the early 21st century: new manifestations and challenges. Curr. Opin. Pediatr. 2016;28(1):107–113. doi: 10.1097/MOP.0000000000000303. https://pubmed.ncbi.nlm.nih.gov/26709690/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao Z., Wang Z., Lin X., Wang S., Wang H., Yoshida H., Xu A., Song Y. One-year Survey of human enteroviruses from sewage and the factors affecting virus adsorption to the suspended solids. Sci. Rep. 2016;6 doi: 10.1038/srep31474. 31474-31474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cattell L., Reeves W., Vallance J., Thornton C. 2016. The Potential of Wastewater Testing for Rapid Assessment of Opioid Abuse (Research Brief) 2016. [Google Scholar]
- 10.Duvallet C., Hayes B., Erickson T., Chai P., Matus M. Mapping community opioid exposure through wastewater-based epidemiology as a means to engage pharmacies in harm reduction efforts. Prev. Chronic Dis. 2020;17 doi: 10.5888/pcd17.200053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olesen S.W., Imakaev M., Duvallet C. Making waves: defining the lead time of wastewater-based epidemiology for COVID-19. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peccia J., Zulli A., Brackney D.E., Grubaugh N.D., Kaplan E.H., Casanovas-Massana A., Ko A.I., Malik A.A., Wang D., Wang M., et al. SARS-CoV-2 RNA concentrations in primary municipal sewage sludge as a leading indicator of COVID-19 outbreak dynamics. medRxiv. 2020 2020.2005.2019.20105999. [Google Scholar]
- 13.National Institute of Standards and Technology . 2020. A NIST-Hosted Webinar on Measuring SARS-CoV-2 in Wastewater and Fecal Material: A Call for Standards. [Google Scholar]
- 14.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140444. 140444-140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah S., Gwee S.X.W., Ng J.Q.X., Lau N., Koh J., Pang J. Wastewater surveillance to infer COVID-19 transmission: a systematic review. Sci. Total Environ. 2021;804 doi: 10.1016/j.scitotenv.2021.150060. 150060-150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran H.N., Le G.T., Nguyen D.T., Juang R.S., Rinklebe J., Bhatnagar A., Lima E.C., Iqbal H.M.N., Sarmah A.K., Chao H.P. SARS-CoV-2 coronavirus in water and wastewater: a critical review about presence and concern. Environ. Res. 2021;193 doi: 10.1016/j.envres.2020.110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groseclose S.L., Buckeridge D.L. Public health surveillance systems: recent advances in their use and evaluation. Annu. Rev. Publ. Health. 2017;38(1):57–79. doi: 10.1146/annurev-publhealth-031816-044348. [DOI] [PubMed] [Google Scholar]
- 18.National Institute of Standards and Technology . National Institute of Standards and Technology; 2021. DHS/NIST workshop: standards to support an enduring capability in wastewater surveillance for public health.https://www.nist.gov/news-events/events/2021/06/dhsnist-workshop-standards-support-enduring-capability-wastewater [Google Scholar]
- 19.Lin N.J., Servetas S.L., Jackson S.A., Lippa K.A., Parratt K.H., Mattson P., Beahn C., Gutierrez S., Focazio M., Smith T., et al. NIST Special Publication. National Institute of Standards and Technology; 2022. Report on the DHS/NIST Workshop on Standards for an Enduring Capability in Wastewater Surveillance for Public Health (SWWS Workshop) [DOI] [Google Scholar]
- 20.Yeager R., Holm R., Saurabh K., Fuqua J., Talley D., Bhatnagar A., Smith T. Wastewater sample site selection to estimate geographically resolved community prevalence of COVID-19: a sampling protocol perspective. GeoHealth. 2021;5 doi: 10.1029/2021GH000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed W., Bivins A., Bertsch P.M., Bibby K., Choi P.M., Farkas K., Gyawali P., Hamilton K.A., Haramoto E., Kitajima M., et al. Surveillance of SARS-CoV-2 RNA in wastewater: methods optimization and quality control are crucial for generating reliable public health information. Curr. Opin. Environ. Sci. Health. 2020;17:82–93. doi: 10.1016/j.coesh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castiglioni S., Bijlsma L., Covaci A., Emke E., Hernández F., Reid M., Ort C., Thomas K.V., van Nuijs A.L.N., de Voogt P., et al. Evaluation of uncertainties associated with the determination of community drug use through the measurement of sewage drug biomarkers. Environ. Sci. Technol. 2013;47(3):1452–1460. doi: 10.1021/es302722f. [DOI] [PubMed] [Google Scholar]
- 23.Ort C., Lawrence M.G., Rieckermann J., Joss A. Sampling for pharmaceuticals and personal care products (PPCPs) and illicit drugs in wastewater systems: are your conclusions valid? A critical review. Environ. Sci. Technol. 2010;44(16):6024–6035. doi: 10.1021/es100779n. [DOI] [PubMed] [Google Scholar]
- 24.United States Environmental Protection Agency Septic Systems Overview. 2022. https://www.epa.gov/septic/septic-systems-overview
- 25.Pecson B.M., Darby E., Haas C.N., Amha Y.M., Bartolo M., Danielson R., Dearborn Y., Di Giovanni G., Ferguson C., Fevig S., et al. Vol. 7. 2021. Reproducibility and Sensitivity of 36 Methods to Quantify the SARS-CoV-2 Genetic Signal in Raw Wastewater: Findings from an Interlaboratory Methods Evaluation in the U.S. Environmental Science: Water Res. Technol. pp. 504–520. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips P. Abnormal laboratory results: pitfalls in interpreting laboratory results. Aust. Prescr. 2009;32:43–46. [Google Scholar]
- 27.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 28.Taylor S.C., Nadeau K., Abbasi M., Lachance C., Nguyen M., Fenrich J. The ultimate qPCR experiment: producing publication quality, reproducible data the first time. Trends Biotechnol. 2019;37(7):761–774. doi: 10.1016/j.tibtech.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Huggett J.F., The dMIQE Group The digital MIQE guidelines update: minimum information for publication of quantitative digital PCR experiments for 2020. Clin. Chem. 2020;66(8):1012–1029. doi: 10.1093/clinchem/hvaa125. [DOI] [PubMed] [Google Scholar]
- 30.Borchardt M.A., Boehm A.B., Salit M., Spencer S.K., Wigginton K.R., Noble R.T. The environmental microbiology minimum information (EMMI) guidelines: qPCR and dPCR quality and reporting for environmental microbiology. Environ. Sci. Technol. 2021;55(15):10210–10223. doi: 10.1021/acs.est.1c01767. [DOI] [PubMed] [Google Scholar]
- 31.Filipić A., Dobnik D., Tušek Žnidarič M., Žegura B., Štern A., Primc G., Mozetič M., Ravnikar M., Žel J., Gutierrez Aguirre I. Inactivation of pepper mild mottle virus in water by cold atmospheric plasma. Front. Microbiol. 2021;12(14) doi: 10.3389/fmicb.2021.618209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosario K., Symonds E.M., Sinigalliano C., Stewart J., Breitbart M. Pepper mild mottle virus as an indicator of fecal pollution. Appl. Environ. Microbiol. 2009;75(22):7261–7267. doi: 10.1128/AEM.00410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Symonds E.M., Rosario K., Breitbart M. Pepper mild mottle virus: agricultural menace turned effective tool for microbial water quality monitoring and assessing (waste)water treatment technologies. PLoS Pathog. 2019;15(4) doi: 10.1371/journal.ppat.1007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keshaviah A., Hu X.C., Henry M. Developing a flexible national wastewater surveillance system for COVID-19 and beyond. Environ. Health Perspect. 2021;129(4) doi: 10.1289/EHP8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Center for Disease Control and Prevention National Wastewater Surviellance System. 2021. https://www.cdc.gov/healthywater/surveillance/wastewater-surveillance/wastewater-surveillance.html
- 36.Biobot Analytics Nationwide Wastewater Monitoring Network. 2021. https://biobot.io/data
- 37.UC Merced Researchers COVIDPoops19. 2021. https://www.arcgis.com/apps/dashboards/c778145ea5bb4daeb58d31afee389082
- 38.CoSeS Communicating Sewage Surveillance. 2022. https://sites.uwm.edu/coses/
- 39.McClary-Gutierrez J.S., Mattioli M.C., Marcenac P., Silverman A.I., Boehm A.B., Bibby K., Balliet M., de Los Reyes F.L., 3rd, Gerrity D., Griffith J.F., et al. SARS-CoV-2 wastewater surveillance for public health action. Emerg. Infect. Dis. 2021;27(9):1–8. doi: 10.3201/eid2709.210753. [DOI] [PMC free article] [PubMed] [Google Scholar]