Abstract
Objective
Evidence shows that booster shots offer strong protection against the Omicron variant of COVID-19. However, we know little about why individuals would receive a booster compared to the initial decision to vaccinate. We investigate and assess the factors that affect individuals' reported willingness to receive the COVID-19 vaccine booster. This information can aid in tailoring public health messaging to communicate attributes that are associated with individuals’ attitudes toward the COVID-19 booster.
Rationale
Existing research provides little insight into whether the same factors that affect Americans’ likelihood of accepting initial vaccination against COVID-19 also affect booster uptake. Our experiment also examines the influence of contextual information about a novel variant on willingness to receive a booster.
Methods
We administered a conjoint experiment (N = 2740 trials) in a survey of fully vaccinated US adults that had not yet received a COVID-19 booster (N = 548) to assess the impact of varied vaccine attributes on willingness to receive a booster.
Results
The most important factors associated with higher willingness to receive a booster were efficacy, manufacturer, and the size of a financial incentive. Protection duration and protection against future variants vs. only current variants had modest influence. A contextual prime reporting that some public health experts believe the Omicron variant is more contagious, but less lethal than those previously seen, significantly increased favorability toward boosters. This provides potential motivation and guidance for vaccination campaigns to emphasize these variant-specific traits.
Conclusion
With several vaccines with varying degrees of efficacy available to consumers, emphasizing boosters with a high efficacy would likely improve attitudes toward boosters. Financial incentives and predispositions toward manufacturers also matter. Concerns about more contagious variants may spur uptake, even if such variants are less lethal.
Keywords: COVID-19, Vaccine, Booster, Public acceptance, Conjoint experiment
1. Introduction
On November 19, 2021, with COVID-19 vaccinations plateauing, growing evidence that initial vaccine immunity was waning, and a new COVID-19 variant—Omicron—emerging, the United States expanded eligibility of the COVID-19 vaccine booster to the entire adult population. COVID-19 vaccine boosters offered an effective means of increased protection for the vaccinated population with studies revealing that the protection offered by COVID-19 vaccines had attenuated by about 8–15% (Bruxvoort et al., 2021), and infection risk increased with time from last vaccine dose (Goldberg et al., 2021). A 3-month difference in the administration of a previous COVID-19 dose was associated with a 2-fold increase in COVID-19 infection risk (Mizrahi et al., 2021). The COVID-19 death rate in populations with an additional COVID-19 vaccine dose was 0.16 per 100,000 compared with 2.89 for those without (Abbasi, 2022).
In response to the mounting evidence that boosters are a critical tool to protect against Omicron, governments embraced various policies to spur booster uptake. In Italy, for example, individuals were required to receive a booster shot within six months of completing their initial vaccination cycle to have the “super green pass,” which allowed access to bars, restaurants, cinemas, and cultural spaces, and vaccination, including boosters, became mandatory for those 50 and older. However, booster uptake continues to lag in many countries. As of May 2022, just over 46% of fully vaccinated Americans are estimated to have received a booster (CDC, 2022). As a result, understanding the basis of attitudes toward boosters is critical to accelerate lagging public health campaigns.
A growing literature has examined the factors associated with COVID-19 vaccine acceptance, including concerns about side effects and safety (Dror et al., 2020; Fisher et al., 2020; Freeman et al., 2021), trust in government and public health officials, and demographic characteristics such as age, gender, race/ethnicity, political partisanship/ideology, and educational attainment (Dror et al., 2021; Lin et al., 2021). Additional studies, employing a range of methodologies, have examined the influence of vaccine attributes themselves on public acceptance. These attributes include efficacy (Kaplan and Milstein, 2021; Kreps et al., 2020; Motta, 2021; Schwarzinger et al., 2021), technology (Dror et al., 2021; Motta, 2021), cost/financial incentives (Campos-Mercade et al., 2021; Carpio et al., 2021; Kreps et al., 2021), and manufacturer (Kreps et al., 2021).
While public attitudes on initial vaccine acceptance have been well-studied, the COVID-19 booster presents a novel setting where multiple doses may be required over time. The dynamics underlying willingness to receive a COVID-19 booster might differ from those underlying acceptance of the original shot and more closely resemble those for other boosters, regimented vaccines, or annually recommended vaccines. Research on boosters for tetanus and diphtheria have evaluated cross-national booster vaccination rates (Slifka et al., 2021), although such studies focus on national policies rather than public attitudes toward and uptake of these boosters. Others have studied regimented vaccines such as human papillomavirus (HPV) (de Bekker-Grob et al., 2010). However, the COVID-19 boosters vary from the HPV sequence because of the evolving nature of the COVID-19 virus (e.g., variants) and potential for waning immunity of the initial vaccine doses. COVID-19 boosters may share more of the behavioral and epidemiological characteristics of the influenza vaccine, for which demographics are thought to play an important role in uptake (de Bekker-Grob et al., 2018), except that the flu has a higher degree of predictable seasonality and less uncertainty regarding the transmissibility and virulence than COVID-19 variants.
While recent studies have examined the factors associated with public receptiveness toward COVID-19 boosters, focusing on demographics, trust, vaccine literacy, and safety fears (Lai et al., 2021; Lennon et al., 2021; Rzymski et al., 2021), these studies do not examine whether vaccine attributes examined in prior analyses of vaccine uptake have the same effect on booster acceptance. Moreover, findings from earlier studies may not translate into a new environment dominated by concerns about a novel variant. The evolution of the pandemic has introduced important new attributes that might affect vaccine acceptance, including whether a booster is likely to protect against future variants.
In this research, we deploy a choice-based experiment among 548 fully vaccinated but not yet boosted participants to understand the factors associated with greater willingness to receive a COVID-19 booster among adult Americans. We investigate how features of the booster, including efficacy, protection duration, likely protection against future variants, and manufacturer, and policy instruments such as financial incentives affect likelihood of receiving the booster. The timing of the survey was within weeks of the first detection of the Omicron variant. Combined with an experimental manipulation describing the possible features of the variant, this study offers a unique opportunity to examine booster preference formation in a moment of considerable scientific uncertainty.
2. Methods
2.1. Data
Between December 14–17, 2021, 2398 American adults were contacted and 2241 were successfully recruited to complete a 10-minute survey. We recruited respondents through Lucid, which uses quota-based sampling to approximate nationally representative samples in terms of demographics. As a result, the demographics of Lucid samples tend to more closely reflect the demographics of the nation as a whole, compared to, for example, MTurk samples, which tend to skew younger and more liberal and to under-represent blacks and Hispanics (Berinsky et al., 2012). Research has shown that randomized experimental effects are comparable to those observed in national probability surveys (Coppock and McClellan, 2019). Recent research on Lucid samples replicating earlier experiments also fielded via online quota-based samples found some evidence of increasing inattentiveness during the pandemic, and reduced effect sizes, but no fundamental threat to generalizability (Peyton et al., 2021). Please see Supplemental Table S1 for a comparison of the data used in this study to nationally representative benchmarks.
2.2. Conjoint experiment design
To evaluate the influence of stated preferences on vaccine attributes, we employed a conjoint experiment design (Hainmueller et al., 2014), which randomly varied five attributes, yielding 288 unique vaccine profiles (for a summary of all attributes and levels, see Supplemental Table S2). Conjoint experiments leverage randomization of attribute-levels and, in our study, multiple vaccine profiles (treatments) to provide causal estimates without a prohibitively large design.
After providing informed consent on the initial screen, respondents answered a question about their vaccination status: whether they had (i) chosen not to receive a vaccine, (ii) received the first of a two-shot sequence, or (iii) were fully vaccinated with one shot of the J&J/Janssen or two of the Pfizer or Moderna vaccine. Those who responded that they were fully vaccinated were then asked whether they had already received a COVID-19 booster.
From these initial screening questions, 548 respondents indicated that they had been fully vaccinated against COVID-19 but had not yet received a booster shot. Our main analyses focus on this group. We additionally gathered demographic information from respondents and Table 1 displays the composition of our study sample.
Table 1.
Demographic characteristics of survey sample by vaccination status.
| Characteristic | Full Sample, N = 2121 |
Vaccinated, Unboosted, N = 548 |
Unvaccinated, N = 619 |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 1108 (52) | 333 (61) | 358 (58) |
| Male | 990 (47) | 213 (39) | 248 (40) |
| Prefer not to say | 23 (1) | 2 (0) | 13 (2) |
| Age, n (%) | |||
| 18-29 | 431 (20) | 113 (21) | 182 (29) |
| 30-44 | 667 (31) | 135 (25) | 225 (36) |
| 45-59 | 459 (21) | 161 (29) | 126 (20) |
| 60+ | 564 (27) | 139 (25) | 86 (14) |
| Educational attainment, n (%) | |||
| Less than HS | 67 (3) | 18 (3) | 29 (4) |
| High school/GED | 531 (25) | 140 (26) | 222 (36) |
| Some college | 483 (23) | 137 (25) | 165 (27) |
| 2-year college degree | 240 (11) | 65 (12) | 60 (10) |
| 4-year college degree | 534 (25) | 132 (24) | 102 (16) |
| Master's degree | 190 (9) | 44 (8) | 32 (5) |
| Doctoral degree | 28 (1) | 7 (1) | 2 (0) |
| Professional degree | 48 (2) | 5 (1) | 7 (1) |
| Race, n (%) | |||
| Black | 277 (13) | 63 (12) | 103 (17) |
| Latino | 181 (9) | 56 (10) | 55 (9) |
| Income (USD), n (%) | |||
| <20,000 | 430 (20) | 114 (21) | 174 (28) |
| 20,000–39,000 | 460 (22) | 133 (24) | 163 (26) |
| 40,000–59,000 | 395 (19) | 113 (21) | 108 (17) |
| 60,000–79,000 | 241 (11) | 69 (13) | 60 (10) |
| 80,000–99,999 | 191 (9) | 44 (8) | 38 (6) |
| 100,000< | 404 (19) | 75 (14) | 76 (12) |
| Political party, n (%) | |||
| Democrat (including leaners) | 1012 (47) | 241 (44) | 194 (31) |
| Republican (including leaners) | 659 (33) | 190 (35) | 251 (41) |
| Work type, n (%) | |||
| In-person, essential | 662 (31) | 171 (31) | 190 (31) |
| Work from home | 452 (21) | 93 (17) | 164 (26) |
| In-person, remote capable | 151 (7) | 49 (9) | 35 (6) |
Note: Sample recruited through Lucid from 12/14/21 to 12/17/21. Details on study methods and survey prompts available in Supplemental Information. Respondents were asked their COVID-19 vaccination status before experimental survey design. Vaccinated, non-boosted respondents are categorized as such if they had received 1 dose of the J&J/Janssen or 2 doses of the Moderna/Pfizer vaccine but had not yet received an additional dose. Totals do not sum to 100% for Race, Political Party, and Work Type. Race presents respondents who listed Black and Latino as one of their self-reported race identifiers. Political Party omits non-partisan respondents. Work type omits respondents who were not employed at the time of our survey.
Before the conjoint tasks, we also randomized assignment to a contextual prime about the likely transmissibility and lethality of the new omicron variant. An extensive literature in both marketing and related disciplines has shown that exposure to certain attributes may make these salient and affect the receptiveness to a particular product. In our case, we were interested in whether information about the emerging variant would affect the relative weight respondents place on different attributes when making choices (Yi, 1990). At the beginning of the survey respondents were shown a prompt that read “[…] cases of a new variant of COVID-19, the Omicron variant, have appeared across the globe [ …].” Half of the sample was told that “Public health experts do not yet know whether this variant spreads more or less quickly or is more or less deadly than previous variants.” The other half of the sample was randomly given additional information that public health experts “[…] suggest the new variant may spread more quickly, but be less deadly than previous variants.”
The survey then presented respondents with five tasks. While research suggests that the number of tasks has little effect on response quality (Bech et al., 2011), we limited the number to five to minimize cognitive burden. In each task, participants evaluated a hypothetical vaccine. Based on respondents' vaccination status, unvaccinated respondents' tasks referred to a hypothetical vaccine. All others’ tasks referred to a hypothetical vaccine booster. Randomization ensured that attributes were orthogonal and allowed for estimation of the marginal contribution for each attribute-level. Supplemental Table S2 shows the attributes used in our vaccine profiles and their levels.
Hypothetical bias is a major concern of any survey experiment; the estimated treatment effects of various factors on individuals’ stated preferences may not accurately reflect the effects of the same factors on actual health behaviors. While important questions remain, recent research suggests that the results from choice-based experiments reflect real-world choices quite well across a range of settings (Hainmueller et al., 2015), including health behaviors (Haghani et al., 2021; Quaife et al., 2018).
2.3. Outcome measure
The primary outcome variable in this study was a measure of respondents’ willingness to receive a candidate vaccine profile. Respondents were asked a binary (Yes/No) question if they would receive a given vaccine profile.
2.4. Covariates
Table 1 lists the descriptive statistics of all control variables. The full questionnaire is provided in the Supplemental Information. The covariates for “Political Party” stem from the following two questions: A. “In politics, as of today, do you consider yourself a Republican, a Democrat, or an Independent?” with the following response categories: “Republican (1), Democrat (2), Independent (3), Other/don't know (4).” B. If question A was answered with “Independent”, the following question B. was asked: “As of today, do you lean more toward the Democratic Party or the Republican Party?” Because those who “lean” toward a political party often have opinions and exhibit behaviors that closely resemble those of self-identified partisans (Petrocik, 2009), the Democratic and Republican indicator variables combine those who identified with either party in question A with those who “leaned” toward the party in question B.
2.5. Analysis
To estimate the effect of each attribute-level on willingness to receive the booster, we used an ordinary least squares (OLS) regression with robust standard errors clustered on respondent. In each case, the dependent variable is an indicator coded 1 for those who would be willing to receive a given vaccine profile and 0 for those who would not. The independent variables of interest are a series of indicator variables identifying assignment to each attribute-level in the conjoint (Table S2) as well as an indicator coded 1 for those who received the opening prompt that Omicron may be more transmissible, but less lethal and 0 for those who received the prompt saying that the relative transmissibility and lethality of omicron are unknown. Additional OLS regressions also control for the demographic factors listed in Table 1. The resulting regression coefficients for the conjoint attribute-levels are the average marginal component effects (AMCEs). AMCEs represent the mean difference in a respondent choosing a vaccine when comparing two attribute values averaged across all possible combinations of the other vaccine attribute values. AMCEs are nonparametrically identified under a modest set of assumptions, many of which – such as randomization of attribute-levels – were determined by design (Kaplan and Milstein, 2021; Kreps et al., 2020; Motta, 2021). All analysis was conducted in STATA 15.1. To graphically illustrate the effect of hypothetical attributes, marginal means – measuring the level of favorability toward a vaccine for each attribute-level averaging across all other features – were calculated (Leeper et al., 2020).
3. Results
Respondents evaluated each vaccine profile – five total per respondent – with a binary (Yes/No) response on whether they would be willing to receive the hypothetical vaccine. The impact of varied attribute-levels on willingness to revceive a booster are presented in Table 2 and illustrated as marginal means in Fig. 1 . Precise point estimates and standard errors for each marginal mean are presented in Supplemental Table S3.
Table 2.
OLS regression results of attributes on booster acceptance.
| (1) | (2) | |
|---|---|---|
| Efficacy: 70% | 0.103*** (0.024) |
0.099*** (0.023) |
| Efficacy: 90% | 0.237*** (0.024) |
0.231*** (0.024) |
| Duration: 1 year | 0.048** (0.022) |
0.042* (0.022) |
| Duration: 2 years | 0.035 (0.023) |
0.037 (0.023) |
| Protection: Protects against future variants | 0.043** (0.018) |
0.038** (0.017) |
| Manufacturer: Moderna | −0.051** (0.024) |
−0.050** (0.024) |
| Manufacturer: Johnson & Johnson | −0.178*** (0.023) |
−0.176*** (0.023) |
| Incentive: Paid day off work | 0.031 (0.027) |
0.037 (0.027) |
| Incentive: $100 incentive | 0.085*** (0.028) |
0.092*** (0.028) |
| Incentive $1000 incentive | 0.164*** (0.027) |
0.175*** (0.026) |
| Likely more contagious, less lethal treatment | 0.076*** (0.029) |
0.074*** (0.028) |
| Controls | ||
| Democrat | 0.070* (0.038) |
|
| Republican | −0.086** (0.040) |
|
| Female | −0.031 (0.028) |
|
| Age (in 10s) | −0.033*** (0.010) |
|
| Black | −0.069 (0.050) |
|
| Latino | −0.020 (0.049) |
|
| Education | 0.010 (0.010) |
|
| Income | 0.007 (0.010) |
|
| Work from home | −0.076* (0.045) |
|
| Work in-person, remote capable | −0.064 (0.054) |
|
| Work in-person, essential | −0.057 (0.037) |
|
| Constant | 0.407*** (0.036) |
0.494*** (0.088) |
| Observations | 2740 | 2740 |
| R-squared | 0.090 | 0.121 |
Note: ***p < 0.01, **p < 0.05, *p < 0.1. OLS regressions on willingness to receive booster among fully vaccinated, but non-boosted respondents. Base categories for each attribute are: Efficacy-50%; Protection duration-6 months; Future variants: May require a booster against future variants; Manufacturer-Pfizer; Incentive-$10. The dependent variable in these regressions is a binary variable indicating willingness to receive a hypothetical vaccine booster. Robust standard errors in parentheses.
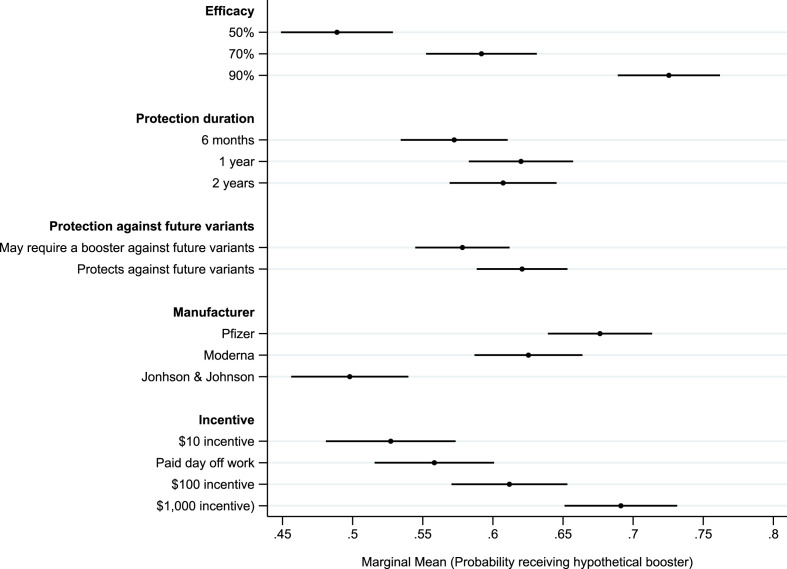
Fig. 1.
Marginal Means of Booster Acceptance by COVID-19 Vaccine Attributes
Note: Marginal means obtained from OLS regression in Table 2, Model (1).
3.1. Vaccinated, unboosted respondents
Our primary analyses focus on respondents who were fully vaccinated but had not received a booster at the time of the survey. Fig. 1 plots the marginal mean likelihood of individuals’ willingness to receive the booster at each attribute-level. These estimates are obtained from an OLS model regressing booster acceptance on each attribute-level as well as an indicator variable identifying assignment to one of the two opening contextual treatments describing the likely lethality/transmissibility of the omicron variant (Table 2, Model (1)). The most important predictor of likelihood of receiving a booster in this survey sample was efficacy. The marginal mean willingness to receive the booster was just 0.49 (95% CI: 0.45–0.53) for a booster that was 50% effective in preventing symptomatic infection. This increased to 0.59 (β = 0.10, p < 0.01) for a booster that was 70% effective and to 0.73 (β = 0.24, p < 0.01) for a booster that is 90% effective. Marginal means at all three levels of efficacy are significantly different from one another (p < 0.05, two-tailed test).
Protection duration against current COVID-19 variants had only a modest impact on willingness to receive the booster. The marginal mean willingness of receiving a booster that offered six months of protection was 0.57 (95% CI: 0.53–0.61). This increased for a booster that offered one (0.62; β = 0.05, p < 0.05) or two (0.61; β = 0.04, p > 0.10) years of protection. However, the only statistically significant difference in marginal means was between the six-month and one-year levels. Protection against future variants also had only a modest effect on booster uptake. The marginal mean willingness to receive a booster that was unlikely to protect against future variants was 0.58 (95% CI: 0.54–0.61). This increased modestly to 0.62 (β = 0.04, p < 0.05) for a booster that was likely to protect against future variants.
The vaccine manufacturer also significantly influenced willingness to receive the booster. The marginal mean willingness was highest for one produced by Pfizer (0.68; 95% CI: 0.66–0.70), followed by Moderna (0.63; β = −0.05, p < 0.05), and finally Johnson & Johnson (0.50; β = −0.18, p < 0.01). Marginal means across all three manufacturers are significantly different from one another (p < 0.05, two-tailed test).
Financial incentives had varying effects on booster uptake. The marginal mean willingness to receive a hypothetical booster that came with a $10 incentive was 0.53 (95% CI: 0.48–0.57); this increased to 0.56 (β = 0.03, p > 0.10) for a booster with a financial incentive of a paid day off work, but the increase is not statistically significant. A booster with a $100 incentive had a marginal mean likelihood of being received of 0.61 (β = 0.09, p < 0.01) and was significantly higher than both the $10 incentive and paid day off level. This increased to 0.69 (β = 0.16, p < 0.01) for a booster with a $1000 financial incentive, which was significantly higher than for all other incentive levels.
Model (1) of Table 2 shows that the contextual prime – suggesting that the potential variant could prove to be more contagious, but less lethal than previous variants – significantly increased the willingness to receive a booster (β = 0.08, p < 0.01). This effect is both substantively large and statistically significant. While the contextual prime had a direct effect on the likelihood of receiving a booster, there is little evidence that it significantly moderated the influence of vaccine attributes on willingness to receive the booster (see Supplemental Table S4).
Model (2) of Table 2 augments Model (1) and includes a set of control variables in a multivariate regression framework to attempt to adjust for the simultaneous impact of potentially correlated predictors – such as age and political affiliation – on the willingness to receive a booster. After including these control variables, the attribute-specific estimates align with the magnitude of the unadjusted model shown in Fig. 1.
Model (2) shows that a sizable partisan gap in willingness to receive a booster persists even among those already fully vaccinated. The partisan gap between Democrats (β = 0.07, p < 0.10) and Republicans (β = −0.09, p < 0.05) is substantively large and highly statistically significant [two-tailed Wald test, p < 0.001]. Political independents were the omitted baseline category.
Older respondents in our sample were significantly more likely to be fully vaccinated than younger respondents. However, older Americans in our study were less likely to receive a booster, all else being equal, than younger respondents. Finally, this sample's respondents in the workforce reported being significantly less willing to receive a booster, all else equal, and the mode of work – specifically the ability to work remotely – had no impact on the willingness to receive a booster.
3.2. Unvaccinated participants
Respondents who reported not yet having received a single dose of a COVID-19 vaccine in the initial screening questions participated in the same experiment, but the hypothetical profile was described as a vaccine rather than a booster. Additional analyses reported fully in Supplemental Table S5 show that most vaccine attributes had little effect on willingness to receive the vaccine among this group. The only exceptions were that a 90% effective vaccine increased acceptance from a 50% effective baseline (β = 0.07, p < 0.01); a protection duration of two years increased the likelihood of accepting the vaccine from a 6-month protection duration baseline (β = 0.04, p < 0.05); and a $1000 incentive significantly increased uptake from the $10 incentive baseline (β = 0.06, p < 0.01). No other attribute-level had a statistically significant effect on uptake from the baseline. Marginal means at each attribute-level are presented in Supplemental Figure S1.
Finally, unvaccinated respondents were no more or less willing to receive the vaccine in the contextual treatment providing information that the variant may be more contagious, but less lethal than prior variants than in the control group, which suggested that both factors remained unknown.
4. Discussion
To our knowledge, this research is the first that carries out a randomized choice-based analysis to evaluate how booster attributes affect willingness to receive a COVID-19 vaccine booster. We assess the causal impact of booster attributes on reported willingness among the critical subgroup of fully vaccinated, but un-boosted respondents. We contrast these findings to the effects of the same vaccine attributes on the willingness of unvaccinated individuals to receive a first dose of a COVID-19 vaccine in light of an emergent variant.
Our study offers causal estimates of the willingness to accept a COVID-19 booster in a period of high uncertainty. Several of our findings are consistent with adjacent literature on vaccination. For example, we find that efficacy of the booster with respect to the new variant strongly affects inclination toward the booster, consistent with prior research that points to the strong effect of efficacy on vaccine preferences (Determann et al., 2014; Kreps et al., 2020). For unvaccinated individuals, efficacy must be considerably higher to incline individuals to receive the vaccine, even in the context of the emerging Omicron variant.
To be sure, the public health recommendations that follow from this finding are challenging. Efficacy data for boosters vis-à-vis Omicron and now the subvariants continues to emerge, yet in ways that may be confusing to the public in terms of how efficacy is presented (rate ratios versus proportionate reduction in disease among the vaccinated or boosted group), the specific population studied (Abu-Raddad et al., 2022; Bar-On et al., 2021; Moreira et al., 2022), and now the efficacy of a second booster (Regev-Yochay et al., 2022). Our analysis suggests that boosters with higher efficacy will likely improve attitudes toward boosters.
Our results also indicate the limited importance of protection duration against both current and potential future variants. Longer temporal protection duration, as well as protection against future variants did modestly, but significantly, increase willingness to receive the booster. Indeed, the prospect of taking an Omicron-specific booster, as the CEO of Pfizer has posited (Kimball, 2021), or even receiving a booster every year could dampen enthusiasm for the third dose. However, even a short protection duration against current variants – combined with the probability that new boosters may be needed to protect against future variants – depressed willingness to receive a booster only modestly.
Monetary incentives, particularly for the already-vaccinated to get a booster – also affected willingness. Although early research on monetary incentives for COVID-19 vaccination showed that incentives had little effect on vaccine preferences (Kreps et al., 2021) and research on previous vaccines showed that individuals were willing to pay for the vaccine (Carpio et al., 2021), growing research has shown that incentives can help overcome reluctance get the COVID-19 vaccine (Campos-Mercade et al., 2021). The same appears to be true regarding boosters.
Finally, while the manufacturer had no effect on the unvaccinated, it strongly affected vaccinated individuals’ attitudes about the booster. Johnson & Johnson/Janssen reduced the likelihood of receiving the booster compared to either Pfizer or Moderna. Those preferences about manufacturers have become salient compared to earlier studies showing public agnosticism toward the manufacturer (Kreps et al., 2021).
Among the demographic variables associated with willingness to receive a booster, the persistence of a partisan gap is notable. Previous research has shown stark partisan divides in willingness to receive initial vaccination against COVID-19 and called for messages targeted toward Republicans skeptical of vaccination (Barry et al., 2021; Pink et al., 2021). The continued presence of a partisan gap speaks to the need to continue targeted outreach to overcome important pockets of booster hesitancy.
4.1. Limitations
As is the case with all between-subjects analysis, this study represents a snapshot in time; in this case, it was a point where Omicron was emerging as an uncertain variant of concern. However, we have no reason to believe that the new variant would change the relative importance of attributes in influencing vaccine preferences. Indeed, our contextual experimental primes about Omicron affected baseline willingness to receive a booster but did not significantly moderate attribute treatment effects. It might, however, influence the way people value the booster versus antiviral treatments, or their willingness to pay for greater protection against COVID-19.
Most fundamentally, the survey only examines self-reported willingness to vaccinate. Individuals might diverge in their self-reported willingness and actual vaccination behavior, yet this study is unable to measure the possible delta that previous research has identified (Ding et al., 2005; Johnston et al., 2017). Future studies should update demand estimates to broaden understanding of this divergence between self-reported vaccination willingness and booster uptake, particularly with respect to future variants’ transmissibility and lethality, and newly available boosters.
Further, one structural limitation of studies like ours is the question of external validity. Although our research design randomizes vaccine attributes and explicitly instructs participants to refer to hypothetical vaccines, we cannot exclude that participants’ priors about efficacy or safety, for example, had an impact on their stated willingness to receive a booster. However, the experiment took place at a time when, at least for boosters, the relevant attributes such as efficacy and duration of protection were unknown. We acknowledge, however, that prior beliefs of fully vaccinated participants may nevertheless affect their stated hypothetical preferences.
In addition, future research should also consider using probability-based survey samples to replicate the results obtained with this quota-based Lucid sample. However, prior research using conjoint experiments to assess the influence of COVID-19 vaccine attributes on vaccine acceptance using Lucid and probability-based samples yielded substantively similar effects (Kaplan and Milstein, 2021; Kreps et al., 2020). Moreover, future research could also examine cross-national contexts to study if these findings hold beyond the case of the United States.
Credit author statement
Shyam Raman: Conceptualization, Study design, Data collection, Formal analysis, Writing; Doug Kriner: Conceptualization, Study design, Formal analysis, Writing; Nicolas Ziebarth: Conceptualization, Study design Writing; Kosali Simon: Conceptualization, Study design, Writing; Sarah Kreps: Conceptualization, Study design, Writing
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.socscimed.2022.115277.
Appendix ASupplementary data
The following is the Supplementary data to this article:
Data availability
All data and code needed to replicate the analysis are publicly available via the Harvard Dataverse: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/DMIMUL
References
- Abbasi J. Studies suggest COVID-19 vaccine boosters save lives. JAMA. 2022;327:115. doi: 10.1001/jama.2021.23455. [DOI] [PubMed] [Google Scholar]
- Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., AlMukdad S., Yassine H.M., Al-Khatib H.A., Smatti M.K., Tang P., Hasan M.R., Coyle P., Al-Kanaani Z., Al-Kuwari E., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Abdul-Rahim H.F., Nasrallah G.K., Al-Kuwari M.G., Butt A.A., Al-Romaihi H.E., Al-Thani M.H., Al-Khal A., Bertollini R. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N. Engl. J. Med. 2022;386:1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Alroy-Preis S., Ash N., Huppert A., Milo R. Protection against covid-19 by BNT162b2 booster across age groups. N. Engl. J. Med. 2021;385:2421–2430. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C.L., Anderson K.E., Han H., Presskreischer R., McGinty E.E. Change over time in public support for social distancing, mask wearing, and contact tracing to combat the COVID-19 pandemic among US adults, april to november 2020. Am. J. Publ. Health. 2021;111:937–948. doi: 10.2105/ajph.2020.306148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech M., Kjaer T., Lauridsen J. Does the number of choice sets matter? Results from a web survey applying a discrete choice experiment. Health Econ. 2011;20:273–286. doi: 10.1002/hec.1587. [DOI] [PubMed] [Google Scholar]
- Berinsky A.J., Huber G.A., Lenz G.S. Evaluating online labor markets for experimental research: amazon.com's mechanical Turk. Polit. Anal. 2012;20:351–368. doi: 10.1093/pan/mpr057. [DOI] [Google Scholar]
- Bruxvoort K.J., Sy L.S., Qian L., Ackerson B.K., Luo Y., Lee G.S., Tian Y., Florea A., Aragones M., Tubert J.E., Takhar H.S., Ku J.H., Paila Y.D., Talarico C.A., Tseng H.F. Effectiveness of mRNA-1273 against delta, mu, and other emerging variants of SARS-CoV-2: test negative case-control study. BMJ. 2021;375 doi: 10.1136/bmj-2021-068848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Mercade P., Meier A.N., Schneider F.H., Meier S., Pope D., Wengström E. Monetary incentives increase COVID-19 vaccinations. Science. 2021;374:879–882. doi: 10.1126/science.abm0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpio C.E., Coman I.A., Sarasty O., García M. COVID-19 vaccine demand and financial incentives. Appl. Health Econ. Health Pol. 2021;19:871–883. doi: 10.1007/s40258-021-00687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cdc, U.D. of H. and H.S 2022. https://covid.cdc.gov/covid-data-tracker COVID Data Tracker [WWW Document]. COVID-19 Data Tracker.
- Coppock A., McClellan O.A. Validating the demographic, political, psychological, and experimental results obtained from a new source of online survey respondents. Res. Pol. 2019;6:1–14. doi: 10.1177/2053168018822174. [DOI] [Google Scholar]
- de Bekker-Grob E.W., Hofman R., Donkers B., van Ballegooijen M., Helmerhorst T.J.M., Raat H., Korfage I.J. Girls' preferences for HPV vaccination: a discrete choice experiment. Vaccine. 2010;28:6692–6697. doi: 10.1016/j.vaccine.2010.08.001. [DOI] [PubMed] [Google Scholar]
- de Bekker-Grob E.W., Veldwijk J., Jonker M., Donkers B., Huisman J., Buis S., Swait J., Lancsar E., Witteman C.L.M., Bonsel G., Bindels P. The impact of vaccination and patient characteristics on influenza vaccination uptake of elderly people: a discrete choice experiment. Vaccine. 2018;36:1467–1476. doi: 10.1016/j.vaccine.2018.01.054. [DOI] [PubMed] [Google Scholar]
- Determann D., Korfage I.J., Lambooij M.S., Bliemer M., Richardus J.H., Steyerberg E.W., De Bekker-Grob E.W. Acceptance of vaccinations in pandemic outbreaks: a discrete choice experiment. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M., Grewal R., Liechty J. Incentive-aligned conjoint analysis. J. Mar. Res. 2005;42:67–82. doi: 10.1509/jmkr.42.1.67.56890. [DOI] [Google Scholar]
- Dror A.A., Daoud A., Morozov N.G., Layous E., Eisenbach N., Mizrachi M., Rayan D., Bader A., Francis S., Kaykov E., Barhoum M., Sela E. Vaccine hesitancy due to vaccine country of origin, vaccine technology, and certification. Eur. J. Epidemiol. 2021;36:709–714. doi: 10.1007/s10654-021-00758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror A.A., Eisenbach N., Taiber S., Morozov N.G., Mizrachi M., Zigron A., Srouji S., Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol. 2020;35:775–779. doi: 10.1007/s10654-020-00671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine : a survey of U.S. adults. Ann. Intern. Med. 2020;173:964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D., Loe B.S., Yu L.M., Freeman J., Chadwick A., Vaccari C., Shanyinde M., Harris V., Waite F., Rosebrock L., Petit A., Vanderslott S., Lewandowsky S., Larkin M., Innocenti S., Pollard A.J., McShane H., Lambe S. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public Health. 2021;6:e416–e427. doi: 10.1016/S2468-2667(21)00096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., Milo R., Alroy-Preis S., Ash N., Huppert A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghani M., Bliemer M.C.J., Rose J.M., Oppewal H., Lancsar E. Hypothetical bias in stated choice experiments: Part I. Macro-scale analysis of literature and integrative synthesis of empirical evidence from applied economics, experimental psychology and neuroimaging. J. Choice Model. 2021;41 doi: 10.1016/j.jocm.2021.100309. [DOI] [Google Scholar]
- Hainmueller J., Hangartner D., Yamamoto T. Validating vignette and conjoint survey experiments against real-world behavior. Proc. Natl. Acad. Sci. USA. 2015;112:2395–2400. doi: 10.1073/pnas.1416587112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainmueller J., Hopkins D.J., Yamamoto T. Causal inference in conjoint analysis: understanding multidimensional choices via stated preference experiments. Polit. Anal. 2014;22:1–30. doi: 10.1093/pan/mpt024. [DOI] [Google Scholar]
- Johnston R.J., Boyle K.J., Adamowicz W., Vic), Bennett J., Brouwer R., Cameron T.A., Hanemann W.M., Hanley N., Ryan M., Scarpa R., Tourangeau R., Vossler C.A. Contemporary guidance for stated preference studies. J. Assoc. Environ. Resour. Econ. 2017;4:319–405. doi: 10.1086/691697. [DOI] [Google Scholar]
- Kaplan R.M., Milstein A. Influence of a COVID-19 vaccine's effectiveness and safety profile on vaccination acceptance. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2021726118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball S. CNBC; 2021. Pfizer CEO Says Fourth Covid Vaccine Doses May Be Needed Sooner than Expected Due to Omicron.https://www.cnbc.com/2021/12/08/omicron-pfizer-ceo-says-we-may-need-fourth-covid-vaccine-doses-sooner-than-expected.html [WWW Document] accessed 12.22.21. [Google Scholar]
- Kreps S., Prasad S., Brownstein J.S., Hswen Y., Garibaldi B.T., Zhang B., Kriner D.L. Factors associated with US adults' likelihood of accepting COVID-19 vaccination. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps S.E., Dasgupta N., Brownstein J.S., Hswen Y., Kriner D.L. 2021. Public Attitudes toward COVID-19 Vaccination: the Role of Vaccine Attributes, Incentives, and Misinformation. Npj Vaccines 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X., Zhu H., Wang J., Huang Y., Jing R., Lyu Y., Zhang H., Feng H., Guo J., Fang H. Public perceptions and acceptance of COVID-19 booster vaccination in China: a cross-sectional study. Vaccines Cold Spring Harb. Lab. Press. 2021;9:1461. doi: 10.3390/vaccines9121461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper T.J., Hobolt S.B., Tilley J. Measuring subgroup preferences in conjoint experiments. Polit. Anal. 2020;28:207–221. doi: 10.1017/pan.2019.30. [DOI] [Google Scholar]
- Lennon R.P., Block R., Schneider E.C., Zephrin L., Shah A. 2021. Underserved Population Acceptance of Combination Influenza-COVID-19 Booster Vaccines. Vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C., Tu P., Beitsch L.M. 2021. Confidence and Receptivity for Covid‐19 Vaccines: A Rapid Systematic Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi B., Lotan R., Kalkstein N., Peretz A., Perez G., Ben-Tov A., Chodick G., Gazit S., Patalon T. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-26672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira E.D., Kitchin N., Xu X., Dychter S.S., Lockhart S., Gurtman A., Perez J.L., Zerbini C., Dever M.E., Jennings T.W., Brandon D.M., Cannon K.D., Koren M.J., Denham D.S., Berhe M., Fitz-Patrick D., Hammitt L.L., Klein N.P., Nell H., Keep G., Wang X., Koury K., Swanson K.A., Cooper D., Lu C., Türeci Ö., Lagkadinou E., Tresnan D.B., Dormitzer P.R., Şahin U., Gruber W.C., Jansen K.U. Safety and efficacy of a third dose of BNT162b2 covid-19 vaccine. N. Engl. J. Med. 2022;386:1910–1921. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta M. Can a COVID-19 vaccine live up to Americans' expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc. Sci. Med. 2021;113642 doi: 10.1016/j.socscimed.2020.113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrocik J.R. Measuring party support: leaners are not independents. Elect. Stud. 2009;28:562–572. doi: 10.1016/j.electstud.2009.05.022. [DOI] [Google Scholar]
- Peyton K., Huber G.A., Coppock A. The generalizability of online experiments conducted during the COVID-19 pandemic. J. Exp. Polit. Sci. 2021:1–16. doi: 10.1017/XPS.2021.17. [DOI] [Google Scholar]
- Pink S.L., Chu J., Druckman J.N., Rand D.G., Willer R. Elite party cues increase vaccination intentions among Republicans. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2106559118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaife M., Terris-Prestholt F., Di Tanna G.L., Vickerman P. How well do discrete choice experiments predict health choices? A systematic review and meta-analysis of external validity. Eur. J. Health Econ. 2018;19:1053–1066. doi: 10.1007/s10198-018-0954-6. [DOI] [PubMed] [Google Scholar]
- Regev-Yochay G., Gonen T., Gilboa M., Mandelboim M., Indenbaum V., Amit S., Meltzer L., Asraf K., Cohen C., Fluss R., Biber A., Nemet I., Kliker L., Joseph G., Doolman R., Mendelson E., Freedman L.S., Harats D., Kreiss Y., Lustig Y. Efficacy of a fourth dose of covid-19 mRNA vaccine against omicron. N. Engl. J. Med. 2022;386:1377–1380. doi: 10.1056/NEJMc2202542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzymski P., Poniedziałek B., Fal A. Willingness to receive the booster covid‐19 vaccine dose in Poland. Vaccines Cold Spring Harb. Lab. Press. 2021;9:1286. doi: 10.3390/vaccines9111286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzinger M., Watson V., Arwidson P., Alla F., Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021;6 doi: 10.1016/S2468-2667(21)00012-8. e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka A.M., Park B., Gao L., Slifka M.K. Incidence of tetanus and diphtheria in relation to adult vaccination schedules. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021;72:285. doi: 10.1093/cid/ciaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Y. The effects of contextual priming in print advertisements. J. Consum. Res. 1990;17:215. doi: 10.1086/208551. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and code needed to replicate the analysis are publicly available via the Harvard Dataverse: https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/DMIMUL