Abstract
The com operon of naturally transformable streptococcal species contains three genes, comC, comD, and comE, involved in the regulation of competence. The comC gene encodes a competence-stimulating peptide (CSP) thought to induce competence in the bacterial population at a critical extracellular concentration. The comD and comE genes are believed to encode the transmembrane histidine kinase and response regulator proteins, respectively, of a two-component regulator, with the comD-encoded protein being a receptor for CSP. Here we report on the genetic variability of comC and comD within Streptococcus pneumoniae isolates. Comparative analysis of sequence variations of comC and comD shows that, despite evidence for horizontal gene transfer at this locus and the lack of transformability of many S. pneumoniae strains in the laboratory, there is a clear correlation between the presence of a particular comC allele and the cognate comD allele. These findings effectively rule out the possibility that the presence of noncognate comC and comD alleles may be responsible for the inability to induce competence in many isolates and indicate the importance of a functional com pathway in these isolates. In addition, we describe a number of novel CSPs from disease-associated strains of S. mitis and S. oralis. The CSPs from these isolates are much more closely related to those from S. pneumoniae than to most CSPs previously reported from S. mitis and S. oralis, suggesting that these particular organisms may be a potential source of DNA in recombination events generating the mosaic structures commonly reported in genes of S. pneumoniae that are under strong selective pressure.
Many members of the oral streptococci are naturally transformable, being able to take up naked DNA from the extracellular environment (16). Homologous recombination of foreign DNA into the host chromosome following transformation is believed to play a major role in the evolution of these bacteria. This notion is illustrated by both the rapid emergence of penicillin resistance following the acquisition of low-affinity penicillin binding proteins (4) and evidence for the occurrence of frequent recombination events in the evolution of virulence factors in Streptococcus pneumoniae (5, 20). Competence for transformation in streptococci is not constitutive, as it is in Neisseria species (24), but is regulated by genes of the recently characterized com locus (18). The com operon contains three genes, comC, comD, and comE, encoding a competence-stimulating peptide (CSP), histidine kinase, and a response regulator, respectively (1, 7, 9, 19). Two genes located elsewhere on the chromosome, comA and comB, encode proteins responsible for the export of CSP from the cell (11, 27). CSP is thought to induce competence when a critical extracellular concentration is reached. The comD-encoded transmembrane histidine kinase is believed to be a receptor for the CSP and to phosphorylate a comE-encoded transcription regulator, producing an active form that up regulates both the comCDE operon and, presumably, a number of other genes involved in competence development.
Despite the fact that many S. pneumoniae strains appear untransformable under laboratory conditions, the CSP-encoding gene, comC, is thought to be ubiquitous (22). Sequencing studies recently identified two distinct alleles encoding S. pneumoniae CSP, comC1 and comC2 (21). It appears that the vast majority of strains carrying the comC1 allele cannot be induced to competence with the comC2-encoded peptide CSP-2 and vice versa (21). Recently, it was suggested that all members of the mitis and anginosus phylogenetic groups of streptococci possess homologues of the comCDE operon (10). The CSP-encoding genes of several such isolates have been sequenced, revealing a range of structurally related but biologically distinct peptides both between species and among organisms recognized as a single species, such as S. mitis (9, 10, 21). A complete understanding of the specificity of competence induction is crucial for understanding the biology of S. pneumoniae and other naturally transformable streptococci, especially with regard to the horizontal transfer of antibiotic resistance and virulence markers within these organisms.
Here we describe a study designed to confirm and extend current knowledge of the genetic diversity of comC and the corresponding regulatory genes, comD and comE. The aims of the study were as follows. First, we aimed to characterize the comC genes of a diverse range of S. pneumoniae isolates in order to extend understanding of the genetic variation of the CSP, to link these findings to competence phenotype, and to provide a well-characterized set of isolates for further studies. Second, we aimed to characterize the 5′ region of comD (encoding the CSP receptor motif) from the same strains; while comC variation has been examined previously with a limited number of strains of S. pneumoniae, there are currently no data describing genetic variation of the corresponding comD genes. One feasible explanation for the reported lack of ability to transform at least 50% of strains tested with synthetic CSP (21), which has yet to be addressed, is the possibility of noncognate comC alleles and comD alleles in particular strains. A recent report that horizontal gene transfer may occur between the com operons of distinct streptococcal species, generating mosaic genes (10), highlights the need to examine this possibility. We therefore performed a comparative analysis of comC and comD alleles within a set of diverse S. pneumoniae isolates. Third, as an understanding of interspecies signalling may have important implications for understanding the population biology of the naturally transformable streptococci, we also identified a number of novel comC alleles from isolates of oral streptococci that are apparently closely related to pneumococci and that may act as donors in horizontal gene transfer events with pneumococci. Finally, we analyzed the complete comCDE region from a limited number of strains with variant comC alleles in order to add to the limited knowledge of diversity across the whole com operon.
MATERIALS AND METHODS
Strains.
The designations and sources of the S. pneumoniae strains used in this study are shown in Table 1. Strains were routinely cultured on brain heart infusion (BHI) agar containing 5% (vol/vol) defibrinated sheep blood at 37°C in 5% CO2. Strains were stored frozen at −80°C in BHI broth with 15% (vol/vol) glycerol until needed. The non-S. pneumoniae streptococcal strains, used in screening for a comC PCR product, were as follows (sources of strains are available on request): mitis group—S. mitis NCTC10712, NS51T, NCTC3166, NCTC1080, NCTC3165, HV51, PP53, K208, NCTC7864, NCTC11189, Col15, Col16, Col17, and Col18, S. oralis NCTC11427T, 20070NS, 20003NS, Col19, Col21, Col22, and Col24, S. sanguis 12088NS, 2397, NCTC7863T, and 13, S. parasanguis 55895, and S. cristae CR311 and CC5A; anginosus group—S. milleri NCTC10708, S. constellatus F436 and NMH4, S. anginosus NCTC11062, and S. intermedius 415-87, AM6425, and HW7, salivarius group—S. vestibularis NCTC12166T and S. salivarius NCTC8618T; and other groups—group G streptococcus strains 91.2153, 91.2388, and 11555, group K streptococcus strain NCTC11389, S. iniae NCFB5389, S. adjacens X193, S. cricetus NCFB2720, S. ferus NCFB2721, S. rattus NCFB2723, S. defectivus DA4, S. ceocorum NCFB2674, and S. sobrinus NCFB2724.
TABLE 1.
Designations, sources, and serotypes of bacterial isolates used in this study
| Straina | Source (date) of isolationb | Serotypec | Alleled |
|---|---|---|---|
| VA1 | United States (1983) | 19 | comC1 |
| Pn60 (143G) | Spain (1993) | 19A | comC1 |
| Pn16 (110K/70) | Papua New Guinea (NK) | 42 | comC1 |
| Pn12 (53139/72) | Papua New Guinea (1972) | 6 | comC1 |
| 960 | Oxford, UK (1994) | 6B | comC1 |
| 670 | Spain (1988) | 6B | comC1 |
| 954 | Oxford, UK (1994) | 6B | comC1 |
| 964 | Oxford, UK (1995) | 6B | comC1 |
| 950 | Oxford, UK (1995) | 18C | comC1 |
| Pn17 (46/68) | Papua New Guinea (1968) | 46 | comC1 |
| 878 | Kenya (1990) | 10F | comC1 |
| Pn15 (N943/69) | Papua New Guinea (1969) | 12 | comC1 |
| 871 | Kenya (1990) | 18C | comC1 |
| 952 | Oxford, UK (1995) | 22F | comC1 |
| 29044 | Czechoslovakia (1987) | 14 | comC1 |
| R6 | United States (1930) | NT | comC1 |
| Rst7 | NK (NK) | NK | comC1 |
| Pn6 (9/122) | Brighton, UK (1988) | NK | comC1 |
| Col14 | UK (1993) | NT | comC1 |
| Col6 | UK (1993) | NT | comC1 |
| 881 | Kenya (1990) | NT | comC1 |
| Col8 | UK (1993) | NT | comC1 |
| Pn109 | UK (1995) | 1 | comC1 |
| 873 | Kenya (1990) | 8 | comC1 |
| Pn112 | Cambridge, UK (1995) | 3 | comC1 |
| Pn111 | Ashford, UK (1995) | 3 | comC1 |
| 7731 | Equine isolate (1982) | 3 | comC1 |
| Pn148 | Equine isolate (1987) | 3 | comC1 |
| Pn146 | Equine isolate (1987) | 3 | comC1 |
| 41G | NK (NK) | NK | comC2.1 |
| 9858 | Brighton, UK (1988) | NK | comC2.1 |
| Col12 | UK (1993) | NT | comC2.1 |
| Pn5 (9/121) | Brighton, UK (1988) | NK | comC2.1 |
| Col11 | UK (1993) | NT | comC2.1 |
| Col7 | UK (1993) | NT | comC2.1 |
| Col11 | UK (1993) | NT | comC2.1 |
| Col13 | UK (1993) | NT | comC2.1 |
| 872 | Kenya (1990) | 15A | comC2.1 |
| 967 | Oxford, UK (1994) | 35F | comC2.1 |
| Pn107 | Oxford, UK (1995) | 1 | comC2.1 |
| Pn108 | Oxford, UK (1995) | 1 | comC2.1 |
| Pn110 | Norwich, UK (1995) | 3 | comC2.1 |
| 875 | Kenya (1990) | 16F | comC2.1 |
| 880 | Kenya (1990) | 13 | comC2.1 |
| 953 | Oxford, UK (1994) | 6A | comC2.1 |
| 951 | Oxford, UK (1994) | 6A | comC2.1 |
| 85G | Mexico (1992) | 23 | comC2.1 |
| 859 | Liverpool, UK (1996) | 23F | comC2.1 |
| 81G | Mexico (1992) | 23F | comC2.1 |
| 3G | Mexico (1993) | 23F | comC2.2 |
| Pn8 (DN87/629) | Oldham, UK (1987) | 23 | comC2.2 |
| Pn24 (264) | Spain (NK) | 23 | comC2.2 |
| 577 | UK (1987) | 23 | comC2.2 |
| SP1 | Spain (1989) | 23 | comC2.2 |
| Pn25 (267) | Spain (1994) | 23 | comC2.2 |
| F5 | France (1992) | 6B | comC2.2 |
| Pn59 (138G) | Spain (1993) | NK | comC3 |
| Pn13 (Kagnane/73) | Papua New Guinea (1973) | 14 | comC4 |
| 101/87 | Spain (1987) | NT | comC5 |
| 874 | Kenya (1990) | NT | comC6.1 |
| Col19 | UK (1993) | comC6.2 | |
| Col15 | UK (1992) | comC7 | |
| Col18 | UK (1993) | comC7 | |
| Col16 | UK (1993) | comC8 | |
| NCTC10712 | comC9 |
In the vast majority of cases, both comC and 5′ comD sequences were examined. However, in the case of strains shown in boldface, only the sequence of comC was determined. All strains were S. pneumoniae except for Col19 (S. oralis) and Col15, Col18, Col16, and NCTC10712 (S. mitis).
NK, not known. UK, United Kingdom.
NT, nontypeable.
The comC allele of each strain, as determined by sequencing, is indicated. Both alleles comC2 and comC6 are subdivided by a coding change within the leader peptide.
Purification of streptococcal chromosomal DNA.
Chromosomal DNA from each strain was obtained by harvesting the confluent overnight growth from two or three heavily inoculated agar plates into 1 ml of 50 mM Tris-HCl–10 mM EDTA (pH 8.0). Cell lysates were obtained by sequential addition (at 37°C) of 5 μl of lysozyme (10 mg ml−1), (10 min), 5 μl of proteinase K (10 mg ml−1) (30 min), and 40 μl of 20% (wt/vol) Sarkosyl. The clear viscous lysates were extracted once with phenol and once with chloroform and precipitated in 2.5 volumes of ethanol with 10% (vol/vol) 3 M sodium acetate (pH 5.2). The resulting DNA was washed with 70% (vol/vol) ethanol, resuspended in 10 mM Tris-HCl–1 mM EDTA (pH 7.5), and stored at −20°C.
Induction of competence by a CSP.
Stimulation of competence by a synthetic CSP was tested essentially as described by Pozzi et al. (21). Bacteria were grown overnight on BHI agar supplemented with 4% (vol/vol) sterile defibrinated sheep blood at 37°C in an atmosphere of 5% CO2 before being resuspended in BHI broth to an optical density at 620 nm of approximately 0.01. The cultures were incubated at 37°C until the optical density at 620 nm reached 0.4 to 0.5 (mid-exponential to late exponential phase) and were frozen in 15% glycerol at −80°C. For transformation, the frozen cultures were diluted 1:20 in C+y medium (25) containing 0.16% (wt/vol) bovine serum albumin, 0.01% (wt/vol) CaCl2, and 100 ng of synthetic CSP-1 or CSP-2 ml−1 (both generously provided by D. Morrison, University of Illinois). Chromosomal DNA from a pneumococcus carrying a spectinomycin resistance cassette (14) was added to a concentration of 1 μg ml−1. The transformation reaction mixture was kept at 37°C for 150 min before samples were plated on BHI blood agar supplemented with spectinomycin at 200 ng μl−1. Approximately 5 × 107 cells were plated from each transformation reaction, and the induction of competence was judged to have taken place by the appearance of significant numbers (>500) of spectinomycin-resistant transformants on the selective plates after overnight incubation at 37°C.
Analysis of comC and comD allelic variations.
The sequences of the oligonucleotides used as PCR primers in this study are shown in Table 2. The sequence flanking comC in strain R6 was obtained by performing inverse PCR to amplify a PCR product from a DraI-generated chromosomal digest by use of primers 1 and 2 designed against the strain Rx CSP-encoding sequence (7). PCR products were cloned in pTAg (R & D Systems) according to the manufacturer’s instructions. Plasmids were purified from the resulting clones with Wizard Plus Minipreps (Promega), and inserts were sequenced by use of primers corresponding to the vector sequence and novel primers to “walk” along the sequence. The comC-flanking sequence obtained was then used to design primers 3 and 4, which were used to amplify a comC-containing PCR product from chromosomal DNA. PCR was performed with standard parameters at an annealing temperature of 48°C for 32 cycles. PCR products were purified by passage through Microcon 100 columns (Amicon) and were sequenced directly by the cycle sequencing method with an ABI 373A automated sequencing system. Later, when the comD sequence had been determined, primer 3 was used in conjunction with primer 5 to amplify a region encompassing both comC and the 5′ variable region of comD, and the 5′ sequence of comD was determined directly in the same manner.
TABLE 2.
Sequences of primers used in this study
| Primer | Primer sequence (5′→3′) | Location or use |
|---|---|---|
| 1 | CAAAGCTACAAATCGTTCCAAT | Inverse PCR |
| 2 | GATGAGGTTGTCAAAATTCTTC | Inverse PCR |
| 3 | TGACAGTTGAGAGAATCTT | 5′ to comC |
| 4 | CTTTTCTATTTATTTGACCT | 3′ to comC |
| 5 | TAGTTCCAAATGGAAATA | 5′ of comD |
| 6 | CATAGCTCAGCTGGATAGAGCA TTCGCCTTC | Arg-tRNA gene |
| 7 | GGCGGTGTCTTAACCCCTTGAC CAACGGACC | Glu-tRNA gene |
| 8 | WGAAATIGGWSAACGAT | 3′ of comE |
Cloning and sequencing of the comCDE operon.
PCR products corresponding to the entire comC gene and the entire comD gene and most or all of comE were obtained with forward primer 6, corresponding to the upstream Arg-tRNA gene (9), or primer 3, located just upstream of comC, in conjunction with primer 7, corresponding to the downstream Glu-tRNA gene (9), or primer 8, located in the 3′ region of the comE gene. PCR products obtained with these primers were cloned in pTAg as described above, and the sequence of the entire insert was obtained by use of flanking primers corresponding to the vector sequence and a series of internal primers.
Phylogenetic analysis.
Preliminary analysis and alignment of sequences were performed with the DNAStar package. Phylogenetic analysis and tree construction were performed with the program MEGA (15). Trees were constructed by the UPGMA method with the Jukes-Cantor correction (12), and the bootstrap confidence level of internal branches was estimated from 500 resamplings of the data.
Nucleotide sequence accession numbers.
The EMBL accession numbers for the sequences reported in this paper are AJ240738 to AJ240795.
RESULTS
Sequences of S. pneumoniae comC alleles.
When this work was being performed, the coding sequence of only a single comC gene was available (7). In order to obtain the flanking sequences of comC, thus facilitating the amplification and sequencing of the entire comC gene from multiple isolates of pneumococci, inverse PCR was performed with strain R6. By use of primers 1 and 2, a PCR product of approximately 2.6 kb was obtained from self-ligated, DraI-digested chromosomal DNA and cloned into pTAg, and regions flanking comC were sequenced by use of both primers 1 and 2 and primers for the flanking vector sequence. On the basis of this sequence, primers 3 and 4 were designed in order to attempt to amplify comC from a diverse range of S. pneumoniae strains and from closely related streptococcal species.
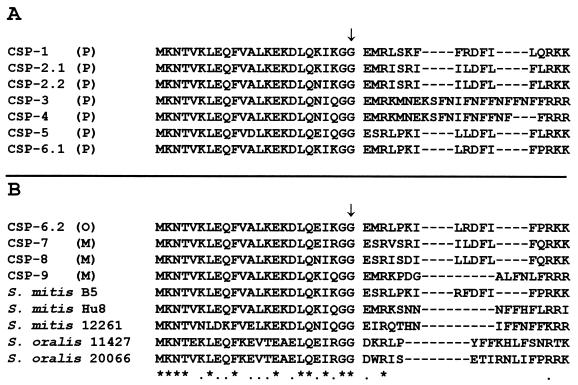
PCR amplifications with primers 3 and 4 were performed with DNA isolated from a range of geographically, serotypically, and temporally diverse S. pneumoniae isolates (Table 1). A PCR product of about the expected size (337 bp) was obtained from all 60 S. pneumoniae strains examined. PCR products were sequenced in full, and six distinct mature CSPs, designated CSP-1 to CSP-6 (encoded by comC1 to comC6, respectively), were identified. The predicted amino acid sequences of the CSPs are shown in Fig. 1A. The vast majority of strains of S. pneumoniae contained one of the two major alleles, comC1 (29 of 60) or comC2 (27 of 60), corresponding to CSP-1 or CSP-2, respectively, as previously described (21). However, four other alleles were detected, although only one example of each of these alleles was seen in the strains examined. The comC3 allele of strain Pn59, isolated from Spain in 1993, is identical to a third pneumococcal allele recently reported (22). Pn13, an isolate from Papua New Guinea, possesses a unique but closely related allele (comC4) encoding a CSP with a deletion of a 3-amino-acid repeat relative to Pn59. The comC5 allele was found only in strain 101/87, a Spanish atypical bile-insoluble S. pneumoniae strain (2), the comC6 allele was found only in strain 874, a Kenyan strain isolated from an human immunodeficiency virus-seropositive, asymptomatic pneumococcal carrier.
FIG. 1.
Alignments of the predicted amino acid sequences of the nine distinct CSPs characterized in this study. Asterisks represent amino acids conserved across all sequences, while dots represent conservative amino acid substitutions. Gaps in the alignment are represented by dashes. The predicted cleavage point of the mature peptide from the leader sequence, based on homology with other double-glycine-type leader peptides, is indicated by an arrow. The CSP-2 and CSP-6 groups are subdivided by coding changes in the leader peptide. The species of origin was S. oralis (O), S. pneumoniae (P), or S. mitis (M). (A) Sequences of the six distinct CSPs obtained from S. pneumoniae isolates. (B) Sequences obtained from isolates characterized as S. mitis or S. oralis alongside the sequences of three S.mitis CSPs and two S. oralis CSPs characterized by Håvarstein et al. (10).
Induction of competence by a CSP.
The ability of synthetic CSP-1 and CSP-2 to induce competence in a subset of strains containing comC1 or comC2 was examined by monitoring the ability of each CSP to induce transformation to spectinomycin resistance (Table 3). As reported previously (21), the vast majority of strains could be induced to competence only by their congruent CSP, although two strains (Pn16 and VA1) appeared to develop competence in the presence of either CSP. However, and again as reported previously (21), less than 50% of strains were found to be rendered transformable even in the presence of their specific CSP.
TABLE 3.
Induction of competence in strains containing comC1 and comC2 by artificial CSPsa
| Strain | Allele | CSP1 | CSP2 |
|---|---|---|---|
| Col8 | comC1 | + | − |
| 29044 | comC1 | + | − |
| R6 | comC1 | + | − |
| Pn15 | comC1 | + | − |
| 670 | comC1 | − | − |
| 7731 | comC1 | − | − |
| Pn109 | comC1 | − | − |
| Pn111 | comC1 | − | − |
| Pn112 | comC1 | − | − |
| Pn146 | comC1 | − | − |
| Pn148 | comC1 | − | − |
| Pn60 | comC1 | − | − |
| Pn6 | comC1 | − | − |
| Col14 | comC1 | − | − |
| VA1 | comC1 | + | + |
| Pn16 | comC1 | + | + |
| 81G | comC2 | − | + |
| Sp1 | comC2 | − | + |
| 3G | comC2 | − | + |
| Pn5 | comC2 | − | + |
| Pn25 | comC2 | − | − |
| Pn8 | comC2 | − | − |
| 41G | comC2 | − | − |
| Col11 | comC2 | − | − |
| Pn107 | comC2 | − | − |
| Pn108 | comC2 | − | − |
| Pn110 | comC2 | − | − |
| Col12 | comC2 | − | − |
| 577 | comC2 | − | − |
The ability of a CSP to induce competence was monitored by the ability to induce the uptake of a spectinomycin resistance marker (+, competence induced, −, competence not induced).
Analysis of comC and comD allelic variations.
As described above and as demonstrated previously (21), at least 50% of the strains appeared untransformable in the laboratory even in the presence of their specific CSP. There are many potential explanations for this phenomenon, notably, encapsulation (23, 26). However, one possible explanation, in view of the occurrence of multiple CSPs in S. pneumoniae and which has not been formally examined, is noncongruence (and therefore lack of binding) between the comC-encoded CSP and the comD-encoded CSP receptor protein within an individual strain. The data of Håvarstein et al. (10), indicating that horizontal gene transfer occurs between the com loci of distinct streptococcal species, provides supportive evidence that such a situation could arise. We investigated this possibility of noncognate comC and comD alleles within individual strains by examining the 5′ 384 bp of the comD sequence (encoding the putative CSP binding domain) of most of the strains from which the comC sequence had already been determined. This examination facilitated a comparative analysis of comC and comD sequences within a large number of diverse pneumococcal isolates.
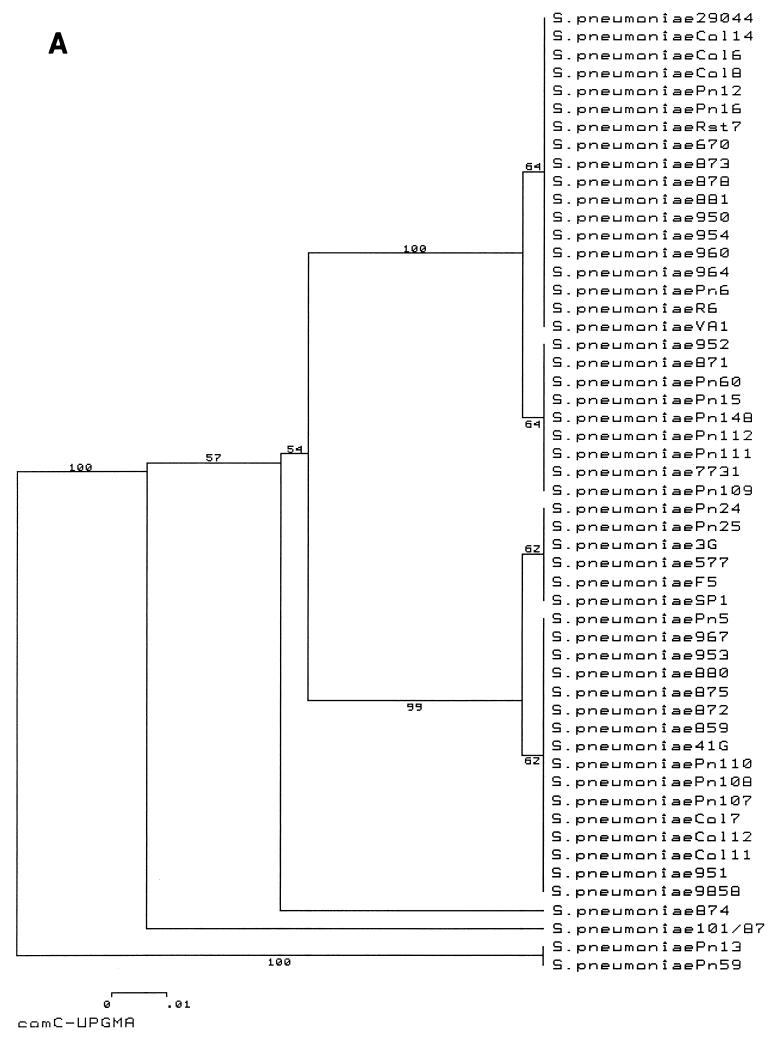
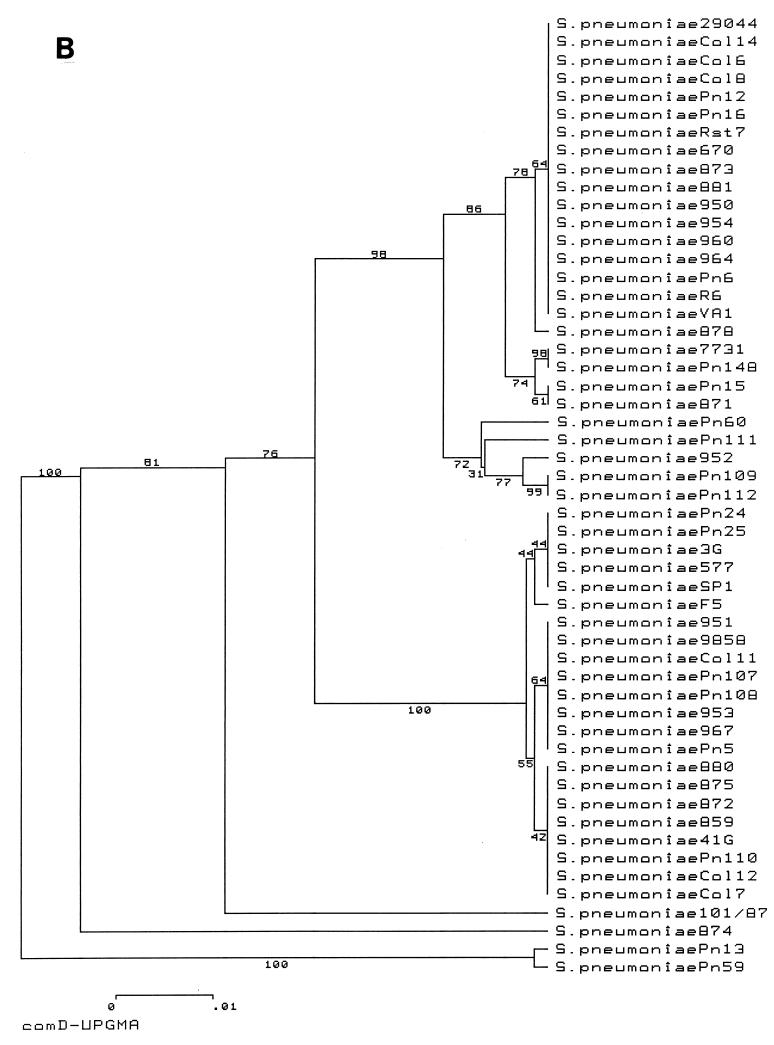
The results of this analysis are presented in the form of two phylogenetic trees representing the sequences of the entire comC gene (Fig. 2A) and the 5′ end (first 384 bp) of the comD gene (Fig. 2B). The comC tree illustrates the relationship between the two major alleles, comC1 (upper half of tree) and comC2 (lower half of tree), which are subdivided by one silent change and one coding change (in the leader peptide), respectively; the amino acid sequences of the mature peptides are unaltered. The remaining sequences, representing strains containing comC3 to comC6, are rather divergent from those of strains containing the major alleles. For the two major groupings, representing alleles comC1 and comC2, allele groups for comC and comD remain entirely congruent: there is no mixing of the 27 comC1 strains with the 22 comC2 strains within the comD tree. Two distinct groups of comD sequences, comD1 and comD2, are apparent; these groups are entirely congruent with the comC1 and comC2 groups. Like the comC sequences, the comD sequences of strains with comC3 to comC6 are all divergent from the two major groupings, comD1 and comD2. Therefore, in spite of previous reports of evidence of horizontal gene transfer within the com locus (10), comC1 and comD1 group sequences and comC2 and comD2 group sequences are always paired within the diverse collection of S. pneumoniae strains examined in this study. Thus, the lack of transformability of many strains is due to factors other than horizontal gene transfer resulting in mismatched alleles of comC and comD.
FIG. 2.
UPGMA trees constructed with DNA sequence data from comC (A) and the 5′ 384 bp of comD (B) illustrating the complete congruence of CSP (comC) and receptor (comD) sequences. The numbers at internal branches represent the bootstrap confidence levels of particular branches estimated from 500 resamplings of the data set.
Screening of other streptococci for comC homologues.
It is thought that the horizontal gene transfer of DNA from oral streptococcal species to S. pneumoniae has played an important role in the evolution of this organism. We therefore used our primer set to screen isolates of a range of oral and other streptococci to investigate organisms with CSPs closely related to those of pneumococci, which may have an increased likelihood of acting as DNA donors. The results of this screening are summarized in Table 4. Only a small proportion of the S. mitis and S. oralis isolates screened yielded comC PCR products with this primer set, suggesting considerable intraspecies diversity within the com operon. PCR products were sequenced from four S. mitis strains (NCTC10712, Col15, Col16, and Col18) and a single S. oralis strain (Col19). The strains with the prefix Col were obtained from A. Efstratiou of the Central Public Health Laboratory, Colindale, England, and were unusual oral streptococci in that they were associated with chest infections and/or pneumonia. The strains were typed on the basis of their reactions with optochin, bile insolubility, quellung reaction, and biochemical profile. The predicted amino acid sequences of the CSPs are shown in Fig. 1B. Three distinct alleles were seen in the S. mitis strains. Strains Col15 and Col18 possess a novel allele, comC7. Strain Col16 possesses a unique and novel allele, comC8. However, S. mitis NCTC10712 was found to possess a CSP, encoded by comC9, unique in this study but identical to that previously reported from S. mitis B6 (10). The single CSP identified in an S. oralis strain (Col19) was found to be identical, apart from one amino acid change in the leader sequence, to that seen in pneumococcal isolate 874 described above (CSP-6).
TABLE 4.
Amplification of comC by PCR from streptococcal strainsa
| Groupb | No. of PCR-positive strains/ total no. of strains tested |
|---|---|
| Mitis | |
| S. pneumoniae | 60/60 |
| S. mitis | 4/14 |
| S. oralis | 1/7 |
| S. sanguis | 0/4 |
| S. parasanguis | 0/1 |
| S. cristae | 0/2 |
| Anginosus | 0/6 |
| Salivarius | 0/2 |
| Other | 0/15 |
Purified streptococcal DNA was screened with primers 3 and 4.
Full details of the strains examined are given in Materials and Methods.
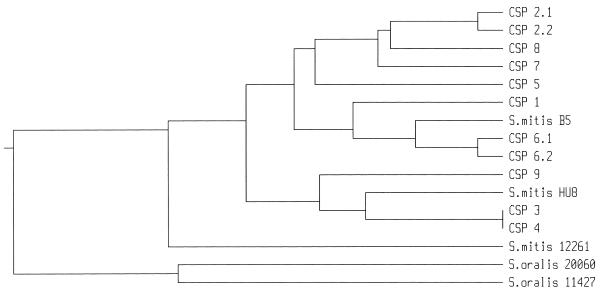
CSP-7 and CSP-8, from organisms classified as S. mitis, are distinct from any of four CSPs recently reported from S. mitis strains (10), as demonstrated in the alignments shown in Fig. 1B. Likewise, Col19 is typed as S. oralis but has a CSP very different from the two previously reported S. oralis CSPs (10). The relationships between these sequences are demonstrated in the dendrogram shown in Fig. 3, constructed from CSP amino acid sequences. This dendrogram is not intended to be a phylogenetic interpretation but is intended to be used merely as a simple visual representation of sequence relationships. Thus, it can be seen that CSP-6, CSP-7, and CSP-8 are all much more closely related to the common pneumococcal CSPs, CSP-1 and CSP-2, than to most of the previously described S. mitis or S. oralis CSPs. One other previously reported S. mitis CSP, from strain B5 (10), also falls within this group. Likewise, pneumococcal CSP-3 and CSP-4 which, at least in our sample, appear much less common, appear most closely related to some S. mitis CSPs.
FIG. 3.
UPGMA tree demonstrating the relationships between ComC (CSP) proteins characterized in this study (CSP-1 and CSP-9) and previously identified CSPs.
Variations across the whole comCDE locus.
Primers corresponding to conserved flanking regions were used to amplify a PCR product representing the complete comC and comD genes and a large portion of the comE gene from six strains with divergent CSPs. The samples were cloned and sequenced in full in order to examine the nature and extent of genetic variations in the comD and comE genes of these strains. The strains examined were S. pneumoniae F5, containing the second major comC allele (comC2) and from which the sequences of comD and comE have not yet been reported; S. pneumoniae Pn59, S. pneumoniae Pn13, and atypical bile-insoluble S. pneumoniae 101/87 (2), each of which contains atypical pneumococcal CSPs; and S. oralis Col19 and S. mitis NCTC10712.
The levels of sequence divergence vary significantly across the comD and comE genes. As might be expected, the region encoding the N-terminal membrane-spanning domain of ComD, presumably containing the CSP receptor, is the most variable part of the comD gene, with up to 10.24% nucleotide divergence seen in a comparison of the 5′ 420 bp of the S. pneumoniae sequences. The region encoding the C-terminal kinase domain is much more conserved, displaying a maximum of 1.77% variation in the remaining 3′ 906 bp of comD in a comparison of the same sequences. The distribution of coding changes is illustrated by an alignment (Fig. 4) of the six sequences characterized here with the published S. pneumoniae Rx ComD sequence. Transmembrane segments and the possible topology of the ComD protein were determined with the program TmPred (25a). This analysis suggested that the ComD protein most likely contains seven transmembrane helices, in agreement with the structure predicted for the ComD protein of Streptococcus gordonii (9). The approximate locations (amino acids) of the transmembrane helices were predicted to be as follows: 3 to 20 (outside-inside), 31 to 51 (in-out), 47 to 74 (out-in), 85 to 104 (in-out), 121 to 140 (out-in), 161 to 180 (in-out), and 188 to 204 (out-in). The vast majority of the coding variation in comD is confined to regions corresponding to amino acids 1 to 63 and 114 to 151, suggesting that the ComD receptor motif is located within a surface-exposed region contained within these sequences. When the ComD sequences of strains containing the two major S. pneumoniae comC alleles, comC1 and comC2, were compared virtually all variation was located in the region from amino acids 4 to 59; at least in strains containing these two major alleles, this result appears to narrow the location of the receptor segment to within this region. As might be expected, the comE-encoded response regulator protein is highly conserved even in strains with divergent comC and comD alleles (data not shown). Nucleotide divergence within comE ranged from 0.16 to 1.27% in the four S. pneumoniae strains examined in this study.
FIG. 4.
Alignment of the predicted amino acid sequences of the ComD proteins from streptococcal strains in comparison to the published S. pneumoniae Rx ComD sequence (19). Only residues which differ from the Rx sequence are shown. Identical residues are shown by dots. The active-site histidine residue of ComD is indicated by a number sign, while the predicted stop codon is indicated by an asterisk.
Horizontal gene transfer between com loci.
A clear example of a recombination event generating mosaic structure can be seen by comparison of the sequences of comCDE of S. pneumoniae Pn13 and Pn59 with the previously published sequence of strain Rx. Figure 5 shows an alignment of polymorphic residues seen in a comparison of these sequences. It can readily be seen that the sequences of the Pn13 and Pn59 comC and 5′ comD regions are very similar to each other and divergent from the Rx sequence. However, beyond nucleotide 621 (located within the comD gene), the sequences of Pn13 and Pn59 diverge such that the Pn13 sequence is characteristic of the Rx sequence. This observed mosaic structure, indicative of horizontal gene transfer, is significant at a P level of <0.01 (chi-square test) (17). Similarly, a comparison of the divergent comCD regions of Pn13 and Pn59 with the comCD region of S. mitis (NCTC10712) also suggests that the polymorphisms seen in Pn13 and Pn59 may be the result of horizontal transfer of DNA encoding both the CSP and the congruent receptor region of ComD from an S. mitis strain. These data support the previous observation that horizontal gene transfer, involving acquisition of the CSP and congruent receptor protein, has occurred during the evolution of the com locus (10).
FIG. 5.
Distribution of polymorphic sites among the sequences of the com operons of S. pneumoniae Rx, Pn59, and Pn13. Numbering, shown above the sequence, begins at the comC start codon, with residues identical to those in strain Rx shown by dots and gaps in the sequence alignment shown by dashes.
DISCUSSION
The data presented here characterize nine distinct CSP variants; five of these represent novel CSP variants not previously reported. However, all the CSP variants conform to previously described characteristics, being small cationic peptides with a negatively charged N terminus, with a positively charged C terminus, and possessing double-glycine-type leader peptides (6, 8). The leader peptides are highly conserved among all the CSPs, with almost every residue being identical or showing a conservative substitution (Fig. 1). Similarly, the negatively charged N-terminal amino acid and the arginine residue of the mature peptide are completely conserved as, to a large extent, are the three C-terminal arginine and/or lysine residues. In contrast, the central regions of the mature peptide are highly variable, suggesting that these are the regions which confer peptide specificity and which interact with the ComD receptor, while the flanking regions are functionally constrained.
Six distinct CSPs were found in isolates which have been classified as S. pneumoniae. The vast majority of S. pneumoniae isolates possess one of two major CSP variants reported previously (CSP-1 or CSP-2). However, the occurrence of closely related CSP-3 and CSP-4 in strains which were isolated about 20 years apart in different parts of the world but which are, as far as we are aware, representative of typical pneumococci suggests that a significant subpopulation of pneumococci which possess these very distinct CSPs may exist. CSP-3 was reported in a single South African isolate of a distinct serotype (22), indicative of probable long-term and worldwide prevalence of such isolates. The biological significance of these isolates is unclear, although one might expect that some genetic isolation could develop because of the possibility of reduced genetic exchange with other pneumococci.
Two other strains, 101/87 and 874, classified as S. pneumoniae and possessing distinct CSPs, CSP-5 and CSP-6, may represent either atypical or incorrectly classified organisms. In this respect, one should remember that distinguishing pneumococci and other members of the oral streptococci in the clinical microbiology laboratory can be problematic. Strain 874 was obtained by us as part of a study of the population genetic structure of carried pneumococcal isolates and was classified as S. pneumoniae by conventional criteria, including bile solubility, optochin sensitivity, and the results of an Accuprobe S. pneumoniae culture identification test (Gen-Probe). However, almost all housekeeping genes examined in this organism are distinct from those of other pneumococci that we have examined (unpublished data), raising doubts about the classification of this strain as S. pneumoniae. Demonstration that the CSP carried by this organism is virtually identical to that carried by an S. oralis isolate, Col19, supports such doubts. Strain 101/87 is a well-studied organism isolated from the blood of a patient with pneumonia; although initially classified as S. mitis on the basis of biochemical tests indicating a nontypeable alpha-hemolytic strain resistant to bile and optochin, it was eventually classified as an atypical S. pneumoniae strain on the basis of the use of specific DNA probes (2). Given the background of these strains, it was therefore not surprising to isolate novel CSPs from them.
There have been no previous studies of the genetic diversity of the comD gene of S. pneumoniae. Sequencing of the 5′ region of comD from 53 strains for which comC had already been characterized allowed a comparative analysis of the relationship between the alleles of these two genes. The region sequenced encompasses the most variable segment of comD and indeed contains virtually all of the diversity seen when the complete comD sequences of strains containing the two major comC alleles, comC1 and comC2, are examined (Fig. 4). Thus, the approach of sequencing only the 5′ region of comD is a valid one. Despite the inability in both this study and previous studies to induce competence even with the cognate synthetic CSP in up to 50% of strains, a complete correlation between comC alleles and apparently matching comD alleles was found in these strains. All CSP-1-containing strains had closely related comD alleles (comD1 group) which were substantially different from the comD alleles found in all CSP-2-containing strains (comD2 group). Thus, the inability to induce competence in many strains cannot be due to the lack of a ComD receptor protein which is cognate for the CSP. Other factors must therefore be responsible for the observed nontransformability of many strains; these could include capsule production (23, 26) or nonoptimal in vitro culture conditions.
In spite of the complete congruence of the comC and comD alleles seen here (i.e., comC1 with the comD1 group and comC2 with the comD2 group), both this study and previous studies have revealed data illustrating that horizontal gene transfer was involved in the evolution of the com locus. Håvarstein et al. (10) recently examined the comCDE operon from several representatives of the mitis and anginosus phylogenetic groups of streptococci (13) and reported three instances of apparent mosaic structure in comparisons of distinct Streptococcus gordonii or distinct milleri group isolates, indicative of horizontal gene transfer. In agreement with the findings reported above of cognate comC and comD alleles in all strains examined, all of the putative recombination events appeared to involve the transfer of comC and the 5′ region of comD (i.e., the receptor-encoding segment) in conjunction. Presumably, horizontal gene transfer events generating noncongruent comC and comD alleles must occur in nature but are either rare or rapidly selected against.
In the second part of this study, we used our primer set flanking comC to screen isolates of streptococcal species for organisms which might produce closely related CSPs. The rationale behind this strategy was that the horizontal gene transfer of DNA from other streptococcal species is believed to play an important role in generating the genetic diversity of S. pneumoniae. Mosaic structures which result from horizontal gene transfer have been demonstrated in a number of genes. For example, pbp genes, encoding penicillin binding proteins, display mosaic structures which impart β-lactam resistance and which are thought to result from the horizontal transfer of DNA from oral streptococci to pneumococci (4). Similarily, a number of genes encoding putative virulence factors of S. pneumoniae possess mosaic structures believed to result from the horizontal transfer of DNA originating in oral streptococci (5, 20).
In some of the instances of horizontal gene transfer reported above, the exact DNA donor has not been identified (3). Organisms containing closely related CSPs might be expected to act as donors in such events simply because their own CSP might be able to induce some degree of competence in S. pneumoniae, thus increasing the probability of uptake of their DNA. Despite the fact that Håvarstein et al. (10) have recently reported the presence of the com operon in all members of the mitis and anginosus phylogenetic groups, the primer set used here appeared to successfully amplify PCR products only from strains with very closely related com operons. Thus, for example, Håvarstein et al. (10) amplified the com operon by using primers to flanking tRNA genes from strains such as Streptococcus oralis NCTC11427 and Streptococcus sanguis NCTC7863, which were PCR negative in our study. A comparison of the recently published sequences of these com operons demonstrates that these sequences have only 58% homology and 74% homology, respectively, with the primer 3 sequence; thus, no PCR product would be expected under the rather stringent amplification conditions used in this study. However, our PCR primers did successfully yield PCR products from a small subset of S. mitis and S. oralis isolates and, as predicted, the comC sequences obtained from these organisms were generally more closely related to S. pneumoniae sequences than to other, previously determined S. mitis and S. oralis sequences. This apparent subset of S. mitis and S. oralis isolates may represent an important group of organisms, and it is possible that they are a source of DNA generating mosaic genes following horizontal transfer of DNA. We are now actively examining this possibility. These findings also support the growing belief that S. mitis and S. oralis may actually be rather poorly defined species containing a wide range of rather disparate organisms.
ACKNOWLEDGMENTS
This work was supported by grants from the Wellcome Trust, the Medical Research Council (United Kingdom), and the National Institutes of Health (United States).
We are grateful to Paul Pickerill and Maggie Yeo for expert technical assistance and to A. Efstratiou of the Central Public Health Laboratory, Colindale, United Kingdom, for providing isolates of S. oralis and S. mitis.
REFERENCES
- 1.Cheng Q, Campbell E A, Naughton A M, Johnson S, Masure H R. The com locus controls genetic transformation in Streptococcus pneumoniae. Mol Microbiol. 1997;23:683–692. doi: 10.1046/j.1365-2958.1997.2481617.x. [DOI] [PubMed] [Google Scholar]
- 2.Diaz E, López R, García J L. Role of the major pneumococcal autolysin in the atypical response of a clinical isolate of Streptococcus pneumoniae. J Bacteriol. 1992;174:5508–5515. doi: 10.1128/jb.174.17.5508-5515.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dowson C G, Coffey T J, Kell C, Whiley R A. Evolution of penicillin resistance in Streptococcus pneumoniae; the role of Streptococcus mitis in the formation of a low affinity PBP2B in S. pneumoniae. Mol Microbiol. 1993;9:635–643. doi: 10.1111/j.1365-2958.1993.tb01723.x. [DOI] [PubMed] [Google Scholar]
- 4.Dowson C G, Coffey T J, Spratt B G. Origin and molecular epidemiology of penicillin-binding-protein-mediated resistance to β-lactam antibiotics. Trends Microbiol. 1994;2:361–366. doi: 10.1016/0966-842x(94)90612-2. [DOI] [PubMed] [Google Scholar]
- 5.Dowson C G, Barcus V, King S, Pickerill P, Whatmore A, Yeo M. Horizontal gene transfer and the evolution of resistance and virulence determinants in Streptococcus. J Appl Microbiol. 1997;83:42S–51S. doi: 10.1046/j.1365-2672.83.s1.5.x. [DOI] [PubMed] [Google Scholar]
- 6.Håvarstein L S, Holo H, Nes I F. The leader peptide of colicin V shares consensus sequences with leader peptides that are common among peptide bacteriocins produced by Gram positive bacteria. Microbiology. 1994;140:2383–2389. doi: 10.1099/13500872-140-9-2383. [DOI] [PubMed] [Google Scholar]
- 7.Håvarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Håvarstein L S, Diep D B, Nes I F. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol Microbiol. 1995;16:229–240. doi: 10.1111/j.1365-2958.1995.tb02295.x. [DOI] [PubMed] [Google Scholar]
- 9.Håvarstein L S, Gaustad P, Nes I F, Morrison D A. Identification of the streptococcal competence-pheromone receptor. Mol Microbiol. 1996;21:863–869. doi: 10.1046/j.1365-2958.1996.521416.x. [DOI] [PubMed] [Google Scholar]
- 10.Håvarstein L S, Hakenbeck R, Gaustad P. Natural competence in the genus Streptococcus: evidence that streptococci can change pherotype by interspecies recombinational exchanges. J Bacteriol. 1997;179:6589–6594. doi: 10.1128/jb.179.21.6589-6594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui F, Morrison D A. Competence for transformation in Streptococcus pneumoniae: nucleotide sequence analysis shows comA, a gene required for competence induction, to be a member of the bacterial ATP-dependent transport protein family. J Bacteriol. 1991;173:372–381. doi: 10.1128/jb.173.1.372-381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press, Inc.; 1969. pp. 21–132. [Google Scholar]
- 13.Kawamura Y, Hou X-G, Sultana F, Miura H, Ezaki T. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int J Syst Bacteriol. 1995;45:406–408. doi: 10.1099/00207713-45-2-406. [DOI] [PubMed] [Google Scholar]
- 14.Kell C M, Sharma U K, Dowson C G, Town C, Balganesh T S, Spratt B G. Deletion analysis of the essentiality of penicillin-binding proteins 1A, 2B and 2X of Streptococcus pneumoniae. FEMS Microbiol Lett. 1993;106:171–176. doi: 10.1111/j.1574-6968.1993.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetic Analysis software for microcomputers. CABIOS. 1993;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz M G, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maynard Smith J. Analysing the mosaic structure of genes. J Mol Evol. 1992;34:126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- 18.Morrison D A. Streptococcal competence for genetic transformation: regulation by peptide pheromones. Microb Drug Resist. 1997;3:27–37. doi: 10.1089/mdr.1997.3.27. [DOI] [PubMed] [Google Scholar]
- 19.Pestova E V, Håvarstein L S, Morrison D A. Regulation of competence for genetic transformation in Streptococcus pneumoniae by an auto-induced peptide pheromone and a two-component regulatory system. Mol Microbiol. 1996;21:853–862. doi: 10.1046/j.1365-2958.1996.501417.x. [DOI] [PubMed] [Google Scholar]
- 20.Poulson K, Reinholdt J, Jespersgaard C, Boye K, Brown T A, Hauge M, Kilian M. A comprehensive genetic study of streptococcal immunoglobulin A1 proteases: evidence for recombination within and between species. Infect Immun. 1998;66:181–190. doi: 10.1128/iai.66.1.181-190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pozzi G, Masala L, Iannelli F, Manganelli R, Håvarstein L S, Piccoli L, Simon D, Morrison D A. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J Bacteriol. 1996;178:6087–6090. doi: 10.1128/jb.178.20.6087-6090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramirez M, Morrison D A, Tomasz A. Ubiquitous distribution of the competence related genes comA and comC among isolates of Streptococcus pneumoniae. Microb Drug Resist. 1997;3:39–52. doi: 10.1089/mdr.1997.3.39. [DOI] [PubMed] [Google Scholar]
- 23.Ravin A W. Reciprocal capsular transformation of pneumococci. J Bacteriol. 1959;77:296–309. doi: 10.1128/jb.77.3.296-309.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solomon J M, Grossman A D. Who’s competent and when: regulation of natural genetic competence in bacteria. Trends Genet. 1996;12:150–155. doi: 10.1016/0168-9525(96)10014-7. [DOI] [PubMed] [Google Scholar]
- 25.Tomasz A, Hotchkiss R D. Regulation of the transformability of pneumococcal cultures by macromolecular cell products. Proc Natl Acad Sci USA. 1964;51:480–487. doi: 10.1073/pnas.51.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Worley, K. C. BCM Search Launcher. 18 September 1998, revision date. [Online.] TmPred program. http://dot.imgen.bcm.tmc.edu:9331/seq-search/struc-predict.html. [9 April 1999, last date accessed.]
- 26.Yother J, McDaniel L S, Briles D E. Transformation of encapsulated Streptococcus pneumoniae. J Bacteriol. 1986;168:1463–1465. doi: 10.1128/jb.168.3.1463-1465.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou L, Hui F M, Morrison D A. Competence for genetic transformation in Streptococcus pneumoniae: organisation of a regulatory locus with homology to two lactococcin A secretion genes. Gene. 1995;153:25–31. doi: 10.1016/0378-1119(94)00841-f. [DOI] [PubMed] [Google Scholar]