Abstract
Scientists from around the world are studying the effects of microgravity and cosmic radiation via the “off-Earth” International Space Station (ISS) laboratory platform. The ISS has helped scientists make discoveries that go beyond the basic understanding of Earth. Over 300 medical experiments have been performed to date, with the goal of extending the knowledge gained for the benefit of humanity. This paper gives an overview of these numerous space medical findings, critically identifies challenges and gaps, and puts the achievements into perspective toward long-term space traveling and also adding benefits to our home planet. The medical contents are trifold structured, starting with the well-being of space travelers (astronaut health studies), followed by medical formulation research under space conditions, and then concluding with a blueprint for space pharmaceutical manufacturing. The review covers essential elements of our Earth-based pharmaceutical research such as drug discovery, drug and formulation stability, drug–organ interaction, drug disintegration/bioavailability/pharmacokinetics, pathogen virulence, genome mutation, and body’s resistance. The information compiles clinical, medicinal, biological, and chemical research as well as fundamentals and practical applications.
Keywords: Space, Medicine, Earth, Chemistry, Biology, Clinical
With the developments in advanced materials and technology, space exploration has now become more feasible than it has ever been before. With that motivation, scientists are pushing all boundaries of technology and science limitations to explore space. Yet, many aspects of space discoveries remain hidden and are waiting to be revealed.
Our vista to space has changed. Space is no longer the empty starry sky it might have been in the 1950s. It offers unique opportunities for mankind and has opened a space economy. Space provides a vantage point (communication, global positioning system, space data collection), harbors space resources, offers an ideal physical environment through microgravity, and is possibly part of the human destination (see Figure 1). The latter two are relevant for this publication and are seen as part of the “new space economy”, as compared to the old space economy with its satellites, rockets, propulsion fuels, etc.
Figure 1.

Usage domains (present, future) of space investigations with economic potential.
This paper will report on the pivotal problems to be solved in all kinds of space, i.e., the “Grand Challenges”, which is a broader and easier way for humans to access space while being able to conduct experiments as comfortably as they do on Earth. Here is where the problem sits: space has been known to be the harshest environment that people have experienced, as evidenced by factors such as extreme heating–cooling cycles, vacuum, cosmic radiation, and microgravity.1 There are many physiological adaptations and changes that occur in the human body during space flights, including fluid shifts,2 cardiovascular deconditioning,3 muscle and bone loss, immune system dysregulation, and gastrointestinal and metabolic disturbances.4,5 Space is not the environment in which humans are comfortable—one could say it is hostile.6
While there are means to shield subjects from temperature and vacuum, space radiation is the first fundamental property of space that is hostile for astronauts.7,8 Humans exposed to space radiation can have an increased risk of developing chronic health diseases, tissue degradation, and acute radiation syndrome. Because of these, meticulous space mission planning, mission timeline, and radiation protection methods are required for space exploration.8
The second hostile property of space for humans is microgravity. Since the human body is designed to live and function in the Earth’s vertical gravity force (1 Gz), prolonged exposure to weightlessness alters the functional capacity of the body systems and the materials that humans rely on, including medications.9
The third and last challenge to humans in space journeys is their isolation10 and an altered way of living, for example, not following the normal day/night cycle, courses of food uptake, balance between working and privacy, and so on. Humans have emotions and social needs, which make it necessary to create an environment in which humans feel comfortable. Related to this concern, studies have shown an increased likelihood of disease predisposition for humans on Earth in remote areas such as submarines11 and stations in Antarctica12 via research on probability per crew member, number of illnesses, and frequency of medicine intake. Data from research on-board space shuttles show that those threats are intensified.13 This threat holds even more so on the International Space Station (ISS) because of the prolonged exposure to the “hostile space environment”. The use of medicines, the most common intervention in healthcare, has been practiced in preventing, treating, and/or managing many illnesses or conditions. As a matter of fact, medicines have been used by 21–55% of all ISS crew members.13 Typical space adaptation symptoms that have been reported include loss of sleep, body pain, congestion/allergy, skin rash, headache, and more.14
The good news about these threats is that we have an advanced space laboratory that allows us to perform all kinds of testing on human health under real space conditions, to bring the average human safely into space. The ISS has facilitated many scientific achievements in the past 20 years, including foremost medicinal studies. Thousands of experiments have been conducted on the ISS, more than 300 of which are in health sciences.15 In this way, space researchers from space agencies, private companies, and universities are provided with the unique opportunity to access the testing facilities on the ISS U.S. National Laboratory.16 The ISS allows us to investigate space adaptation syndrome and to analyze the efficacy of medicines (pharmacokinetics/pharmacodynamics) in space.
The review given herein will report on how the unique settings of the ISS experiments are propelled by the usage of the most modern disruptive technologies for medicinal investigations, such as advanced microfluidics, tissue engineering, and nanoformulations. For instance, by combining three-dimensional (3D) printing technology and advanced microfluidics, researchers are now able to fabricate microphysiological systems (MPSs) or “organs-on-a-chip” to send to space.5,17 Research with these systems is also more ethical and sustainable, as it shifts from animal-based to cell-based investigations. Sustainability is a paramount prerequisite of any kind of space research and manufacturing.
Space medicine has become an essential part of human space exploration.18 Space medicine has been regularly used to improve the health state of all crew members.19
As mentioned earlier, the two prime “hostile sources” in space, being microgravity and radiation, impact not only humans but also organic matter such as plants, food, and medicines. As a matter of fact, drugs decompose faster and perhaps differently in space than on Earth.20 Plants are impacted by all kinds of space radiation (alpha, beta, gamma, ultraviolet (UV)), especially at high doses. Studies have shown significant changes in drug pharmacokinetics in humans during space flights;21,22 however, the essential data is limited and insufficient to draw conclusions. Many causes could account for the changes in drug efficacy, such as altered disintegration/dissolution and absorption through cell membranes. Other factors include convection-absence and density-irrelevant gastrointestinal digestion, circulatory blood flow, and drug–target (antibody) complexation. To date, the current knowledge on spaceflight-induced drug efficacy is still vague.23
This review aims to illustrate and critically evaluate the current state of the art of space life sciences research. This involves providing ISS research highlights and additionally pinpointing the difficulties associated with space research. The paper is divided into three parts: (1) space effects on the traveler’s well-being (astronaut health), (2) space effects on medicinal formulations and their drugs, and (3) the visionary concept of space pharmaceutical manufacturing (see Figure 2).
Figure 2.
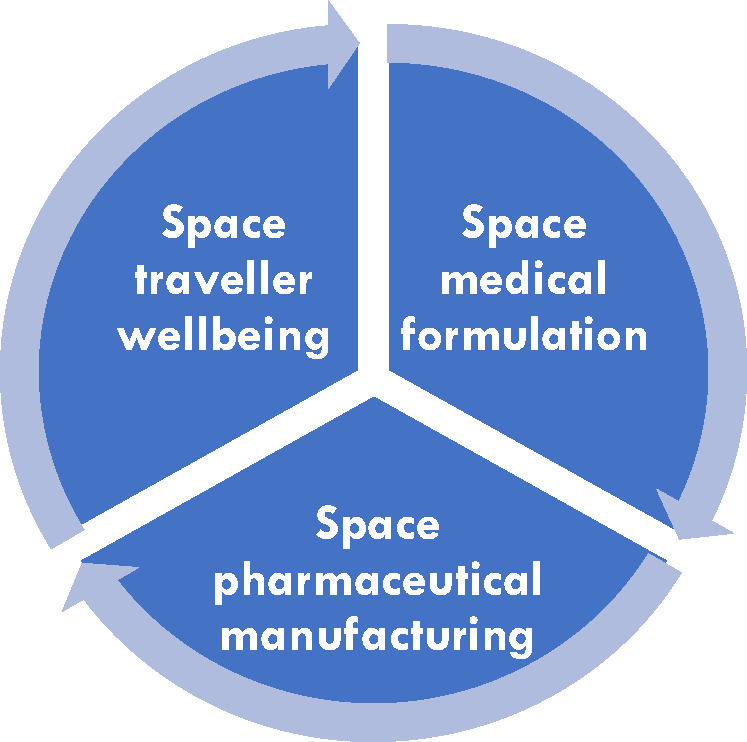
Trifold structure of this review: from human health (disease) and medicine efficacy (healing) to medication supply (manufacturing)—all in space.
1. Space Effects on Travelers’ Well-Being
Long exposures to weightlessness and space radiation can have some deleterious effects on human health (see Figure 3). The extreme environment induces a myriad of physiological reactions and adaptations.9 For example, microgravity-induced weightlessness experienced by space travelers fuels physiological changes across the cardiovascular system, where hydrostatic forces exerted on the heart and blood vessels are increased. This leads to structural changes in the heart, potential changes to the pumping action, and fluid redistribution of the blood and interstitial fluid centrally as opposed to the wider extremities of the body.25 Furthermore, astronauts often suffer discomfort from these changes and develop symptoms such as Space Adaptation Syndrome (SAS),26 which impacts 70% of astronauts.27 Consequently, many studies on the human and biological impacts of space flight have been conducted on ISS and previously on space shuttles or other spacecraft. These include studies on the shift of fluids inside the human body, resulting in swelling,9 distorted vision,28 and loss in taste and smell.29 The deconditioning of some physiological systems results, e.g., in musculoskeletal atrophy30 and dysregulation in human innate and adaptive immune systems.31 To elaborate, in the absence of countermeasures, astronauts’ bone mineral density decreases are estimated to be 1.5% at trochanter and around 1% at femoral neck and spine per month, respectively.250 Even changes in the human genome have been reported.32 These problems do not act alone but rather interact in an integrated human response, which may significantly damage a space traveler’s health.
Figure 3.
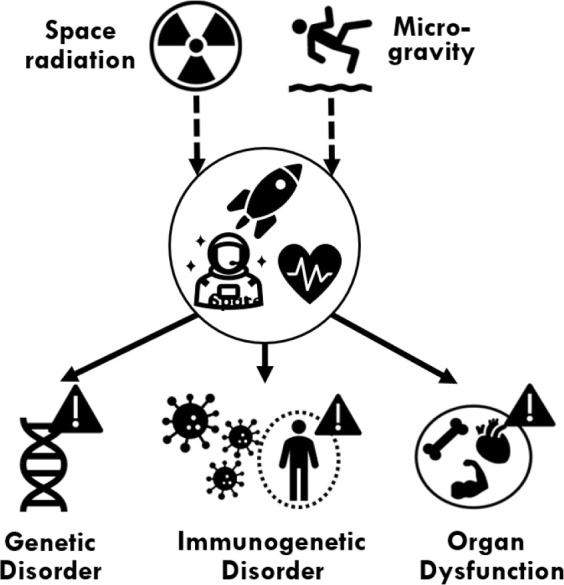
Effects of long-duration space travels on humans’ well-being.
Such studies help scientists map systematic and physiological health risks of pre, during, and post space flight. By doing this, the health risk of space travelers can be characterized and protected by reducing risks even after completing long-term space missions.33 A holistic healthcare provision for sustained human presence in space exploration is needed.
1.1. Health Risks from Space Dust
As part of extraterrestrial exploration, astronauts will be exposed to particles of planetary and interstellar dust. Astronauts reported having hay-fever-like symptoms when exposed to space dust during the Apollo missions. There is evidence that the exposure is hazardous and needs to be accounted for in the risk to human health.34 These claims are supported by the study reported in ref (35), where in mice, prolonged exposure to Mars and lunar dust simulants led to chronic inflammatory changes in the lungs. This is further corroborated by ref (36), which indicated that lunar soil simulants cause cytotoxicity as well as genotoxicity on neuronal and lung-derived cells.
1.2. Genetic Health Disorders in Space
Cosmic radiation affects the health of astronauts and can cause genetic disorders.7,8 Humans exposed to space radiation have an increased risk of chronic health diseases, tissue degradation, and acute radiation syndrome.8 With the current technology, astronauts’ trips are relatively safe for short-duration space missions. As far as future space travels are concerned, more countermeasures in radiation protection methods will be necessary, for instance, for a mission to Mars, far from the shielding of Earth’s magnetic field.37
Genetic damage was studied in astronaut twins on Earth and in space. Researchers can examine both genetic and environmental influences through twin studies, which provide important information on health and psychology.38 The study of identical twins allows researchers to identify the genetic and environmental factors contributing to a particular trait.38 Prior to the National Aeronautics and Space Administration (NASA) twin study, it was difficult to integrate the effects of the environment on human performance and health of previous space travelers due to the paucity of data.32 Through the twin study, NASA was able to continuously measure human biological “cues” to the hostile environment for up to a mission year. This revealed the vulnerability of humans in space travel and suggested countermeasures to extend human exploration into deep space.32
A twin study was conducted in which one twin brother was an astronaut who spent a year in space while the other twin brother stayed on Earth.32 The astronaut twin, Scott Kelly, was monitored before, during, and after a 1-year (total 25 months) mission onboard the ISS, while his twin, retired astronaut Mark Kelly, who genetically matches the astronaut, was monitoried to compare as a “ground control experiment”. Ten research teams worked together to observe the physiological, molecular, and cognitive changes that resulted from space flight.32
The assessments aimed to disclose space-flight-specific changes, such as body mass loss, telomere elongation, genome instability, carotid artery distension, ocular structure alteration, transcriptional and metabolic changes, cognitive decline post-flight, and more. In particular, the genetic assessment of the space-traveling twin, astronaut Scott Kelly, revealed dynamic changes in telomeres’ length expressed in CD4 and CD8 and lymphocyte-depleted cells during and post-flight. Telomeres are special features at the ends of each strand of DNA that protect the chromosomes. The finding of short telomeres points at an accelerated aging syndrome, which is normally considered to be due to inheritable gene mutations. However, these changes returned to near pre-flight levels within 6 months after return to Earth.32
1.3. Microbial Analysis of Pathogen Infection
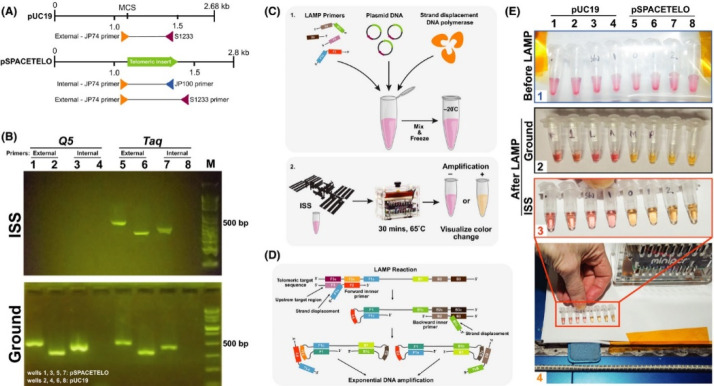
It is inevitable that astronauts are exposed to microbes during their mission, as occurs on Earth every day and everywhere. Yet the problem might be escalated by the facts that microgravity causes dysregulation in the immune system31 and that the immune response might be weakened in space. Therefore, pathogen studies are required to understand the cause and potential risk of microbial exposure during space flight. A fast and accurate microbial analysis is essential to human health because it provides vital information regarding a disease’s cause, enabling the patient to receive effective treatment based on the exact cause, thereby improving the outcome of patients,39 especially when they live in remote areas, e.g., the ISS. In this field, a series of NASA-supported pathological studies, known as Biomolecule Sequencer and Genes in Space, have been conducted.40 These studies provide real-time analysis and inform real-time decisions and remediation strategies during a space mission by using colorimetric loop-mediated isothermal amplification or rDNA nanopore sequencing (see Figure 4). These experiments help to promote health and safety in space travel, particularly in view of the diversity of the new space environments, ranging from the ISS cabin to extraterrestrial land, e.g., on the moon or Mars.41,42
Figure 4.
Pathogen studies to monitor microbial exposure: nucleic acid detection aboard the International Space Station by colorimetric loop-mediated isothermal amplification. Reproduced with permission from ref (42). Copyright 2020 Wiley-VCH.
Besides the efforts to identify the threat of viral infection by pathogens, it is necessary to ensure that the human immune response is strong enough to prevent hostile attack by those pathogenic microbes. In this sense, studies have been performed to show possible dysregulation in the human innate and adaptive immune system during space flight.31 The innate immune system is the body’s first line of defense against germs entering the body. For this reason, the U.S. National Institutes of Health (NIH) and the ISS U.S. National Laboratory have collaborated to perform three studies addressing the effects of microgravity on the immune response, as follows.
1.3.1. Immunogenic Response for Aging
As we plan for longer-lasting human exposure in space, the impact of aging in space on the dysregulation of the immune system needs to be determined. Tissue regeneration is the key parameter to be investigated. With that motivation, the effects on aging and corresponding changes in the immune system were studied by investigating late differentiation in CD8+ T-cells under microgravity. The focus was on the effects of bone healing and vascular regeneration for generative-specific stem cells, which is considered as the aging factor. In a translational way, the research provides insight into reducing the pro-inflammatory effect on elderly individuals on Earth.43 In-flight results are yet to be published.
1.3.2. Immunogenic Response for Lung Infection
The human immune response to a viral infection is complex. A clinical study investigated the immune response of lung cells to a Pseudomonas aeruginosa bacterial infection using MPSs. The innate immune system response of lung–bone–marrow cell tissue was studied under microgravity.44 In-flight results are yet to be published.
1.3.3. Immunogenic Response for Post-Traumatic Injury Recovery
Injuries are another condition that require a human immune response. As an example, post-traumatic osteoarthritis (PTOA) was studied using cartilage–bone–synovium MPSs. The research aimed to investigate the potential of an osteoarthritis drug to give a therapeutic effect on inflammatory cytokines and suppress the degradation of cartilage. This will facilitate to development of a therapeutic strategy for the inflammatory response to PTOA pathology. In the long run, the research may help solve the problem of chronic pain of osteoarthritis for humans on Earth and long-term space flights.45 In-flight results are yet to be published.
1.4. Immunogenic Response for Typhoid Fever Infection, Taken as the Monitor for the General Strength of the Human Immune System
Crew health needs to be defended against virulence-enhancing effects of microbes in space flight and can guide antimicrobial strategies on Earth.46 In this context, microbiology studies help to develop countermeasures against microscopic pathogenic organisms. In one study, the bacterial pathogen Salmonella typhimurium was grown aboard Space Shuttle and compared with identical ground control cultures. These bacterial causes typhoid fever in mice and its variants are considering as serious human pathogens. Mice infected with bacteria from the flight cultures displayed a decreased time to death increased percent mortality at each infection dosage, and a decreased LD50 value compared with those infected with ground controls. The space flight environment could provide insights into the mechanistic role in the regulation of microbial responses, in particular, concerning the role of Hfq as a conserved bacterial RNA-binding protein with complex roles in post-transcriptional gene regulation.47
The T-cells’ repertoires are surface proteins expressed exclusively by T-cells, which are white blood cells and essential parts of the human immune system.48 The T-cell’s repertoires function to recognize potential pathogens. Samples from spaceflight and ground control were analyzed before and within 10 days after trivalent flu immunization. The samples were taken at three time points: pre-flight, flight, and post-flight. CD4 and CD8 T-cells were analyzed from frozen peripheral blood mononuclear cells from the ISS. The ratio of CD4 cells to CD8 cells is a measure of how strong one’s immune system is and helps in predicting the sensitivity to infection. A lack of CD4 cells typically causes more affinity for infections. No significant differences in the percentage of unique CD4 or CD8 T-cell receptor sequences (vaccination-responsive clones) were found. Thus, this suggests that vaccination in space and on Earth occurs similarly, at least within the experimental settings of the vaccination response experiment considered.32
1.5. Microphysiological Systems—The In Vitro Equivalent to the Human Organs and Body
MPSs are also known as organs-on-a-chip technology and are a microfabricated platform technology designed to regain functional units of human organs in vitro. They provide realistic mock-up studies of the human body functioning via in vitro tissue and organ models. They are particularly powerful when combined with organoids, which represent miniaturized organs with a three-dimensional structure and multiple cell layers. Organ anatomic microstructure and basic organ functions are better maintained by this representation than any two-dimensional analogs. Studies with organoids embedded into an MPS can thus mirror the in vivo microenvironment (see Figure 5).17
Figure 5.
Organs-on-a-chip and their ISS studies.
Compared to the conventional 3D extracellular matrix, the MPS can reveal how medicines and nutrients interact with targeted cells in microgravity. These interactions provide information on drug efficacy and toxicity levels.17,49 With the MPS, researchers can address health conditions experienced by astronauts, such as musculoskeletal deconditioning, kidney stones, or fluid shifts. Furthermore, stem cells taken from space travelers can be used to create a unique MPS, facilitating personalized treatment for the individual.
This section aims to summarize current and recent organs-on-a-chip experiments on the ISS platform. Yet, our knowledge is limited here. The in-flight results of many studies have yet to be published, and there is a lack of both technical and procedural validation of experiments to guarantee reproducibility and recapitulation of results. Still, there is a common belief about the large potential MPS systems have to model human physiology diseases in vitro. Relevant experiments are summarized in Table 1.
Table 1. MPS Research Summary.
| function | cell type | maturity | ref |
|---|---|---|---|
| immune | late-differentiated CD8+ T-cells | mature | (43) |
| lung epithelial cells | mature | (44) | |
| musculoskeletal | primary proximal tubule epithelial cells (PTECs) | mature | (50) |
| distal tubule epithelial cell model | mature | (50) | |
| human primary muscle cells | mature | (51) | |
| cardiovascular | hiPSC-derived cardiomyocytes | premature | (52), 53 |
| blood–brain barrier | microvascular brain endothelial cells | mature | (54) |
| gut | colonic epithelial cells | mature | (55) |
| lamina propria-derived resident immune cells | mature | (55) | |
1.5.1. MPS Testing of Musculoskeletal Functions in Space
The absence of Earth’s gravity in space decreases the vertical loading on the musculoskeletal system. Astronauts experience decreased loading on the weight-bearing bones after long space flights. This could lead to resorption of calcium into the blood, leading to higher concentrations of calcium in the urinary tract, which may cause kidney stones.56 Furthermore, the bones and muscles undergo deleterious adaptations, which lead to conditions such as space-flight-induced osteopenia and muscular atrophy. These adaptations put the astronaut at risk of weakness and injury upon return to a gravity field.57 Rigorous exercise can mitigate these problems to some extent.5
To this end, an MPS was manufactured using proximal tubule epithelial cells to imitate the altered physiological functions of the kidney in microgravity, including ion transport and vitamin D bioactivation. The MPS study included also the simulation of the formation of kidney stones by growing oxalate microcrystals. This can provide insight into the mechanisms of stone formation in patients on Earth.50 In-flight results are yet to be published, but the kidney MPS system has been implemented in the study about CYP3P5, an enzyme that, when expressed, contributes to renal disorder.58 In this study, the MPS was used to gauge the safety of antisense oligonucleotides treatments on proximal tubule epithelial cells. The results suggest that both neutral phosphorodiamidate morpholino oligomers and negative-charged phosphorothioate oligomers for human papillomavirus treatments are well tolerated in the MPS when dosed at 1 μM for 5 days.58
1.5.2. MPS Testing of Muscle Atrophy (Loss of Muscle Tissue) in Space
Research on microgravity-related muscle atrophy on human specimens has been performed in recent years. Human myocyte samples from healthy volunteers aged under 40 and over 60 years were taken to investigate the effect of microgravity on cells or tissues in real time. Muscle contraction is stimulated by tiny electrodes integrated into the MPS that simulates a muscle, and a microscopic camera is used to capture real-time images and data of such stimulation.51
1.5.3. MPS Testing of Cardiac Functions in Space
Without the influence of Earth’s gravity, the fluids in the human body shift upward toward the chest and head. The upward shift of body fluids increases the pressure on the organs in these areas. As a result, space travelers are exposed to a greater risk of developing conditions such as space-flight-associated cardiovascular diseases. Cardiac MPS chips are being developed to study the effects of microgravity on cardiac tissue. These studies aim to provide an understanding of the progression of cardiovascular disease in vitro in short- and long-term space missions, with overviews given in refs (52 and 53). In this context, an MPS chip was developed to identify, monitor, and screen the prevention of phenotypic changes in microgravity, resembling ischemic cardiomyopathy.52 Another study investigated therapeutic strategies to provide insight into the progression of chronic heart disease on Earth.53 In-flight results are yet to be published.
1.5.4. MPS Testing of Neuron Functions in Space
In humans, organs are protected from chemicals or pathogens by barriers such as the skin, gut wall, or blood–brain barrier.5 The integrity of epithelial cell layers is critical for the organs’ immune response against harmful influences.59 MPS studies in this field help to identify the mechanism of neurodegenerative diseases like Alzheimer’s disease, Parkinson’s disease, and viral infections.54,55 In this context, Emulate, Inc., a provider for advanced in vitro models, used MPS technology to create two sets of organ-on-a-chip systems simulating the epithelial mucosa of the gut and the blood–brain barrier. The MPS samples were closely monitored and assessed to provide insights on the effects of the space environment travel stressor and bacteria on the function of the targeted organs in microgravity. Although the in-flight results are yet to be published, Earth-based research using similar blood–brain barrier chips has revealed key aspects of the mechanisms of endothelial dysfunctions and Parkinson’s disease.60
2. Space Effects on Medicines and Their Formulation
The above reports make it clear that diseases, which are a regular part of life on Earth, are unavoidable and likely intensified in space. Accordingly, astronauts need to have a well-stocked kit of medicines during their journeys in space, ideally on a standard even better than on Earth. A number of space medicine issues need to be solved, and some of them are space-specific (see Figure 6).
Figure 6.
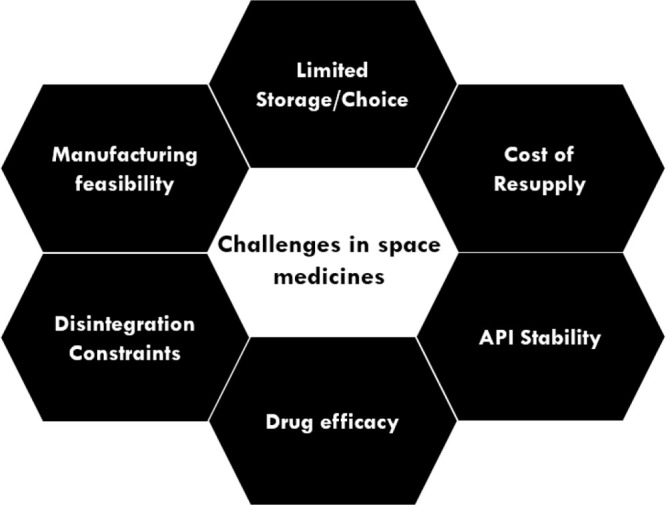
Challenges in space medicines.
These issues concern both the efficacy and stability of the space medicines. On today’s short missions, which rarely extend beyond 6 months per astronaut, the typical shelf life of medicines of 1–2 years is deemed to be sufficient. Yet, experimental confirmation is needed for the above claim. On top of that, with the current technological limitations on mass, volume, and power per flight, space exploration will have limits regarding drug storage and drug safety. Over and above, for a long space journey, such as a 2-year Mars mission (comprising two times 9-month trips traveling the shortest distance between Earth and Mars and a half-year stay), resupply is not feasible at all. And even if we could supply the mission with small payloads, current medicine kits would go beyond their expiry date. Previous studies have all been conducted in lower Earth orbit (LEO) and suffered from a lack of appropriate controls61,62 or sample size. There remains a need for conclusive data in LEO, and this is the time to begin studies beyond the LEO. Solutions to extending shelf life and/or manufacturing medication in space should be explored. We need to be able to fabricate medicines in space, which is further explained in section 3 of this review. Beyond all of that, the question remains as to how space investigations of medicines and their formulations can give us guidance for better medicines on Earth.
2.1. Space Radiation and Medicine Stowage
The efficacy of the active pharmaceutical ingredient (API) is a crucial performance quantity to be assured for space exploration. Medications stored at the ISS have been shown to be compromised compared to samples kept on Earth. This is because of a shorter shelf life of the space-located drugs.62−65 Besides microgravity and the repackaging of the drugs to fit into medical kits for space missions, space radiation is seen as one of the underlying causes of the instability of medicines is space, meaning the drugs and their formulations.66 It might even create decomposition products not found on Earth and potentially of higher toxicity than on Earth. Vice versa, studying the effects of space radiation can refine our understanding of medicine stowage and radiation protection on Earth.
Indeed, space radiation studies at the ISS have shown that the environment beyond the LEO is filled with radiation. Space radiation can come in various forms, including high-intensity photons, high-energy protons, neutrons, electrons, gamma rays, and heavy particles. Thanks to the Earth’s unique magnetosphere, the majority of heavy-charged radiation is deflected, and the radiation risk is remarkably reduced. However, there is still a significant amount of energy trapped in the Earth’s magnetic field (protons and electrons) as well as secondary radiation that also need to be accounted for.7,67−70 Thus, a human presence in the LEO and the more remote outer space requires protection against the effects of radiation.
One radiation study monitored the magnitude of radiation dose differences between the ISS and ground (Earth) (see Figure 7). The space-related dose increased largely with time and after 28 months was cumulatively about 20 times higher.65
Figure 7.
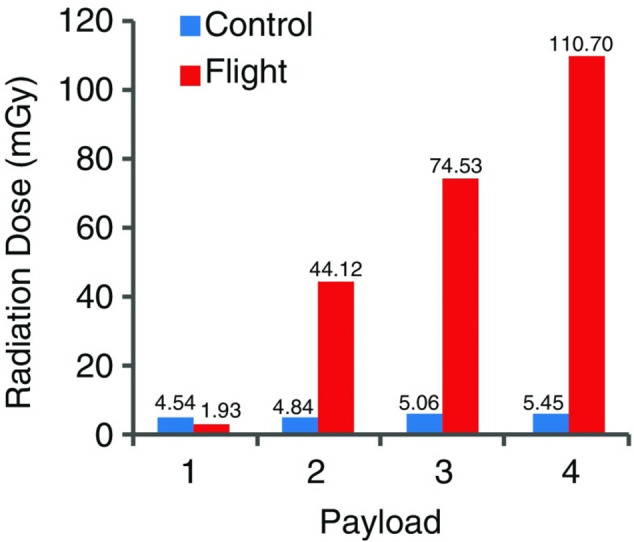
Cosmic ray dose at the ISS (flight) and on the ground (control). In cumulative order, the four payloads amount to a total exposure time of 28 months; payloads 1–4 amount to 13, 354, 597, and 881 days, respectively. Reproduced with permission from ref (65). Copyright 2011 Springer.
Liquid formulations are more prone to indirect ionization than solid ones.66 This is due to the high-energy radiation-induced radiolysis of water molecules to hydrogen and hydroxyl.71 These molecules will then diffuse themselves inside other molecules, which causes the destruction of API molecules.
2.2. History of Drug-Related Stability Studies on the ISS
Several APIs within drugs have been studied in space, and all over the past decade—meaning aboard the ISS (see Table 2).66 Yet, the vast majority has been studied in one mission with 35 drugs altogether, while the other five missions investigated only 2–9 APIs, including multivitamins. The studies included “API mixtures” (multivitamin tablets). Tablets are the prevalent dosage design form investigated on the ISS. The APIs on the ISS are designated as dietary supplements or prescription medications. Key findings were the following: (i) a vitamin B1 formulation is subjected to marginal degradation after 4 months of ISS storage;64 (ii) space-flight formulations undergo physical changes such as discoloration, liquefaction, or phase separation with higher rates than ground control counterparts;65 (iii) there are higher degradation rates in space flight than on ground for photolabile drugs;65 (iv) the degradation rate of promethazine, a photolabile drug, in a liquid formulation is double that of ground control samples;65 (v) some medications still meet United States Pharmacopeia (USP) requirements 5–8 months after their listed expiration date for space flight, while one failed to meet the USP standard after 11 months, before its expiration;62 (vi) riboflavin, vitamin A, and vitamin C in a multivitamin tablet decreased in API content by 10–35% after 880 days storage in space.63 Some of the studies given in Table 2 are discussed in detail below.
Table 2. History of Drug-Related Stability Studies on the ISS.
| group | name | formulation | comment | ref |
|---|---|---|---|---|
| vitamin | Centrum Silver multivitamin/multimineral supplements (Wyeth) | tablet | neglectable differences in API degradation between flight and ground control sample after 4 months storage in space | (64) |
| OneADay Women’s (Bayer) | tablet | formulation subject to marginal degradation after storage on ISS for 4 months | ||
| Centrum Silver multivitamin/multimineral supplements (Wyeth) | tablet | riboflavin, vitamin A, and vitamin C in the multivitamin decreased 10–35% after 880 days of storage in space | (63) | |
| Nature’s Way vitamin D supplements | tablet | neglectable differences in API degradation between flight and ground control sample after 880 days in space | ||
| nasal cobalamine | gel | formulation fails both physical and chemical potency standards after space storage | (65) | |
| antibiotic/antifungal/antiviral | acyclovir | tablet | formulation fails physical standard after space storage | (65) |
| amoxicillin/clavulanate | tablet | formulation fails both physical and chemical potency standards after space storage | ||
| azithromycin | tablet | neglectable differences in API degradation between flight and ground control sample after 880 days in space | ||
| cefadroxil | capsule | neglectable differences in API degradation between flight and ground control sample after 880 days in space | ||
| ciprofloxacin | tablet | formulation fails both physical and chemical potency standards after space storage | ||
| fluconazole | tablet | formulation fails both physical and chemical potency standards after space storage | ||
| imipenem/cilastatin | powder | formulation fails physical standard after space storage | ||
| levothyroxine | tablet | formulation fails both physical and chemical potency standards after space storage | ||
| metsronidazole | tablet | formulation fails physical standard after space storage | ||
| sulfametshoxazole/trimetshoprim | tablet | formulation fails both physical and chemical potency standards after space storage | ||
| ciprofloxacin | ointment | formulation fails both physical and chemical potency standards after space storage | ||
| clotrimazole | cream | formulation fails both physical and chemical potency standards after space storage | ||
| mupirocin | ointment | formulation fails both physical and chemical potency standards after space storage | ||
| silver sulfadiazine | cream | formulation fails both physical and chemical potency standards after space storage | ||
| ciprofloxacin | ophthalmic solution | formulation fails physical standard after space storage | ||
| immune system | levothyroxine | tablet | formulation fails chemical potency standard after space storage | (65) |
| loratadine | tablet | formulation meets the former USP requirements 5 months after its listed expiration yet fails to meet updated USP standard | (62) | |
| promethazine | semi solid | formulation fails both physical and chemical potency standards after space storage | (65) | |
| promethazine | injection | formulation fails chemical potency standard after space storage | ||
| NSAID | acetaminophen | tablet | formulation meets the former USP requirements 5 months after its listed expiration yet fails to meet updated USP standard | (62) |
| aspirin | tablet | formulation meets the former USP requirements 9 months after its listed expiration | ||
| ibuprofen | tablet | neglectable differences in API degradation between flight and ground control sample after 880 days in space | (65) | |
| ibuprofen | tablet | formulation meets the former USP requirements 3 months before its listed expiration | (62) | |
| loperamide | tablet | formulation meets the former USP requirements 5 months after its listed expiration yet fails to meet updated USP standard | ||
| triamcinolone | cream | formulation fails physical standard after space storage | (65) | |
| musculoskeletal | furosemide | tablet | formulation fails chemical potency standard after space storage | (65) |
| risedronate | tablet | formulation fails chemical potency standard after space storage | ||
| cardiovascular | atorvastatin | tablet | neglectable differences in API degradation between flight and ground control sample after 880 days in space | (65) |
| metoprolol succinate | tablet | formulation fails chemical potency standard after space storage | ||
| pseudoephedrine | tablet | formulation meets the former USP requirements 9 months after its listed expiration | (62) | |
| central nervous system | dextroamphetamine | tablet | formulation fails both physical and chemical potency standards after space storage | (65) |
| phenytoin | capsule | formulation fails chemical potency standard after space storage | ||
| progestin/estrogen | pack | formulation fails chemical potency standard after space storage | ||
| sertraline | tablet | formulation fails chemical potency standard after space storage | ||
| temazepam | capsule | formulation fails chemical potency standard after space storage | ||
| melatonin | tablet | formulation failed both API and degradants/impurities assays 11 months after its listed expiration | (62) | |
| modafinil | tablet | formulation meets the former USP requirements 2 months before its listed expiration | ||
| zolpidem | tablet | Formulation meets the former USP requirements 9 months after its listed expiration | ||
| epinephrine | injection | formulation fails chemical potency standard after space storage | (65) | |
| lidocaine | injection | formulation fails chemical potency standard after space storage | ||
2.3. Cosmic Ray Stability Study of the ISS Medical Kit, with 35 API/Formulations
One experiment investigated the long-term cosmic stability of formulations that were stowed for 28 months in the ISS medical kit in space and compared their physicochemical changes to ground control samples on Earth.65 The actual ISS medical kit itself, with its 35 formulations, was investigated, which gives the investigations authenticity. The research team included members from diverse clinical laboratories, universities, and NASA. Four payloads corresponded to four different stowage periods: 13, 354, 597, and 881 days, respectively (the latter being about 28 months). In that period, the radiation dose increased cumulatively with time in space. Temperature and humidity were kept similar to those on the ground to focus solely on the radiation dose effect.
The study delivered extensive insight into the cosmic-ray-induced decay of the drug formulations. First, while Earth samples exhibited alterations, space conditions accelerated medicine degradation: 6 medications aboard the ISS and 2 of matching ground control samples changed measurably. The second question is if space and Earth medicines can still be used in legislative terms, which is a matter of providing a threshold API content: 9 medications from the ISS and 17 from the ground met the USP acceptance criteria. A higher percentage of medications from each flight kit had lower API content than the respective ground controls. Also, and as expected, the API content decreased as a function of time in space. This seems to be decoupled from the Earth expiration date, which means that medicines with supposed long expiry dates also decreased in API content under space conditions. The API content is not the only relevant physicochemical quality. In fact, dissolution is also very much relevant. The investigated solid dosage forms met the USP standard for dissolution after storage in space.
In another study of the same motivation, nine medications were analyzed after a stay on ISS.62 Eight of the medications still contained their API within acceptable limits.
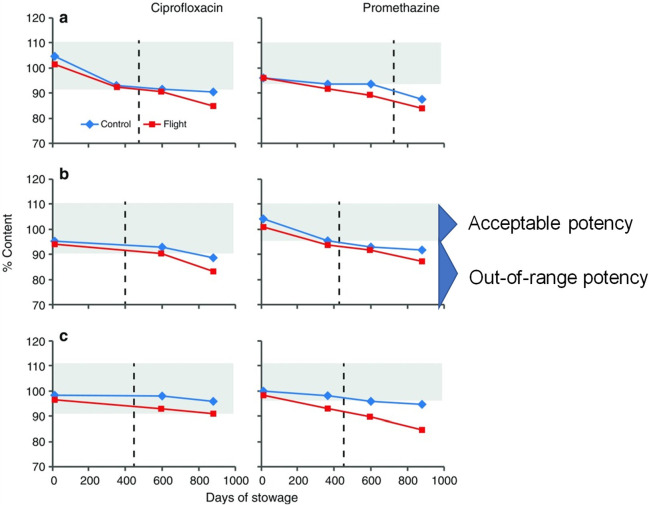
Coming back to the above-mentioned signature study,65 the stability of medicines as per dosage design form was investigated. Solid formulations were generally found to be more stable than liquid ones, with semi-solid ranging in between. Figure 8 shows the performance for two APIs and also after which time of exposure their stability has run out of the potency specifications.
Figure 8.
Cosmic ray dose at the ISS (flight) and on the ground (control) in cumulative order for four payloads, amounting to a total exposure time of 28 months. Payloads 1–4 amount to 13, 354, 597, and 881 days, respectively. Reproduced with permission from ref (65). Copyright 2011 Springer.
2.4. Role of Diffusion in Crystal Formation and Absence of Convection
The crystal size and shape of a drug are of crucial importance in formulation manufacturing and the drug’s bioavailability (including disintegration/dissolution).72 On top of that, drugs occur in different kinds of crystal polymorphism, meaning different intermolecular arrangements for the same type of molecule.73 Drug polymorphs exhibit different dissolution and bioavailability.74 In a word, having control over the crystal’s characteristics and its formation process is essential to define the function of a drug and a formulation.
Crystal formation is largely governed by mass transfer, and the control of diffusion and convection are the major tools here.75 In this way, the seed formation and crystal growth are decided. Crystal formation is also driven by heat transfer and the exact setting of cooling rates, having uniform temperature profiles.75
In microgravity, however, molecular processes that on Earth support the matter distribution such as natural convection are absent, e.g., facilitated by buoyancy or density convection.76 This absence actually is ideal for crystal formation, as it leaves diffusion as the only driving force, which is a theoretically very well understood physical process with uniform mass-transfer settings.77 Convection, in contrast, while being effective in speeding up the mass transfer, is a complex matter-moving process77 in which mass transfer varies in space and time. Hence, convection leads to non-uniform “crystal formation histories” 78 and non-uniform crystals. As a result, a strict diffusion-ruled environment is deemed “ideal” for processing of crystals. While we can stop forced convection (by the absence of stirring and shaking), we cannot eliminate natural convection. Therefore, space provides the ultimate diffusion control.
2.5. Pembrolizumab Crystal Formation and Processing Consequences
Merck Company investigated their cancer immunotherapy drug Pembrolizumab (Keytruda) and found that the crystals formed in space were monomodal, while the crystals formed on Earth were bimodal, meaning the latter showed two bands of size distributions with two maxima.79 The bimodal maxima were larger and smaller, respectively, than the monomodal maximum. A good API should have a well-defined monomodal distribution of spherical shape, for both the space and Earth samples, and small size.
The Merck studies also provided meaningful insight into the formulation processing capabilities of the crystal suspensions, seen also in glasses for future pharmaceutical manufacturing in space. Viscosity is a key quantity here, ruled by the crystal size and shape. The space crystals were less viscous and, accordingly, sedimented more uniformly than the ground-based crystalline suspensions. This space development could thus guide the production of crystalline suspensions on Earth, concerning the choice of rotational mixers as suitable equipment to reduce sedimentation and temperature gradients for improved crystallization. As an outcome, Earth-made uniform crystalline suspensions (1–5 μm) with acceptable viscosity (<12 cP) were prepared which met the rheological and syringe-ability specifications. This was deemed suitable for injection formulations, with increased storage and drug delivery options for patients on Earth as well as astronauts.79
The physical state of the formulation is also important. It is proposed that liquid pharmaceutical formulations are more susceptible to degradation than solid formulations.66 This is because drugs in a dissolved state are more prone to radiation activation than those in a solid state. Yet, then the matter of administration and its speed becomes an issue, which can be steered by choosing the type of formulation: solid, semisolid, or liquid. Solid formulations such as tablets are on the slower side for administration, this being a matter of the speeds of disintegration and dissolution which majorly determine the time it takes until the drug reaches the blood circulatory system.
2.6. Role of Proteins in Drug Discovery
Protein crystallization is a central process in drug discovery, as it reveals the macromolecular fingerprint and template of the disease. It helps elucidate the crystalline structure of antibodies and other relevant protein sites which are deemed to be the “lock” to which the API (drug) is the “key”. It enables the elemental molecular understanding of the origin of the disease and how diseases can be antagonized. It is the basis for a rational design of drugs.
As outlined in section 2.4, the microgravity environment of the ISS is ideal for crystal formation due to allowing diffusion to be the only physical mechanism for the transport of matter. Thus, for the past 30 years, researchers have been using microgravity to improve the quality and uniformity of crystals. To date, more than 10,000 protein samples have been investigated on the ISS. Understanding protein crystallization provides insight into health and disease treatment.80−82
2.7. Big Crystal Formation in Space to Facilitate Structure Elucidation
In microgravity, the exclusive presence of diffusion slows down mass transport, and, as a consequence, a lower number of seeds is formed in the first stage of crystal growth. This means bigger crystals are formed from the same mass of material. To expand on this finding, the Japanese Aerospace Exploration Agency (JAXA) focused on the formation of bigger crystals and brought the larger crystals back to Earth for the elucidation of the crystal structure, since larger and more consistently shaped crystals provide more analytical insight.83 The first investigations were made in 2003 and continued in 2009 using the “Kibo” cell unit in collaboration with the Russian space agency (ROSCOSMOS) and the National Aeronautics and Space Administration (NASA).
The study utilized the counter diffusion method for crystallization in capillary tubes at 20 °C. The space-manufactured protein crystals had outstanding properties, e.g., concerning the crystallization yield, protein purity, and protein 3D structure (see Figure 9).84−86 As shown in Figure 9, the protein crystals grown in space had greater degree of order and perfection compared to those grown on Earth.80
Figure 9.
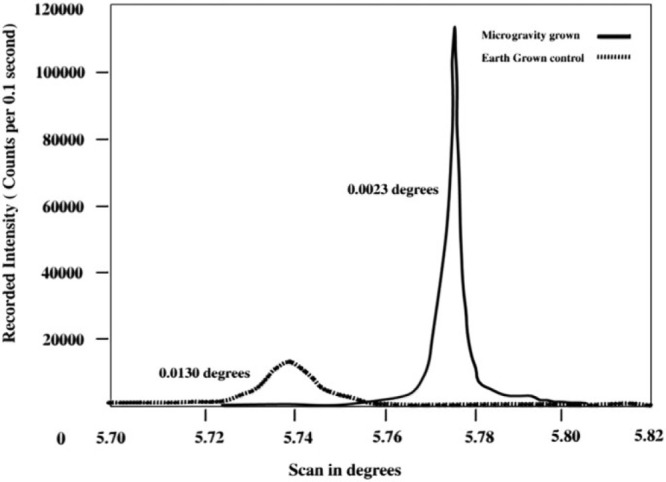
Protein crystals produced on the ground and in space. Reproduced with permission from ref (80). Copyright 2015 Springer Nature.
2.8. Monoclonal Antibody Crystal Growth in Space
Merck has developed sets of space experiments termed “Microgravity Growth of Crystalline Monoclonal Antibodies for Pharmaceutical Applications (CASIS-PCG)”, focusing on protein crystal growth, to gain a better understanding of therapeutic agents and target diseases including cancer. The experimental results from Reichert et al.79 showed the positive effect of microgravity on the uniformity of producing protein crystal particle size distributions compared to their Earth counterparts. The result was then applied to produce uniform crystal suspensions suitable for injectable formulations, which has increased storage and drug delivery options for patients on Earth as well as for astronauts.79
2.9. Artificial Retina Crystal Growth in Space
Using the knowledge of protein crystal growth in space, LambdaVision aims to develop a method to grow artificial protein-based retina crystal for retinal implantation. This would open a treatment option for patients suffering from blindness. The aim is to implant the space-manufactured bacteriorhodopsin-based protein crystal in a subretinal position to replace degenerated cells. The LambdaVision study targets the mechanism of light activation in proteins, particularly bacteriorhodopsin. The use of the photosensitive molecule bacteriorhodopsin is investigated for fabricating artificial retina implants. Microgravity allows researchers to perform crystallization of the individual layers of the artificial retina homogeneously and with few imperfections. In addition to the health benefits, space-manufactured retinal implants could reduce the manufacturing costs of visual aids87 and, by extension, have applications in 3D data storage, chemical sensing, and photovoltaic devices.
2.10. Tablet Disintegration and Dissolution under Strict Diffusion Control in Space
While the absence of diffusion helps form perfect crystals, it is detrimental to some processes where the speed of matter transfer counts. Convection is very helpful here on Earth but absent in space. Knowing this, we need to learn, in the future, how to design tablets and formulations in space that operate purely on diffusion. Vice versa, pure diffusion-controlled tablet disintegration/dissolution studies might provide fundamental insight which is inaccessible on Earth.
Eli Lilly Company has performed an ISS-based suspension dissolution experiment to study the effects of surface wettability and buoyancy in dissolution on pharmaceutical products, from a starting point of a disintegrating tablet in an aqueous solution. While the official report is not yet published, preliminary results show that dissolution in microgravity is significantly slower than anticipated. That was expected since, in space, buoyancy or density convection does not assist the dissolution of particulates. A key observation was the formation of a gel interface between the tablet and solution, which does not occur to the same extent on Earth. The studies were deemed to show potential for the innovation of formulation design in space and on Earth.88
2.11. Bioavailability Studies in Space
Pharmacokinetics and pharmacodynamics have been studied on the ISS for the absorption of paracetamol and scopolamine. Both APIs changed in flight as a function of time and API.89,90 The physiological changes due to the space environment affect drug absorption, distribution, metabolism, excretion, or transport (ADMET), the elements of pharmacokinetics.4 Pharmacology is an important area in which to establish the role of human adaptation, as well as pharmacogenomics and the environmental role of pharmaceuticals. Furthermore, some of the body’s tissues undergo changes during space missions. For instance, bone and muscle undergo atrophy, which can reduce the targets for paracetamol, with the potential to alter drug efficacy. The existing bioavailability data from space flights are dated, fragmentary, and inconclusive.4 Data from deep-space missions are entirely lacking. With ISS studies reporting on the dissolution of solid formulations, the next logical step would be to undergo studies that are more comprehensive on the whole disintegration/dissolution/bioavailability process.
As another piece of evidence based on space analog studies demonstrating space medicine effectivity, pharmacokinetics was determined for acetaminophen formulated in tablets and capsules using healthy volunteers after single peroral administration, both under Earth living conditions and during long-term ISS presence.91 The absorption of acetaminophen was delayed in the ISS onboard studies and differed substantially from the Earth routine (see Figure 10).
Figure 10.
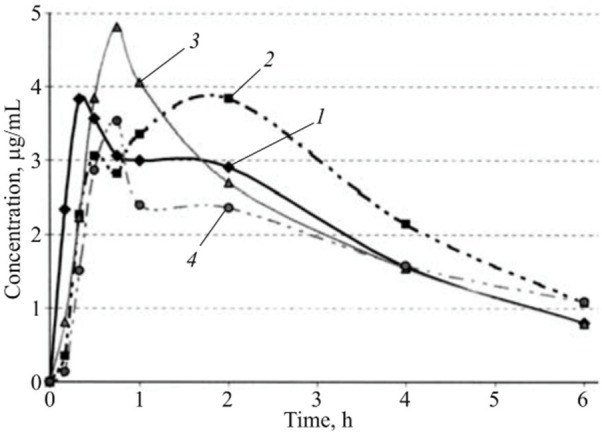
Average pharmacokinetic curves of acetaminophen: tablets, ground control (1); tablets, space flight (2); capsules, ground control (3); capsules, space flight (4). Reproduced with permission from ref (91). Copyright 2009 Wiley-VCH.
The drug concentration in ISS volunteers was below the threshold for a regular API efficacy. The research compared the dosage design form of paracetamol as tablet and capsule formulations. Under microgravity, the rate of drug absorption from tablets was substantially lowered, while the bioavailability increased to the same extent. The capsules showed in line with the tablet measurements, a reduced absorption time change while exhibiting the same bioavailability on Earth as in space.91
2.12. Controlled Drug Delivery in Space
Drug administration can be done in various ways, and the main concern on Earth is controlling its speed, meaning how long it takes for the drug to reach the circulatory system. This can be steered by choosing the type of formulation: solid, semisolid, or liquid. Solid formulations such as tablets are on the slower side, while liquid formulations are delivered faster, as they allow direct injection into the blood circulatory system or muscle tissues close to it. On the other hand, sometimes the disease demands a long-lasting, slow release of the drug, e.g., over days to months. A controlled release for drug delivery is demanded. The question is how that all operates “in space”.
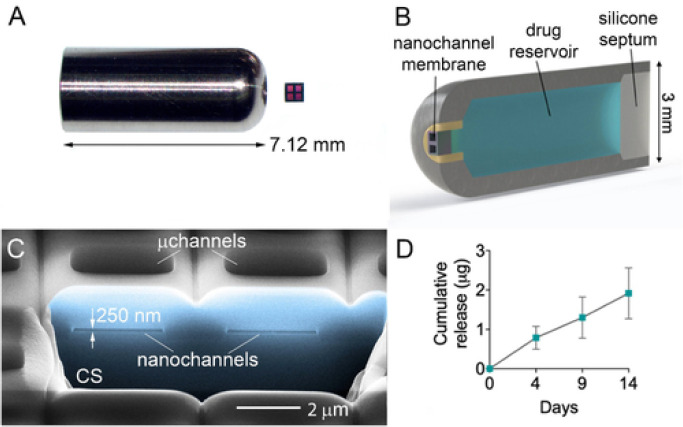
Nano-implantable medical devices are cutting-edge solutions for controlled drug delivery in the medical field.92,93 A study facilitated by the ISS U.S. National Laboratory treated muscle loss with continuous delivery of a low dose of medicine to a patient system through a tiny implantable unit with a channel that is in the nanometer range94 (see Figure 11). The concept was tested on healthy mice in a real space environment in the Rodent Research 6 mission. In this study, (i) a mechanism of controlled-release profile for formoterol through the nanofluidic membrane in microgravity condition was established (136 μg of formoterol per day); (ii) the sustained release at a low dose of formoterol induced an anabolic effect on muscles on healthy mice without causing cardiac hypertrophy; and (iii) the prevention of muscle atrophy in mice at the ISS using the nano-implantable device was not as effective as compared to the ground control unit. In essence, the nano-implantable device delivered a substantial dose of API, which amounts to a third of the subcutaneous injection dose. Thereby, the daily injection dose can be at least reduced. These results lay the foundation for future drug delivery systems and benefit the health of astronauts and space travelers in the future.94
Figure 11.
Nanofluidic implantable drug delivery device. Reproduced with permission from ref (94). Copyright 2020 Wiley-VCH.
3. In-Space Manufacturing
To mitigate the risk of medication-related problems, safe and effective medication management via evidence-based systems and interventions needs to be developed and implemented. Medication management has been carefully planned out to ensure a sufficient supply of medication for each space mission. However, for extended space journeys, resupply missions may not be feasible, and the medicine itself may exceed its safe shelf-life. A potential solution here is in-space manufacturing. Manufacturing medicine in space is a challenge. The cargo capacity is restricted to carry drugs and their formulations, which restricts finally the choice of drugs available. We cannot take a full “pharmacy” to space, and it would be expensive to bring an entire pharmaceutical factory to space. Even so, this would works only when using pre-assembled modular units which then are finally erected in space to build the whole pharmaceutical plant—like the container plants on Earth, known for flexible campaign manufacturing (see Figure 12). It makes sense to do that exercise on a bench-scale format and to learn from that on the main performance issues, one of which is flexibility in producing medicines for individual use. It is also important that these innovations consider encompassing an international harmonization of appropriate drug licensing, regulation, and quality assurance structures appropriate for the space sector.
Figure 12.
Assets of on-board space manufacturing.
A miniaturized portable system for on-demand pharmaceutical formulation has been studied, called Bio-MOD, or Biologic Medications on Demand (see Figure 13). Bio-MOD represents the concept of lab-on-a-chip, which can move a small number of chemicals from one operation to another in small microfluidic channels/chambers to make the final product, which is the API. The goal is one big and demanding step further, namely to make personalized medicines in a short response time—the authors quoted less than 24 h.95 In this sense, the Bio-MOD was used to study on automatic end-to-end manufacture of His-tagged Granulocyte colony-stimulating factor protein, which includes production, purification, and collection of products.96 In this study, the Bio-MOD was shown to have an ability to process 2–10 mL of lysate per 8.5 h run.96 Although the production quality, yield, and product uniformity are not comparable to industrial processes, this has confirmed the feasibility of in situ manufacturing of medication.
Figure 13.
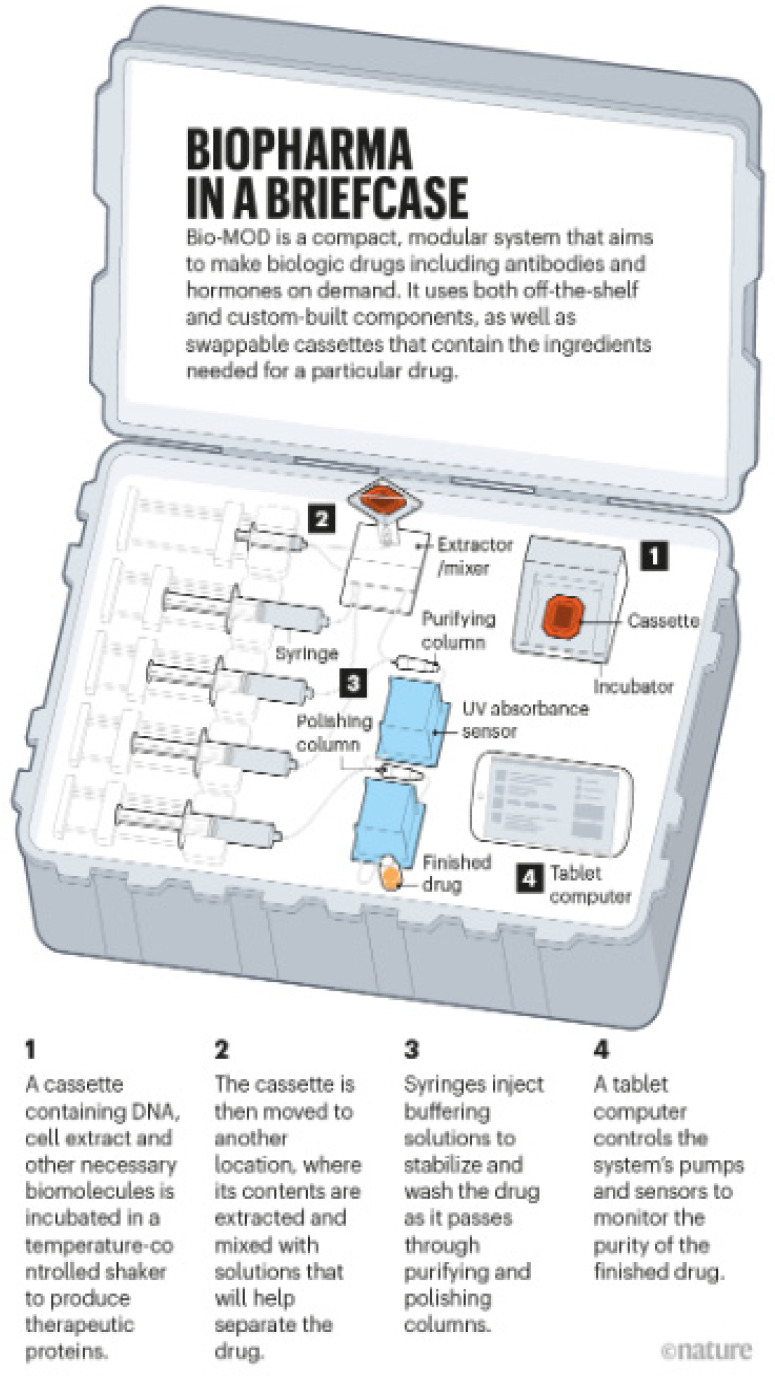
Bio-MOD briefcase on the pharmaceutical formulation. Reproduced with permission from ref (97). Copyright 2019 Nature.
Since 2014, 3D printing has been a part of space exploration.98 In an isolated environment like space, 3D printing is a perfect solution. The technology allows on-site, on-demand manufacturing in space. Advancement in 3D printing technology has created the possibility of 3D printing medicinal tablets.99 This makes it possible for individualized medicine, where the patient’s dose can be customized according to the patient’s physical condition.99 Additionally, advances in 3D printing tablet technology also allow printed tablets to be formed into specific shapes and structures that are designed to control the release rate.99 The future of drug manufacturing and delivery could be transformed by this technology.
The miniaturized system discussed in the previous paragraphs still needs to be fed with raw materials supplied from Earth as payload. However, for long space missions, all digestible contents such as food and medicines will need to be renewed as done on Earth. This ultimately requires manufacturing using local materials, taken from celestial bodies such as the moon and Mars.
A running ISS experiment aims to study the concept of tablet manufacturing solely from “moon simulants”, which are inorganic materials known to exist abundantly on the moon. Strictly speaking, these “moon simulants” hold only for their chemical identity, while other parameters like particle size distribution might be different. A mix of six lunar-simulant materials was taken as pharmaceutical excipients, forming a tablet, in which ibuprofen and vitamin C were distributed as APIs. Ibuprofen was chosen as the second goal of the ISS experiment is a long-duration study of the effects of cosmic rays as well as formulation-based countermeasures to enhance its stability. To the authors’ best knowledge, ibuprofen is one of the best APIs to study photodegradation, as much is known about the photodecomposition products and kinetics.100
Three shielding concepts were applied against the cosmic radiation: (i) the mere dilution of the API in the excipient matrix, (ii) a coating of the tablet with assumed cosmic ray shielding material, and (iii) a molecular complexation of the ibuprofen with the excipients.
The “lunar-made” tablet research is in two running ISS-NASA experiments, investigating the long-duration stability of ibuprofen in the tablets under real cosmic irradiation. In total, 60 and 6 tablet samples were compounded and sent to the ISS for 9 and 12 months in real-space conditions inside and outside the ISS, respectively (see Figure 14). First, on-Earth radiation results on alpha, beta, and X-ray (gamma) decomposition of the tablet specimens are at hand yet are not published and thus are not discussed here. The on-Earth radiation dose equals the cumulative space condition.100
Figure 14.

(Left) MISSE-14 installation on the MISSE-FF lifting the long-duration cosmic-ray stability experiment of the University of Adelaide toward outer space exposure, outside of the ISS (right). The same ISS experiment with the ibuprofen tablet samples inside the ISS U.S. National Laboratory facility. Photo credit to NASA.
To sum up, space experimentation under the surveillance of microgravity provides an outstanding opportunity for scientists to conduct unique health research to improve the well-being of astronauts in long space flights. Impressive records of human benefits have been produced by space research and exploration. This critical review provides a refined collection of some recent advancements in medical research over the past few years and aims to make their essence in innovation transparent by putting the experimental findings into a structured format. This review outlines how this can lead to positive effects in improving the quality of life for humans on Earth.
We also identified gaps in the research and knowledge related to medication management, especially in in-space manufacturing, formulation, and drug stability. Space investigation is conducted in a relatively short time with limitations in laboratory footprint and research personnel. Hence, the intensity of the research is not on par with what is known for Earth-based research. As a result, we largely refer to single results, giving an isolated view. Also, the repeatability and reproducibility were hardly practiced, further complicated by a lack of a comprehensive research program. This is especially evident for the testing of space medicines and their formulations, whereas human health issues and impacts of space pathogens are better documented in space research and literature. For instance, in a study on the cosmic radiation stability of drugs, there is a hypothesis that drug profiles change during space flights due to the radiation effect. However, the effect happens in a complex manner with many variables, like type of drug, drug formulation, flight duration, and possibly local radiation conditions, which make it difficult to prove the claim. This asks for a large, holistic future space research program, and thus highlights the need for a greater quantity of experimental payload studies with appropriate ground controls to produce reliable and consistent results.62,65
Seen on the positive side, the experimentation on the ISS has provided basic information on the crystal size of drugs and proteins, drug-formulation disintegration, dissolution, absorption (bioavailability), and drug pharmacology. Some results seem to have the potential for groundbreaking developments, both in space and on Earth. The number of experiments is steadily increasing, and major global pharmaceutical groups are investing in ISS experiments.
The conceptualization of space-specific medicines and space medication is just at the onset. As of now, astronauts are still treated by Earth’s medical solutions. Finally, it is unlikely that space exploration will occur without the onboard manufacturing of space medicines. Here, a substantial groundwork still needs to be done. To accomplish this, the above-mentioned knowledge on ISS medicinal testing and the even larger learnings about human health in space need to be brought together. This waits for its uptake.
4. Outlook
Investing in space in the research and development of medicines has gained a lot of interest as space is now affordable and accessible to private companies. The Center for the Advancement of Science in Space (CASIS) data from various corporations, private companies, medical centers, and governmental services shows the rising interest in space medicinal research in 2021. A large portion of 35% of the market investments was made in research equipment for the International Space Station (ISS). Bioengineering solutions for astronauts and technologies for the biotechnology industry were equally at 25%. And the remaining portion of the market investment was equally split between the three sectors, i.e., longevity study, telemedicine, and research design.101
Furthermore, while this review aims to show the breadth of space medicinal investigations and the value of that research, it goes without saying that access to space experiments is limited and costly and will stay that way in the next years. In contrast to Earth conditions, there are more limitations in experimentation procedures in space, due to the smaller laboratories, shorter time, and fewer personnel. In view of this, the question is if space-simulated medicinal research on Earth may add to or complement authentic in-space experimentation.
Space microgravity research is challenging and highly competitive due to insufficient flight opportunities. One way out of this dilemma is to lower the cost of the launch system and reduce the weight. A possible solution here is miniaturized engineered systems. Miniaturized engineered systems are known to provide a compact footprint and low weight, and even to facilitate automation, which is needed to ease the lack of operating personnel in space.5 However, such systems are not available commercially. Intensive R&D is needed to develop such systems that will be able to give an accredited result. Solving the challenge of those space-tested micro-engineered systems will help to have better automation standards of similar systems on Earth.
Another problem of space experimentation refers to the difficulty of controlling experimental parameters such as variations in radiation, vibrations, and humidity.61 Therefore, to understand the sole effect of an individual factor, it is important that analog studies are conducted in which other variables can be controlled. At present, there are several Mars analog environments on Earth that aim to evaluate the health and performance of analog astronauts.102−104 However, it is difficult to create a high-fidelity environment for medication manufacturing, which needs further research.
The alternative is to operate on Earth, e.g., with simulated microgravity methods, as provided by parabolic flights, falling towers, rotating wall vessels, or random positioning machines.105 Yet, these methods might only be able to give an approximate result compared to those in real space. Similar constraints about the validity of analog testing are given for on-Earth radiation studies, which aim to simulate space radiation. Current terrestrial radiation methods emit radiation events at a significantly higher flux (a few mGy/mins) compared to the low-flux dose (a few μGy/days) measured inside the ISS having a cosmic radiation shield. High-energy radiation must not be detrimental under those conditions. There is reason to assume that radiation can simply penetrate through pharmaceutical samples without causing significant damage to the target being tested.66
Glossary
Abbreviations
- ISS
International Space Station
- MPS
micro-physiological system
- UV
ultraviolet
- SAS
Space Adaptation Syndrome
- NASA
National Aeronautics and Space Administration
- NIH
National Institutes of Health
- PTOA
post-traumatic osteoarthritis
- LEO
lower Earth orbit
- API
active pharmaceutical ingredient
- USP
United States Pharmacopeia
- JAXA
Japan Aerospace Exploration Agency
- ROSCOSMOS
Russian space agency
- CASIS-PCG
microgravity growth of crystalline monoclonal antibodies for pharmaceutical applications
- ADMET
drug absorption, distribution, metabolism, excretion, or transport
- Bio-MOD
Biologic Medications on Demand
- CASIS
Center for the Advancement of Science in Space
The authors declare no competing financial interest.
References
- Finckenor M. M.; de Groh K. K.. A researcher’s guide to: space environmental effects. ISS Researcher’s Guide Series; National Aeronautics and Space Administration, March 2015, revised Sept 2020.
- Hargens A. R.; Richardson S. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respiratory Physiol. Neurobiol. 2009, 169, S30–S33. 10.1016/j.resp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Hughson R. L.; Robertson A. D.; Arbeille P.; Shoemaker J. K.; Rush J. W.; Fraser K. S.; Greaves D. K. Increased postflight carotid artery stiffness and inflight insulin resistance resulting from 6-mo spaceflight in male and female astronauts. Ame. J. Physiol. Heart Circulatory Physiol. 2016, 310 (5), H628–38. 10.1152/ajpheart.00802.2015. [DOI] [PubMed] [Google Scholar]
- Kast J.; Yu Y.; Seubert C. N.; Wotring V. E.; Derendorf H. Drugs in space: Pharmacokinetics and pharmacodynamics in astronauts. Eur. J. Pharmaceutical Sci.: Off. J. Eur. Federation Pharmaceutical Sci. 2017, 109s, S2–s8. 10.1016/j.ejps.2017.05.025. [DOI] [PubMed] [Google Scholar]
- Low L. A.; Giulianotti M. A. Tissue Chips in Space: Modeling Human Diseases in Microgravity. Pharm. Res. 2020, 37 (1), 8. 10.1007/s11095-019-2742-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernikos J. Human exploration of space: why, where, what for?. Hippokratia 2008, 12 (Suppl 1), 6–9. [PMC free article] [PubMed] [Google Scholar]
- Bourrieau J.; Flbaz C.; Faugere J. F.; Paillous A. Space and Spacecraft Environment. IFAC Proc. Volumes 1970, 3 (1), 239–244. 10.1016/S1474-6670(17)68781-0. [DOI] [Google Scholar]
- Chancellor J. C.; Scott G. B. I.; Sutton J. P. Space Radiation: The Number One Risk to Astronaut Health beyond Low Earth Orbit. Life (Basel) 2014, 4 (3), 491–510. 10.3390/life4030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis G. C.; Germani M. M.; Caiani E. G.; Barravecchia I.; Passino C.; Angeloni D. Human Pathophysiological Adaptations to the Space Environment. Front. Physiol. 2017, 8, 547. 10.3389/fphys.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel J. I.; Choukèr A. Effects of isolation and confinement on humans-implications for manned space explorations. J. Appl. Physiol. 2016, 120 (12), 1449–1457. 10.1152/japplphysiol.00928.2015. [DOI] [PubMed] [Google Scholar]
- Beardslee L. A.; Casper E. T.; Lawson B. D. Submarine medicine: An overview of the unique challenges, medical concerns, and gaps. Undersea Hyperb. Med. 2021, 48 (3), 263–278. 10.22462/05.06.2021.7. [DOI] [PubMed] [Google Scholar]
- Moiseyenko Y. V.; Sukhorukov V. I.; Pyshnov G. Y.; Mankovska I. M.; Rozova K. V.; Miroshnychenko O. A.; Kovalevska O. E.; Madjar S. A.; Bubnov R. V.; Gorbach A. O.; Danylenko K. M.; Moiseyenko O. I. Antarctica challenges the new horizons in predictive, preventive, personalized medicine: preliminary results and attractive hypotheses for multi-disciplinary prospective studies in the Ukrainian “Akademik Vernadsky” station. EPMA J. 2016, 7 (1), 11. 10.1186/s13167-016-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotring V. E. Medication use by U.S. crewmembers on the International Space Station. FASEB J. 2015, 29 (11), 4417–4423. 10.1096/fj.14-264838. [DOI] [PubMed] [Google Scholar]
- Souvestre P. A.; Landrock C. K.; Blaber A. P. Reducing incapacitating symptoms during space flight: is postural deficiency syndrome an applicable model?. Hippokratia 2008, 12 (Suppl 1), 41–48. [PMC free article] [PubMed] [Google Scholar]
- Witze A.Astronauts have conducted nearly 3,000 science experiments aboard the ISS. Nature, Nov 3, 2020. [DOI] [PubMed]
- Joseph C.; Wood D. Analysis of the Microgravity Research Ecosystem and Market Drivers of Accessibility. New Space 2021, 9 (2), 123–138. 10.1089/space.2020.0044. [DOI] [Google Scholar]
- Bai J.; Wang C. Organoids and Microphysiological Systems: New Tools for Ophthalmic Drug Discovery. Front. Pharmacol. 2020, 11, 407. 10.3389/fphar.2020.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodkinson P. D.; Anderton R. A.; Posselt B. N.; Fong K. J. An overview of space medicine. Br. J. Anaesthesia 2017, 119, i143–i153. 10.1093/bja/aex336. [DOI] [PubMed] [Google Scholar]
- Wotring V. E. Medication use by U.S. crewmembers on the International Space Station. Faseb j 2015, 29 (11), 4417–23. 10.1096/fj.14-264838. [DOI] [PubMed] [Google Scholar]
- Wotring V. E.Risk of Therapeutic Failure Due to Ineffectiveness of Medication, NASA/JSC-CN-32122; National Aeronautics and Space Administration, Oct 10, 2011.
- Putcha L.; Cintrón N. M. Pharmacokinetic consequences of spaceflight. Ann. N.Y. Acad. Sci. 1991, 618, 615–618. 10.1111/j.1749-6632.1991.tb27292.x. [DOI] [PubMed] [Google Scholar]
- Kovachevich I. V.; Kondratenko S. N.; Starodubtsev A. K.; Repenkova L. G. Pharmacokinetics of acetaminophen administered in tablets and capsules under long-term space flight conditions. Pharmaceutical Chem. J. 2009, 43 (3), 130–133. 10.1007/s11094-009-0255-6. [DOI] [Google Scholar]
- Blue R. S.; Bayuse T. M.; Daniels V. R.; Wotring V. E.; Suresh R.; Mulcahy R. A.; Antonsen E. L. Supplying a pharmacy for NASA exploration spaceflight: challenges and current understanding. npj Microgravity 2019, 5, 14. 10.1038/s41526-019-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandarpa K.; Schneider V.; Ganapathy K. Human health during space travel: An overview. Neurol. India 2019, 67, S176–s181. 10.4103/0028-3886.259123. [DOI] [PubMed] [Google Scholar]
- Jennings T. Space adaptation syndrome is caused by elevated intracranial pressure. Med. Hypotheses 1990, 32 (4), 289–291. 10.1016/0306-9877(90)90108-Q. [DOI] [PubMed] [Google Scholar]
- Russomano T.; da Rosa M.; Dos Santos M. A. Space motion sickness: A common neurovestibular dysfunction in microgravity. Neurol. India 2019, 67, S214–s218. 10.4103/0028-3886.259127. [DOI] [PubMed] [Google Scholar]
- Clément G.; Skinner A.; Lathan C. Distance and Size Perception in Astronauts during Long-Duration Spaceflight. Life (Basel) 2013, 3 (4), 524–537. 10.3390/life3040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olabi A. A.; Lawless H. T.; Hunter J. B.; Levitsky D. A.; Halpern B. P. The Effect of Microgravity and Space Flight on the Chemical Senses. J. Food Sci. 2002, 67 (2), 468–478. 10.1111/j.1365-2621.2002.tb10622.x. [DOI] [PubMed] [Google Scholar]
- Furukawa S.; Chatani M.; Higashitani A.; Higashibata A.; Kawano F.; Nikawa T.; Numaga-Tomita T.; Ogura T.; Sato F.; Sehara-Fujisawa A.; Shinohara M.; Shimazu T.; Takahashi S.; Watanabe-Takano H. Findings from recent studies by the Japan Aerospace Exploration Agency examining musculoskeletal atrophy in space and on Earth. npj Microgravity 2021, 7 (1), 18. 10.1038/s41526-021-00145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T.; Horie K.; Hinoi E.; Hiraiwa M.; Kato A.; Maekawa Y.; Takahashi A.; Furukawa S. How does spaceflight affect the acquired immune system?. npj Microgravity 2020, 6 (1), 14. 10.1038/s41526-020-0104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T.; Van Loon J. J.W.A.; Bloomfield S.; Vico L.; Chopard A.; Rittweger J.; Kyparos A.; Blottner D.; Vuori I.; Gerzer R.; Cavanagh P. R. Towards human exploration of space: the THESEUS review series on muscle and bone research priorities. npj Microgravity 2017, 3, 8. 10.1038/s41526-017-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Bakelman F. E.; Darshi M.; Green S. J.; Gur R. C.; Lin L.; Macias B. R.; McKenna M. J.; Meydan C.; Mishra T.; Nasrini J.; Piening B. D.; Rizzardi L. F.; Sharma K.; Siamwala J. H.; Taylor L.; Vitaterna M. H.; Afkarian M.; Afshinnekoo E.; Ahadi S.; Ambati A.; Arya M.; Bezdan D.; Callahan C. M.; Chen S.; Choi A. M. K.; Chlipala G. E.; Contrepois K.; Covington M.; Crucian B. E.; De Vivo I.; Dinges D. F.; Ebert D. J.; Feinberg J. I.; Gandara J. A.; George K. A.; Goutsias J.; Grills G. S.; Hargens A. R.; Heer M.; Hillary R. P.; Hoofnagle A. N.; Hook V. Y. H.; Jenkinson G.; Jiang P.; Keshavarzian A.; Laurie S. S.; Lee-McMullen B.; Lumpkins S. B.; MacKay M.; Maienschein-Cline M. G.; Melnick A. M.; Moore T. M.; Nakahira K.; Patel H. H.; Pietrzyk R.; Rao V.; Saito R.; Salins D. N.; Schilling J. M.; Sears D. D.; Sheridan C. K.; Stenger M. B.; Tryggvadottir R.; Urban A. E.; Vaisar T.; Van Espen B.; Zhang J.; Ziegler M. G.; Zwart S. R.; Charles J. B.; Kundrot C. E.; Scott G. B. I.; Bailey S. M.; Basner M.; Feinberg A. P.; Lee S. M. C.; Mason C. E.; Mignot E.; Rana B. K.; Smith S. M.; Snyder M. P.; Turek F. W. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364 (6436), eaau8650. 10.1126/science.aau8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afshinnekoo E.; Scott R. T.; MacKay M. J.; Pariset E.; Cekanaviciute E.; Barker R.; Gilroy S.; Hassane D.; Smith S. M.; Zwart S. R.; Nelman-Gonzalez M.; Crucian B. E.; Ponomarev S. A.; Orlov O. I.; Shiba D.; Muratani M.; Yamamoto M.; Richards S. E.; Vaishampayan P. A.; Meydan C.; Foox J.; Myrrhe J.; Istasse E.; Singh N.; Venkateswaran K.; Keune J. A.; Ray H. E.; Basner M.; Miller J.; Vitaterna M. H.; Taylor D. M.; Wallace D.; Rubins K.; Bailey S. M.; Grabham P.; Costes S. V.; Mason C. E.; Beheshti A. Fundamental Biological Features of Spaceflight: Advancing the Field to Enable Deep-Space Exploration. Cell 2020, 183 (5), 1162–1184. 10.1016/j.cell.2020.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisova T. Express assessment of neurotoxicity of particles of planetary and interstellar dust. npj Microgravity 2019, 5 (1), 2. 10.1038/s41526-019-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C.-W.; James J. T.; Latch J. N.; Hamilto R. F. Jr.; Holian A. Pulmonary Toxicity of Simulated Lunar and Martian Dusts in Mice: II. Biomarkers of Acute Responses after Intratracheal Instillation. Inhalation Toxicol. 2002, 14 (9), 917–928. 10.1080/08958370290084692. [DOI] [PubMed] [Google Scholar]
- Caston R.; Luc K.; Hendrix D.; Hurowitz J. A.; Demple B. Assessing Toxicity and Nuclear and Mitochondrial DNA Damage Caused by Exposure of Mammalian Cells to Lunar Regolith Simulants. GeoHealth 2018, 2 (4), 139–148. 10.1002/2017GH000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta F. A.; Kim M. H.; Chappell L. J.; Huff J. L. How safe is safe enough? Radiation risk for a human mission to Mars. PLoS One 2013, 8 (10), e74988. 10.1371/journal.pone.0074988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu M.; Prasuna J. G. Twin Studies: A Unique Epidemiological Tool. Indian J. Community Med. 2016, 41 (3), 177–182. 10.4103/0970-0218.183593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.; Zakaria S.; Esmaeili Samani S.; Chang Y.; Filipe C. D. M.; Soleymani L.; Brennan J. D.; Liu M.; Li Y. Functional Nucleic Acids for Pathogenic Bacteria Detection. Acc. Chem. Res. 2021, 54 (18), 3540–3549. 10.1021/acs.accounts.1c00355. [DOI] [PubMed] [Google Scholar]
- Gaskill M.First DNA Sequencing in Space a Game Changer. Space Daily; National Aeronautics and Space Administration, Aug 29, 2016.
- Boguraev A.-S.; Christensen H. C.; Bonneau A. R.; Pezza J. A.; Nichols N. M.; Giraldez A. J.; Gray M. M.; Wagner B. M.; Aken J. T.; Foley K. D.; Copeland D. S.; Kraves S.; Alvarez Saavedra E. Successful amplification of DNA aboard the International Space Station. npj Microgravity 2017, 3 (1), 26. 10.1038/s41526-017-0033-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfien J.; Atabay K. D.; Nichols N. M.; Tanner N. A.; Pezza J. A.; Gray M. M.; Wagner B. M.; Poppin J. N.; Aken J. T.; Gleason E. J.; Foley K. D.; Copeland D. S.; Kraves S.; Alvarez Saavedra E. Nucleic acid detection aboard the International Space Station by colorimetric loop-mediated isothermal amplification (LAMP). FASEB BioAdvances 2020, 2 (3), 160–165. 10.1096/fba.2019-00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Microgravity as model for immunological senescence and its impact on tissue stem cells and regeneration. https://reporter.nih.gov/search/ACWKH3GbGEeiqc7Pb4O56A/project-details/10005510 (accessed March 16, 2022).
- Lung Host Defense in Microgravity. https://reporter.nih.gov/project-details/9403062 (accessed March 16, 2022).
- Cartilage-Bone-Synovium MPS: Musculoskeletal Disease Biology in Space. https://reporter.nih.gov/project-details/9402868#details (accessed March 16, 2022).
- Taylor P. W. Impact of space flight on bacterial virulence and antibiotic susceptibility. Infect Drug Resist 2015, 8, 249–262. 10.2147/IDR.S67275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. W.; Ott C. M.; Höner zu Bentrup K.; Ramamurthy R.; Quick L.; Porwollik S.; Cheng P.; McClelland M.; Tsaprailis G.; Radabaugh T.; Hunt A.; Fernandez D.; Richter E.; Shah M.; Kilcoyne M.; Joshi L.; Nelman-Gonzalez M.; Hing S.; Parra M.; Dumars P.; Norwood K.; Bober R.; Devich J.; Ruggles A.; Goulart C.; Rupert M.; Stodieck L.; Stafford P.; Catella L.; Schurr M. J.; Buchanan K.; Morici L.; McCracken J.; Allen P.; Baker-Coleman C.; Hammond T.; Vogel J.; Nelson R.; Pierson D. L.; Stefanyshyn-Piper H. M.; Nickerson C. A. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (41), 16299–16304. 10.1073/pnas.0707155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B. V.; Connors T. J.; Farber D. L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48 (2), 202–213. 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y.; Han X.; Wang Y.; Chen Z.; Lu Y.; Liu T.; Wu Z.; Jin Y.; Luo Y.; Zhang X. Drug Toxicity Evaluation Based on Organ-on-a-chip Technology: A Review. Micromachines (Basel) 2020, 11 (4), 381. 10.3390/mi11040381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effects of microgravity on the structure and function of proximal and distal tubule MPS. https://reporter.nih.gov/search/I0AP06wbnUCPP5X-CgXRhQ/project-details/9946987 (accessed March 16, 2022).
- Electrical Stimulation of Human Myocytes in Microgravity: An In Vitro Model to Evaluate Therapeutics to Counteract Muscle Wasting. https://reporter.nih.gov/search/I0AP06wbnUCPP5X-CgXRhQ/project-details/10262954 (accessed March 16, 2022).
- Effect of Microgravity on Drug Responses Using Engineered Heart Tissues. https://reporter.nih.gov/project-details/9644885 (accessed March 16, 2022).
- A Human iPSC-based 3D Microphysiological System for Modeling Cardiac Dysfunction in Microgravity. https://reporter.nih.gov/project-details/9644709 (accessed March 16, 2022).
- Organs-on-Chips as a Platform for Studying Effects of Microgravity on Human Physiology: Blood-Brain Barrier-Chip in Health and Disease. https://reporter.nih.gov/project-details/9402972 (accessed March 16, 2022).
- Organ-Chips as a Platform for Studying Effects of Space on Human Enteric Physiology: Interactions of Epithelial Mucosa with Sensory Neurons and Microbiome. https://reporter.nih.gov/project-details/9645490 (accessed March 16, 2022).
- Smith S. M.; Heer M.; Shackelford L. C.; Sibonga J. D.; Spatz J.; Pietrzyk R. A.; Hudson E. K.; Zwart S. R. Bone metabolism and renal stone risk during International Space Station missions. Bone 2015, 81, 712–720. 10.1016/j.bone.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Lang T.; Van Loon J. J. W. A.; Bloomfield S.; Vico L.; Chopard A.; Rittweger J.; Kyparos A.; Blottner D.; Vuori I.; Gerzer R.; Cavanagh P. R. Towards human exploration of space: the THESEUS review series on muscle and bone research priorities. npj Microgravity 2017, 3 (1), 8. 10.1038/s41526-017-0013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidberg K. A.; Annalora A. J.; Jozic M.; Elson D. J.; Wang L.; Bammler T. K.; Ramm S.; Monteiro M. B.; Himmelfarb J.; Marcus C. B.; Iversen P. L.; Kelly E. J. Antisense oligonucleotide development for the selective modulation of CYP3A5 in renal disease. Sci. Rep. 2021, 11 (1), 4722. 10.1038/s41598-021-84194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens E.; Veldhoen M. Epithelial barrier biology: good fences make good neighbours. Immunology 2012, 135 (1), 1–8. 10.1111/j.1365-2567.2011.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pediaditakis I.; Kodella K. R.; Manatakis D. V.; Le C. Y.; Hinojosa C. D.; Tien-Street W.; Manolakos E. S.; Vekrellis K.; Hamilton G. A.; Ewart L.; Rubin L. L.; Karalis K. Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. Nat. Commun. 2021, 12 (1), 5907. 10.1038/s41467-021-26066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P.; Bhayani D. Impact of space environment on stability of medicines: Challenges and prospects. J. Pharm. Biomed. Anal. 2017, 136, 111–119. 10.1016/j.jpba.2016.12.040. [DOI] [PubMed] [Google Scholar]
- Wotring V. E. Chemical Potency and Degradation Products of Medications Stored Over 550 Earth Days at the International Space Station. AAPS J. 2016, 18 (1), 210–6. 10.1208/s12248-015-9834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart S. R.; Kloeris V. L.; Perchonok M. H.; Braby L.; Smith S. M. Assessment of nutrient stability in foods from the space food system after long-duration spaceflight on the ISS. J. Food Sci. 2009, 74 (7), H209–17. 10.1111/j.1750-3841.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- Chuong M. C.; Prasad D.; Leduc B.; Du B.; Putcha L. Stability of vitamin B complex in multivitamin and multimineral supplement tablets after space flight. J. Pharm. Biomed. Anal. 2011, 55 (5), 1197–200. 10.1016/j.jpba.2011.03.030. [DOI] [PubMed] [Google Scholar]
- Du B.; Daniels V. R.; Vaksman Z.; Boyd J. L.; Crady C.; Putcha L. Evaluation of physical and chemical changes in pharmaceuticals flown on space missions. AAPS J. 2011, 13 (2), 299–308. 10.1208/s12248-011-9270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue R. S.; Chancellor J. C.; Antonsen E. L.; Bayuse T. M.; Daniels V. R.; Wotring V. E. Limitations in predicting radiation-induced pharmaceutical instability during long-duration spaceflight. npj Microgravity 2019, 5 (1), 15. 10.1038/s41526-019-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissarenko N. F.Radiation Environment During the Long Space Mission (Mars) Due to Galactic Cosmic Rays. In Biological Effects and Physics of Solar and Galactic Cosmic Radiation: Part B; Swenberg C. E., Horneck G., Stassinopoulos E. G., Eds.; Springer US: Boston, MA, 1993; pp 1–14. [Google Scholar]
- Furukawa S.; Nagamatsu A.; Nenoi M.; Fujimori A.; Kakinuma S.; Katsube T.; Wang B.; Tsuruoka C.; Shirai T.; Nakamura A. J.; Sakaue-Sawano A.; Miyawaki A.; Harada H.; Kobayashi M.; Kobayashi J.; Kunieda T.; Funayama T.; Suzuki M.; Miyamoto T.; Hidema J.; Yoshida Y.; Takahashi A. Space Radiation Biology for “Living in Space”. Biomed. Res. Int. 2020, 2020, 4703286. 10.1155/2020/4703286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor J. C.; Blue R. S.; Cengel K. A.; Auñón-Chancellor S. M.; Rubins K. H.; Katzgraber H. G.; Kennedy A. R. Limitations in predicting the space radiation health risk for exploration astronauts. npj Microgravity 2018, 4 (1), 8. 10.1038/s41526-018-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirsk R.; Kuipers A.; Mukai C.; Williams D. The space-flight environment: the International Space Station and beyond. CMA J. 2009, 180 (12), 1216–1220. 10.1503/cmaj.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Caër S. Water Radiolysis: Influence of Oxide Surfaces on H2 Production under Ionizing Radiation. Water 2011, 3 (1), 235–253. 10.3390/w3010235. [DOI] [Google Scholar]
- Hadjittofis E.; Isbell M. A.; Karde V.; Varghese S.; Ghoroi C.; Heng J. Y. Y. Influences of Crystal Anisotropy in Pharmaceutical Process Development. Pharm. Res. 2018, 35 (5), 100. 10.1007/s11095-018-2374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistyakov D.; Sergeev G. The Polymorphism of Drugs: New Approaches to the Synthesis of Nanostructured Polymorphs. Pharmaceutics 2020, 12 (1), 34. 10.3390/pharmaceutics12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censi R.; Di Martino P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules (Basel, Switzerland) 2015, 20 (10), 18759–18776. 10.3390/molecules201018759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z.; Rohani S.; Gong J.; Wang J. Recent Developments in the Crystallization Process: Toward the Pharmaceutical Industry. Engineering 2017, 3 (3), 343–353. 10.1016/J.ENG.2017.03.022. [DOI] [Google Scholar]
- Nijhuis J.; Schmidt S.; Tran N. N.; Hessel V. Microfluidics and Macrofluidics in Space: ISS-Proven Fluidic Transport and Handling Concepts. Front. Space Technol. 2022, 10.3389/frspt.2021.779696. [DOI] [Google Scholar]
- Ruocco G.Mass Transfer by Diffusion and Convection. Introduction to Transport Phenomena Modeling: A Multiphysics, General Equation-Based Approach; Springer International Publishing: Cham, 2018; pp 201–239. [Google Scholar]
- Lee C. P.; Chernov A. A. Solutal convection around growing protein crystals and diffusional purification in Space. J. Cryst. Growth 2002, 240, 531–544. 10.1016/S0022-0248(02)00909-0. [DOI] [Google Scholar]
- Reichert P.; Prosise W.; Fischmann T. O.; Scapin G.; Narasimhan C.; Spinale A.; Polniak R.; Yang X.; Walsh E.; Patel D.; Benjamin W.; Welch J.; Simmons D.; Strickland C. Pembrolizumab microgravity crystallization experimentation. npj Microgravity 2019, 5 (1), 28. 10.1038/s41526-019-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson A.; DeLucas L. J. Microgravity protein crystallization. npj Microgravity 2015, 1 (1), 15010. 10.1038/npjmgrav.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucas L. J.; Smith C. D.; Smith H. W.; Vijay-Kumar S.; Senadhi S. E.; Ealick S. E.; Carter D. C.; Snyder R. S.; Weber P. C.; Salemme F. R.; et al. Protein crystal growth in microgravity. Science 1989, 246 (4930), 651–654. 10.1126/science.2510297. [DOI] [PubMed] [Google Scholar]
- Benz K. W.; Dold P. Crystal growth under microgravity: present results and future prospects towards the International Space Station. J. Cryst. Growth 2002, 237–239, 1638–1645. 10.1016/S0022-0248(01)02358-2. [DOI] [Google Scholar]
- Boyko K. M.; Timofeev V. I.; Samygina V. R.; Kuranova I. P.; Popov V. O.; Koval’chuk M. V. Protein crystallization under microgravity conditions. Analysis of the results of Russian experiments performed on the International Space Station in 2005–2015. Crystallogr. Rep. 2016, 61 (5), 718–729. 10.1134/S1063774516050059. [DOI] [Google Scholar]
- Tanaka H.; Sasaki S.; Takahashi S.; Inaka K.; Wada Y.; Yamada M.; Ohta K.; Miyoshi H.; Kobayashi T.; Kamigaichi S. Numerical model of protein crystal growth in a diffusive field such as the microgravity environment. J. Synchrotron Radiat. 2013, 20 (6), 1003–1009. 10.1107/S0909049513022784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranova I. P.; Smirnova E. A.; Abramchik Y. A.; Chupova L. A.; Esipov R. S.; Akparov V. K.; Timofeev V. I.; Kovalchuk M. V. Crystal growth of phosphopantetheine adenylyltransferase, carboxypeptidase t, and thymidine phosphorylase on the international space station by the capillary counter-diffusion method. Crystallogr. Rep. 2011, 56 (5), 884. 10.1134/S1063774511050154. [DOI] [Google Scholar]
- Inaka K.; Takahashi S.; Aritake K.; Tsurumura T.; Furubayashi N.; Yan B.; Hirota E.; Sano S.; Sato M.; Kobayashi T.; Yoshimura Y.; Tanaka H.; Urade Y. High-Quality Protein Crystal Growth of Mouse Lipocalin-Type Prostaglandin D Synthase in Microgravity. Cryst. Growth Des. 2011, 11 (6), 2107–2111. 10.1021/cg101370v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D. LambdaVision Receives $5 Million Award From NASA for Additional ISS Research. https://www.issnationallab.org/iss360/lambdavision-space-tango-5-million-award-from-nasa-additional-retinal-research/.
- National Aeronautics and Space Administration Science in Short: Eli Lilly-Hard to Wet Surfaces. https://blogs.nasa.gov/ISS_Science_Blog/2016/10/18/science-in-short-eli-lilly-hard-to-wet-surfaces/.
- Cintron N.; Putcha L.; Chen Y.; Vanderploeg J.. Inflight salivary pharmacokinetics of scopolamine and dextroamphetamine. Results of the life sciences DSOs conducted aboard the space shuttle 1981–1986; National Aeronautics and Space Administration, 1987; pp 25–29. [Google Scholar]
- Cintron N.; Putcha L.; Vanderploeg J.. Inflight pharmacokinetics of acetaminophen in saliva. Results of the life sciences DSOs conducted aboard the space shuttle 1981–1986; National Aeronautics and Space Administration, 1987; pp 19–23. [Google Scholar]
- Kovachevich I.; Kondratenko S.; Starodubtsev A.; Repenkova L. Pharmacokinetics of acetaminophen administered in tablets and capsules under long-term space flight conditions. Pharmaceutical Chem. J. 2009, 43, 130–133. 10.1007/s11094-009-0255-6. [DOI] [Google Scholar]
- Chua C. Y. X.; Liu H.-C.; Di Trani N.; Susnjar A.; Ho J.; Scorrano G.; Rhudy J.; Sizovs A.; Lolli G.; Hernandez N.; Nucci M. C.; Cicalo R.; Ferrari M.; Grattoni A. Carbon fiber reinforced polymers for implantable medical devices. Biomaterials 2021, 271, 120719. 10.1016/j.biomaterials.2021.120719. [DOI] [PubMed] [Google Scholar]
- Silvestri A.; Di Trani N.; Canavese G.; Motto Ros P.; Iannucci L.; Grassini S.; Wang Y.; Liu X.; Demarchi D.; Grattoni A. Silicon Carbide-Gated Nanofluidic Membrane for Active Control of Electrokinetic Ionic Transport. Membranes 2021, 11 (7), 535. 10.3390/membranes11070535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini A.; Chua C. Y. X.; Rhudy J.; Susnjar A.; Di Trani N.; Jain P. R.; Laue G.; Lubicka D.; Shirazi-Fard Y.; Ferrari M.; Stodieck L. S.; Cadena S. M.; Grattoni A. Counteracting Muscle Atrophy on Earth and in Space via Nanofluidics Delivery of Formoterol. Adv. Therapeutics 2020, 3 (7), 2000014. 10.1002/adtp.202000014. [DOI] [Google Scholar]
- Arnold C. Who shrank the drug factory? Briefcase-sized labs could transform medicine. Nature 2019, 575, 274–277. 10.1038/d41586-019-03455-x. [DOI] [PubMed] [Google Scholar]
- Adiga R.; Al-adhami M.; Andar A.; Borhani S.; Brown S.; Burgenson D.; Cooper M. A.; Deldari S.; Frey D. D.; Ge X.; Guo H.; Gurramkonda C.; Jensen P.; Kostov Y.; LaCourse W.; Liu Y.; Moreira A.; Mupparapu K.; Peñalber-Johnstone C.; Pilli M.; Punshon-Smith B.; Rao A.; Rao G.; Rauniyar P.; Snovida S.; Taurani K.; Tilahun D.; Tolosa L.; Tolosa M.; Tran K.; Vattem K.; Veeraraghavan S.; Wagner B.; Wilhide J.; Wood D. W.; Zuber A. Point-of-care production of therapeutic proteins of good-manufacturing-practice quality. Nature Biomed. Eng. 2018, 2 (9), 675–686. 10.1038/s41551-018-0259-1. [DOI] [PubMed] [Google Scholar]
- Arnold C. Who shrank the drug factory? Briefcase-sized labs could transform medicine. Nature 2019, 575 (7782), 274–278. 10.1038/d41586-019-03455-x. [DOI] [PubMed] [Google Scholar]
- Prater T.; Werkheiser N.; Ledbetter F.; Timucin D.; Wheeler K.; Snyder M. 3D Printing in Zero G Technology Demonstration Mission: complete experimental results and summary of related material modeling efforts. International J. Adv. Manufacturing Technol. 2019, 101 (1), 391–417. 10.1007/s00170-018-2827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Li H.; Huang L.; Zhang M.; Fan W.; Cui L. 3D printing promotes the development of drugs. Biomed. Pharmacotherapy 2020, 131, 110644. 10.1016/j.biopha.2020.110644. [DOI] [PubMed] [Google Scholar]
- Tran Q. D.; Tran N.; Hessel V.; Thavarajah S. R.; Rahman S.; Stoudemire J.; Zeckovic O.; Fisk I.. Long-duration stability study of medicines on the International Space Station providing data for potential future on-orbit manufacturing, 43rd COSPAR Scientific Assembly. Jan 28–Feb 4, 2021; p 1835.
- SpaceTech Analytics . Space Medicine and Human Longevity in Space Q3 2021; Sept 2021.
- Groemer G.; Losiak A.; Soucek A.; Plank C.; Zanardini L.; Sejkora N.; Sams S. The AMADEE-15 Mars simulation. Acta Astronautica 2016, 129, 277–290. 10.1016/j.actaastro.2016.09.022. [DOI] [Google Scholar]
- Luger T. J.; Stadler A.; Gorur P.; Terlevic R.; Neuner J.; Simonsen O.; Sansone P.; Toferer E.; Luger M. F.; Winter E.; Beck T. Medical preparedness, incidents, and group dynamics during the analog MARS2013 mission. Astrobiology 2014, 14 (5), 438–50. 10.1089/ast.2013.1128. [DOI] [PubMed] [Google Scholar]
- Tafforin C. The Mars-500 crew in daily life activities: An ethological study. Acta Astronautica 2013, 91, 69–76. 10.1016/j.actaastro.2013.05.001. [DOI] [Google Scholar]
- van Loon J. J. W. A. Some Challenges in Gravity Related Research. Front. Space Technol. 2020, 1, 3. 10.3389/frspt.2020.00003. [DOI] [Google Scholar]