Abstract
The NLRP3 inflammasome is a multiprotein complex that facilitates activation and release of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 in response to infection or endogenous stimuli. It can be inappropriately activated by a range of danger signals resulting in chronic, low-grade inflammation underlying a multitude of diseases, such as Alzheimer’s disease, Parkinson’s disease, osteoarthritis, and gout. The discovery of potent and specific NLRP3 inhibitors could reduce the burden of several common morbidities. In this study, we identified a weakly potent triazolopyrimidone hit (1) following an in silico modeling exercise. This was optimized to furnish potent and selective small molecule NLRP3 inflammasome inhibitors. Compounds such as NDT-30805 could be useful tool molecules for a scaffold-hopping or pharmacophore generation project or used as leads toward the development of clinical candidates.
Keywords: NLRP3, inflammasome, interleukin-1, inflammation, innate immunity
An inflammasome is a multiprotein complex of the innate immune system that functions to activate caspase-1 in response to the detection of infection and danger signals.1 Active caspase-1 catalyzes the conversion of inactive pro-IL-1β and pro-IL-18 into active inflammatory cytokines IL-1β and IL-18. Furthermore, caspase-1 is responsible for the proteolysis and activation of gasdermin D (GSDMD), a process that results in the formation of pores in the cell membrane and a form of lytic cell death known as pyroptosis.
The NLRP3 (NOD-like receptor, Leucine-rich Repeat, and Pyrin-domain-containing protein 3) inflammasome is unique among inflammasomes in that it is assembled in response to a diverse range of endogenous and exogenous danger signals, termed DAMPs (danger-associated molecular patterns) and PAMPs (pathogen-associated molecular patterns). The activation of the NLRP3 inflammasome is a two-step process. The first step (priming) occurs in response to the binding of endogenous cytokines or microbial-derived molecules (e.g., lipopolysaccharide (LPS)) to extracellular receptors such as toll-like receptors (TLRs), IL-1 receptors, and tumor necrosis factor (TNF) receptors.2 Priming upregulates the transcription and production of NLRP3, pro-IL-1β, and pro-IL-18 through the activation of transcription factor NF-κB. The second step (activation) occurs following the detection of a second stimulus such as a DAMP or PAMP. Activation results in the assembly of the NLRP3 inflammasome and the cleavage of procaspase-1 into the bioactive form of caspase-1, ultimately leading to the release of proinflammatory cytokines IL-1β and IL-18 into extracellular space.
The discovery of a wide range of danger signals that can activate the NLRP3 inflammasome infers a link to a multitude of human diseases. PAMPs include peptidoglycan and both viral and bacterial DNA and RNA. Some examples of DAMPs are adenosine triphosphate (ATP),3 amyloid-β,4 tau,5,6 α-synuclein,7 cholesterol crystals,8 monosodium urate (MSU) crystals,9 calcium pyrophosphate crystals,9 hydroxyapatite,10 fatty acids,11 ceramides,12 asbestos, and silica.13 Further evidence of the impact of inappropriate NLRP3 activation in humans comes from the study of several gain-of-function mutations causing conditions collectively known as cryopyrin-associated periodic syndromes (CAPS).14 In these autoinflammatory diseases, the NLRP3 inflammasome exists in an activated state, which leads to dysregulated inflammation. Symptoms vary in severity but may include urticaria on the skin (particularly when exposed to cold), episodic fevers and chills, joint pain or destruction, arthritis, or chronic meningitis leading to neurological damage.
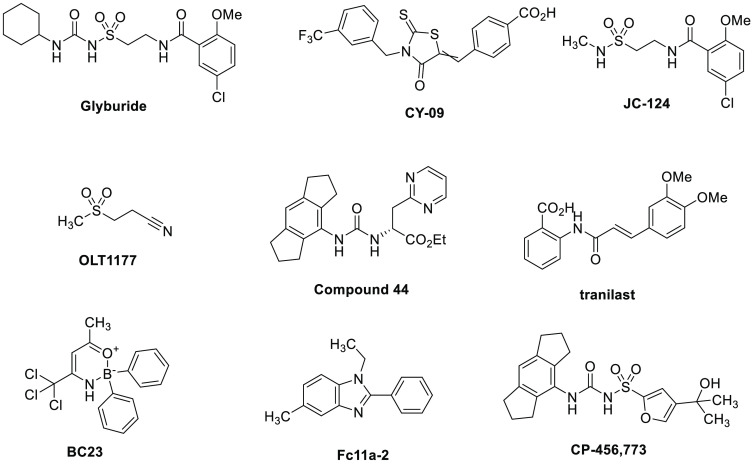
Many small molecules have been reported to inhibit cytokine release via disruption of the NLRP3 inflammasome pathway, the structures of some of which are shown in Figure 1. Glyburide (glibenclamide) is a widely prescribed sulfonylurea antidiabetic drug, which was shown to inhibit the conversion of pro-IL-1β into its active form.15 Further studies revealed that glyburide disrupted the formation of the NLRP3 inflammasome.16 CY-09, a rhodanine-based molecule, blocks NLRP3 inflammasome activation by directly binding to the NLRP3 NACHT domain.17 A molecule with some identical structural features to glyburide, JC-124, was identified as an NLRP3 inhibitor, which showed an effect in a mouse model of Alzheimer’s disease.18,19 OLT-1177 (dapansutrile) is a low-molecular-weight β-sulfonylnitrile compound that has been tested in a phase IIa clinical trial for acute gout flares.20 It is reported to be a selective NLRP3 inhibitor and exhibited an anti-inflammatory effect in mouse in vivo studies.21 Ester 44 was recently disclosed among a series of selective NLRP3 inflammasome inhibitors, displaying excellent potency in a whole blood assay and high permeability.22 Other molecules with reported inhibition of the NLRP3 inflammasome include the natural product tranilast,23 the oxazaborine complex BC23,24 and the benzimidazole Fc11a-2.25 Several reviews have been published detailing these and further NLRP3 inflammasome-disrupting molecules.26−32
Figure 1.
Structures of small molecule NLRP3 inflammasome inhibitors.
The most widely studied NLRP3 inhibitor is CP-456,773 (CRID3/MCC950). It was designed to be an IL-1β inhibitor in the late 1990s, prior to the discovery of the inflammasome, using glyburide as the starting point.33 Subsequent studies by Coll and co-workers showed this to be a specific NLRP3 inhibitor.34 Recently, two groups have published crystal structures illustrating that CP-456,773 and related sulfonylurea inhibitor NP3–146 bind close to the NACHT domain of the NLRP3 complex, stabilizing its inactive conformation.35,36
CP-456,773 has been tested in numerous in vitro and in vivo models that have revealed disease-relevant effects in multiple conditions such as Alzheimer’s disease,37 Parkinson’s disease,38 chronic kidney disease,39 gout,34 inflammatory bowel disease,40 rheumatoid arthritis,41 and asthma.42 These discoveries serve to validate NLRP3 inflammasome inhibition as a promising strategy for the treatment of wide-ranging diseases related to inflammation.
Although several clinical trials for small molecule inhibitors of the NLRP3 inflammasome have been reported, none have reached market approval. Since the MCC950 disclosure by Coll and co-workers, there has been great interest in the search for novel NLRP3 inflammasome inhibitors. Several drug discovery programs have used CP-456,773 as the chemical starting point, yet owing to the acidic center and high polar surface area, the physicochemical properties of this molecule and related sulfonylureas (such as the acidic center and high polar surface area) are suboptimal for facile membrane permeability and CNS penetration. Accordingly, there remains a need for selective NLRP3 inflammasome inhibitors that are structurally distinct from the sulfonylureas, as these could possess different properties and therefore have potentially different applications. Furthermore, the discovery of more diverse compounds could contribute to a greater understanding of the pharmacophoric requirements for NLRP3 inflammasome inhibitors.
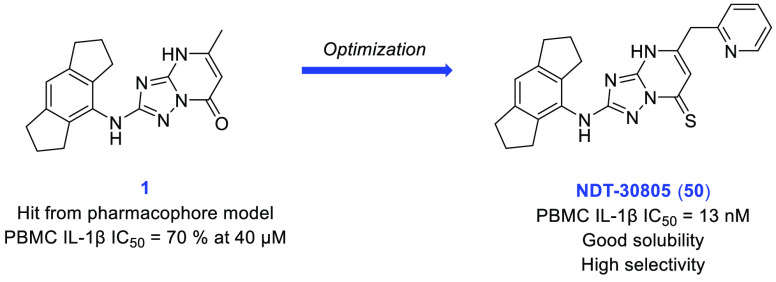
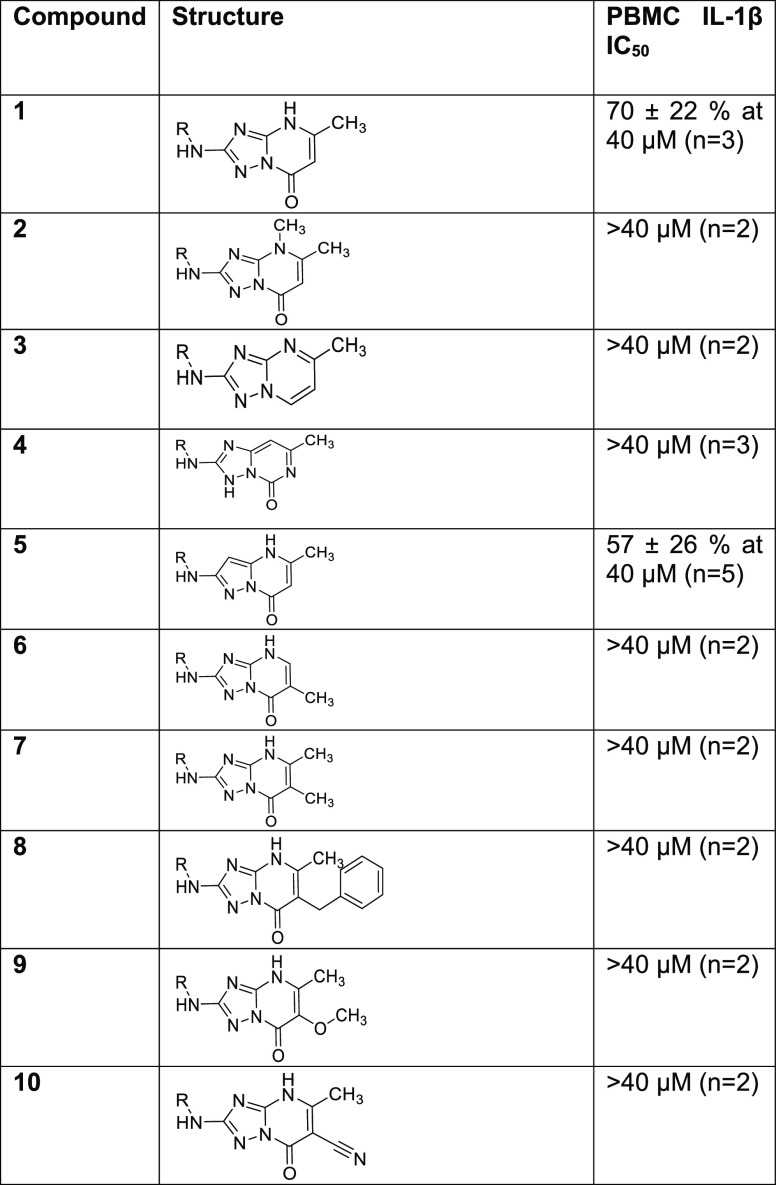
We began our search for novel NLRP3 inflammasome inhibitors by building a pharmacophore model based on proprietary molecules. We used a range of structurally diverse, selective NLRP3 inflammasome inhibitors and refined the model with data from our in-house “active” and “inactive” molecules to improve the outcome. An in silico screening of various molecules identified a low-molecular-weight (321 Da) triazolopyrimidinone hit (1). This was screened in our primary assay, a peripheral blood mononuclear cell (PBMC) assay that measured IL-1β release following stimulation with lipopolysaccharide (LPS) and adenosine triphosphate (ATP) and showed a consistent inhibition of IL-1β release of 70% at 40 μM. Following the discovery of this weak hit, a structure–activity relationship investigation was undertaken to improve upon the potency of 1, the results of which are shown in Table 1.
Table 1. Initial Screening Hit and SAR.
Initially, some fundamental changes to the bicyclic core were explored. Methylation of the 4-nitrogen (2) and removal of the carbonyl (3) resulted in a complete loss of inhibition (defined as having an IC50 greater than 40 μM in the PBMC assay), as did the change to a structurally isomeric triazolopyrimidinone core (4). Replacement of the 3-nitrogen with carbon to give 5 resulted in a small reduction in activity. We chose to retain the 4H,7H-[1,2,4]triazolo[1,5-a]pyrimidin-7-one core and probed the SAR by introducing various substituents. At the 6-position, the addition of a methyl group either with the 5-methyl removed (6), or retained (7), ablated activity. Similarly, substitution with 6-benzyl (8), 6-methoxy (9), or 6-cyano (10) gave inactive compounds.
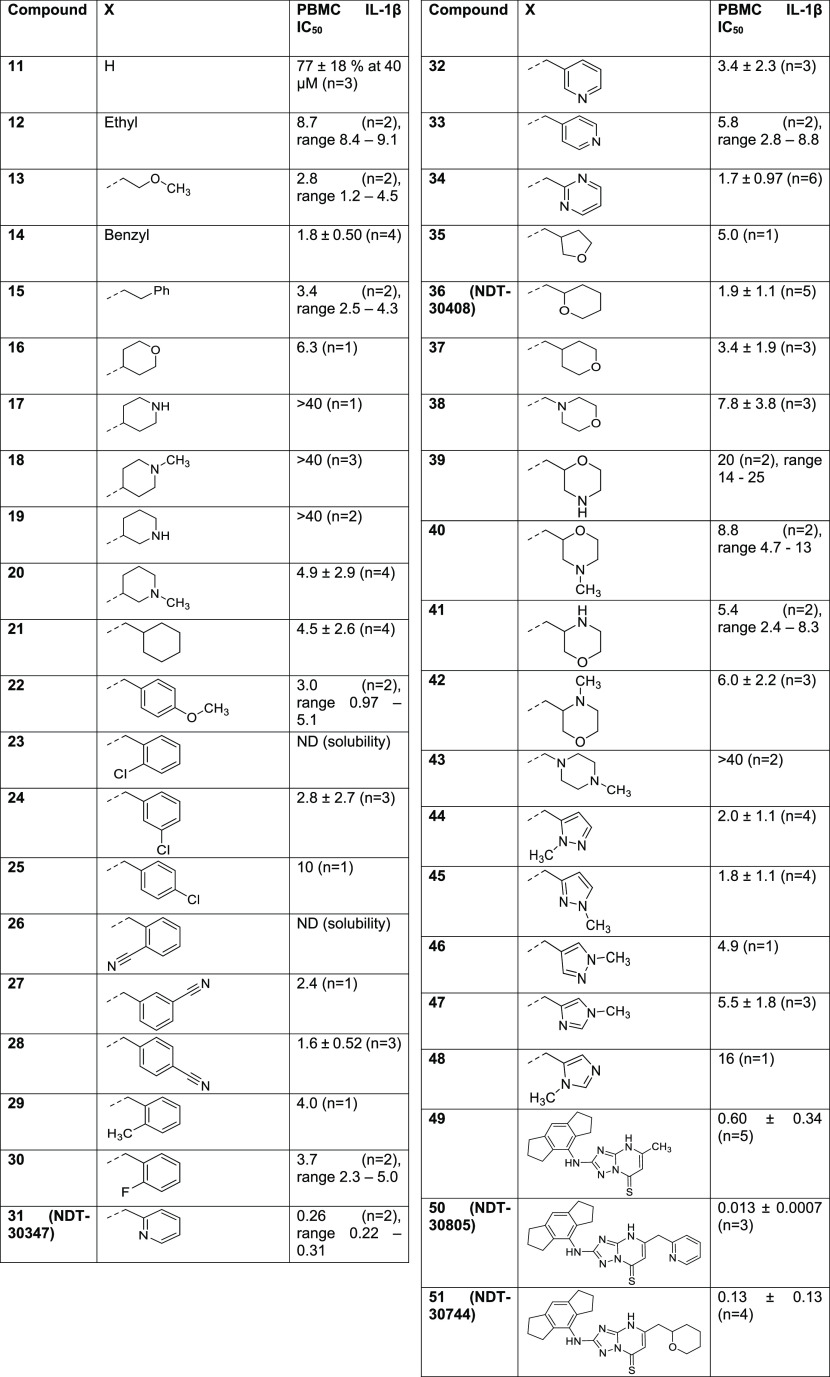
Next, we turned our attention to the 5-position. Primary assay data for the synthesized compounds are shown in Table 2. Replacement of the 5-methyl with hydrogen (11) had little effect on IL-1β inhibition. Exchange of the methyl for various larger substituents including ethyl (12), methoxyethyl (13), benzyl (14), phenethyl (15) and 4-tetrahydropyranyl (16) all resulted in compounds with single-digit micromolar IC50 values. The tolerance for basic groups in the 5-position was less encouraging, with 4-piperidinyl (17), 1-methyl-4-piperidinyl (18), and 3-piperidinyl (19) analogues not inhibiting at the top concentration, although 5-(1-methyl-3-piperidine) substitution (20) did result in an improvement in potency compared to compound 1. Of the compounds profiled thus far, 14 displayed the best potency. Encouraged by this, a more focused set of analogues containing a methylene-spaced cyclic group in the 5-position (21 to 48) were investigated. The cyclohexyl analogue 21 was slightly less potent than the benzyl equivalent, and a range of benzyl analogues scanning ortho-, meta-, and para-substitution (22 to 30) did not identify any compounds more potent than the unsubstituted matched pair. A scan of methylene-linked pyridyl analogues (31 (NDT-30347) to 33) led to a further boost in potency; the 2-pyridyl group was shown to be favored over the other two isomers, with PBMC activity an order of magnitude greater than that of the benzyl 14. The 2-pyrimidinyl 34 was synthesized as a close analogue of the potent 2-pyridyl lead, but the additional nitrogen was detrimental to potency. Profiling of analogues bearing both basic and nonbasic saturated heterocycles (35 to 43) brought mixed results. No potency improvements over benzyl 14 were made, yet the equipotent 2-tetrahydropyranyl compound, 36 (NDT-30408), offered potential advantages owing to its greater sp3 character, lower lipophilicity, and reduced aromatic ring count. These parameters are commonly associated with improved developability characteristics such as solubility. Finally, an investigation of azoles (44 to 48) revealed that the 1-methyl-3-pyrazolyl was equipotent to benzyl 14, but no superior compounds were identified.
Table 2. 5-Position Modifications and SAR.
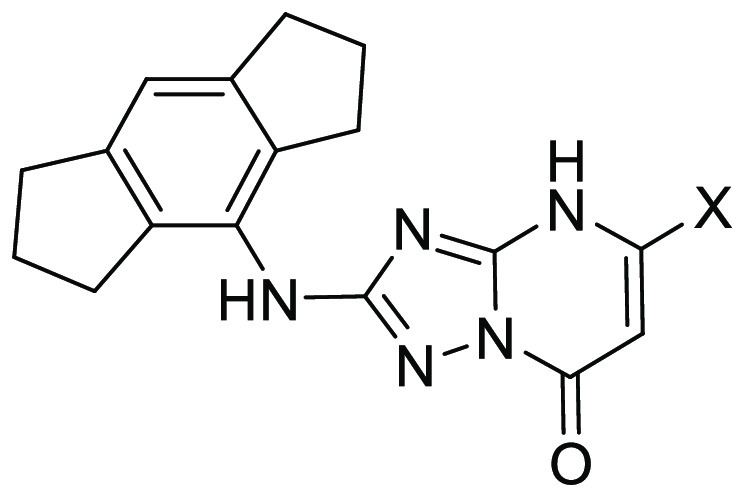
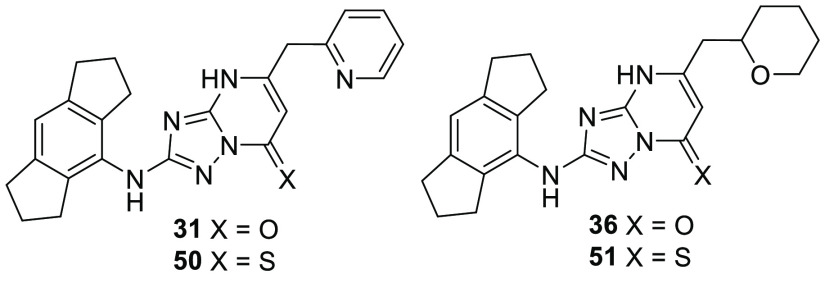
In addition to the extensive SAR investigation into the 5-position, we returned to hit 1 and synthesized the corresponding thiocarbonyl analogue 49. To our surprise, this single atom change greatly increased the potency. Gratifyingly, applying this switch from carbonyl to thiocarbonyl on two additional molecules (31 and 36, resulting in 50 (NDT-30805) and 51 (NDT-30744), respectively) had a similar potency boosting effect. Both thiocarbonyl molecules were an order of magnitude more potent than the carbonyl matched pairs in the PBMC assay.
With a structure–activity relationship now established and several molecules identified as interesting leads, we selected the two most potent thiocarbonyl compounds 50 (NDT-30805) and 51 (NDT-30744) for further profiling, along with the corresponding carbonyl matched pairs 31 (NDT-30347) and 36 (NDT-30408). The synthetic route to 31, 36, 50, and 51 is shown in Scheme 1. The aminotriazole ring was formed in two steps starting from the corresponding aniline. Reaction with dimethyl cyanocarbonimidodithioate gave the thiourea derivative, which was subsequently cyclized with hydrazine hydrate. The aminotriazole was condensed with a β-keto ester by refluxing in acetic acid to give the triazolopyrimidone compounds 31 and 36. The corresponding thiocarbonyl derivatives were then prepared using phosphorus pentasulfide.
Scheme 1. Synthesis of Compounds 31, 36, 50, and 51.
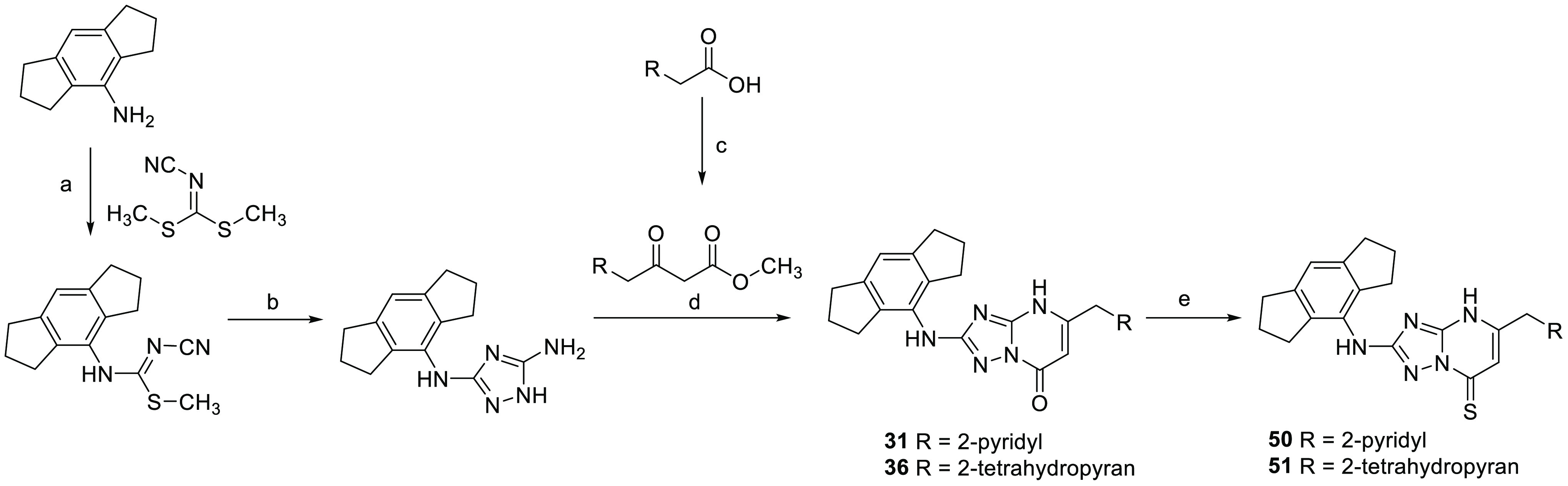
Reagents and conditions: (a) NaH, DMF, 70 °C; (b) hydrazine hydrate, EtOH, 110 °C; (c) potassium 3-methoxy-3-oxopropanoate, CDI, MgCl2, MeCN, 0 °C; (d) AcOH, 110 °C; (e) phosphorus pentasulfide, dioxane, 70 °C.
Some calculated properties and additional in vitro data for the four lead molecules are shown in Table 3. All four molecules are classified as lipophilic, with log P values at the upper end or above the desirable range for orally bioavailable drugs. The lipophilic character of the compounds contributes to very high protein binding, with free fractions in human plasma for 31, 36, and 51 at or below 0.2%. Compound 50 has the largest free fraction, which is 1.9% in human plasma. The thermodynamic solubility varies; the carbonyl compounds 31 and 36 have low aqueous solubility, while the corresponding thiocarbonyls 50 and 51 possess high solubility. Compounds 31, 50, and 51 have very low membrane permeability, and 36 has a permeability of 0.38 nm/s, as measured in a PAMPA assay.
Table 3. Profiling of Lead Molecules.
| compound | 31 (NDT-30347) | 36 (NDT-30408) | 50 (NDT-30805) | 51 (NDT-30744) |
|---|---|---|---|---|
| MW | 398 | 405 | 414 | 421 |
| c log P (ChemAxon) | 4.8 | 4.4 | 5.1 | 5.2 |
| tPSA | 85 | 78 | 68 | 64 |
| PAMPA, nm/s | <0.18 | 0.38 | <0.18 | <0.004 |
| thermodynamic solubility, mg/mL [μM] | 0.0009 [2.4] | 0.0097 [24] | 0.61 [1500] | 0.31 [730] |
| PPB, % | Hu = 99.8 | Hu = 99.9 | Hu = 98.1 | Hu = 99.9 |
| Rat = 100 | Rat = 100 | Rat = 98.5 | Rat = 99.8 | |
| Mu = 99.8 | Mu = 99.6 | Mu = 99.6 | ||
| PBMC IL-1β IC50, μM | 0.26 (n = 2), range 0.22–0.31 | 1.9 ± 1.1(n = 5) | 0.013 ± 0.0007(n = 3) | 0.13 ± 0.13(n = 4) |
| WB IL-1β IC50, μM | >40 (n = 2) | >40 (n = 1) | 1.5 ± 0.63(n = 3) | 8.9 ± 7.6(n = 4) |
| ASC speck inh IC50, μM | 0.24 (n = 1) | 1.3 (n = 1) | 0.034 (n = 1) | 0.19 (n = 1) |
| PBMC IL-6 IC50, μM | >40 (n = 1) | >40 (n = 1) | >40 (n = 1) | >20 (n = 1) |
| PBMC TNFα IC50, μM | >40 (n = 1) | >40 (n = 1) | >40 (n = 1) | >20 (n = 1) |
In addition to the cellular potency assay, the activity of each lead compound was measured in human whole blood (WB), following a similar protocol to the PBMC assay. The IC50 of a compound in this assay is affected by protein binding. The highly bound nature of 31, 36, 50, and 51 results in a large attenuation between PBMC and WB potency. NDT-30805 (50) is the most active compound from this series and is approximately 2-fold more potent than the widely studied NLRP3 inflammasome inhibitor CP-456,773 in both PBMC and whole blood assays (reported IC50 = 0.030 and 2.9 μM, respectively).22
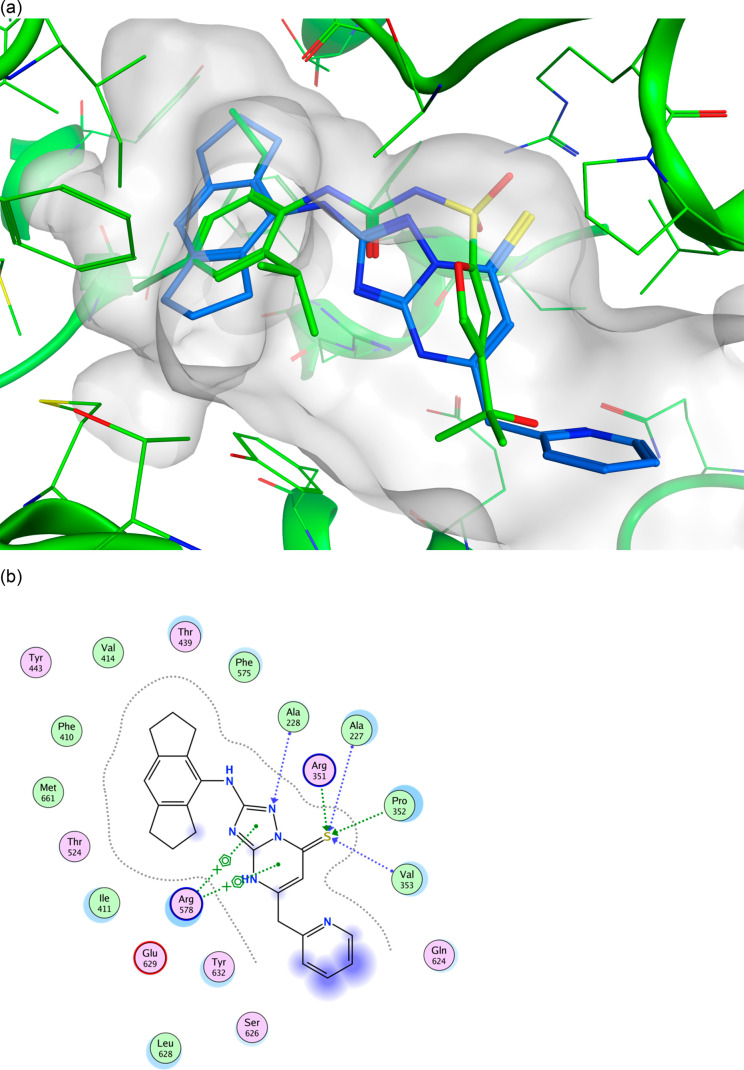
The genesis of the above four compounds is from the original triazolopyrimidone hit (1), itself derived from our pharmacophore model. Figure 2 shows compound 50 docked into the NLRP3 NACHT domain of the recently disclosed cocrystal structure of the sulfonylurea NP3–146.36 The hexahydroindacene moiety is buried in a lipophilic pocket coplanar with the aromatic ring of NP3–146, with the anilinic NH also overlaid. The 2-nitrogen of the triazolo ring may act as an H-bond acceptor of Ala228; the thiocarbonyl is in the H-bonding range of several residues as illustrated in the ligand interaction plot (Figure 2b). The methylene-spaced 2-pyridyl substituent occupies similar space to the tertiary alcohol of NP3–146 but projects further out of the binding pocket and into solvent space. The favorable docking pose of 50, which fits well into the ligand binding pocket occupied by NP3–146, affords a rationale for the good cellular potency.
Figure 2.
(a) Docked pose of one tautomer of NDT-30805 (50) shown in blue in the NLRP3 NACHT domain structure 7ALV binding site. The 7ALV ligand NP3–146 is shown in green for comparison. (b) Ligand interaction plot of the docked pose of NDT-30805 (50).
Further mechanistic evidence of selective NLRP3 inflammasome inhibition was obtained from an ASC speck assay and IL-6/TNFα selectivity assays. The ASC speck assay uses green fluorescent protein (GFP)-tagged ASC, a component of the NLRP3 inflammasome. The activation of the inflammasome with LPS and nigericin results in fluorescence, the disruption of which is measured by confocal microscopy. The selectivity assays look at downstream cytokines IL-6 and TNFα, neither of which should be affected by a selective inhibitor during the time frame of the assay. All four molecules 31, 36, 50, and 51 show a mechanistic profile consistent with a selective disruptor of NLRP3 inflammasome formation, as evidenced by the inhibition of ASC speck formation and lack of effect on inflammatory cytokines IL-6 and TNFα.
In summary, an in silico pharmacophore model was generated by overlaying several structurally diverse NLRP3 inflammasome inhibitors and subsequent refinement with proprietary screening data. Using this model, we were able to discover a weakly potent triazolopyrimidine hit, 1. Subsequent optimization led to the identification of a novel chemotype of NLRP3 inflammasome inhibitors, as exemplified by NDT-30805 (50). This is a potent, selective, and highly soluble molecule. Its advantages over CP-456,773 are superior potency and that it is uncharged at physiological pH (compared with the acidic characteristic of the sulfonylurea). Its disadvantages are high protein binding and low permeability, which in part may derive from the high lipophilicity (c log P = 5.1) and aromatic character (four aromatic rings, fsp3 = 0.30). The SAR around the hexahydroindacene moiety was not explored during this work, so this would be a potentially fruitful area for future exploration. The molecules described in this study could further refine the NLRP3 inflammasome inhibitor pharmacophore and provide potential starting points for further optimization toward the discovery of clinical candidates for the treatment of inflammatory diseases.
Acknowledgments
The authors would like to thank Matthew Boyton, Enrique Garcia-Alvarez, and their colleagues at Charles River Discovery Services in Portishead, UK, for generating much of the screening data. We also acknowledge the role of Han Xuejun and co-workers at WuXi AppTec in Tianjin, China, for the synthesis and analysis of many compounds. We thank Adam Keeney, President of NodThera Inc., for supporting the research that led to this manuscript.
Glossary
Abbreviations
- NLRP3
NOD-like receptor, Leucine-rich Repeat, and Pyrin-domain-containing protein 3
- PAMP
pathogen-associated molecular pattern
- DAMP
danger-associated molecular pattern
- PBMC
peripheral blood mononuclear cell
- ASC
apoptosis-associated speck-like protein containing a CARD
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.2c00242.
Assay protocols for PBMC IL-1β, whole blood IL-1β, PBMC IL-6, PBMC TNFα, ASC-speck, PAMPA, and thermodynamic solubility assays, details of docking studies, and synthesis and spectral data for compounds 1 to 51 (PDF)
The work contained within this manuscript was funded entirely by NodThera, Inc.
The authors declare no competing financial interest.
Supplementary Material
References
- Martinon F.; Burns K.; Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of pro IL-1β. Mol. Cell 2002, 10 (2), 417–426. 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- He Y.; Hara H.; Nuñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 2016, 41, 1012–1021. 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S.; Weiss D. S.; Newton K.; McBride J.; O’Rourke K.; Roose-Girma M.; Lee W. P.; Weinrauch Y.; Monack D. M.; Dixit V. M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 2006, 440 (7081), 228–232. 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Halle A.; Hornung V.; Petzold G. C.; Stewart C. R.; Monks B. G.; Reinheckel T.; Fitzgerald K. A.; Latz E.; Moore K. J.; Golenbock D. T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9 (8), 857–865. 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancu I.-C.; Cremers N.; Vanrusselt H.; Couturier J.; Vanoosthuyse A.; Kessels S.; Lodder C.; Brône B.; Huaux F.; Octave J.-N.; Terwel D.; Dewachter I. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropath. 2019, 137, 599–617. 10.1007/s00401-018-01957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising C.; Venegas C.; Zhang S.; Scheiblich H.; Schmidt S. V.; Vieira-Saecker A.; Schwartz S.; Albasset S.; McManus R. M.; Tejera D.; Griep A.; Santarelli F.; Brosseron F.; Opitz S.; Stunden J.; Merten M.; Kayed R.; Golenbock D. T.; Blum D.; Latz E.; Buée L.; Heneka M. T. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. 10.1038/s41586-019-1769-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codolo G.; Plotegher N.; Pozzobon T.; Brucale M.; Tessari I.; Bubacco L.; de Bernard M. Triggering of Inflammasome by Aggregated α–Synuclein, an Inflammatory Response in Synucleinopathies. PLOS One. 2013, 8 (1), e55375. 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duewell P.; Kono H.; Rayner K. J.; Sirois C. M.; Vladimer G.; Bauernfeind F. G.; Abela G. S.; Franchi L.; Núñez G.; Schnurr M.; Espevik T.; Lien E.; Fitzgerald K. A.; Rock K. L.; Moore K. J.; Wright S. D.; Hornung V.; Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F.; Pétrilli V.; Mayor A.; Tardivel A.; Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Jin C.; Frayssinet P.; Pelker R.; Cwirka D.; Hu B.; Vignery A.; Eisenbarth S. C.; Flavell R. A. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl. Acad. Sci. U.S.A. 2011, 108 (36), 14867–14872. 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H.; Gris D.; Lei Y.; Jha S.; Zhang L.; Huang M. T-H.; Brickey W. J.; Ting J. P-Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiblich H.; Schlütter A.; Golenbock D. T.; Latz E.; Martinez-Martinez P.; Heneka M. T. Activation of the NLRP3 inflammasome in microglia: the role of ceramide. J. Neurochem. 2017, 143, 534–550. 10.1111/jnc.14225. [DOI] [PubMed] [Google Scholar]
- Dostert C.; Pétrilli V.; Van Bruggen R.; Steele C.; Mossman B. T.; Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320 (5876), 674–677. 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman H. M.; Mueller J. L.; Broide D. H.; Wanderer A. A.; Kolodner R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001, 29 (3), 301–305. 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaux D. G.; McNiff P.; Laliberte R.; Hawryluk N.; Peurano H.; Stam E.; Eggler J.; Griffiths R.; Dombroski M. A.; Gabel A. A. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 2001, 299 (1), 187–197. [PubMed] [Google Scholar]
- Lamkanfi M.; Mueller J. L.; Vitari A. C.; Misaghi S.; Fedorova A.; Deshayes K.; Lee W. P.; Hoffman H. M.; Dixit V. M. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell. Biol. 2009, 187 (1), 61–70. 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.; He H.; Chen Y.; Huang W.; Cheng J.; Ye J.; Wang A.; Tao J.; Wang C.; Liu Q.; Jin T.; Jiang W.; Deng X.; Zhou R. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp Med. 2017, 214 (11), 3219–3238. 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.; Zhao F.; Chojnacki J. E.; Fulp J.; Klein W. L.; Zhang S.; Zhu X. NLRP3 Inflammasome Inhibitor Ameliorates Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55 (3), 1977–1987. 10.1007/s12035-017-0467-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulp J.; He L.; Toldo S.; Jiang Y.; Boice A.; Guo C.; Li X.; Rolfe A.; Sun D.; Abbate A.; Wang X.-Y.; Zhang S. Structural Insights of Benzenesulfonamide Analogues as NLRP3 Inflammasome Inhibitors: Design, Synthesis, and Biological Characterization. J. Med. Chem. 2018, 61 (12), 5412–5423. 10.1021/acs.jmedchem.8b00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klück V.; Jansen T. L. A.; Janssen M.; Comarniceanu A.; Efdé M.; Tengesdal I. W.; Schraa K.; Cleophas M. C. P.; Scribner C. L.; Skouras D. B.; Marchetti C.; Dinarello C. A.; Joosten L. A. B. Dapansutrile, an oral selective NLRP3 inflammasome inhibitor, for treatment of gout flares: an open-label, dose-adaptive, proof-of-concept, phase 2a trial. Lancet Rheumatol. 2020, 2 (5), e270–e280. 10.1016/S2665-9913(20)30065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C.; Swartzwelter B.; Gamboni F.; Neff C. P.; Richter K.; Azam T.; Carta S.; Tengesdal I.; Nemkov T.; D’Alessandro A.; Henry C.; Jones G. S.; Goodrich S. A.; St. Laurent J. P.; Jones T. M.; Scribner C. L.; Barrow R. B.; Altman R. D.; Skouras D. B.; Gattorno M.; Grau V.; Janciauskiene S.; Rubartelli A.; Joosten L. A. B.; Dinarello C. A. OLT1177, a β-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. U.S.A. 2018, 115 (7), e1530–e1539. 10.1073/pnas.1716095115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.; Boutard N.; Brzozka K.; Bugaj M.; Chmielewski S.; Cierpich A.; Doedens J. R.; Fabritius C-H. R. Y.; Gabel C. A.; Galezowski M.; Kowalczyk P.; Levenets O.; Mroczkowska M.; Palica K.; Porter R. A.; Schultz D.; Sowinska M.; Topolnicki G.; Urbanski P.; Woyciechowski J.; Watt A. P. Discovery of a series of ester-substituted NLRP3 inflammasome inhibitors. Bioorg. Med. Chem. Lett. 2020, 30 (23), 127560. 10.1016/j.bmcl.2020.127560. [DOI] [PubMed] [Google Scholar]
- Huang Y.; Jiang H.; Chen Y.; Wang X.; Yang Y.; Tao J.; Deng X.; Liang G.; Zhang H.; Jiang W.; Zhou R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018, 10 (4), e8689. 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. G.; Rivers-Auty J.; Daniels M. J. D.; White C. S.; Schwalbe C. H.; Schilling T.; Hammadi H.; Jaiyong P.; Spencer N. G.; England H.; Luheshi N. M.; Kadirvel M.; Lawrence C. B.; Rothwell N. J.; Harte M. K.; Bryce R. A.; Allan S. M.; Eder C.; Freeman S.; Brough D. Boron-based inhibitors of the NLRP3 inflammasome. Cell Chem. Biol. 2017, 24 (11), 1321–1335. 10.1016/j.chembiol.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Guo W.; Wu J.; Luo Q.; Tao F.; Gu Y.; Shen Y.; Li J.; Tan R.; Xu Q.; Sun Y. A novel benzo[d]imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem. Pharmacol. 2013, 85 (10), 1504–1512. 10.1016/j.bcp.2013.03.008. [DOI] [PubMed] [Google Scholar]
- El-Sharkawy L.; Brough D.; Freeman S. Inhibiting the NLRP3 Inflammasome. Molecules 2020, 25 (23), 5533. 10.3390/molecules25235533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan M. S. J.; Olhava E. J.; Roush W. R.; Seidel H. M.; Glick G. D.; Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discovery 2018, 17, 588–606. 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- Zahid A.; Li B.; Kombe Kombe A. J.; Jin T.; Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front. Immunol. 2019, 10, 2538. 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xu A.; Lv J.; Zhang Q.; Ran Y.; Wei C.; Wu J. Development of small molecule inhibitors targeting NLRP3 inflammasome pathway for inflammatory diseases. Eur. J. Med. Chem. 2020, 185, 111822. 10.1016/j.ejmech.2019.111822. [DOI] [PubMed] [Google Scholar]
- Schwaid A. G.; Spencer K. B. Strategies for Targeting the NLRP3 Inflammasome in the Clinical and Preclinical Space. J. Med. Chem. 2021, 64 (1), 101–122. 10.1021/acs.jmedchem.0c01307. [DOI] [PubMed] [Google Scholar]
- Platnich J. M.; Muruve D. A. NOD-like receptors and inflammasomes: A review of their canonical and non-canonical signaling pathways. Arch. Biochem. Biophys. 2019, 670, 4–14. 10.1016/j.abb.2019.02.008. [DOI] [PubMed] [Google Scholar]
- Coll R. C.; Schroder K.; Pelegrin P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43 (8), 653–668. 10.1016/j.tips.2022.04.003. [DOI] [PubMed] [Google Scholar]
- Laliberte R. E.; Perregaux D. G.; Hoth L. R.; Rosner P. J.; Jordan C. K.; Peese K. M.; Eggler J. F.; Dombroski M. A.; Geoghegan K. F.; Gabel C. A. Glutathione S-transferase Omega 1–1 Is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1β posttranslational processing. J. Biol. Chem. 2003, 278 (19), 16567–16578. 10.1074/jbc.M211596200. [DOI] [PubMed] [Google Scholar]
- Coll R. C.; Robertson A. A. B.; Chae J. J.; Higgins S. C.; Muñoz-Planillo R.; Inserra M. C.; Vetter I.; Dungan L. S.; Monks B. G.; Stutz A.; Croker D. E.; Butler M. S.; Haneklaus M.; Sutton C. E.; Núñez G.; Latz E.; Kastner D. L.; Mills K. H. G.; Masters S. L.; Schroder K.; Cooper M. A.; O’Neill L. A. J. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochheiser I. V.; Pilsl M.; Hagelueken G.; Moecking J.; Marleaux M.; Brinkschulte R.; Latz E.; Engel C.; Geyer M. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature 2022, 604, 184–189. 10.1038/s41586-022-04467-w. [DOI] [PubMed] [Google Scholar]
- Dekker C.; Mattes H.; Wright M.; Boettcher A.; Hinniger A.; Hughes N.; Kapps-Fouthier S.; Eder J.; Erbel P.; Stiefl N.; Mackay A.; Farady C. J. Crystal Structure of NLRP3 NACHT Domain With an Inhibitor Defines Mechanism of Inflammasome Inhibition. J. Mol. Biol. 2021, 433 (24), 167309. 10.1016/j.jmb.2021.167309. [DOI] [PubMed] [Google Scholar]
- Dempsey C.; Rubio Araiz A.; Bryson K. J.; Finucane O.; Larkin C.; Mills E. L.; Robertson A. A. B.; Cooper M. A.; O’Neill L. A. J.; Lynch M. A. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav. Immun. 2017, 61, 306–316. 10.1016/j.bbi.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Gordon R.; Albornoz E. A.; Christie D. C.; Langley M. R.; Kumar V.; Mantovani S.; Robertson A. A. B.; Butler M. S.; Rowe D. B.; O’Neill L. A.; Kanthasamy A. G.; Schroder K.; Cooper M. A.; Woodruff T. M. Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci. Transl. Med. 2018, 10 (465), eaah4066. 10.1126/scitranslmed.aah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Portugall I.; Bartok E.; Dhana E.; Evers B. D. G.; Primiano M. J.; Hall J. P.; Franklin B. S.; Knolle P. A.; Hornung V.; Hartmann G.; Boor P.; Latz E.; Kurts C. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 2016, 90 (3), 525–539. 10.1016/j.kint.2016.03.035. [DOI] [PubMed] [Google Scholar]
- Perera A. P.; Fernando R.; Shinde T.; Gundamaraju R.; Southam B.; Sohal S. S.; Robertson A. A. B.; Schroder K.; Kunde D.; Eri R. MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 2018, 8, 8618. 10.1038/s41598-018-26775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.; Fu R.; Wang S.; Huang Y.; Li X.; Zhou M.; Zhao J.; Yang N. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin. Exp. Immunol. 2018, 194 (2), 231–243. 10.1111/cei.13167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R. Y.; Pinkerton J. W.; Essilfie A. T.; Robertson A. A. B.; Baines K. J.; Brown A. C.; Mayall J. R.; Ali M. K.; Starkey M. R.; Hansbro N. G.; Hirota J. A.; Wood L. G.; Simpson J. L.; Knight D. A.; Wark P. A.; Gibson P. G.; O’Neill L. A. J.; Cooper M. A.; Horvat J. C.; Hansbro P. M. Role for NLRP3 Inflammasome–mediated, IL-1β–Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196 (3), 283–297. 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.