Abstract
Background
Respiratory syncytial virus (RSV) can cause serious illness in those aged <5 years in the United States, but uncertainty remains around which populations receive RSV testing. We conducted a systematic literature review of RSV testing patterns in studies published from 2000 to 2021.
Methods
Studies of RSV, medically attended RSV lower respiratory tract infections (LRTIs), and bronchiolitis were identified using standard methodology. Outcomes were clinical decisions to test for RSV, testing frequency, and testing incidence proportions in inpatient (IP), emergency department (ED), outpatient (OP), and urgent care settings.
Results
Eighty good-/fair-quality studies, which reported data from the period 1988–2020, were identified. Twenty-seven described the clinical decision to test, which varied across and within settings. Two studies reported RSV testing frequency for multiple settings, with higher testing proportions in IP (n = 2, range: 83%–85%, 1996–2009) compared with ED (n = 1, 25%, 2006–2009) and OP (n = 2, 15%–25%, 1996–2009). Higher RSV testing incidence proportions were observed among LRTI infant populations in the ED (n = 1, 74%, 2007–2008) and OP (n = 2, 54%–69%, 1995–2008). Incidence proportions in LRTI populations were not consistently higher in the IP setting (n = 13). Across studies and time, there was heterogeneity in RSV testing patterns, which may reflect varying detection methods, populations, locations, time periods, and healthcare settings.
Conclusions
Not all infants and children with LRTI are tested for RSV, highlighting underestimation of RSV burden across all settings.
Keywords: bronchiolitis, children, incidence, infants, laboratory testing, PCR, pediatric, respiratory syncytial virus, RSV, systematic literature review
Respiratory syncytial virus (RSV) affects nearly all infants and children aged <5 years and can cause serious illness including lower respiratory tract infections (LRTIs) such as bronchiolitis and pneumonia [1]. A retrospective cohort study based on nationally representative datasets of United States (US) infant hospitalizations and emergency department (ED) encounters in 2011–2019 found that annual average infant RSV LRTI hospitalizations and ED visits were 56 927 (range, 43 845–66 155) and 131 999 (range, 89 809–177 680), respectively [2]. Additionally, RSV remains the leading cause of US infant hospitalizations for the past 2 decades [3–7], indicating the ongoing burden RSV poses on the infant and the health system.
However, the epidemiology of RSV in infants and children outside of the inpatient (IP) hospital setting is understudied [8]. RSV laboratory testing patterns are also not systematically summarized in the current literature; it is unclear at the population level which infants and children are being tested for RSV or why they are tested. Hence, this systematic literature review (SLR) describes RSV laboratory testing patterns, testing frequency, and testing incidence across all healthcare settings (IP, ED, outpatient [OP], urgent care) for US infants and children aged <5 years.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed [9]. The protocol for this SLR was registered with the International Prospective Register of Systematic Reviews (PROSPERO identifier CRD42020162991) before the study began.
Eligibility Criteria
Study population, exposure, comparator, outcomes, and study design (PECOS) criteria were used to identify studies of US infants and children aged <5 years (population) with RSV and bronchiolitis (exposure) [10]. A comparator was not relevant for this review. Studies reporting outcomes of interest (predetermined to be RSV laboratory testing practices, testing frequency, and testing incidence proportion) were included. RSV laboratory testing practices were described as the study-reported clinical decisions to test for RSV (eg, physician judgment, presentation of symptoms such as fever, cough, and wheezing during the medical encounter, bronchiolitis diagnosis). RSV testing frequency was defined as the percentage tested for RSV among the enrolled population. RSV laboratory testing incidence proportion was defined as the percentage of RSV-positive infants and children among those tested for RSV. When available, outcomes reported for LRTI populations were summarized, given that RSV is one of the leading causes of medically attended LRTI infections [4, 11]. Outcomes stratified by sociodemographic and clinical variables such as chronological age, weeks’ gestational age (wGA), race/ethnicity, and insurance payer were summarized when available.
Randomized controlled trials (RCTs) and observational studies (surveillance, cohort, case-control, and cross-sectional) were included. Case reports with <20 cases, studies not published in English, and studies not meeting the PECOS criteria were excluded. To identify additional studies not captured by the literature searches, reference lists of relevant reviews were checked to ensure that all studies meeting the PECOS criteria were identified and included.
Study Identification, Screening, and Abstraction
Literature searches in the PubMed, Embase, and Web of Science databases were conducted to capture RSV and bronchiolitis literature published from 1 January 2000 to 11 June 2021. Literature search terms are provided in Supplementary Table 1. DistillerSR [12] was used to de-duplicate the search results and conduct the review. One reviewer examined the titles and abstracts using the predefined PECOS criteria. The articles deemed to be relevant at the abstract stage were reviewed for full text by 2 reviewers independently. Data were abstracted from the included full-text studies in DistillerSR; abstraction elements included study characteristics (eg, design, time period, location, setting), population characteristics (eg, sample size, age, wGA, sex, race/ethnicity), and outcomes (overall and by sociodemographic variables when available). After one reviewer abstracted the data elements, a second reviewer checked them independently; all conflicts were resolved by the senior reviewers. Data visualizations were done using Microsoft Excel for Mac (version 16.56).
Risk of Bias
For observational studies, a modified version of the Newcastle-Ottawa Scale was used to evaluate the study quality. The Cochrane Risk of Bias (RoB) tool was used to determine the study quality of the RCTs. Detailed description of the assessments and study quality determination is reported elsewhere [8]. This SLR considered good- and fair-quality studies and did not include the poor-quality studies.
RESULTS
Article Identification
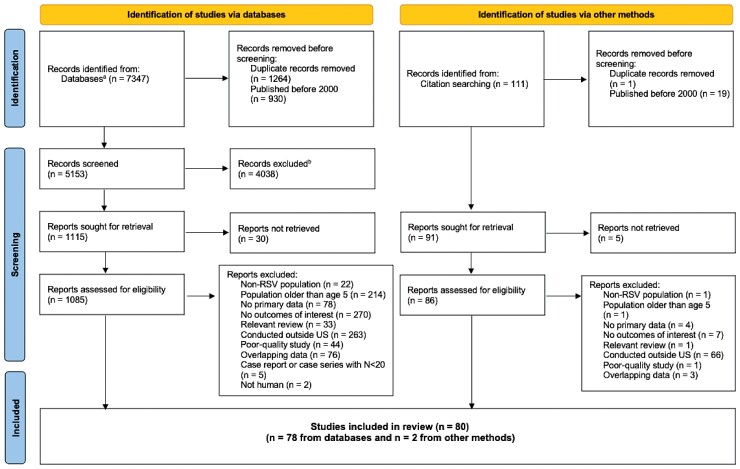
The PRISMA study flow diagram is presented in Figure 1, and the PRISMA checklist is provided in the Supplementary Materials. At the title and abstract level, 5153 publications were screened. References cited in 34 relevant reviews were reviewed, and 91 additional publications were identified. At the full-text level, 1206 publications were reviewed. Of the 1126 publications eliminated at the full-text stage, 23 were non-RSV populations, 215 had populations aged ≥5 years, 82 had no primary data, 277 had no outcomes of interest, 34 were reviews, 329 were conducted outside the US, 45 were poor-quality studies, 79 had overlapping data, 5 were excluded study designs (ie, case reports or case series with ≤20 cases), 2 were not human, and 35 were unable to be obtained as full-text articles. Eighty good- and fair-quality studies with testing data were identified.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of the study selection process. aPubMed, Embase, and Web of Science databases. bExcluded for not meeting the predefined eligibility criteria. Abbreviations: RSV, respiratory syncytial virus; US, United States.
Risk of Bias of Included Studies (n = 80)
Of the 3 RCTs identified, 1 was good quality and 2 were fair quality as assessed with the Cochrane RoB tool (Supplementary Table 2). Among the 77 observational studies (75 cohort studies, 2 case-control), 39 and 38 studies were of good and fair quality, respectively. RoB scores for the 75 cohort studies are summarized in Supplementary Figure 1. RoB was apparent in the comparability of cohorts on the basis of design or analysis (45% did not control for >1 factor) and adequacy of follow-up (11% did not describe losses to follow-up or losses were >10%, when described).
Study and Population Characteristics
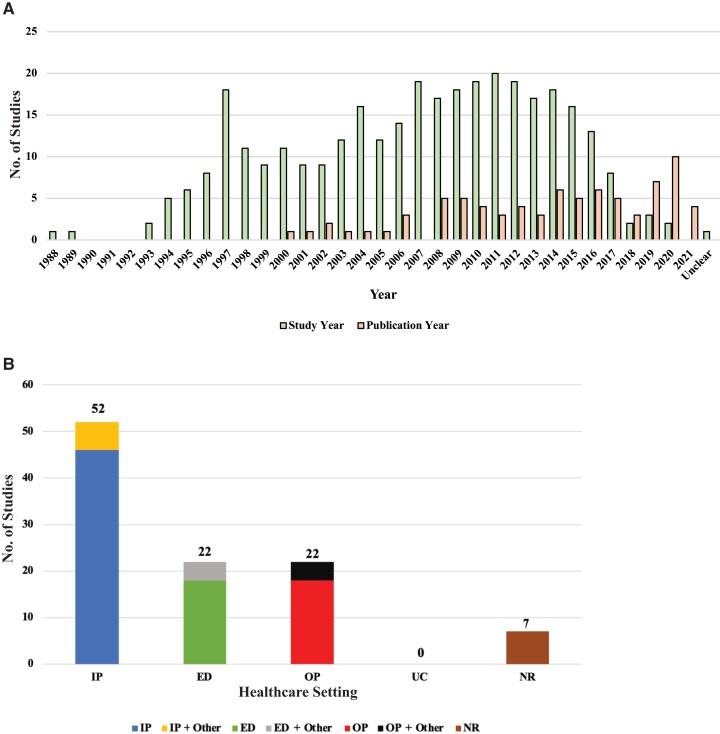
The 80 included studies were published between January 2000 and June 2021 and reported 1 or more years of data from 1988 to 2020 (Figure 2A). Eight studies (10%) reported data between 2015 and 2020, while 13 (16%) provided data that included years earlier than 2015 and up to 2020 (Supplementary Table 2). Eleven studies were surveillance, 38 were prospective cohorts, 24 were retrospective cohorts, 2 were case-control, 1 was a study of passive surveillance and prospective cohort design, 1 was a post hoc analysis of surveillance, and 3 were RCTs (Supplementary Table 2). Studies were conducted in various states across the US (Supplementary Table 2). More than half of the studies (n = 52 [65%]) provided data from the IP setting or IP combined with other settings (Figure 2B). Eighteen studies presented ED-specific data, and 4 studies provided ED data combined with other settings. Eighteen studies provided data specific to the OP setting, whereas 4 studies presented OP data combined with other settings. There were no data available for urgent care.
Figure 2.
Histograms of included studies (n = 80). A, Data years vs publication years. B, By healthcare setting. Numbers do not sum to 80 because studies including multiple settings were counted more than once. Healthcare setting is based on the testing outcomes reported in each study. Abbreviations: ED, emergency department; IP, inpatient; NR, not reported; OP, outpatient; UC, urgent care.
RSV Laboratory Testing Practices: Clinical Decisions to Test for RSV
Twenty-seven studies [13–39] provided descriptive data on the clinical decisions to test for RSV in various settings: 16 provided data for IP [13–16, 18–29], 1 for IP and for ED [17], 9 for ED [30–38], and 1 for OP [39] (Supplementary Table 3A and 3B). Thirteen studies describing laboratory testing practice for combined settings (ie, IP with other settings, ED with other settings, or OP with other settings) are listed in Supplementary Table 3C. These studies reported that RSV laboratory testing was done as part of routine care, at the discretion of the provider, based on symptoms, or per institutional guidelines. However, the clinical decision to test for RSV was not consistent across or within settings.
RSV Laboratory Testing Frequency in the IP, ED, and OP Settings: RSV Testing Among the Enrolled Populations
Seven studies reported testing frequency for a single setting (ie, IP, ED, or OP) [15, 22, 34, 40–43]; due to differences in geographical locations, time periods, population characteristics, and test types, comparisons across studies and settings could not be done, and thus, they were not described further (see Supplementary Table 4 for additional details).
Only 2 retrospective cohort studies conducted in infants and children enrolled in the Kaiser Permanente Northern California health system reported RSV testing frequency for multiple settings [44, 45] (Table 1). One Kaiser study identified 717 bronchiolitis episodes in the IP setting, 425 in the ED setting, and 9269 in the OP setting from 2006 to 2009 among infants aged 0–12 months using International Classification of Diseases, Ninth Revision (ICD-9) codes 466.11, 480.1, and 466.19 [44]. Eighty-three percent of the IP bronchiolitis episodes were laboratory tested for RSV, whereas 29% and 25% of the ED and OP bronchiolitis episodes, respectively, were tested. Another Kaiser study conducted from 1996 to 2004 among infants and children aged <2 years described similar trends in testing by setting; this study reported RSV testing frequency among IP and OP bronchiolitis episodes (ICD-9 codes 466.1, 466.1x, 466.0, 480.0–480.2, 079.0, and 079.6) with and without antibiotic use to examine the association between RSV testing and antibiotic use [45]. Testing frequency was 85% for the IP bronchiolitis episodes regardless of antibiotic use and 15% for the OP bronchiolitis episodes regardless of antibiotic use [45].
Table 1.
Respiratory Syncytial Virus Laboratory Testing Frequency (ie, Number of Infants and Children Tested Among the Enrolled Study Populations) for Studies That Reported Data for Multiple Settings, United States Infants and Children Aged <5 Years (n = 2)
| Study, First Author (Year) [Reference] | Data Source, Location | Setting | Time Period | Underlying Respiratory Condition of the Study Populationa | Study Size, No. | Age | Test Type | Testing Frequency | AHRQ Quality Score |
|---|---|---|---|---|---|---|---|---|---|
| Turi (2018) [44] | Kaiser Permanente Northern CA | IP | 2006–2009 | LRTI | 717 bronchiolitis episodes | <2 y | Antigen; culture; RT-PCR | 83% tested | Fair |
| ED | 2006–2009 | LRTI | 425 bronchiolitis episodes | <2 y | Antigen; culture; RT-PCR | 29% tested | Fair | ||
| OP | 2006–2009 | LRTI | 9269 bronchiolitis episodes | <2 y | Antigen; culture; RT-PCR | 25% tested | Fair | ||
| Flaherman (2010) [45] | Kaiser Permanente Northern CA | IP | 1996–2004 | LRTI | 926 bronchiolitis episodes without antibiotic use; 1110 with antibiotic use | <2 y | DFA | Without antibiotic use: 85% tested | Fair |
| With antibiotic use: 85% tested | |||||||||
| OP | 1996–2004 | LRTI | 15 173 bronchiolitis episodes without antibiotic use; 6539 with antibiotic use | <2 y | DFA | Without antibiotic use: 15% tested | Fair | ||
| With antibiotic use: 15% tested |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; CA, California; DFA, direct fluorescent antibody; ED, emergency department; IP, inpatient; LRTI, lower respiratory tract infection; OP, outpatient; RT-PCR, real-time polymerase chain reaction.
As reported by the study authors.
In Turi et al [44], antigen, culture, or polymerase chain reaction (PCR) tests were used from 2006 to 2009; Flaherman et al [45] used direct fluorescent antibody tests from 1996 to 2004, indicating utilization changes in RSV test types across time.
RSV Laboratory Testing Incidence Proportion in the IP Setting: Percentage of RSV-Positive Results Among Those Tested for RSV
In the IP setting, 12 studies provided RSV laboratory testing incidence proportion data for LRTI populations only [14, 41, 46–55] (Table 2). One study reported data for LRTI and upper respiratory illness populations separately [56]. One study combined data for LRTI with other respiratory distress [13], 6 studies grouped data by acute respiratory infections (ARI) or symptomatic (eg, fever) populations [40, 57–61], 1 study included healthy infants [62], and 4 studies provided information among populations of unknown respiratory conditions [24, 63–65]. Higher RSV testing incidence proportions among studies with LRTI populations were not consistently observed in the IP setting compared with studies among ARI, symptomatic, healthy, or unknown respiratory condition populations, likely due to differences in study designs, time periods, test types, locations, and other population characteristics (Table 2).
Table 2.
Respiratory Syndrome Virus (RSV) Laboratory Testing Incidence Proportion (ie, Percentage of RSV-Positive Results Among Infants and Children Tested for RSV) in the Inpatient Setting, United States Infants and Children Aged <5 Years (n = 25)
| Study: First Author, Publication Year [Reference] | Data Source and Location | Time Period | Underlying Respiratory Condition of the Study Populationa | Age | Laboratory Test Method | RSV Incidence Proportion | AHRQ Quality Score |
|---|---|---|---|---|---|---|---|
| Tsou (2020) [14] | Children’s hospital: TX | 2015–2017 | LRTI (bronchiolitis) | <2 y | RT-PCR | 66% (179/270) | Fair |
| General pediatric unit: 59% (79/135) | |||||||
| PICU: 74% (100/135) | |||||||
| El Assal (2020) [46] | Hospital | 2014–2017 | LRTI (bronchiolitis) | <2 y | NR | 51% (133/260) | Fair |
| Hasegawa (2019) [47] | MARC-30; MARC-35: multisite | MARC-30: 2007–2010; MARC-35: 2011–2014 | LRTI | <1 y | Single or duplex RT-PCR | 77% (2228/2912) | Fair |
| Luthe (2018) [48] | 27 sites across US | 2007–2014 | LRTI | <1 y | RT-PCR | RSV-A: 49% (included coinfections, numbers NR) | Fair |
| RSV-B: 29% (included coinfections, numbers NR) | |||||||
| Bruden (2015) [41] | YKD regional hospital and Alaska Native medical center: AK | 1994–2012 | LRTI | <3 y | EIA; culture; DIA | 40% (1903/4744) | Fair |
| Suárez-Arrabal (2015) [49] | Children’s hospital: OH | 2010–2011 | LRTI (bronchiolitis) | <2 y | DFA; rapid antigen; PCR | 88% (136/154) | Fair |
| Mansbach (2012)b [50] | MARC: multisite | 2007–2010 | LRTI | <2 y | RT-PCR | 72% (1589/2207) | Fair |
| De Hoyos (2012) [51] | Hospitals: 16 centers | 2007–2010 | LRTI (bronchiolitis) | <2 y | PCR | 73% (1611/2207) | Fair |
| Miernyk (2011) [52] | Yukon-Kuskokwim hospital: AK | 2005–2007 | LRTI | <3 y | Rapid antigen; RT-PCR | All, 0 to <3 y: 25% (79/311) | Good |
| <1 y: 27% (58/213) | |||||||
| 1–3 y: 21% (21/98) | |||||||
| Mella (2010) [53] | Children’s hospital: OH | 2009 | LRTI (bronchiolitis) | <2 y | NR | 74% (92/125) | Good |
| Singleton (2006) [54] | YKD regional hospital: AK | 1993–2004 | LRTI | <3 y | Rapid antigen | 0 mo: 37% | Good |
| 1–5 mo: 38% | |||||||
| 6–11 mo: 40% | |||||||
| <1 y: 39% | |||||||
| 1 y: 30% | |||||||
| 2 y: 22% | |||||||
| Numbers NR. Percentages obtained from text. | |||||||
| Bockova (2002) [55] | Navajo hospitals | 1997–2000 | LRTI | <2 y | EIA | All, <2 y: 50% (913/1837) | Good |
| <1 y: 49% (642/1322) | |||||||
| 1 to <2 y: 53% (271/515) | |||||||
| Miller (2013)c [56] | TCRI: Children’s hospital, TN | 2004–2008 | LRTI, URI (symptoms) | <2 y | RT-PCR | Bronchiolitis: 79% (310/392) | Fair |
| URI: 10% (3/29) | |||||||
| Bronchiolitis: 0–6 mo: 59% (214/360) | |||||||
| RSV alone | |||||||
| Bronchiolitis: 6–12 mo: 42% (30/71); RSV alone | |||||||
| Shutes (2021) [13] | Academic medical center PICU: OH | 2014–2017 | LRTI, other (respiratory distress) | <1 y | PCR | 42% (417/984) | Fair |
| Rha (2020) [57] | NVSN: NY, OH, TN, MO, TX, WA, CA | 2015–2016 | ARI | <5 y | RT-PCR | All, <5 y: 35% (1043/2969) | Good |
| 0–2 mo: 46% (342/743) | |||||||
| 3–5 mo: 60% (184/305) | |||||||
| 6–11 mo: 38% (178/472) | |||||||
| <1 y: 46% (704/1520) | |||||||
| 1 to <2 y: 28% (199/702) | |||||||
| 2 to <5 y: 19% (140/747) | |||||||
| Hall (2009) [58] | NVSN: TN, NY, OH | 2000–2004 | ARI | <5 y | RT-PCR; culture | 0–5 mo: 24% (328/1370) | Good |
| 6–11 mo: 24% (97/403) | |||||||
| 12–23 mo: 18% (99/540) | |||||||
| 24–59 mo: 7% (40/579) | |||||||
| Jain (2015) [40] | CDC EPIC: TN, UT | 2010–2012 | ARI, symptoms | <5 y | PCR | All, <5 y: 37% (574/1539) | Fair |
| <2 y: 42% (412/980) | |||||||
| 2–4 y: 29% (162/559) | |||||||
| Suryadevara (2011)d [59] | Medical center: NY | 2007–2010 | Fever | <2 y | EIA; viral cultures | 58% (108/denominator NR) | Good |
| Muñiz (2009) [60] | NR | 1988–2007 | Symptoms | <1 y | RSV antigen | <90 d: 39% (70/180) | Fair |
| Golombek (2004)e [61] | NY | 2000–2001 | Symptoms | <1 y | EIA | 24% (17/70) | Good |
| O’Brien (2015) [62] | RCT: southwestern US | 2004–2007 | Healthy | <1 y | RT-PCR | ITT placebo: 11% (80/710) | Good |
| Per-protocol placebo: 13% (73/571) | |||||||
| Vendetti (2016) [63] | Premier Perspective database: 14 hospitals | 2009–2013 | NR | <1 y | PCR; EIA; culture | 0.3% (31/11418) | Fair |
| Bender (2014) [64] | Kaiser Permanente network: Southern CA | 2010–2011 | NR | <1 y | RVP: multiplex PCR | All, 1–90 d: 32% (79/245) | Good |
| Bennett (2012) [65] | 2 NICUs: NY | 2009 | NR | <1 y | RVP: multiplex PCR | 30% (15/50) | Good |
| Durani (2008) [24] | Children’s hospital: DE | 2002 | NR | <5 y | Rapid antigen; viral culture | 64% (126/197) | Fair |
Abbreviations: AHRQ, Agency for Healthcare Research and Quality; ARI, acute respiratory infection; AK, Alaska; CA, California; CDC EPIC, Centers for Disease Control and Prevention Etiology of Pneumonia in the Community Study; DE, Delaware; DFA, direct fluorescent antibody; DIA, direct immunofluorescence assay; EIA, enzyme-linked immunoassay; ITT, intention to treat; LRTI, lower respiratory tract infection; MARC, Multicenter Airway Research Collaboration; MARC-30, 30th Multicenter Airway Research Collaboration; MARC-35, 35th Multicenter Airway Research Collaboration; MD, Maryland; NICU, neonatal intensive care unit; NR, not reported; NVSN, New Vaccine Surveillance Network; NY, New York; OH, Ohio; PCR, polymerase chain reaction; PICU, pediatric intensive care unit; RCT, randomized controlled trial; RSV, respiratory syncytial virus; RT-PCR, real-time polymerase chain reaction; RVP, respiratory viral panel; TCRI, Tennessee Children’s Respiratory Initiative; TN, Tennessee; TX, Texas; URI, upper respiratory infection; US, United States; UT, Utah; WA, Washington; YKD, Yukon-Kuskokwim Delta.
As reported by the authors. Designation of ARI and LRTI were specified by the study authors. For infant populations with symptoms, the study authors reported fever, cough, runny nose, nasal congestion, and wheezing as common symptoms.
Mansbach et al [50] reported an incidence of 72% in the abstract. However, the numbers provided in the text add up to 73%.
Miller et al [56] also reported in the text that RSV was detected in 63% of the infants aged <6 months and 42% of the infants aged 6–12 months.
Suryadevara et al [59] enrolled 201 infants and children aged <2 years and reported an incidence of 58%. However, the denominator is not clear as multiple numbers are reported in the results.
The denominator is not clear in Golombek et al [61] as multiple numbers are reported.
RSV Laboratory Testing Incidence Proportion in the ED Setting: Percentage of RSV-Positive Results Among Those Tested for RSV
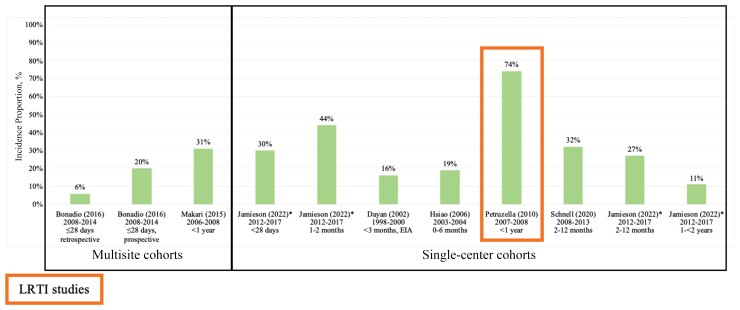
Seven studies [30, 33, 35, 38, 66–68] reported RSV laboratory testing incidence proportion in the ED (Figure 3; Supplementary Table 5A). The highest RSV laboratory testing incidence proportion was observed in infants aged <1 year with LRTI seen at a single ED in Wisconsin (74% among 85 infants, 2007–2008) [35]. Among the 6 studies [30, 33, 38, 66–68] describing populations with symptoms or unknown respiratory conditions, RSV laboratory testing incidence proportion ranged between 6% among 378 febrile infants aged ≤28 days seen at an urban, pediatric ED in New York from 2008 to 2014 [33] and 44% among 82 infants aged 1–2 months intubated for respiratory failure with a suspected infection at an urban, pediatric ED in Ohio from 2012 to 2017 (underlying respiratory condition not reported) [66]. This study of intubated infants also provided testing incidence proportion stratified by chronological age, noting a higher incidence among younger populations (underlying respiratory condition of the population not reported) (<28 days: 31%; 1–2 months: 44%; 2–12 months: 27%; 12–24 months: 11%) [66].
Figure 3.
Respiratory syncytial virus testing laboratory incidence proportion in the emergency department, United States infants and children aged <5 years (n = 7). The x-axis shows the author (publication year) and reporting data years. Studies are presented in increasing age order of the study population in each study. Study references are provided in Supplementary Table 2. Populations across the studies were heterogeneous; thus, testing patterns may not be uniform across the studies. *The Jamieson (2022) study was published electronically in 2020 and was captured in our literature search. Abbreviations: EIA, enzyme immunoassay; LRTI, lower respiratory tract infection.
RSV Laboratory Testing Incidence Proportion in the OP Setting: Percentage of RSV-Positive Results Among Those Tested for RSV
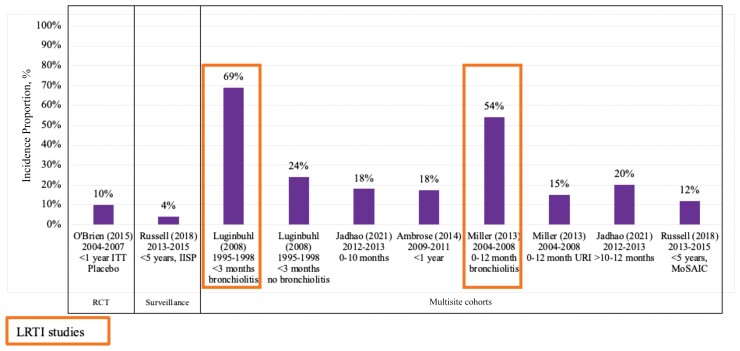
Six studies [39, 42, 56, 62, 69, 70] reported RSV laboratory testing incidence proportions in the OP setting (Figure 4; Supplementary Table 5A). The highest RSV laboratory testing incidence proportion was observed in 2 studies with LRTI infant populations [42, 56]. Incidence proportions of 54% among 63 bronchiolitis infants aged 0–12 months in a 2004–2008 Tennessee Children’s Respiratory Initiative cohort [56] and 69% among 102 bronchiolitis infants aged <3 months seen across 219 practices in 44 states from 1995 to 1998 [42] were observed. Among ARI, symptomatic, or healthy populations, the lowest RSV laboratory testing incidence proportion was 4% among 148 infants and children aged <5 years with influenza-like symptoms seen from 2013 to 2015 in New York [69], and the highest was 24% among 174 infants aged <3 months without bronchiolitis seen across 219 practices in 44 states from 1995 to 1998 [42].
Figure 4.
Respiratory syncytial virus testing laboratory incidence proportion in the outpatient setting, United States infants and children aged <5 years (n = 6). The x-axis shows the author (publication year) and reporting data years. Studies are presented in increasing age order of the study population in each study. Study references are provided in Supplementary Table 2. Populations across the studies were heterogeneous; thus, testing patterns may not be uniform across the studies. Abbreviations: IISP, Influenza Incidence Surveillance Project; ITT, intention to treat; LRTI, lower respiratory tract infection; MoSAIC, Mobile Surveillance for Acute Respiratory Infections and Influenza-like Illness in the Community; RCT, randomized controlled trial; URI, upper respiratory illness.
RSV testing incidence proportion data further stratified by wGA, race/ethnicity, comorbidity conditions, or insurance payer were not provided for any healthcare setting.
Studies describing RSV laboratory testing incidence proportion for combined settings (ie, IP with other settings, ED with other settings, or OP with other settings) are listed in Supplementary Table 5C but were not described further due to the lack of setting-specific data.
DISCUSSION
This SLR reviewed studies published between January 2000 and June 2021 (data from 1988 to 2020), reporting RSV laboratory testing practices, testing frequency, and testing incidence proportion across all healthcare settings in US infants and children aged <5 years. Clinical decision to test for RSV was variable and often unclear across and within settings, with only a proportion of infants and children being laboratory tested, suggesting that those tested for RSV may not be representative of total US infant and pediatric RSV populations. Only 2 studies conducted in Northern California [44, 45] provided RSV laboratory testing frequency data for multiple settings to allow for comparisons across settings and the findings elucidate that testing occurs less frequently in the ED and OP compared to the IP setting. This SLR also showed the changes in RSV testing types over time with earlier studies utilizing tests such as direct fluorescent antibody and cultures while more recent studies reported greater utilization of PCR tests. Moreover, not all infants and children were tested for RSV and this pattern held across all healthcare settings, suggesting there may be heterogeneity in RSV laboratory testing practice with differences in testing frequency by setting that could potentially underestimate RSV. However, the data were >10 years old, not available for all healthcare settings, and pertained to select infant and pediatric populations within a closed health system. Further research using current data and conducted within other health systems in various geographical locations is urgently needed to fill the knowledge gaps identified by this SLR.
Varying pediatric populations by chronological age, setting, respiratory symptoms, time periods, locations, testing practices, and test types were included in the studies identified in this SLR, making it difficult to summarize the RSV laboratory testing frequency and incidence proportion data across studies. Stratified data by sociodemographic and clinical variables such as wGA, insurance status, and comorbidity conditions were also not available by setting. Hence, there are potential uncertainties around assessing the impact of new immunization strategies to prevent RSV due to these data gaps in the existing literature landscape. As new RSV prevention strategies are on the horizon [71], models used to estimate the impact of potential new immunoprophylaxis on the RSV disease burden will need laboratory testing data inputs, overall and by sociodemographic and clinical variables. Specifically, the number of infants and children who test positive for RSV among LRTIs and the number of LRTIs among RSV-positive infants and children for multiple settings should be described. Studies conducted among all infants and children aged <5 years across all settings, with detailed sociodemographic and clinical data collection, will allow for a complete perspective of RSV disease in the US and are urgently needed.
This SLR had several strengths including rigorous study methodology that registered the study protocol a priori before SLR conduct, adherence to the PRIMSA guidelines, and use of validated RoB tools to evaluate the quality of the included studies. Because the SLR was specific to infants and children aged <5 years in the US, our findings may not be generalizable to those outside of the US or to populations >5 years of age. Furthermore, there were changes in detection methods over time, which were not accounted for, and there is the possibility of testing bias across the studies given the variability in the clinical decisions to test. The impact of coronavirus disease 2019 on RSV epidemiology was not considered in this review.
This SLR highlights the substantial variability in RSV laboratory testing practices, testing frequency, and testing incidence proportions. Furthermore, the limited number of studies detailing RSV laboratory testing frequency emphasizes the lack of routine testing in the US, especially outside of the IP setting. Studies exploring the intersection between RSV laboratory testing results and ICD diagnosis codes are needed to inform the extent of underestimation of RSV. Prospective studies with active, routine testing across all healthcare settings are needed to comprehensively describe the true burden of RSV among infants and children in the US.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Naimisha Movva, EpidStrategies, a Division of ToxStrategies, Rockville, Maryland, USA.
Mina Suh, EpidStrategies, a Division of ToxStrategies, Rockville, Maryland, USA.
Lauren C Bylsma, EpidStrategies, a Division of ToxStrategies, Rockville, Maryland, USA.
Jon P Fryzek, EpidStrategies, a Division of ToxStrategies, Rockville, Maryland, USA.
Christopher B Nelson, Sanofi, Swiftwater, Pennsylvania, USA.
Notes
Acknowledgments . Editorial assistance was provided by inScience Communications (Philadelphia, Pennsylvania). This work was performed in accordance with current Good Publication Practice guidelines and was supported/funded by Sanofi.
Financial support . This collaborative study with EpidStrategies was supported/funded by Sanofi and AstraZeneca.
Supplement sponsorship . This article appears as part of the supplement “Respiratory Syncytial Virus Disease Among US Infants,” sponsored by Sanofi and AstraZeneca.
References
- 1. Centers for Disease Control and Prevention . RSV in infants and young children.https://www.cdc.gov/rsv/high-risk/infants-young-children.html. Accessed November 2021.
- 2. Suh M, Movva N, Jiang X, et al. Respiratory syncytial virus burden and healthcare utilization in United States infants <1 year of age: study of nationally representative databases, 2011–2019. J Infect Dis 2022; 226(S2):S184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suh M, Movva N, Jiang X, et al. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009–2019: a study of the national (nationwide) inpatient sample. J Infect Dis 2022; 226(S2):S154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr 2003; 143:S127–32. [DOI] [PubMed] [Google Scholar]
- 5. Holman RC, Curns AT, Cheek JE, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics 2004; 114:e437–44. [DOI] [PubMed] [Google Scholar]
- 6. Sangaré L, Curtis MP, Ahmad S. Hospitalization for respiratory syncytial virus among California infants: disparities related to race, insurance, and geography. J Pediatr 2006; 149:373–7. [DOI] [PubMed] [Google Scholar]
- 7. Yorita KL, Holman RC, Sejvar JJ, Steiner CA, Schonberger LB. Infectious disease hospitalizations among infants in the United States. Pediatrics 2008; 121:244–52. [DOI] [PubMed] [Google Scholar]
- 8. Suh M, Movva N, Bylsma LC, et al. A systematic literature review of the burden of respiratory syncytial virus and health care utilization among United States infants younger than 1 year. J Infect Dis 2022; 226(S2):S195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021; 18:e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Version 6.3. Chichester (UK): Cochrane Collaboration, 2022.
- 11. Driscoll AJ, Arshad SH, Bont L, et al. Does respiratory syncytial virus lower respiratory illness in early life cause recurrent wheeze of early childhood and asthma? Critical review of the evidence and guidance for future studies from a World Health Organization–sponsored meeting. Vaccine 2020; 38:2435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evidence Partners. DistillerSR version 2.35.https://www.evidencepartners.com. Accessed September 2019 to October 2021.
- 13. Shutes BL, Patel AB, Moore-Clingenpeel MD, Mejias A, Karsies TJ. Relationship of viral detection with duration of ventilation in critically ill infants with lower respiratory tract infection. Ann Am Thorac Soc 2021; 18:1677–84. [DOI] [PubMed] [Google Scholar]
- 14. Tsou P, Vadivelan A, Kovvuri M, et al. Association between multiple respiratory viral infections and pediatric intensive care unit admission among infants with bronchiolitis. Arch Pediatr 2020; 27:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arriola CS, Kim L, Langley G, et al. Estimated burden of community-onset respiratory syncytial virus–associated hospitalizations among children aged <2 years in the United States, 2014–15. J Pediatric Infect Dis Soc 2020; 9:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson EJ, DeVincenzo JP, Simões EAF, et al. SENTINEL1: two-season study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks’ gestational age not receiving immunoprophylaxis. Am J Perinatol 2020; 37:421–9. [DOI] [PubMed] [Google Scholar]
- 17. Fine J, Bray-Aschenbrenner A, Williams H, Buchanan P, Werner J. The resource burden of infections with rhinovirus/enterovirus, influenza, and respiratory syncytial virus in children. Clin Pediatr (Phila) 2019; 58:177–84. [DOI] [PubMed] [Google Scholar]
- 18. Cerone JB, Santos RP, Tristram D, et al. Incidence of respiratory viral infection in infants with respiratory symptoms evaluated for late-onset sepsis. J Perinatol 2017; 37:922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arbefeville S, Ferrieri P. Epidemiologic analysis of respiratory viral infections mainly in hospitalized children and adults in a midwest university medical center after the implementation of a 14-virus multiplex nucleic acid amplification test. Am J Clin Pathol 2017; 147:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grindeland CJ, Mauriello CT, Leedahl DD, Richter LM, Meyer AC. Association between updated guideline-based palivizumab administration and hospitalizations for respiratory syncytial virus infections. Pediatr Infect Dis J 2016; 35:728–32. [DOI] [PubMed] [Google Scholar]
- 21. Litwin CM, Bosley JG. Seasonality and prevalence of respiratory pathogens detected by multiplex PCR at a tertiary care medical center. Arch Virol 2014; 159:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J 2012; 31:1221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beneri C, Ginocchio CC, Manji R, Sood S. Comparison of clinical features of pediatric respiratory syncytial virus and human metapneumovirus infections. Infect Control Hosp Epidemiol 2009; 30:1240–1. [DOI] [PubMed] [Google Scholar]
- 24. Durani Y, Friedman MJ, Attia MW. Clinical predictors of respiratory syncytial virus infection in children. Pediatr Int 2008; 50:352–5. [DOI] [PubMed] [Google Scholar]
- 25. Levin DL, Garg A, Hall LJ, Slogic S, Jarvis JD, Leiter JC. A prospective randomized controlled blinded study of three bronchodilators in infants with respiratory syncytial virus bronchiolitis on mechanical ventilation. Pediatr Crit Care Med 2008; 9:598–604. [DOI] [PubMed] [Google Scholar]
- 26. Aldous WK, Gerber K, Taggart EW, Rupp J, Wintch J, Daly JA. A comparison of Thermo Electron RSV OIA to viral culture and direct fluorescent assay testing for respiratory syncytial virus. J Clin Virol 2005; 32:224–8. [DOI] [PubMed] [Google Scholar]
- 27. Katz BZ, Lo J, Sorrentino M. Costs of respiratory syncytial virus infection at a tertiary-care children’s hospital. Pharm Ther 2003; 28:343–5. [Google Scholar]
- 28. Buckingham SC, Quasney MW, Bush AJ, DeVincenzo JP. Respiratory syncytial virus infections in the pediatric intensive care unit: clinical characteristics and risk factors for adverse outcomes. Pediatr Crit Care Med 2001; 2:318–23. [DOI] [PubMed] [Google Scholar]
- 29. Altman CA, Englund JA, Demmler G, et al. Respiratory syncytial virus in patients with congenital heart disease: a contemporary look at epidemiology and success of preoperative screening. Pediatr Cardiol 2000; 21:433–8. [DOI] [PubMed] [Google Scholar]
- 30. Schnell J, Schroeder L, Sinclair K, Patel L, Dowd D. The effect of early knowledge of respiratory syncytial virus positivity on medical decision making and throughput time within the pediatric emergency department. Pediatr Emerg Care 2020; 36:134–7. [DOI] [PubMed] [Google Scholar]
- 31. Leonardi GP. Evaluation of rapid, molecular-based assays for the detection of respiratory syncytial virus. Intervirology 2019; 62:112–5. [DOI] [PubMed] [Google Scholar]
- 32. Nicholson EG, Schlegel C, Garofalo RP, et al. Robust cytokine and chemokine response in nasopharyngeal secretions: association with decreased severity in children with physician diagnosed bronchiolitis. J Infect Dis 2016; 214:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bonadio W, Huang F, Nateson S, et al. Meta-analysis to determine risk for serious bacterial infection in febrile outpatient neonates with RSV infection. Pediatr Emerg Care 2016; 32:286–9. [DOI] [PubMed] [Google Scholar]
- 34. Akenroye AT, Baskin MN, Samnaliev M, Stack AM. Impact of a bronchiolitis guideline on ED resource use and cost: a segmented time-series analysis. Pediatrics 2014; 133:e227–34. [DOI] [PubMed] [Google Scholar]
- 35. Petruzella FD, Gorelick MH. Duration of illness in infants with bronchiolitis evaluated in the emergency department. Pediatrics 2010; 126:285–90. [DOI] [PubMed] [Google Scholar]
- 36. Bourgeois FT, Valim C, McAdam AJ, Mandl KD. Relative impact of influenza and respiratory syncytial virus in young children. Pediatrics 2009; 124:e1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bourgeois FT, Valim C, Wei JC, McAdam AJ, Mandl KD. Influenza and other respiratory virus-related emergency department visits among young children. Pediatrics 2006; 118:e1–8. [DOI] [PubMed] [Google Scholar]
- 38. Dayan P, Ahmad F, Urtecho J, et al. Test characteristics of the respiratory syncytial virus enzyme-linked immunoabsorbent assay in febrile infants < or = 60 days of age. Clin Pediatr (Phila) 2002; 41:415–8. [DOI] [PubMed] [Google Scholar]
- 39. Jadhao SJ, Ha B, McCracken C, et al. Performance evaluation of antibody tests for detecting infant respiratory syncytial virus infection. J Med Virol 2021; 93:3439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372:835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bruden DJT, Singleton R, Hawk CS, et al. Eighteen years of respiratory syncytial virus surveillance: changes in seasonality and hospitalization rates in southwestern Alaska Native children. Pediatr Infect Dis J 2015; 34:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Luginbuhl LM, Newman TB, Pantell RH, Finch SA, Wasserman RC. Office-based treatment and outcomes for febrile infants with clinically diagnosed bronchiolitis. Pediatrics 2008; 122:947–54. [DOI] [PubMed] [Google Scholar]
- 43. DePorre A, Williams DD, Schuster J, et al. Evaluating the impact of implementing a clinical practice guideline for febrile infants with positive respiratory syncytial virus or enterovirus testing. Hosp Pediatr 2017; 7:587–94. [DOI] [PubMed] [Google Scholar]
- 44. Turi KN, Wu P, Escobar GJ, et al. Prevalence of infant bronchiolitis-coded healthcare encounters attributable to RSV. Health Sci Rep 2018; 1:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flaherman V, Li S, Ragins A, Masaquel A, Kipnis P, Escobar GJ. Respiratory syncytial virus testing during bronchiolitis episodes of care in an integrated health care delivery system: a retrospective cohort study. Clin Ther 2010; 32:2220–9. [DOI] [PubMed] [Google Scholar]
- 46. El Assal O, Brandt H, Weichler K, Besunder J, Pollauf L. Does perceived responsiveness to bronchodilator therapy in patients admitted for bronchiolitis predict future development of asthma? Pediatrics 2020; 146:195. [Google Scholar]
- 47. Hasegawa K, Goto T, Hirayama A, et al. Respiratory virus epidemiology among US infants with severe bronchiolitis: analysis of 2 multicenter, multiyear cohort studies. Pediatr Infect Dis J 2019; 38:e180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luthe SK, Hirayama A, Laham FR, et al. Respiratory virus epidemiology of infants with severe bronchiolitis: prospective multicenter studies. Acad Emerg Med 2018; 25:S16. [Google Scholar]
- 49. Suárez-Arrabal MC, Mella C, Lopez SM, et al. Nasopharyngeal bacterial burden and antibiotics: influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J Infect 2015; 71:458–69. [DOI] [PubMed] [Google Scholar]
- 50. Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med 2012; 166:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Hoyos P, Mansbach JM, Piedra PA, et al. A multicenter study to predict continuous positive airway pressure and intubation for children hospitalized with bronchiolitis. Acad Emerg Med 2012; 19:S82. [Google Scholar]
- 52. Miernyk K, Bulkow L, DeByle C, et al. Performance of a rapid antigen test (Binax NOW RSV) for diagnosis of respiratory syncytial virus compared with real-time polymerase chain reaction in a pediatric population. J Clin Virol 2011; 50:240–3. [DOI] [PubMed] [Google Scholar]
- 53. Mella C, Mejias A, Muszynski J, Hall MW, Ramilo O. Relationships between viral pathogen, bacterial co-infection, and outcomes from bronchiolitis in critically ill children. Crit Care Med 2010; 38:U81. [Google Scholar]
- 54. Singleton RJ, Bruden D, Bulkow LR, Varney G, Butler JC. Decline in respiratory syncytial virus hospitalizations in a region with high hospitalization rates and prolonged season. Pediatr Infect Dis J 2006; 25:1116–22. [DOI] [PubMed] [Google Scholar]
- 55. Bockova J, O’Brien KL, Oski J, et al. Respiratory syncytial virus infection in Navajo and White Mountain Apache children. Pediatrics 2002; 110:e20. [DOI] [PubMed] [Google Scholar]
- 56. Miller EK, Gebretsadik T, Carroll KN, et al. Viral etiologies of infant bronchiolitis, croup and upper respiratory illness during 4 consecutive years. Pediatr Infect Dis J 2013; 32:950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus–associated hospitalizations among young children: 2015–2016. Pediatrics 2020; 146:e20193611. [DOI] [PubMed] [Google Scholar]
- 58. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Suryadevara M, Cummings E, Bonville CA, et al. Viral etiology of acute febrile respiratory illnesses in hospitalized children younger than 24 months. Clin Pediatr (Phila) 2011; 50:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Muñiz AE. Respiratory syncytial virus is not protective of urinary tract infections in febrile infants less than 90 days old. Ann Emerg Med 2009; 54:S105–6. [Google Scholar]
- 61. Golombek SG, Berning F, Lagamma EF. Compliance with prophylaxis for respiratory syncytial virus infection in a home setting. Pediatr Infect Dis J 2004; 23:318–22. [DOI] [PubMed] [Google Scholar]
- 62. O’Brien KL, Chandran A, Weatherholtz R, et al. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: a phase 3 randomised double-blind placebo-controlled trial. Lancet Infect Dis 2015; 15:1398–408. [DOI] [PubMed] [Google Scholar]
- 63. Vendetti N, Gerber JS, Sammons JS, Fisher BT, Zaoutis TE, Coffin SE. Administration of palivizumab in the NICU. Hosp Pediatr 2016; 6:354–8. [DOI] [PubMed] [Google Scholar]
- 64. Bender JM, Taylor CS, Cumpio J, et al. Infants 1–90 days old hospitalized with human rhinovirus infection. J Clin Lab Anal 2014; 28:349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bennett NJ, Tabarani CM, Bartholoma NM, et al. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr 2012; 161:814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jamieson N, Akande M, Karsies T, Smith RM, Kline D, Spencer SP. Respiratory pathogen detection in pediatric patients intubated for presumed infection. Pediatr Emerg Care 2022; 38:e398– 403. [DOI] [PubMed] [Google Scholar]
- 67. Makari D, Staat MA, Henrickson KJ, Wu X, Ambrose CS. The underrecognized burden of respiratory syncytial virus among infants presenting to US emergency departments. Clin Pediatr (Phila) 2015; 54:594–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hsiao AL, Chen L, Baker MD. Incidence and predictors of serious bacterial infections among 57- to 180-day-old infants. Pediatrics 2006; 117:1695–701. [DOI] [PubMed] [Google Scholar]
- 69. Russell KE, Fowlkes A, Stockwell MS, et al. Comparison of outpatient medically attended and community-level influenza-like illness-New York City, 2013–2015. Influenza Other Respir Viruses 2018; 12:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ambrose CS, Anderson EJ, Simões EA, et al. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32–35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J 2014; 33:576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Karron RA. Preventing respiratory syncytial virus (RSV) disease in children. Science 2021; 372:686–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.