Abstract
Manganese-56 (56Mn) was one of the dominant neutron-activated radionuclides during the first hours following the atomic-bombing of Hiroshima and Nagasaki. The radiation spectrum of 56Mn and the radiation emission from excited levels of 56Fe following 56Mn beta-decay include gamma-quanta, beta-particles, Auger electrons and X-rays. The dispersion of neutron activated 56Mn in the air can lead to entering of radioactive microparticles into the lungs. The investigation of spatial microdistribution of an internal dose in biological tissue exposed to 56Mn is an important matter with regards to the possible elevated irradiation of the lung alveoli and alveolar ducts. The Monte Carlo code (MCNP-4C) was used for the calculation of absorbed doses in biological tissue around 56Mn dioxide microparticles. The estimated absorbed dose has a very essential gradient in the epithelium cells of lung alveoli and alveolar duct: from 61 mGy/decay on the surface of simple squamous cells of epithelium to 0.15 mGy/decay at distance of 0.3 μm, which is maximal cell thickness. It has been concluded that epithelial cells of these pulmonary microstructures are selectively irradiated by low-energy electrons: short-range component of beta-particles spectrum and Auger electrons. The data obtained are important for the interpretation of biological experiments implementing dispersed neutron-activated 56Mn dioxide powder.
Keywords: A-bombing, internal irradiation, 56Mn radioactive microparticles, lungs, alveoli, radiation dose microdistribution
INTRODUCTION
The radionuclide 56Mn (T1/2 = 2.58 h) was one of the dominant neutron activated emitters during the first hours following the neutron irradiation as a result of A-bombing of Hiroshima and Nagasaki [1–6]. The radiation spectrum of 56Mn and radiation from excited levels of 56Fe following 56Mn beta-decay, include gamma-quanta, beta-particles, Auger electrons and x-rays [7]. Dispersion of 56Mn dioxide in the air in a form of dust can lead to entering of radioactive microparticles into the lung’s alveolar duct and alveoli, when the dispersed powder of this material is inhaled. Taking into account the existence of short-range component of beta-spectrum and electrons as a result of 56Mn decays and radiation from excited levels of 56Fe, the investigation of spatial micro distribution of internal dose in biological tissue exposed by neutron activated 56Mn dioxide microparticles is important matter with regards to possible elevated exposure of lung’s microstructures—in comparison with organ-average internal doses. The data obtained are important for the interpretation of the results from biological experiments using dispersed neutron activated 56Mn powder in experimental animals—rats and mice [6, 8–12].
MATERIAL AND METHODS
The absorbed dose was calculated in spherical layers of biological tissue around the 56Mn microparticle as a function of the radial distance from the surface of the microparticle. The 56Mn microparticle is located in the center of the surrounding spherical layers and assumed to be as an isotropic radioactive spherical source. Average diameter of Mn dioxide microparticles is equal to 3 μm [8, 9, 11, 12]. In such kind of geometry only one parameter is important for calculation of absorbed dose distribution around 56Mn microparticle—it is the radial distance from the surface of radioactive particle. The spatial absorbed dose distribution around 56Mn microparticle was calculated for radial distances from the surface of 56Mn microparticle ranged from 10−2 μm to 104 μm (see section Results).
For the calculation of the absorbed dose around 56Mn dioxide microparticles the method of stochastic modeling of the interaction of ionizing radiation with matter (Monte-Carlo code MCNP-4C) [13] was used. It should be specially noted that for electron energies less than 10 keV the dose calculation was performed using information about dose point kernels for low-energy electrons presented in the [14].
Radial distribution of absorbed dose versus the distance to the surface of 56Mn dioxide microparticle, surrounded by biological tissue, was estimated with accounting for all components of radioactive emission of 56Mn. Tables 1–5 show all the components of radioactive emission of 56Mn and from excited levels of 56Fe following 56Mn beta-decay (gamma-rays, beta-particles, Auger electrons and X-rays). The contribution to the absorbed dose from 56Mn beta-particles was calculated for each of 20 energy intervals, which were used as discrete approximation of continuous spectrum of all 56Mn beta-particles (Table 3).
Table 1.
Gamma emission from excited levels of 56Fe following 56Mn beta-decay [15]
| Energy of gamma-quanta (MeV) |
Intensity (gammas per decay) |
|---|---|
| 0.8468 | 0.9890 |
| 1.0380 | 0.0004 |
| 1.2380 | 0.0010 |
| 1.8110 | 0.2720 |
| 2.1130 | 0.1430 |
| 2.5230 | 0.0099 |
| 2.5980 | 0.0002 |
| 2.6570 | 0.0065 |
| 2.9600 | 0.0031 |
| 3.3700 | 0.0017 |
Table 5.
X-ray emission from excited levels of 56Fe following 56Mn beta-decay [7]
| Energy (keV) | Intensity (photons per 100 decays) | Relative probability |
|---|---|---|
| 6.39091 | 0.00295 | 0.51 |
| 6.40391 | 0.00578 | 1 |
| 7.05804 | 0.00119 | 0.206 |
Table 3.
Digital version of 56Mn spectrum of all beta-particles approximated by 20 energy intervals of electrons [15]
| Intervals of energy (MeV) |
Intensity (particles per decay) |
|---|---|
| 0.0000–0.1424 | 1.11E-01 |
| 0.1424–0.2848 | 1.26E-01 |
| 0.2848–0.4272 | 1.21E-01 |
| 0.4272–0.5695 | 1.03E-01 |
| 0.5695–0.7119 | 7.91E-02 |
| 0.7119–0.8543 | 6.12E-02 |
| 0.8543–0.9967 | 4.97E-02 |
| 0.9967–1.1391 | 4.65E-02 |
| 1.1391–1.2815 | 4.66E-02 |
| 1.2815–1.4239 | 4.56E-02 |
| 1.4239–1.5663 | 4.32E-02 |
| 1.5663–1.7086 | 3.96E-02 |
| 1.7086–1.8510 | 3.50E-02 |
| 1.8510–1.9934 | 2.96E-02 |
| 1.9934–2.1358 | 2.37E-02 |
| 2.1358–2.2782 | 1.77E-02 |
| 2.2782–2.4206 | 1.18E-02 |
| 2.4206–2.5630 | 6.63E-03 |
| 2.5630–2.7054 | 2.72E-03 |
| 2.7054–2.8477 | 4.17E-04 |
Table 2.
| Mean/max energy (MeV) | Intensity (beta-particles per decay) |
|---|---|
| 0.0736 / 0.2502 | 0.0002 |
| 0.0992 / 0.3257 | 0.0116 |
| 0.1905 / 0.5726 | 0.0004 |
| 0.2553 / 0.7356 | 0.1460 |
| 0.3820 / 1.0379 | 0.2790 |
| 0.6364 / 1.6104 | 0.0006 |
| 1.2170 / 2.8487 | 0.5630 |
Table 6 shows the typical dimension of lung microstructures [16, 17], which were considered as final sites of 56Mn dioxide microparticle penetration into the lungs, when the neutron-activated Mn dioxide powder is inhaled. It was assumed that as a result 56Mn dioxide microparticles are attached to the epithelium. The density of biological tissue was assumed to be equal to 1 g/cm3. Composition of soft tissue was taken from ICRP Publication 89 [18].
Table 6.
Typical dimension of lung’s microstructures [16, 17], which were considered as final sites of 56Mn dioxide microparticles penetration into the lungs
| Component | Thickness of epithelium |
|---|---|
| Alveolar duct | Mostly simple squamous epithelium cells (thickness from 0.05 μm to 0.3 μm) |
| Alveoli | Each alveoli is lined with simple squamous epithelium cells (from 0.05 μm to 0.3 μm thick) and covered over cells by surfactant (about 0.01 μm thick) |
RESULTS
Manganese dioxide particles were considered as spherical isotropic sources of ionizing irradiation from the 56Mn with activity uniformly distributed across their volume. The absorbed doses around the spherical isotropic sources of 56Mn in biological tissue were calculated inside concentric layers, surrounding the microparticles. As a result, radial distribution of absorbed dose was calculated as a function of the distance from the surface of radioactive microparticles (Figs 1 and 2).
Fig. 1.
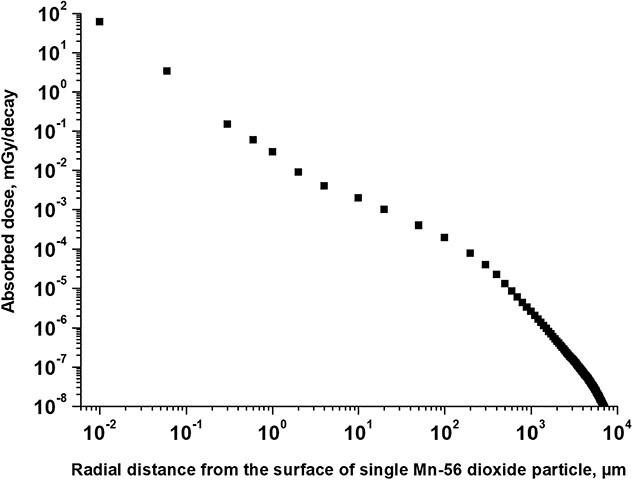
Radial distribution of absorbed dose versus the distance to the surface of single radioactive 56Mn dioxide microparticle, surrounded by biological tissue: irradiation by beta-particles and electrons.
Fig. 2.
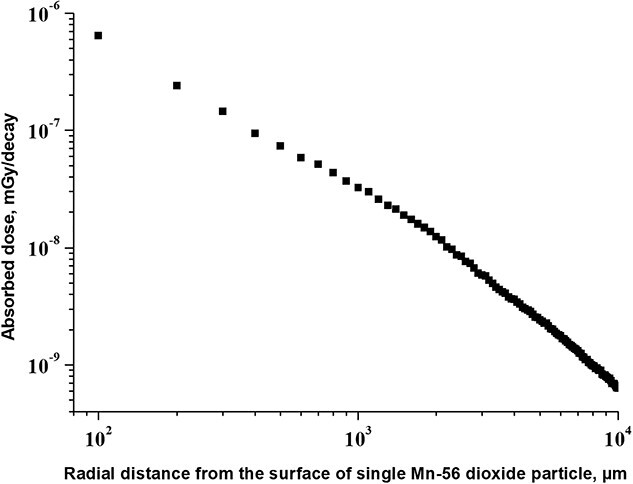
Radial distribution of absorbed dose versus the distance to the surface of single radioactive 56Mn dioxide microparticle, surrounded by biological tissue: irradiation by photons (gamma-rays and X-rays).
Figure 1 shows that exposure to beta-particles as a result of 56Mn decay and electrons emitted from excited levels of 56Fe following 56Mn beta-decay has a significant distance-dependent gradient effect in the epithelium of lung’s alveolar ducts, and in the epithelium of alveoli. Absorbed dose per one unit decay is equal to: 61 mGy/decay on the surface of simple squamous cells of epithelium (at distance 0.01 μm from the surface of 56Mn microparticle, which is located near epithelium); 3.4 mGy/decay at 0.05 μm distance—on a layer of epithelial cells at the minimal thickness of cells; 0.15 mGy/decay at distance 0.3 μm—on a layer of epithelium cells at the maximal thickness of simple squamous cells (see Table 6 with information about thickness of epithelium).
Figure 2 shows that dose from penetrating photon irradiation from the single radioactive 56Mn dioxide microparticle, embedded within the tissue, gives a lower level of irradiation in comparison with irradiation by beta-particles and electrons. At a distance of 100 μm (the diameter of alveolar duct) from the surface of 56Mn dioxide particle, the dose from gammas is equal to 6.4 × 10−7 mGy/decay in comparison with dose 2.1 × 10−4 mGy/decay observed by beta-particle radiation at the same distance. Importantly, that the data shown in Fig. 2 shows the absorbed dose from single radioactive 56Mn dioxide microparticle. The real mean organ dose could be higher—due to penetration of gammas from other 56Mn dioxide microparticles within the lungs.
Nevertheless, the excess dose from beta-particles is estimated about two orders of magnitude higher compared to that from gamma quanta even at a distance of 1000 μm (twice more than diameter of alveoli) from the 56Mn microparticle: 2.5 × 10−6 mGy/decay for beta-particles versus 3.2 × 10−8 mGy/decay for gamma radiation.
DISCUSSION
These data demonstrate that: (i) exposure to beta-particles as a result of 56Mn decay and electrons emission from excited levels of 56Fe following 56Mn beta-decay has a significant distance-dependent gradient in the simple squamous cells of alveoli and alveolar duct epithelium (Fig. 1); and (ii) absorbed dose from penetrating photon irradiation from a radioactive 56Mn dioxide microparticle, embedded in biological tissue, is much less (by 2–3 orders of magnitude) in biological microstructures compared with irradiation by beta-particles and electrons (Figs 1 and 2).
The main contribution to the dose increase at the level of the biological tissue microstructure is due to the low-energy component of the 56Mn beta-particles spectrum, which is the most intense part of this spectrum (top row in Table 3). Some additional contributions to absorbed dose in tissues at very small distances from 56Mn dioxide particles may be due to emitted Auger electrons (Table 4).
Table 4.
Auger electron emission from excited levels of 56Fe following 56Mn beta-decay [7]
| Electrons | Energy (keV) | Intensity (electrons per 100 decays) | Relative probability |
|---|---|---|---|
| K Auger electrons | |||
| KLL | 5.370-5.645 | 0.0139 | 1 |
| KLX | 6.158-6.400 | 0.00382 | 0.274 |
| KXY | 6.926-7.105 | 0.000261 | 0.0187 |
| L Auger electrons | 0.510-0.594 | 0.0428 | 3.07 |
From these data it has been concluded that epithelial cells of key pulmonary microstructures are selectively irradiated with short-range beta-spectrum component of 56Mn and with electrons emission from excited levels of 56Fe following 56Mn beta-decay.
These data are important for the interpretation of the results of biological experiments using dispersed neutron-activated 56Mn dioxide powder, which was inhaled by experimental animals—rats and mice [6]. It was demonstrated in these experiments [6] that biological effects caused by internal irradiation from inhaled 56Mn dioxide particles are more significant in comparison to external irradiation by 60Co, despite small values of organ averaged internal radiation doses [10, 11]. The values of organ mean doses in experimental mice and rats are presented in [8, 9, 12].
ACKNOWLEDGEMENT
We express our gratitude to Dr. T. Kolizhenkov, Dr. A. Petukhov, Dr. D. Dubov, Dr. T. Lavrova—the personnel of the A. Tsyb Medical Radiological Research Center – Branch of the National Medical Research Center of Radiology of the Ministry of Health of the Russian Federation, who supported this research in a framework of the Institute Research Program AAAA-A18-118062590091-2 and in a framework of bilateral International Agreement on the scientifical cooperation with Hiroshima University.
Contributor Information
Valeriy Stepanenko, A. Tsyb Medical Radiological Research Center – Branch of the National Medical Research Radiological Center of the Ministry of Health of the Russian Federation, Koroleva Str., 4., Obninsk, Kaluga Region 2490036, Russian Federation.
Andrey Kaprin, National Medical Research Radiological Center of the Ministry of Health of the Russian Federation, Koroleva Str., 4., Obninsk, Kaluga Region 2490036, Russian Federation.
Sergey Ivanov, A. Tsyb Medical Radiological Research Center – Branch of the National Medical Research Radiological Center of the Ministry of Health of the Russian Federation, Koroleva Str., 4., Obninsk, Kaluga Region 2490036, Russian Federation.
Peter Shegay, National Medical Research Radiological Center of the Ministry of Health of the Russian Federation, Koroleva Str., 4., Obninsk, Kaluga Region 2490036, Russian Federation.
Viktoria Bogacheva, A. Tsyb Medical Radiological Research Center – Branch of the National Medical Research Radiological Center of the Ministry of Health of the Russian Federation, Koroleva Str., 4., Obninsk, Kaluga Region 2490036, Russian Federation.
Hitoshi Sato, Ibaraki Prefectural University of Health Sciences, 4669-2, Ami-chyo Ami, Inashiki-gun, Ibaraki 300-0394, Japan.
Kazuko Shichijo, Atomic Bomb Disease Institute, Nagasaki University, 1-12-4, Sakamoto, Nagasaki 852-8523, Japan.
Shin Toyoda, Department of Applied Physics, Okayama University of Science, 1-1 Ridai, Kita-ku, Okayama 700-0005, Japan.
Noriyuki Kawano, The Center for Peace Hiroshima University, Higashisenda-machi 1-1-89, Naka-ku, Hiroshima 730-0053, Japan.
Megu Ohtaki, Research Institute for Radiation Biology and Medicine Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8551, Japan.
Nariaki Fujimoto, Research Institute for Radiation Biology and Medicine Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8551, Japan.
Satoru Endo, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-4-1, Kagamiyama, Higashi, Hiroshima 739-8527, Japan.
Nailya Chaizhunusova, Semey State Medical University, Abay Str., 103, Semey, 071400, Kazakhstan.
Dariya Shabdarbaeva, Semey State Medical University, Abay Str., 103, Semey, 071400, Kazakhstan.
Kassym Zhumadilov, Eurasian National University named after L.N. Gumilyov, Munaipasova Str. 13, Astana 010008, Kazakhstan.
Masaharu Hoshi, Research Institute for Radiation Biology and Medicine Hiroshima University, 1-2-3, Kasumi, Minami-ku, Hiroshima 734-8551, Japan.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
This work was supported by the Grants-in-Aid for Scientific Research No. 26257501 and 19H01149, KAKENHI to M. Hoshi, Japan.
SUPPLEMENT FUNDING
This work was supported by JSPS KAKENHI Grant Number JP19H01149.
REFERENCES
- 1. Roesch WC (Ed.). US-Japan Joint Reassessment of Atomic Bomb Radiation Dosimetry in Hiroshima and Nagasaki (DS86). Final Report. Vol. 1. Hiroshima: Radiation Effects Research Foundation, 1987. https://www.rerf.or.jp/library/scidata/scids/ds86/ds86aa.html (30 March 2021, date last accessed) [Google Scholar]
- 2. Young RW, Kerr GD (Eds). Reassessment of the Atomic Bomb Dosimetry for Hiroshima and Nagasaki—Dosimetry System 2002 (DS02) . Hiroshima: Radiation Effects Research Foundation, 2005. https://www.rerf.or.jp/en/library/list-e/scids/ds02-en (25 June 2021, date last accessed) [Google Scholar]
- 3. Kerr GD, Egbert SD, Al-Nabulsi I et al. Workshop report on atomic bomb dosimetry—residual radiation exposure: recent research and suggestions for future studies. Health Phys 2013;105:140–9. [DOI] [PubMed] [Google Scholar]
- 4. Kerr GD, Egbert SD, Al-Nabulsi I et al. Workshop report on atomic bomb dosimetry – review of dose related factors for the evaluation of exposure to residual radiation at Hiroshima and Nagasaki. Health Phys 2015;109:582–600. [DOI] [PubMed] [Google Scholar]
- 5. Weitz R. Reconstruction of beta-particle and gamma-ray doses from neutron activated soil at Hiroshima and Nagasaki. Health Phys 2014;107:S43. [Google Scholar]
- 6. Hoshi M. A long history exploring radiation exposure. Impact 2020:70–2. https://www.ingentaconnect.com/content/sil/impact/2020/00002020/00000003/art00026?crawler=true&mimetype=application/pdf (12 April 2021, date last accessed). [Google Scholar]
- 7. Be M-M, Chiste V, Dulieu C et al. Table of Radionuclides (Vol. 1- a= 1 to 150) . France: Bureau International des Poids et Mesures. Pavillon de Breteuil, F-92310 Servees, 2004. https://www.bipm.org/utils/common/pdf/monographieRI/Monographie_BIPM-5_Tables_Vol1.pdf (12 April 2021, date last accessed). [Google Scholar]
- 8. Stepanenko V, Rakhypbekov T, Otani K et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats—part 1: dosimetry. Radiat Environ Biophys 2016;56:47–54. [DOI] [PubMed] [Google Scholar]
- 9. Stepanenko VF, Rakhypbekov TK, Kaprin AD et al. Irradiation of experimental animals by neutron activated dust: development and realization of the method—first results of international multicenter study. Radiation and Risk 2016;25:111–25. [Google Scholar]
- 10. Shichijo K, Fujimoto N, Uzbekov D et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats-part 2: pathological effects. Radiat Environ Biophys 2017;56:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shichijo K, Takatsuji T, Abishev Zh et al. Impact of local high doses of radiation by neutron activated Mn dioxide powder in rat lungs: protracted pathologic damage initiated by internal exposure. Biomedicine , 2020;8:171. doi: 10.3390/biomedicines8060171. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7345208 (12 April 2021, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stepanenko V, Kaprin A, Ivanov S et al. Internal doses in experimental mice and rats following exposure to neutron-activated 56MnO2 powder: results of an international, multicenter study. Radiat Environ Biophys 2020;59:683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Briemeister JF. MCNP–A General Monte–Carlo N–Particle Transport Code. Version 4C. Los Alamos: Los Alamos National Laboratory, 2000. [Google Scholar]
- 14. Berger MJ. Improved Point Kernels for Electron and Beta-Ray Dosimetry. Washington, DC: Center for Radiation Research, Institute for Basic Standards, National Bureau of Standards; U.S. Atomic Energy Commission, Division of Biomedical and Environmental Research, 1973. [Google Scholar]
- 15. RADAR . The decay data. Available on . https://www.doseinfo-radar.com/RADARDecay.html 12 April 2021, date last accessed. [Google Scholar]
- 16. DukeMedicine . Available on: https://web.duke.edu/histology/NormalBody/Respiratory/Respiratory.html (12 April 2021, date last accessed).
- 17. Medicalplanet . Available on: https://medicalplanet.su/gistologia/alveoli.html (12 April 2021, date last accessed).
- 18. Valentin J (Ed). ICRP publication 89. Basic anatomical and physiological data for use in radiological protection reference values. Ann ICRP , 2002;32:1–265. 10.1177/ANIB_32_3-4 (12 April 2021, date last accessed) [DOI] [PubMed] [Google Scholar]


