Abstract
Background
Limited data are available on the economic costs of respiratory syncytial virus (RSV) infections among infants and young children in the United States.
Methods
We performed a systematic literature review of 10 key databases to identify studies published between 1 January 2014 and 2 August 2021 that reported RSV-related costs in US children aged 0–59 months. Costs were extracted and a systematic analysis was performed.
Results
Seventeen studies were included. Although an RSV hospitalization (RSVH) of an extremely premature infant costs 5.6 times that of a full-term infant ($10 214), full-term infants accounted for 82% of RSVHs and 70% of RSVH costs. Medicaid-insured infants were 91% more likely than commercially insured infants to be hospitalized for RSV treatment in their first year of life. Medicaid financed 61% of infant RSVHs. Paying 32% less per hospitalization than commercial insurance, Medicaid paid 51% of infant RSVH costs. Infants’ RSV treatment costs $709.6 million annually, representing $187 per overall birth and $227 per publicly funded birth.
Conclusions
Public sources pay for more than half of infants’ RSV medical costs, constituting the highest rate of RSVHs and the highest expenditure per birth. Full-term infants are the predominant source of infant RSVHs and costs.
Keywords: economic cost, gestational age, hospitalization, infant, Medicaid, premature, respiratory syncytial virus, RSV, systematic analysis, systematic literature review
Respiratory syncytial virus (RSV) infects almost all children by 2 years of age in the United States [1, 2]. RSV is the leading cause of hospitalizations in infants [3] and the most common respiratory virus detected in multiple health care settings [4]. RSV causes an estimated 2.1 million medically attended infections each year in children under 5 years of age [3]. Annually, RSV is responsible for approximately 500 000 emergency room visits, 1.5 million ambulatory visits [5], and 50 000 hospitalizations [6] in children <24 months of age. Since March 2021, out-of-season RSV hospitalizations (RSVH) have been mounting [7].
The sole approved prophylaxis for RSV, palivizumab, is recommended only for extremely premature and other high-risk infants, comprising <2% of annual birth cohorts [8, 9]. Although a number of studies have documented the costs for premature and other high-risk infants with RSV, there is a dearth of evidence on the costs of RSV infections in full-term infants, which account for approximately 88% of annual births [9].
Information about the cost per RSV illness episode and the cost of RSV illness per birth are critical to payers and public health officials who are developing, potentially funding, and advising on preventive approaches for both mothers and infants. Our objective was to critically assess and analyze the current evidence on the medical costs of RSV infection among US infants and young children to inform policy.
METHODS
Identification of Studies
The systematic literature review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The protocol was submitted to the Prospective Register of Systematic Reviews prior to the review (PROSPERO ID, CRD42020168469). Key databases (PubMed, Scopus, ProQuest Dissertations and Theses, Cochrane Database of Systematic Reviews, Cochrane CENTRAL, EconLit, Health Technology Assessment Database, Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database, and Paediatric Economic Database Evaluation) were searched using terms related to (1) RSV, (2) cost of illness, and (3) quality of life (QOL). The search strategy was based on a combination of medical subject headings, terms, and keywords for each concept, specific to each database (Supplementary Material 1).
The inclusion and exclusion criteria are shown in Supplementary Material 2. We included all studies that reported RSV-related costs in US children aged 0 through 59 months published from 1 January 2014, through 2 August 2021. We then selected the literature focused on economic outcomes of RSV, separating the review of QOL for a separate submission [11]. Two reviewers (K.R.R. and D.H. or R.M.G.) independently evaluated all potential economic articles during the title and abstract screening and full text eligibility phases. Articles were screened for risk of bias using the Drummond checklist modified for a global RSV cost study [12] as shown in Supplementary Material 3. For consistency, this review used the same quality threshold as Zhang et al [13] to include a study in the nationally weighted analysis. We evaluated studies individually as well as trends across all included studies (Supplementary Materials 4 and 5). As our study was based on published aggregate data, informed consent was not applicable.
Data Extraction
We extracted the following characteristics from all included studies: authors, year published, journal, costing methodology, dates of study data, cost categories and specific resources, and cost analysis results. Cost data were sorted based on diagnosis, setting, weeks’ gestational age (wGA) at birth (full-term ≥37 wGA, late preterm 35–36 wGA, early preterm 29–34 wGA, and extremely preterm ≤28 wGA), and insurance type (payer). Studies that reported overlapping wGAs were classified in the category that contained the majority of wGAs.
Statistical Analysis
From each of the selected studies, we extracted the mean and variability of cost and sample size. If no standard deviation (SD) or standard error of the mean (SEM) was reported, we calculated both using a mean coefficient of variation from other studies with similar wGA groups and settings. We converted all cost data to mean, SD, and SEM. Because of the low rate of readmission within the same season [14], all studies reporting inpatient costs by year were counted as 1 hospitalization. We performed all analyses using Microsoft Excel, version 16.38, unless otherwise indicated.
Cost-to-Charge Ratio and Consumer Price Index
For the 4 studies that reported hospital charges rather than costs, we estimated costs using each hospital’s fiscal year-specific cost-to-charge ratio [15–17]. All reported costs from each study’s costing year were adjusted to January 2020 USD using the all-urban consumer price index (CPI) for medical care [18]. For studies that did not report a costing year, we used the midpoint of the study collection dates.
Overlapping Samples
To account for unique studies that used partially overlapping datasets, we adjusted the reported sample sizes by calculating mean lives covered per year and then adjusting the sample size based on the number of overlapping years and number of applicable studies. We used this adjusted sample size when calculating weighted means within each overlapping year.
Within-Study and Across-Study Weighted Analysis
For the within-study weighted analysis, we first calculated within-study mean cost values by pooling smaller subgroups into larger groupings (based on wGA and high-risk populations), weighted by the adjusted sample size of each subgroup. Next, we used these within-study mean cost values to calculate across-study means of our study wGA groupings using adjusted sample sizes [19]. The primary analysis included infants 0–11 months of age, as the greatest number of RSV episodes occurred in the first year of life. Our secondary analysis examined RSV episodes in all children aged 0–59 months.
Nationally Weighted Analysis and Cost per Birth by Payer
We used a nationally representative sample of RSVH data [6] to calculate an overall national weighted hospitalization cost based on the adjusted, weighted across-study mean costs by wGA groups. Using this nationally representative sample and national births for 2018 [20], we calculated aggregate RSVH costs and breakdowns by insurance type (payer) and wGA. We grouped “other” payers with public payers for consistency. For the sampled hospitalizations with unknown payer source, we assumed the distribution was proportional to those with known sources. We derived cost per birth by payer by dividing aggregate costs by numbers of births in 2019 by payer [21].
Additional Statistical Analyses
We performed additional analyses of cost per RSVH by insurance type, diagnosis, and chronological age at the time of the RSV episode across studies that separated costs by these variables. We tested heterogeneity in cost per hospitalization across studies using the I-squared statistic using Stata SE, version 16.1 (StataCorp). We calculated this statistic and its P value for groupings with at least 3 studies or subgroups. To understand aggregate hospitalization costs fully, we also needed to examine rates of RSVH. We therefore calculated the odds ratio (OR) of RSVH by payer using the nationally representative RSVH sample [6] and birth statistics for 2018 with the largest payer category as the reference [20]. We also calculated the mean of the data years, the number of data years, publication years, and lags between data years and publication years.
RESULTS
Study Selection
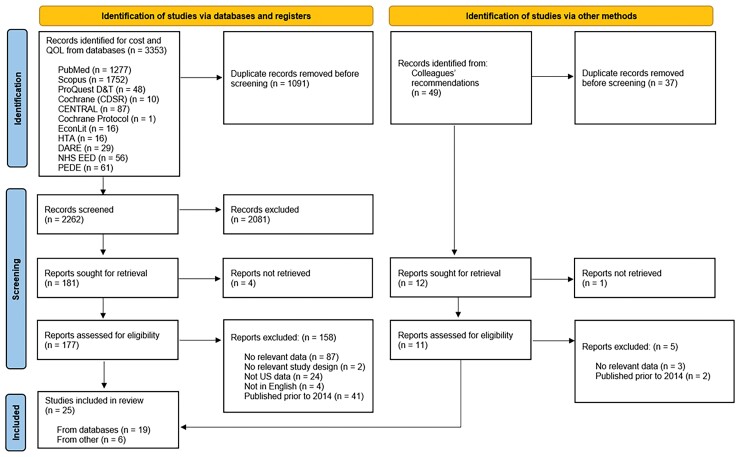
Our initial literature search returned 3353 citations. After eliminating duplicates, applying inclusion criteria, and excluding 4 reports that could not be retrieved, 177 articles remained and were screened at the full text review stage. From these, 158 were excluded leaving 19 studies. Research colleagues identified an additional 49 citations, of which 6 were deemed eligible for inclusion. The PRISMA diagram (Figure 1) and checklist (Supplementary Material 6 [10]) report the results of each phase of review. Of the total 25 studies, 8 were excluded for data quality or data insufficiency, leaving 17 articles that met eligibility criteria for weighted analyses.
Figure 1.
PRISMA diagram. Abbreviations: CDSR, Cochrane Database of Systematic Reviews; DARE, Database of Abstracts of Reviews of Effects; HTA, Health Technology Assessment; NHS EED, National Health Service Economic Evaluation Database; PEDE, Paediatric Economic Database Evaluation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; QOL, quality of life.
Study Characteristics
All included studies reported only direct medical costs. Of the 17 eligible studies, 16 (94%) were retrospective analyses of claims data or hospital records, and 1 (6%) was a noninterventional, observational study [22]. Nearly all studies (16/17; 94%) included infants with an RSV or unspecified bronchiolitis (UB) event in the first year of life, and just 2/17 (12%) studies included only infants aged <6 months at the time of illness. The majority of studies (9/17; 53%) included children with an RSV or UB event occurring at <2 years of age. Although most inpatient studies (10/13; 77%) did not explicitly state whether the RSV episodes occurred during the birth hospitalization or whether nosocomial infections were included, 2/13 (15%) explicitly excluded RSV episodes during birth hospitalizations [23, 24] and 1/13 (8%) explicitly excluded nosocomial infections [25]. More than half (11/17; 65%) of the studies included only RSV-specific diagnoses, whereas 3/17 (18%) studies included only bronchiolitis diagnoses. Nearly one-quarter (4/17; 24%) of studies reported both RSV and UB diagnoses, and 3/17 (18%) studies did not report International Classification of Diseases (ICD) codes or specify diagnostic criteria. See Supplementary Material 7 [22–46] for the characteristics of the included studies.
Most studies reported inpatient hospitalization costs (13/17; 76%). Of the 17 studies, 7 (41%) included wGA at birth, and 5 (29%) reported on full-term infants. Very few studies reported costs for ambulatory settings (2/17; 12%), outpatient visits (1/17; 6%), and emergency department or urgent care centers (4/17; 24%). Half (2/4; 50%) of the studies reporting any ambulatory settings costs also reported wGA at birth. Four studies [23, 32, 39, 44] reported costs of other high-risk groups including children with hemodynamically significant congenital heart disease (HS-CHD), chronic lung disease (CLD), and other major health problems. Many studies reported costs from all payers collectively (5/17; 29%) or did not specify payer (7/17; 41%). Three studies [24, 39, 40] reported costs by Medicaid and commercial payers separately. Two studies reported commercial payer costs alone, and no studies reported Medicaid costs alone. Only 1 study explicitly reported a payer perspective.
The publication years of the 17 pooled studies ranged from 2014 through 2020 with a median of 2017. Their data years ranged from 1997 to 2017 with an average of 7 years of data per study (Supplementary Material 8). On average, there was a lag of 8 years between publication year and the median data year. The mean quality score of the 17 included studies was 9.5 (range 6–12) of 13 (Supplementary Material 7).
Inpatient Hospitalization Costs
Adjusted to 2020 USD, mean inpatient hospitalization costs per episode ranged from $4017 (95% confidence interval [CI], $3991–$10 907) for UB in non–high-risk patients [23] to $214 416 (95% CI, $96 410–$332 421) for RSV lower respiratory tract infections (LRTIs) for commercially insured infants born <29 wGA [39].
Cost per Hospitalization by wGA
Table 1 shows mean RSVH costs by study, wGA group, chronological age, and special populations. Mean cost per RSVH varied from $10 214 (95% CI, $9818–$10 610) for full-term infants (0–11 months) to $57 406 (95% CI, $45 529–$69 282) for extremely premature infants. The overall all-payer mean across all gestational ages for infants (0–11 months) was $11 973 ($11 577–$12 369), and for children (0–59 months) was $12 315 ($11 905–$12 725). The infant mean serves as the basis for the aggregate inpatient costs, discussed below.
Table 1.
Average Cost per RSV Hospitalization for All Payers by Gestational Age and Special Populations Group (2020 US$)
| Study Label | Sample Size, n Original (Adjusted) | Mean (95% CI) |
|---|---|---|
| Full term | ||
| Goldstein, 2018a (I) [24] | 3657 (2438) | 12 418 (11 199–13 637) |
| Krilov, 2020a (I) [38] | 1654 (1115) | 18 473 (16 802–20 143) |
| Ledbetter, 2020a (I) [39] | 19 473 (10 277) | 7242 (6331–8153) |
| McLaurin, 2016a (I) [40] | 38 372 (31 337) | 10 722 (10 251–11 196) |
| Wyffels, 2017 (C) [46] | 722 (523) | 8794 (8200–9388) |
| Full term (≥37 wGA) averageb (I) | 63 156 (45 167) | 10 214 (9818–10 610) |
| Full term (≥37 wGA) averageb (C) | 63 878 (45 690) | 10 198 (9807–10 590) |
| Late preterm | ||
| Anderson, 2017 (I) [25] | 34 (34) | 22 623 (13 810–31 436) |
| Ledbetter, 2020a (I) [39] | 2736 (1824) | 10 799 (9902–11 695) |
| McLaurin, 2016a (I) [40] | 3813 (3050) | 15 117 (13 895–16 339) |
| Late preterm (35–36 wGA) averageb (I) | 6583 (4908) | 13 564 (12 730–14 398) |
| Late preterm (35–36 wGA) averageb (C) | 6583 (4908) | 13 564 (12 730–14 398) |
| Early preterm | ||
| Anderson, 2017a (I) [25] | 149 (149) | 21 813 (17 764–25 863) |
| Goldstein, 2018a (I) [24] | 654 (436) | 26 178 (19 455–32 901) |
| Krilov, 2020a (I) [38] | 186 (125) | 44 088 (21 257–66 919) |
| Ledbetter, 2020a (I) [39] | 2600 (1372) | 19 746 (17 480–22 011) |
| McLaurin, 2016a (I) [40] | 3305 (2699) | 21 484 (19 451–23 517) |
| Wozniak, 2016a (C) [45] | 177 (177) | 11 680 (8928–14 432) |
| Wyffels, 2017 (C) [46] | 282 (204) | 12 019 (10 214–13 825) |
| Early preterm (29–34 wGA) averageb (I) | 6894 (4782) | 22 016 (20 435–23 596) |
| Early preterm (29–34 wGA) averageb (C) | 7353 (5163) | 21 266 (19 796–22 736) |
| Extremely preterm | ||
| Ledbetter, 2020a (I) [39] | 710 (355) | 76 614 (55 933–97 295) |
| McLaurin, 2016a (I) [40] | 787 (630) | 46 575 (32 175–60 975) |
| Extremely preterm (≤28 wGA) averageb (I) | 1497 (985) | 57 406 (45 529–69 282) |
| Extremely preterm (≤28 wGA) averageb (C) | 1497 (985) | 57 406 (45 529–69 282) |
| Overall, and other | ||
| All payer overall averagec (I) | 78 130 (55 842) | 11 973 (11 577–12 369) |
| All payer overall averageb (C) | 79 311 (56 746) | 12 315 (11 905–12 725) |
| Doucette, 2016a (I) [23] | 729 894 (729 894) | 4248 (4230–4265) |
| Gupta, 2016 (C) [35] | 146 357 (146 357) | 16 252 (15 921–16 583) |
| Rivera-Sepulveda, 2017a (C) [41] | 5050 (5050) | 5055 (4947–5163) |
| Shah, 2017 (C) [42] | 19 083 (19 083) | 7204 (6699–7708) |
| Unspecified wGA averageb (I) | 729 894 (729 894) | 4248 (4230–4265) |
| Unspecified wGA averageb (C) | 900 384 (900 384) | 6266 (6209–6324) |
| Chu, 2017a (C) [32] | 4049 (2025) | 66 593 (45 220–87 967) |
| Doucette, 2016a (I) [23] | 45 203 (22 602) | 11 320 (10 476–12 164) |
| Ledbetter, 2020a (I) [39] | 4077 (4077) | 13 047 (10 898–15 196) |
| Walpert, 2018a (C) [44] | 625 (625) | 18 353 (14 824–21 881) |
| Special populations averageb (I) | 49 280 (26 679) | 11 584 (10 797–12 370) |
| Special populations averageb (C) | 53 954 (29 328) | 15 525 (13 876–17 174) |
Abbreviations: C, children aged 0–59 months; CI, confidence interval; I, infants aged 0–12 months; RSV, respiratory syncytial virus; wGA, weeks’ gestational age; 2020 US$, United States dollars at 2020 prices.
Within-study weighted average.
Across-study weighted average from all applicable gestational age group studies, adjusted for overlapping samples.
Across-study weighted average adjusted using national data, excludes unspecified wGA and special populations, adjusted for overlapping samples; weights assume that wGA was missing at random for the 5/704 (0.7%) infants and the 9/903 (1.0%) children in the national sample data with unknown wGA.
Cost per Hospitalization by Insurance Type
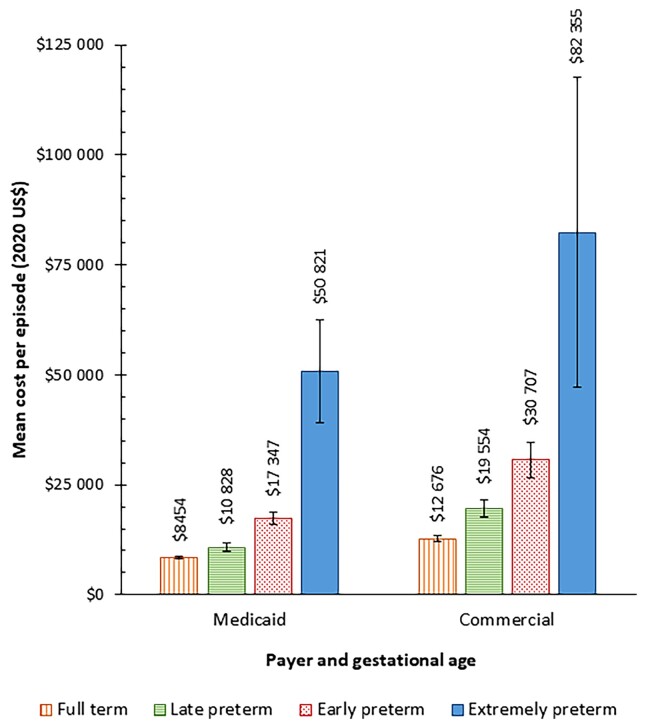
For inpatient costs, the payer perspective was used in 9/13 inpatient studies included, representing 99.7% of the unduplicated hospitalizations. The alternative approach, cost-to-charge ratio, was applied to 0.3% of unduplicated hospitalizations (4 inpatient studies). Reported inpatient costs also varied by insurance (payer) type. Overall, the mean cost per RSVH was 32.0% lower for infants with Medicaid coverage ($10 394; 95% CI, $9869–$10 918) compared with commercially insured children ($15 289; 95% CI, $14 491–$16 086) (Table 2). Figure 2 summarizes the mean cost per RSVH by payer and wGA. Mean costs increased with earlier wGA consistently across payer types. Although the differences in relative costs were smallest among full-term infants (commercial costs 49.9% greater than Medicaid costs) and extremely preterm infants (commercial costs 62.0% greater than Medicaid costs), they were still substantial. The greatest differences in relative costs were seen with preterm infants (commercial costs were 80.6% greater than Medicaid costs) and early preterm infants (commercial costs were 77.0% greater than Medicaid costs).
Table 2.
Mean Cost per RSV Hospitalization in Infants Aged 0–11 Months, by Payer (2020 US$)
| Commercial Payers | Medicaid Payers | |||
|---|---|---|---|---|
| Study Label | Sample Size, n, Original (Adjusteda) | Mean (95% CI) | Sample Size, n, Original (Adjusteda) | Mean (95% CI) |
| Goldstein, 2018b [24] | 981 (654) | 18 083 (15 956–20 211) | 2676 (1784) | 10 342 (8881–11 802) |
| Krilov, 2020b [38] | 1654 (1654) | 18 473 (17 101–19 844) | NA | NA |
| Ledbetter, 2020b [39] | 4842 (2556) | 13 875 (11 598–16 151) | 14 631 (7722) | 5047 (4102–5992) |
| McLaurin, 2016 [40] | 13 885 (11 339) | 12 406 (11 739–13 073) | 24 487 (19 998) | 9770 (9134–10 406) |
| Full term, ≥37 wGA, meanc | 21 362 (13 895) | 12 676 (11 990–13 363) | 41 794 (27 720) | 8454 (7925–8984) |
| Ledbetter, 2020b [39] | 664 (332) | 20 874 (18 161–23 588) | 2072 (1381) | 7570 (6723–8416) |
| McLaurin, 2016 [40] | 1292 (1034) | 19 130 (16 794–21 466) | 2521 (2017) | 13 060 (11 660–14 460) |
| Late preterm, 35–36 wGA, meanc | 1956 (1366) | 19 554 (17 667–21 441) | 4593 (3398) | 10 828 (9924–11 732) |
| Goldstein, 2018b [24] | 163 (109) | 35 891 (24 770–47 012) | 491 (327) | 22 953 (14 819–31 087) |
| Krilov, 2020b [38] | 186 (186) | 44 088 (25 345–62 831) | NA | NA |
| Ledbetter, 2020b [39] | 571 (301) | 41 393 (33 768–49 017) | 2029 (1071) | 13 654 (11 859–15 449) |
| McLaurin, 2016b [40] | 956 (781) | 26 582 (21 837–31 327) | 2349 (1918) | 19 409 (17 305–21 512) |
| Early preterm, 29–34 wGA, meanc | 1876 (1082) | 30 707 (26 660–34 753) | 4869 (2989) | 17 347 (15 849–18 846) |
| Ledbetter, 2020b [39] | 128 (64) | 158 578 (65 062–252 097) | 582 (291) | 58 587 (44 715–72 459) |
| McLaurin, 2016 [40] | 177 (142) | 47 903 (20 512–75 294) | 610 (488) | 46 190 (29 387–62 994) |
| Extremely preterm, ≤28 wGA, meanc | 305 (206) | 82 355 (47 103–117 606) | 1192 (779) | 50 821 (39 087–62 556) |
| Overall meanc | 25 499 (16 548) | 15 289 (14 491–16 086) | 52 448 (34 886) | 10 394 (9869–10 918) |
Abbreviations: CI, confidence interval; NA, not applicable; RSV, respiratory syncytial virus; wGA, weeks’ gestational age; 2020 US$, United States dollars at 2020 prices.
To account for unique studies that used partially overlapping datasets we adjusted the reported sample sizes by first calculating mean lives covered per year by each study, then calculating an adjusted sample size for that study for each year by removing the pro-rata share of overlapping samples based on the number of overlapping studies for that year.
Within-study weighted mean.
Figure 2.
Mean (95% confidence interval) cost per respiratory syncytial virus hospitalization by weeks’ gestational age (wGA) group and payer in infants aged 0–11 months. Group definitions: full term (≥37 wGA), late preterm (35–36 wGA), early preterm (29–34 wGA), and extremely preterm (≤28 wGA).
Additional Analyses
Merging RSV-specific and UB diagnoses lowered the mean RSVH cost by approximately 5% compared with that for RSV alone (Supplementary Materials 9 and 10). In addition, both prematurity and chronological age at the time of RSVH contributed to the variability in mean costs (Supplementary Materials 11 and 12 [24, 38]). We found moderate heterogeneity (48%) for early preterm infant all-payer groupings and considerable heterogeneity (75%–100%) in almost all (9/10) all-payer groupings (Supplementary Material 13).
Ambulatory Setting Costs
Four studies [22, 26, 27, 36] reported RSV-associated costs in ambulatory settings, including emergency or urgent care and outpatient clinics (Table 3). The mean cost for an RSV-associated emergency or urgent care visit across wGAs was $501 (95% CI, $484–$517). The mean cost of RSV-associated emergency or urgent care visits per year was $888 (95% CI, $816–$916). Blake et al [22] reported a mean RSV-associated ambulatory care cost of $73 (95% CI, $41–$105) per visit. Amand et al [27] reported the mean cost of RSV-associated ambulatory care as $1567 (95% CI, $1317–$1816) per year and outpatient care as $1648 (95% CI, $1516–$1781) per year. We calculated the weighted mean RSV-associated cost per year for all 3 settings to be $1446 (95% CI, $1354–$1538).
Table 3.
Outpatient Costs of RSV Episodes (2020 US$)
| Study Label | n | Mean (95% CI) |
|---|---|---|
| Ambulatory episode (reported as cost per visit) | ||
| Blake, 2017 [22] | 15 | 73 (41–105) |
| Emergency and urgent episodes (reported as cost per visit) | ||
| Akenroye, 2014 [26]a | 2929 | 1363 (696–2030) |
| Blake, 2017 [22] | 6 | 137 (51–224) |
| Hasegawa, 2014 [36]a | 914 070 | 498 (481–515) |
| Emergency/urgent averageb | 917 005 | 501 (484–517) |
| Ambulatory, emergency, and outpatient (reported as cost per year) | ||
| Amand, 2018 ambulatory [27]a | 3064 | 1567 (1317–1816) |
| Amand, 2018 emergency [27]a | 3280 | 888 (816–961) |
| Amand, 2018 outpatient [27]a | 7191 | 1648 (1516–1781) |
| Ambulatory, emergency and outpatient averageb | 13 535 | 1446 (1354–1538) |
The terminology is that of the original authors. Blake et al [22] use “ambulatory” to denote outpatient episodes (ie, nonhospitalized and nonemergency).
Abbreviations: CI, confidence interval; RSV, respiratory syncytial virus; 2020 US$, United States dollars at 2020 prices.
Cost is within-study weighted mean.
Cost is across-study weighted mean.
Aggregate Treatment Costs and Utilization Rates by Payer
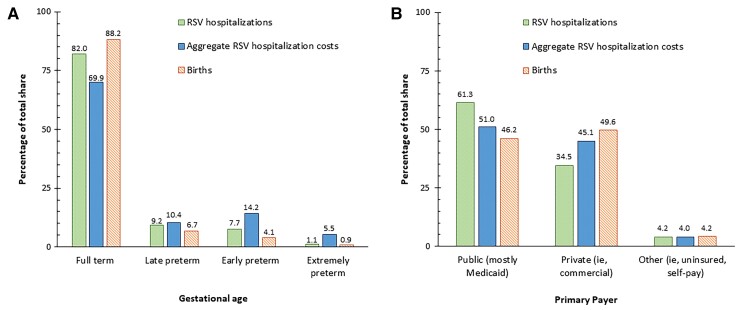
Based on a mean cost per RSVH of $11 973 (95% CI, $11 577–$12 369) and the 39 407 annual RSVH for infants aged 0–11 months, the estimated national annual aggregate RSVH cost was $471.8 million. Full-term infants, who accounted for 88.2% of the 3 791 712 births in 2018 [20], comprised substantial majorities of infant RSVHs (82.0%) and aggregate infant RSVH costs (69.9% or $330 million). Extremely preterm infants accounted for 1.1% of infant RSVHs and 0.9% of births but 5.5% of aggregate costs ($25.9 million) (Figure 3A). Breakdowns between Medicaid and commercial payers showed similar patterns (Supplementary Material 14).
Figure 3.
Distribution of respiratory syncytial virus hospitalization (RSVH) of infants, aggregate infant RSVH costs, and births by (A) gestational age at birth and (B) payer type.
Although Medicaid payments averaged 32.0% less than commercial insurer payments per RSVH, the Medicaid volume was high. Medicaid was the exclusive payer for 61.3% of children aged 0–59 months with RSVH with known insurance status [6]. Medicaid covered 46.2% of all US births in 2018 (Figure 3B) [20].
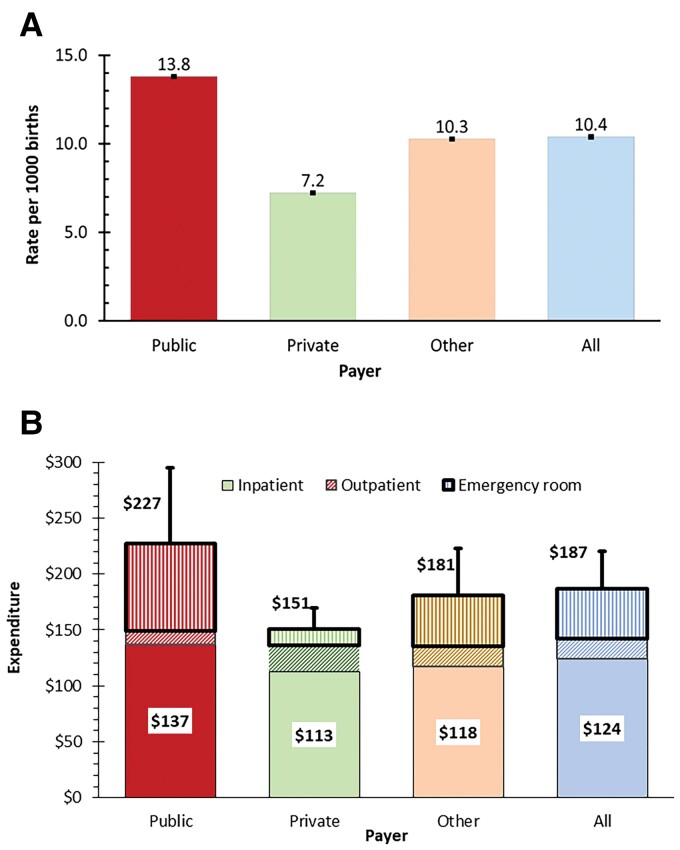
The numbers of annual births in 2018 were 3 791 712 for all payers, 1 751 771 for public payers, 1 880 689 for private payers, and 159 252 for other payers [21]. Rates of hospitalization by payer and treatment expenditure by insurer are provided in Figure 4A and 4B, respectively. Medicaid-insured births were 91% (OR, 1.91) more likely and other (uninsured and self-insured) births were 30% (OR, 0.70) less likely to have an RSVH in the first year of life than commercially (privately) insured infants. Incorporating utilization data from a recent claims analysis [47], estimated annual RSV treatment costs across all settings were $187 per birth and $709.6 million in aggregate. Of this, inpatient care constituted 66.5%, emergency department care 23.9%, and outpatient care 9.6%. Public payers (primarily Medicaid) had the highest rates of hospitalization, hospitalization costs, emergency room costs, and overall costs per insured birth. For outpatient visits and costs, private payers were highest in aggregate costs and cost per birth. These amounts include only medical costs and do not count indirect costs (value of lost time) (Supplementary Material 15).
Figure 4.
Breakdowns by payer (with 95% confidence interval) of (A) rates of respiratory syncytial virus (RSV) hospitalization per 1000 births and (B) expenditure on RSV treatment per birth by setting. The number at the top of each stacked bar shows the total for the payer (eg, $227 for public) and the number in the white box shows the inpatient expenditure for the payer (eg, $187 for public). The stacked bars represent the settings: inpatient (bottom, solid pattern of color representing the payer), outpatient (middle, diagonal stripes, also of color representing the payer), and emergency room (top, vertical stripes, also of color representing the payer).
DISCUSSION
This is the first systematic literature review and nationally weighted analysis to evaluate the current evidence on the cost of RSV in children aged 0–59 months, by insurance type, specific to the United States. Although 1 global systematic literature review included data from the United States, it did not report US results separately [13]. Although most RSV episodes in the United States are treated in ambulatory settings and do not require hospitalization [48], we found minimal literature examining RSV-associated costs in ambulatory settings (4 studies) and no analyses on indirect costs to families and caregivers published since 2014. Most literature focused on inpatient hospitalization costs. By virtue of the retrospective design, we accessed economic data from 55 842 unduplicated hospitalizations.
The cost of an RSVH was heavily dependent on wGA, comorbidities (HS-CHD and CLD), and payer. Infants born extremely premature had a mean cost 5.6 times that of full-term infants. Regardless of the high mean cost of RSV hospitalization for extremely premature term infants, their share of hospitalization cost was relatively small because extremely preterm infants constitute a very small share of overall RSV cases. Full-term infants account for 70% of RSVH costs for Medicaid and commercial payers, despite variations in costs by payer type. The distribution of numbers of infants by wGA and payer in studies that reported these proved reasonably consistent with national data. This finding suggests that our weighted means should be nationally representative.
Another substantial determinant of hospital cost was the type of insurer. Similar to other studies [40, 49], our results show that Medicaid-insured infants are at significantly higher risk for an RSVH than non-Medicaid insured infants. Our finding of lower average cost for Medicaid compared with commercial payers reflects Medicaid’s ability to set relatively low payment rates and does not necessarily reflect lower resource costs by the hospital. These results warrant additional analyses to understand the drivers of this higher level of RSV among the Medicaid populations.
As these cost calculations are based on means, they incorporate high-cost hospitalizations, such as those in the intensive care unit or receiving mechanical ventilation (MV). With occasional exceptions [40], most studies did not separate these subgroups. However, although charges for RSV hospitalizations with MV averaged 10 times more than those without MV, 95.5% of RSV admissions did not have MV. Although MV use undoubtedly raised mean RSV costs, it was not the major cost driver [50].
As RSVH costs constitute two-thirds of RSV treatment costs, this study showed that incorporating costs of nonhospitalized care increases by a half estimates based only on RSVH costs. No studies published in the review’s time period examined indirect costs, out-of-pocket payments, lost time or lost earnings of parents and caregivers, siblings missing school or requiring childcare, or costs of over-the-counter medications, or long-term costs following an RSV episode. More data on these topics would help to estimate the health system’s cost and societal cost of RSV.
Several limitations in this review deserve note. First, variability and incomplete documentation in settings and metrics inhibit generalizing and synthesizing the findings. Although wGA and age are important determinants of cost, only 8/17 (47%) of the studies reported wGA and only 6/17 (32%) studies reported RSVH costs specifically for infants. Heterogeneous reporting (per visit or per year) limited summarizing studies on ambulatory care. Whereas our aggregate RSVH costs were based on a prospective surveillance study of RSVH rates [6], studies using administrative claims gave almost twice the rate of RSVH [51]. Mean costs per RSVH varied significantly among studies, even after controlling for payer. These likely reflect poorly documented differences in the health systems producing those RSVHs.
Second, the absence of routine testing for RSV, especially in ambulatory settings, adds considerable uncertainty to cost studies. We included UB diagnoses to compensate for the lack of routine RSV testing, which may have caused an overestimation of the incidence of RSV and a slight underestimate in the cost of a presumed RSV episode. Third, many of the studies reported the occurrence and cost of services rather than full episodes of care. They lacked follow-up data and were unable to link inpatient encounters to ambulatory visits. Fourth, time lags in retrospective studies are substantial. Our analysis considered papers published from 2014 to 2021 (Supplementary Material 8). Further research on medically attended LRTIs by setting, payer, gestational age, and indirect costs is needed. Fifth, we used the CPI for medical care, which also includes pharmaceuticals, to inflate all reported costs to 2020 levels. This may have overestimated costs by overstating inflation in hospital and ambulatory costs.
In conclusion, hospitalization represents two-thirds of RSV treatment costs. Despite extremely premature infants having the highest mean RSVH costs per episode, full-term infants are the predominant source of infant RSVH costs and RSVHs. Medicaid-insured infants account for over half of RSV inpatient costs, despite accounting for fewer births and lower costs per episode than their commercially insured peers. Although public insurers (primarily Medicaid) have the highest RSV treatment cost per birth, Medicaid paid 32.0% less per RSVH than commercial payers. Medicare and Medicaid were estimated to pay hospitals only 87% of the economic costs of care in 2019, indicating a shortfall of at least 13% [52]. Because hospitals often rely on commercial insurers and donations to help offset shortfalls from public payers, many stakeholders share the costs of treating Medicaid-insured RSVHs. Our analysis highlights the need for further research into indirect costs of RSV. Immunizing term and near-term infants against RSV recently proved efficacious [53] and research on maternal immunization continues [54]. This economic study indicates that effective prevention could generate substantial cost savings for Medicaid and other payers.
Supplementary Material
Contributor Information
Diana M Bowser, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Katharine R Rowlands, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Dhwani Hariharan, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Raíssa M Gervasio, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Lauren Buckley, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Yara Halasa-Rappel, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Elizabeth L Glaser, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Christopher B Nelson, Sanofi, Swiftwater, Pennsylvania, USA.
Donald S Shepard, The Heller School for Social Policy and Management, Brandeis University, Waltham, Massachusetts, USA.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank members of the Sanofi RSV Scientific Advisory Board and Christopher Rizzo for comments on the clinical context; Jon Fryzek, Mina Suh, Heidi Reichert, and Lauren Bylsma for recommendations on meta-analyses; Meghan White and William Crown for constructive comments; and Clare L. Hurley and Barbara Courssaris for editorial assistance. Editorial assistance was also provided by inScience Communications (Philadelphia, PA) funded by Sanofi.
Author contribution. D. M. B. contributed to protocol development and registration, search strategy development, data extraction, statistical and data analysis, review of results, manuscript development, review, and editing. K. R. R. contributed to protocol development and registration, search strategy development, drafting the manuscript, title and abstract review, full-text review, and data extraction. D. H. contributed to data extraction, review of results, manuscript development, review, and editing. R. M. G. contributed to protocol development and registration, search strategy development, title and abstract review, full-text review, and data extraction. L. B. contributed to protocol development and registration, search strategy development and database search. Y. H.-R. contributed to title and abstract review, full-text review, and data extraction. E. L. G. contributed to protocol development and registration, search strategy development, manuscript review, and editing. C. B. N. contributed to securing funding and providing critical comments. D. S. S. contributed to securing funding, protocol development and registration, search strategy development, data extraction, statistical and data analysis, review of results, manuscript development, review, and editing.
Financial support. This work was supported in part by Sanofi, and cosponsored by AstraZeneca, by a grant to Brandeis University.
Supplement sponsorship. This article appears as part of the supplement “Respiratory Syncytial Virus Disease Among US Infants,” sponsored by Sanofi and AstraZeneca.
Presented in part: International Society for Pharmacoeconomics and Outcomes Research Annual Meeting, 17 May 2021, virtual; and ID Week, 21–25 October 2020, virtual.
References
- 1. Walsh EE, Hall Breese C. Respiratory syncytial virus (RSV). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell D and Bennett’s principles and practice of infectious diseases. 8th ed.2015:1948–60.e1943. [Google Scholar]
- 2. Wu P, Hartert TV. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther 2011; 9:731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hall CB, Weinberg GA, Iwane M, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haddadin Z, Rankin DA, Lipworth L, et al. Respiratory virus surveillance in infants across different clinical settings. J Pediat 2021; 234:164–71. [DOI] [PubMed] [Google Scholar]
- 5. Lively JY, Curns AT, Weinberg GA, et al. Respiratory syncytial virus-associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc 2019; 8:284–6. [DOI] [PubMed] [Google Scholar]
- 6. Rha B, Curns AT, Lively JY, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015–2016. Pediatrics 2020; 146:e20193611. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Increased interseasonal respiratory syncytial virus (RSV) activity in parts of the southern United States. Health Alert Network (HAN) 00443, 21 September 2021. https://emergency.cdc.gov/han/2021/han00443.asp. Accessed 3 October 2021.
- 8. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–502. [DOI] [PubMed] [Google Scholar]
- 9. Griffin MP, Yuan Y, Takas T, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020; 383:415–25. [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021; 18:e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glaser EL, Hariharan D, Bowser DM, et al. Impact of respiratory syncytial virus on child, caregiver, and family quality of life in the United States: systematic literature review and analysis. J Infect Dis 2022; 226(S2):S236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drummond MF, Jefferson TO. BMJ Economic Evaluation Working Party. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996; 313:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang S, Akmar LZ, Bailey F, et al. Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis 2020; 222:S680–7. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura MM, Zaslavsky AM, Toomey SL, et al. Pediatric readmissions after hospitalizations for lower respiratory infections. Pediatrics 2017; 140:e20160938. [DOI] [PubMed] [Google Scholar]
- 15. Centers for Medicare and Medicaid Services . Files for FY 2009 final rule and correction notice. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Acute-Inpatient-Files-for-Download-Items/CMS1247872. Accessed 12 June 2020.
- 16. Centers for Medicare and Medicaid Services . Files for FY 2010 final rule and correction notice. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Acute-Inpatient-Files-for-Download-Items/CMS1247873. Accessed 12 June 2020.
- 17. Centers for Medicare and Medicaid Services . Files for FY 2015 final rule and correction notice. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Acute-Inpatient-Files-for-Download-Items/FY2015-Final-Rule-CorrectionNotice-Files. Accessed 12 June 2020.
- 18. US Bureau of Labor Statistics . Consumer price index for all urban consumers: medical care in U.S. city average (CPIMEDSL). Federal Reserve Bank of St. Louis. https://fred.stlouisfed.org/series/CPIMEDSLAccessed 3 January 2022.
- 19. StatsToDo. Combine means and SDs into one group . 2014. https://www.statstodo.com/ResourceIndex_Subjects.php. Accessed 7 Oct 2020.
- 20. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep 2019; 68:1–47. [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Vital Statistics, United States Department of Health and Human Services . About natality 2016–2019 expanded. CDC WONDER. http://wonder.cdc.gov/natality-expanded-current.html. Accessed 25 May 2021.
- 22. Blake SM, Tanaka D, Bendz LM, Staebler S, Brandon D. Evaluation of the financial and health burden of infants at risk for respiratory syncytial virus. Adv Neonatal Care 2017; 17:292–8. [DOI] [PubMed] [Google Scholar]
- 23. Doucette A, Jiang X, Fryzek J, Coalson J, McLaurin K, Ambrose CS. Trends in respiratory syncytial virus and bronchiolitis hospitalization rates in high-risk infants in a United States nationally representative database, 1997–2012. PLoS One 2016; 11:e0152208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldstein M, Krilov LR, Fergie J, et al. Respiratory syncytial virus hospitalizations among U.S. preterm infants compared with term infants before and after the 2014 American Academy of Pediatrics guidance on immunoprophylaxis: 2012–2016. Am J Perinatol 2018; 35:1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anderson EJ, Krilov LR, DeVincenzo JP, et al. SENTINEL1: An observational study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks’ gestational age not receiving immunoprophylaxis. Am J Perinatol 2017; 34:51–61. [DOI] [PubMed] [Google Scholar]
- 26. Akenroye AT, Baskin MN, Samnaliev M, Stack AM. Impact of a bronchiolitis guideline on ED resource use and cost: a segmented time-series analysis. Pediatrics 2014; 133:e227–34. [DOI] [PubMed] [Google Scholar]
- 27. Amand C, Tong S, Kieffer A, Kyaw MH. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: A claims database analysis. BMC Health Serv Res 2018; 18:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ambrose CS, McLaurin KK. The Medicaid cost of palivizumab. J Pediatric Infect Dis Soc 2015; 4:83–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borse RH, Singleton RJ, Bruden DT, Fry AM, Hennessy TW, Meltzer MI. The economics of strategies to reduce respiratory syncytial virus hospitalizations in Alaska. J Pediatric Infect Dis Soc 2014; 3:201–12. [DOI] [PubMed] [Google Scholar]
- 30. Bryan MA, Tyler A, Zhou C, et al. Associations between quality measures and outcomes for children hospitalized with bronchiolitis. Hosp Pediatr 2020; 10:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chirikov VV, Simoes EAF, Kuznik A, Kwon Y, Botteman M. Economic-burden trajectories in commercially insured US infants with respiratory syncytial virus infection. J Infect Dis 2020; 221:1244–55. [DOI] [PubMed] [Google Scholar]
- 32. Chu PY, Hornik CP, Li JS, Campbell MJ, Hill KD. Respiratory syncytial virus hospitalisation trends in children with haemodynamically significant heart disease, 1997–2012. Cardiol Young 2017; 27:16–25. [DOI] [PubMed] [Google Scholar]
- 33. Fergie J, Suh M, Jiang X, Fryzek JP, Gonzales T. Respiratory syncytial virus and all-cause bronchiolitis hospitalizations among preterm infants using the Pediatric Health Information System (PHIS). J Infect Dis 2022; 225:1197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Friedman D, Wong PC. Risk of respiratory syncytial virus hospitalization in the first and second years of life in pediatric patients with congenital heart disease. Pediatr Cardiol 2017; 38:1311–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gupta P, Beam BW, Rettiganti M. Temporal trends of respiratory syncytial virus-associated hospital and ICU admissions across the United States. Pediatr Crit Care Med 2016; 17:e343––51.. [DOI] [PubMed] [Google Scholar]
- 36. Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Temporal trends in emergency department visits for bronchiolitis in the United States, 2006 to 2010. Pediatr Infect Dis J 2014; 33:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayes EA, Hart SA, Gowda C, Nandi D. Hospitalizations for respiratory syncytial virus and vaccine preventable infections following pediatric heart transplantation. J Pediatr 2021; 236:101–7.e103. [DOI] [PubMed] [Google Scholar]
- 38. Krilov LR, Fergie J, Goldstein M, Brannman L. Impact of the 2014 American Academy of Pediatrics immunoprophylaxis policy on the rate, severity, and cost of respiratory syncytial virus hospitalizations among preterm infants. Am J Perinatol 2020; 37:174–83. [DOI] [PubMed] [Google Scholar]
- 39. Ledbetter J, Brannman L, Wade SW, Gonzales T, Kong AM. Healthcare resource utilization and costs in the 12 months following hospitalization for respiratory syncytial virus or unspecified bronchiolitis among infants. J Med Econ 2020; 23:139–47. [DOI] [PubMed] [Google Scholar]
- 40. McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol 2016; 36:990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rivera-Sepulveda A, Garcia-Rivera EJ. Epidemiology of bronchiolitis: a description of emergency department visits and hospitalizations in Puerto Rico, 2010–2014. Trop Med Health 2017; 45:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah A, Narayanan S. Age-specific economic burden of respiratory syncytial virus (RSV) hospitalizations in the United States, 2009–2013: An analysis of healthcare cost and utilization project (HCUP) national inpatient sample (NIS). Value in Health 2017; 20:A75. [Google Scholar]
- 43. Simões EAF, Chirikov V, Botteman M, Kwon Y, Kuznik A. Long-term assessment of healthcare utilization 5 years after respiratory syncytial virus infection in US infants. J Infect Dis 2020; 221:1256–70. [DOI] [PubMed] [Google Scholar]
- 44. Walpert AS, Thomas ID, Lowe MC Jr, Seckeler MD. RSV prophylaxis guideline changes and outcomes in children with congenital heart disease. Congenit Heart Dis 2018; 13:428–31. [DOI] [PubMed] [Google Scholar]
- 45. Wozniak P, Sanchez PJ, Rajah B, et al. Impact of the revised guidelines for respiratory syncytial virus (RSV) prophylaxis: morbidity persists after two seasons! Open Forum Infect Dis 2016; 3(Suppl 1). [Google Scholar]
- 46. Wyffels V, Smulders M, Gavart S, et al. Evaluation of resource utilization among infants, young children and elderly patients diagnosed with RSV infection in the United States. Value in Health 2017; 20:A790–1. [Google Scholar]
- 47. Gomez GB, Nelson CB, Rizzo C, et al. Inequalities in health impact of alternative reimbursement pathways for nirsevimab in the United States. J Infect Dis 2022; 226(S2):S293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fisher RG, Gruber WC, Edwards KM, et al. Twenty years of outpatient respiratory syncytial virus infection: a framework for vaccine efficacy trials. Pediatrics 1997; 99:E7. [DOI] [PubMed] [Google Scholar]
- 49. Greenbaum AH, Chen J, Reed C, et al. Hospitalizations for severe lower respiratory tract infections. Pediatrics 2014; 134:546–54. [DOI] [PubMed] [Google Scholar]
- 50. Shepard DS, Perloff J, Jiang X, et al. Impacts of age and season on rates of hospitalization for respiratory syncytial virus (RSV) in infants in the United States and their use of mechanical ventilation and charges. Poster: 1708. ID Week 2020.Open Fourm Inf Dis 2020; 7(Suppl 1):S837. [Google Scholar]
- 51. McLaughlin JM, Khan F, Schmitt HJ, et al. Respiratory syncytial virus-associated hospitalization rates among US infants: a systematic review and meta-analysis. J Infect Dis 2022; 225:1100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. American Hospital Association . Fact sheet: underpayment by Medicare and Medicaid.https://www.aha.org/fact-sheets/2020-01-07-fact-sheet-underpayment-medicare-and-medicaid. Accessed 6 December 2021.
- 53. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386:837–46. [DOI] [PubMed] [Google Scholar]
- 54. Alonso JA N, Bont LJ, Bozzola E, et al. RSV: perspectives to strengthen the need for protection in all infants. Emerg Themes Epidemiol 2021; 18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.