Abstract
There are two types of exposure to atomic bomb (A-bomb) radiation: exposure to initial radiation released at the time of the detonation of the bomb, and exposure to residual radiation, which remains afterwards. Health hazards caused by exposure from residual radiation have not yet been clarified. The purpose of our study was to reveal the relationships between mortality risk from solid cancer and residual radiation based on data from the early entrants to Hiroshima. It is hard to identify the individual residual radiation doses. However, these are assumed to depend on the date of entry and the entrants’ behavior. Individual behavior is thought to be closely related to gender and age at exposure. We investigated a cohort of 45 809 individuals who were living in Hiroshima Prefecture on 1 January 1970 and were registered on the Database of Atomic Bomb Survivors as entrants after the bombing. Poisson regression methods were used to estimate excess relative risks (ERR) with data cross-classified by sex, age at entry, and date of entry. In males in their 20s, 30s, and 40s at entry and in females less than 10 years old and in their 40s at entry, solid cancer mortality risks were significantly higher among persons who entered the city on the day of the bombing than those who entered three or more days later. With adjustments for the age-dependent sensitivities to radiation exposure, it was extrapolated that middle-aged people who entered the city on the day of the bombing were exposed to higher levels of residual radiation than younger people.
Keywords: atomic bombing, cohort study, early entrants, excess relative mortality risk, residual radiation, solid cancer mortality
INTRODUCTION
The atomic bomb (A-bomb) was dropped on Hiroshima at 8:15 am on 6 August 1945. There are two major cohort data to investigate health effects of radiation, one is a database of A-bomb survivors (ABS) in the Hiroshima Prefecture followed-up by our Institute and the other is the ‘Life span cohort’ followed-up by Radiation Effects Research Foundation (RERF). The former is based on Atomic Bomb Health Handbooks issued officially by Hiroshima City/Prefecture, but the latter is not.
A-bomb radiation exposure can be divided into two major types: exposure to initial radiation released at the time of the detonation of the bombs, and exposure to residual radiation, which happens afterwards. Initial radiation mainly consists of neutrons and gamma rays emitted from the explosions. The doses of the initial radiation were determined on the basis of the distance from the hypocenter and shielding situations in DS02 [1].
On the other hand, residual radiation is secondary radiation resulting from radioactive materials that remain in the environment after nuclear denotations. These can be classified into radioisotopes induced by neutron activation of materials such as soil, building materials, etc., and radioactive fallout known as ‘black rain,’ which fell widely in the cities out to areas several kilometers from the hypocenter in Hiroshima and Nagasaki [2]. Exposure to residual radiation comprises external exposure and internal exposure and dose estimation is exceedingly difficult because it is impossible to obtain records on an individual’s behavior, activity, and their location and duration of stay in the affected city after the atomic bombing. There are a few reports on estimation of external exposures from residual radiation assuming various circumstances concerning individual behavior and activity immediately after the bombing. According to the Life Span Study (LSS) Report 9 by RERF [3], the mean cumulative dose from induced radiation for an infinite period following the bombing in Hiroshima was probably less than 20–30 mGy. Cullings et al. [4] stated that the magnitudes of such doses fall mostly within the margin of uncertainty of direct radiation dose estimates. Imanaka et al. [5] estimated that someone who was at the hypocenter in Hiroshima for 6 hours beginning 1 hour after the blast would have received a cumulative dose of about 210 mGy based on DS02 calculations. In 2014, a workshop was held to discuss and evaluate new studies in detail in order to clarify residual radiation exposure to ABSs in Hiroshima and Nagasaki [6].
According to the LSS report by RERF, there was no evidence of increased mortality among early entrants to Hiroshima [7]. In RERF’s view, the residual radiation doses were low and the health effects of the early entrants were considered negligible [3,7]. On the other hand, despite such low dose estimates of residual radiation for the ABSs and early entrants, there are several reports about acute symptoms, leukemia, and solid cancer observed among entrants [8–12]. Dr. O-ho, a physician living in Hiroshima, conducted a health survey of 3946 survivors 12 years after the A-bomb was dropped [8]. He reported that acute radiation symptoms, such as fever and diarrhea, were found among 30% of the people who entered the central region immediately after the bombing, whereas these symptoms were not found among the entrants who did not. Sutou [9] published O-ho’s results in English. Sawada [10] quantified the effects of residual radiation exposure on the acute symptoms of ABSs based on the results of the study by O-ho. Tonda et al. [11] reported that the risk of leukemia among early entrants who entered the city on 6 August was significantly higher than that among persons who entered on 8 August or later. Using the database of Hiroshima ABSs with follow-ups from 1968–1982, Matsuura et al. [12] reported that those who entered the region within 2 km of the hypocenter on the day of the bombing had a significantly higher risk of mortality due to malignant neoplasm than those who entered thereafter. Though proportional hazards regression models proposed by Cox [13] were used in the study to evaluate cancer mortality risk, it may be difficult to adjust for factors that vary depending on age.
Individual doses of residual radiation to the entrants, comprising external exposure and internal exposure, are assumed to depend mainly on the date of entry, the individual’s behavior or activity for the day, location of entry, and length of stay. Internal exposure is considered to largely depend on an individual’s behavior or activity, and to be closely related to sex and age. Thus, in this study, we tried to evaluate how solid cancer mortality risk among the early entrants to Hiroshima depends on the date of entry, the age at exposure, and sex, to infer the health effects of residual radiation exposure.
MATERIALS AND METHODS
Subjects
ABSs are officially defined as individuals who received an A-bomb Survivors’ Health Handbook by the Japanese government, and they are classified according to their various conditions at the time of the bombing: directly exposed survivors, entrants who entered the region within 2 km from the hypocenter during the first 2 weeks after the bombing, persons engaged in treatment and aid of those suffering bodily injury, and in utero exposed survivors. This study deal with a cohort of 45 749 individuals (25 660 men and 20 149 women) who were living in Hiroshima Prefecture on 1 January 1970 and were registered the Database of Atomic Bomb Survivors [14]. The basic information of gender, date of birth, age at the time of exposure and date of entry into the city listed in the ABS database was used in this study. Death information is based on the Vital Statistics Death Schedules released by the prime minister’s office. Mortality information, including cause of death and data on migration into and out of the city, is updated yearly on the basis of dynamic population statistics provided by Hiroshima’s municipal and prefectural governments.
Method for statistical analysis
We performed descriptive survival analyses for the solid cancer mortality by sex, Age at bombing (ATB), and date of entry into the city using Kaplan–Meier method with Log-rank test, and multivariate analyses for excess relative risk (ERR) using Poisson/Cox regression methods.
Death from malignant neoplasms excluding hematopoietic cancers (referred to as ‘solid cancers’) was defined as the outcome. The time variable is age, with 1 January 1970 as the start of follow-up and the earliest date of death, date of migration out of the area at any time during follow-up, or 31 December 2010 as the end of follow-up. Deaths from causes other than solid cancer, survival to the end of follow-up, and migration were treated as censored cases.
In epidemiological studies of mortality from malignant neoplasms since approximately 1950, numerous investigators have focused on the fact that the age-specific mortality rate 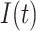 in humans is proportional to a power of age
in humans is proportional to a power of age 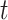 ,
,
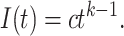 |
(1) |
To explain these observations, Muller and Nordling [15,16] proposed a multistage model of carcinogenesis, which postulates that cancer requires the accumulation of a critical number 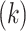 of mutation stages. Later, Armitage and Doll [17], using the multistage model as a basis, sought to develop a mathematical formula to capture the associations between cancer risk and both age and extent of exposure. Pierce [18] proposed a model under which the effect of exposure is equivalent to a change in age. Ohtaki et al. validated the model through a mathematical interpretation in terms of the multistage carcinogenesis hypothesis using the Poisson method [19]. Accordingly, when a subject receives a single exposure of dose D at age
of mutation stages. Later, Armitage and Doll [17], using the multistage model as a basis, sought to develop a mathematical formula to capture the associations between cancer risk and both age and extent of exposure. Pierce [18] proposed a model under which the effect of exposure is equivalent to a change in age. Ohtaki et al. validated the model through a mathematical interpretation in terms of the multistage carcinogenesis hypothesis using the Poisson method [19]. Accordingly, when a subject receives a single exposure of dose D at age 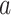 , the hazard of cancer mortality at age
, the hazard of cancer mortality at age 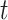 can be expressed as:
can be expressed as:
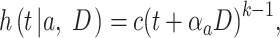 |
(2) |
where 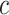 denotes a constant,
denotes a constant, 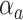 is an exponential function of
is an exponential function of 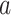 , and
, and 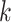 is a positive number to be estimated. Then, the ERR
is a positive number to be estimated. Then, the ERR 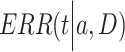 , which is defined by the excess value of the hazard ratio (hazard of exposure to hazard of no exposure) from unity, can be expressed approximately as:
, which is defined by the excess value of the hazard ratio (hazard of exposure to hazard of no exposure) from unity, can be expressed approximately as:
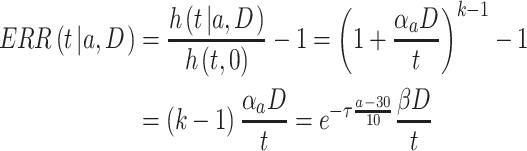 |
(3) |
where 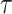 is an unknown parameter representing the effect of age at exposure, and
is an unknown parameter representing the effect of age at exposure, and 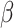 is an unknown parameter representing the coefficient dose–response due to the effect of radiation exposure. We adopted 0.33 as a value of parameter
is an unknown parameter representing the coefficient dose–response due to the effect of radiation exposure. We adopted 0.33 as a value of parameter 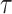 (see Discussion section 4.3).
(see Discussion section 4.3).
In this study, the baseline hazard was defined as the hazard for the reference group of people who first entered the city of Hiroshima between 3 days and 2 weeks after the date of the bombing (i.e. between 9 and 20 August 1945), which is specified as 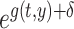 Hereafter, the reference group is referred to as ‘Aug. 9+’. Then, we postulated the following hazard model:
Hereafter, the reference group is referred to as ‘Aug. 9+’. Then, we postulated the following hazard model:
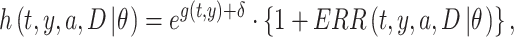 |
(4) |
where 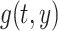 is a 5-degree polynomial in attained age
is a 5-degree polynomial in attained age 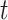 , calendar year
, calendar year 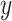 and their cross product
and their cross product 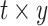 , expressing the log-transformed age- and period-specific solid cancer mortality in all of Japan from 1970–2010 [20]. The variable
, expressing the log-transformed age- and period-specific solid cancer mortality in all of Japan from 1970–2010 [20]. The variable 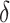 is an unknown parameter estimated by expressing the logarithmic value of the relative background mortality risk of solid cancer for Hiroshima entrants compared to that for the whole of Japan. The ERR for early entrants within 3 days ‘(6, 7 and 8 August 1945) can be specified as:
is an unknown parameter estimated by expressing the logarithmic value of the relative background mortality risk of solid cancer for Hiroshima entrants compared to that for the whole of Japan. The ERR for early entrants within 3 days ‘(6, 7 and 8 August 1945) can be specified as:
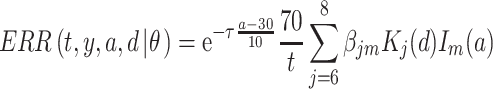 |
(5) |
with 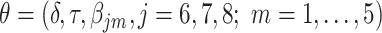 ,where
,where 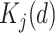 denotes a dummy variable for date-of-entry category defined by
denotes a dummy variable for date-of-entry category defined by
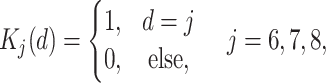 |
and 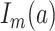 denotes a dummy variable for the age category at the time of exposure defined by:
denotes a dummy variable for the age category at the time of exposure defined by:
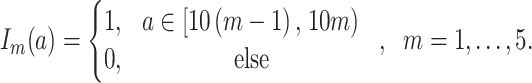 |
Thus, the vector of parameters 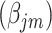 are interpreted as exposure dose due to residual radiation in the city.
are interpreted as exposure dose due to residual radiation in the city.
If the observation period for individual 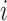 is specified as
is specified as 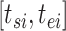 in terms of age, his or her expected mortality rate due to solid cancer is expressed approximately as:
in terms of age, his or her expected mortality rate due to solid cancer is expressed approximately as:
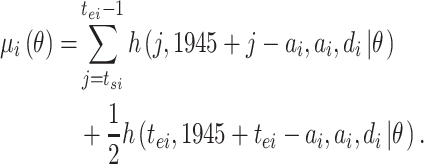 |
(6) |
If 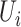 is the censoring indicator variable defined by:
is the censoring indicator variable defined by:
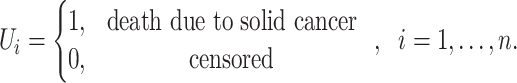 |
Then it is assumed that 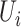 is a Bernoulli random variable specified as:
is a Bernoulli random variable specified as:
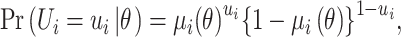 |
(7) |
the log-likelihood function is:
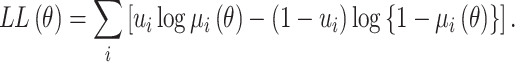 |
(8) |
It should be noted that there is a problem of verifiability between 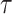 and
and 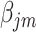 ‘s, as they cannot be estimated simultaneously without any restriction. Therefore, in this study we used the value of
‘s, as they cannot be estimated simultaneously without any restriction. Therefore, in this study we used the value of 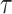 that was given by RERF, 0.33 (see Discussion section 4.3, Effect of age at exposure). The parameter
that was given by RERF, 0.33 (see Discussion section 4.3, Effect of age at exposure). The parameter 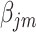 was estimated using an algorithm for optimization with the limited memory Broyden–Fletcher–Goldfarb–Shanno method [21]. The function ‘optim’ in the R software version 3.5.3 was used to carry out the analyses.
was estimated using an algorithm for optimization with the limited memory Broyden–Fletcher–Goldfarb–Shanno method [21]. The function ‘optim’ in the R software version 3.5.3 was used to carry out the analyses.
RESULTS
Descriptive analyses
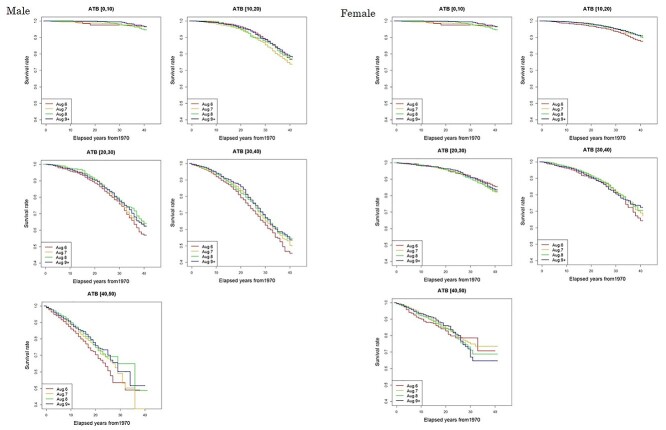
During the follow-up period, the number of people who died due to solid cancer was 4969 (19.4%) among 25 660 male subjects and 2405 (11.9%) among 20 149 female subjects (Table 1). Death rates among entrants who entered the city on the day of the bombing (6 August) were more than 10% higher than the reference entrants’ group (Aug. 9+) among males who were in their 20s, 30s or 40s at the date of entry and among females who were less than 10 years of age or in their 40s at the date of entry. The site-specific frequency of deaths from solid cancers shown in Table A1 in the Appendix, with no major difference in the distribution by date of entry. Figure 1 shows entry-day-specific Kaplan–Meier plots by 10-year categories of age at the date of entry. Log-rank tests were conducted by category of age at entry to evaluate whether or not Kaplan–Meier curves for 6 August entrants and Aug. 9+ entrants were statistically different (Table 2). Among males whose age at the date of entry was in the 30s or 40s, the cumulative survival probability for 6 August entrants was significantly lower than that among those who entered the city on Aug. 9+. No difference was observed in other categories of age at entry. A significant difference was also found with females whose age at entry was less than 10 years old or in the 20s.
Table 1.
Mortality from total solid cancer among entrants by sex and age, by date of entry into the city after the bombing
| Group | Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at bombing (years) | Entry date |
Number of subjects |
Observed person-years |
Number of deaths |
Death rate /105 pyr |
RR (to 9+) |
Number of subjects |
Observed person-years | Number of deaths |
Death rate /105 pyr |
RR (to 9+) |
| [0, 10) | 6 | 144 | 4403 | 8 | 182 | 0.913 | 161 | 4644 | 4 | 86 | 1.063 |
| 7 | 434 | 12 689 | 30 | 236 | 1.188 | 439 | 13 582 | 13 | 96 | 1.182 | |
| 8 | 409 | 12 161 | 29 | 238 | 1.198 | 409 | 12 971 | 17 | 131 | 1.618 | |
| 9+ | 592 | 18 572 | 37 | 199 | 1 | 640 | 19 671 | 16 | 81 | 1 | |
| total | 1579 | 47 825 | 104 | 217 | − | 1649 | 50 868 | 50 | 98 | − | |
| [10, 20) | 6 | 1410 | 44 570 | 253 | 568 | 1.073 | 725 | 25 111 | 77 | 307 | 1.385 |
| 7 | 1467 | 45 144 | 295 | 653 | 1.235 | 1414 | 48 875 | 118 | 241 | 1.090 | |
| 8 | 739 | 23 017 | 131 | 569 | 1.076 | 851 | 29 379 | 71 | 242 | 1.092 | |
| 9+ | 1005 | 31 737 | 168 | 529 | 1 | 1404 | 48 329 | 107 | 221 | 1 | |
| total | 4621 | 144 468 | 847 | 586 | − | 4394 | 151 694 | 373 | 246 | − | |
| [20, 30) | 6 | 1174 | 30 611 | 320 | 1045 | 1.160 | 711 | 22 341 | 79 | 354 | 0.892 |
| 7 | 1496 | 38 909 | 350 | 900 | 0.998 | 1971 | 62 831 | 268 | 427 | 1.076 | |
| 8 | 715 | 19 282 | 164 | 851 | 0.944 | 1311 | 41 231 | 174 | 422 | 1.064 | |
| 9+ | 919 | 24 189 | 218 | 901 | 1 | 1354 | 44 138 | 175 | 396 | 1 | |
| total | 4304 | 112 991 | 1052 | 931 | − | 5347 | 170 541 | 696 | 408 | − | |
| [30, 40) | 6 | 1853 | 34 985 | 470 | 1343 | 1.217 | 646 | 15 300 | 111 | 725 | 1.077 |
| 7 | 2552 | 49 526 | 569 | 1149 | 1.041 | 1960 | 47 297 | 314 | 664 | 0.986 | |
| 8 | 1280 | 24 874 | 284 | 1142 | 1.034 | 1236 | 30 076 | 194 | 645 | 0.958 | |
| 9+ | 1081 | 22 183 | 245 | 1104 | 1 | 1219 | 29 403 | 198 | 673 | 1 | |
| total | 6766 | 131 568 | 1568 | 1192 | − | 5061 | 122 076 | 817 | 669 | − | |
| [40, 50) | 6 | 1855 | 21 155 | 361 | 1706 | 1.327 | 475 | 6572 | 64 | 974 | 1.133 |
| 7 | 3308 | 38 854 | 551 | 1418 | 1.103 | 1583 | 23 395 | 190 | 812 | 0.945 | |
| 8 | 1822 | 21 362 | 272 | 1273 | 0.990 | 892 | 13 294 | 120 | 903 | 1.050 | |
| 9+ | 1405 | 16 635 | 214 | 1286 | 1 | 748 | 11 050 | 95 | 860 | 1 | |
| total | 8390 | 98 006 | 1398 | 1426 | − | 3698 | 54 311 | 469 | 864 | − | |
| Total | 25 660 | 534 858 | 4969 | 929 | − | 20 149 | 549 490 | 2405 | 438 | − | |
Fig. 1.
Survival rate using outcome death from solid cancers with elapsed years from 1970 by date of entry and age at entry. Dates of entry are 6, 7, 8 August and Aug. 9+. Age at entry was grouped into 10-year categories: [0, 10), [10, 20), [20, 30), [30, 40), and [40, 50).
Table 2.
Log-rank tests of difference in Kaplan–Meier curves for 6 August entrants compared to Aug. 9+ entrants, by category of age at the date of entry
| Age at the date of entry | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [0,10) | [10,20) | [20,30) | [30,40) | [40, 50) | ||||||
| χ2 | P value | χ2 | P value | χ2 | P value | χ2 | P value | χ2 | P value | |
| Male | 0.1 | 0.753 | 0.5 | 0.501 | 3.4 | 0.063 | 8.7 | 0.003 | 11.3 | 0.000 |
| Female | 0.0 | 0.913 | 5.1 | 0.024 | 0.6 | 0.443 | 0.6 | 0.444 | 0.7 | 0.419 |
Investigation using the hazard model
The parameters 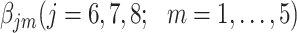 were estimated under
were estimated under 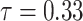 (see Discussion section 4.3, Effect of age at exposure). The resulted point estimates of ERR and 95% confidence intervals by date of entry and 10-year categories of age at entry at age 70 years with the hazard model (5) are shown in Table 3 and illustrated in Figure 2. Among males who entered the city on 6 August, mortality rates were significantly higher than those among Aug. 9+ entrants in their 20s, 30s or 40s at the date of entry. Among females, mortality rates were significantly higher among those who entered on 6 August than among those who entered Aug. 9+ if they were less than 10 years old or in their 40s at the date of entry. Estimated ERRs at age 70 were about 34% among males and 44% among females who were 40 years of age at entry.
(see Discussion section 4.3, Effect of age at exposure). The resulted point estimates of ERR and 95% confidence intervals by date of entry and 10-year categories of age at entry at age 70 years with the hazard model (5) are shown in Table 3 and illustrated in Figure 2. Among males who entered the city on 6 August, mortality rates were significantly higher than those among Aug. 9+ entrants in their 20s, 30s or 40s at the date of entry. Among females, mortality rates were significantly higher among those who entered on 6 August than among those who entered Aug. 9+ if they were less than 10 years old or in their 40s at the date of entry. Estimated ERRs at age 70 were about 34% among males and 44% among females who were 40 years of age at entry.
Table 3.
Estimated ERR under 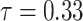 at age 70 years by sex, date of entry, and 10-year categories of age at entry; reference group is Aug. 9+ entrants
at age 70 years by sex, date of entry, and 10-year categories of age at entry; reference group is Aug. 9+ entrants
| Group | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age at exposure (years) | Entry date | ERR | 95%LB | 95%UB | P-value | ERR | 95%LB | 95%UB | P-value |
| [0, 10) | Aug. 6 | 0.554 | −0.058 | 1.167 | 0.038 | 0.685 | 0.112 | 1.257 | 0.010 |
| 7 | 0.938 | 0.719 | 1.157 | 0.000 | 0.151 | −0.525 | 0.826 | 0.331 | |
| 8 | 0.776 | 0.552 | 0.999 | 0.000 | 0.418 | −0.021 | 0.856 | 0.031 | |
| [10, 20) | Aug. 6 | 0.087 | −0.013 | 0.187 | 0.043 | 0.128 | −0.107 | 0.363 | 0.145 |
| 7 | 0.264 | 0.174 | 0.354 | 0.000 | −0.110 | −0.306 | 0.086 | 0.864 | |
| 8 | 0.131 | 0.003 | 0.260 | 0.022 | −0.085 | −0.326 | 0.156 | 0.754 | |
| [20, 30) | Aug. 6 | 0.170 | 0.075 | 0.265 | 0.000 | −0.157 | −0.390 | 0.077 | 0.907 |
| 7 | 0.031 | −0.062 | 0.123 | 0.256 | −0.006 | −0.152 | 0.140 | 0.531 | |
| 8 | −0.061 | −0.186 | 0.063 | 0.829 | −0.013 | −0.184 | 0.158 | 0.560 | |
| [30, 40) | Aug. 6 | 0.177 | 0.096 | 0.259 | 0.000 | 0.181 | −0.036 | 0.399 | 0.051 |
| 7 | 0.007 | −0.071 | 0.085 | 0.432 | 0.075 | −0.071 | 0.222 | 0.159 | |
| 8 | −0.017 | −0.117 | 0.083 | 0.632 | 0.057 | −0.116 | 0.230 | 0.260 | |
| [40, 50) | Aug. 6 | 0.281 | 0.188 | 0.374 | 0.000 | 0.346 | 0.067 | 0.626 | 0.008 |
| 7 | 0.077 | −0.004 | 0.159 | 0.031 | 0.137 | −0.042 | 0.316 | 0.067 | |
| 8 | −0.044 | −0.148 | 0.060 | 0.796 | 0.252 | 0.038 | 0.466 | 0.011 | |
Fig. 2.
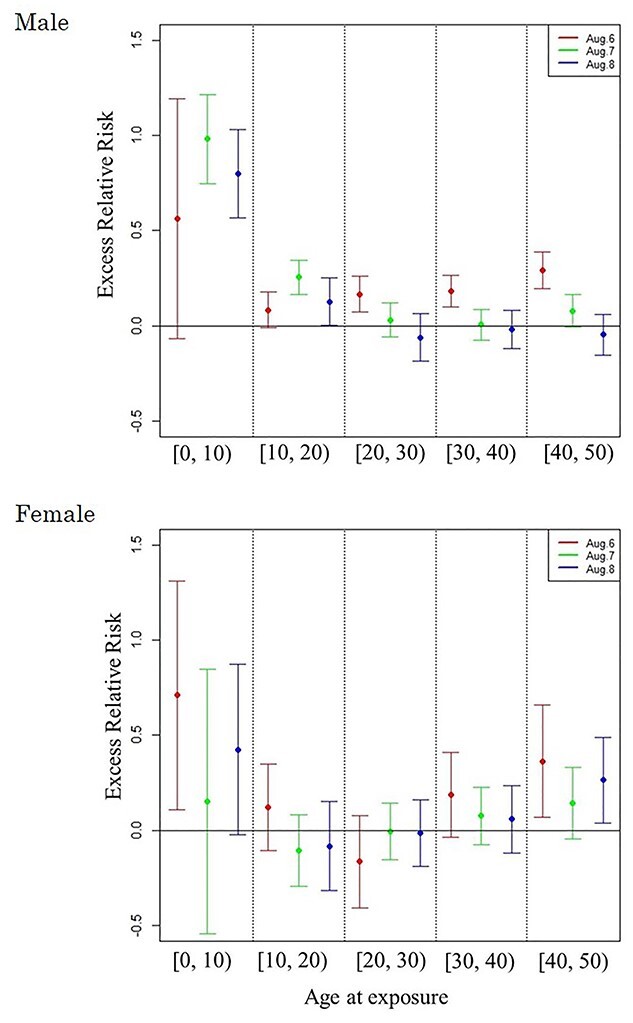
ERR under 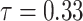 at age 70 years with 95% confidence intervals, by date of entry and age at entry. The Aug. 9+ entrants were used as the reference group.
at age 70 years with 95% confidence intervals, by date of entry and age at entry. The Aug. 9+ entrants were used as the reference group.
In the function 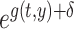 , the values of
, the values of 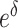 , the relative mortality risk of solid cancer among Aug. 9+ entrants compared with that in the whole of Japan, were estimated as 0.91 and 0.89 in males and females, respectively.
, the relative mortality risk of solid cancer among Aug. 9+ entrants compared with that in the whole of Japan, were estimated as 0.91 and 0.89 in males and females, respectively.
DISCUSSION
Background rates
The mortality risks of solid cancer among Aug. 9+ entrants were estimated less than those in the whole of Japan. It is supposed to be partially due to the beneficial health effects of the Atomic Bomb Survivors’ Health Handbooks [22], which were issued to ABSs by the Japanese government beginning in 1957: Survivors who possess the Handbooks are able to receive free medical checkups twice a year and free medical care for certain designated disorders [23].
Excess relative risk
RERF reported that the ERR/Gy of solid cancer declined monotonically with increasing age at exposure at any attained age [24]. On the other hand, our findings show that under the same attained age, ERRs who were exposed at less than 10 years of age or after the age of 30 had higher ERRs than those in their 20s (see Fig. 2). ERRs among people who were young at exposure are high because young people are expected to be more sensitive to radiation than older people, and ERRs among middle-aged persons are high because they are presumed to have been exposed to additional residual radiation. One possible explanation for this is that the duration of behaviors that led to significant radiation exposure in the city after the date of entry might have been longer with middle-aged persons than with younger people.
Effect of age at exposure
Biologically, young people are more sensitive to radiation than older people; this concept is based on a fundamental premise of radiation biology called Bergonie-Tribondeau’s law [25]. RERF incorporated in their ERR model an effect of age at exposure on solid cancer mortality risk represented as the parametric function 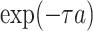 , where
, where  denotes age at exposure and
denotes age at exposure and 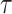 reflects sensitivity to radiation [24, 26]. The parameter
reflects sensitivity to radiation [24, 26]. The parameter 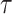 was estimated to be around 0.33 per 10 years in report 14 by RERF based on the LSS [26]. That report showed that those who were exposed at younger ages had a higher relative risk of cancer death. Cullings et al. [4] stated that none of the dosimetry systems used at RERF attempted to provide individual estimates of the dose from residual radiation. If the effects of residual radiation on the risk of solid cancer mortality are real and significant, the value of
was estimated to be around 0.33 per 10 years in report 14 by RERF based on the LSS [26]. That report showed that those who were exposed at younger ages had a higher relative risk of cancer death. Cullings et al. [4] stated that none of the dosimetry systems used at RERF attempted to provide individual estimates of the dose from residual radiation. If the effects of residual radiation on the risk of solid cancer mortality are real and significant, the value of 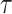 estimated based on dose from only the initial radiation would be expected to change to a lower value.
estimated based on dose from only the initial radiation would be expected to change to a lower value.
Effect of moving out of Hiroshima Prefecture
A stratified Cox regression analysis was performed with the move-out from Hiroshima Prefecture as the event, elapsed time since 1 January 1970, as the time variable, entry date as the explanatory variable and age at exposure by the 10-year-old class as the stratification variable. Figure 3 shows Kaplan–Meier curves adjusted for entry date for each 10-year age group. Except for those who were less than 10 years old at the time of the bombings, the transfer rate remains low and the lifetime retention rate in Hiroshima Prefecture is almost 90% or higher. The relatively high migration rate among those who were less than 10 years old at the time of the atomic bombings is imagined to be due to the fact that marriage or changing jobs often trigger migration. Based on the above, it is assumed that out-migration from the Hiroshima Prefecture does not have a significant impact on solid cancer mortality rates.
Fig. 3.
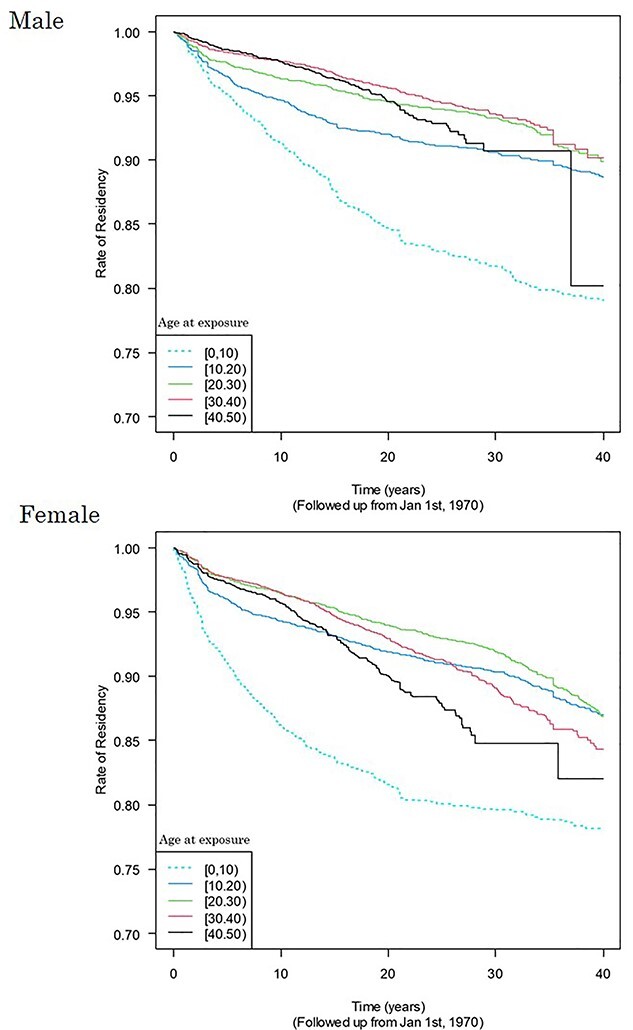
Kaplan–Meier curves for event of moving out of Hiroshima Prefecture adjusted for entry date for each 10-year age group.
Essential exposure factors: candidate radionuclides
The mortality risk from solid cancer among Hiroshima entrants was higher almost in the order of 8, 7 and 6 August entrants than the 9 August or later. There seems to be no other reason to explain this result than residual radiation. The residual radiation sources include neutron-activated radionuclides on the ground surface and radioactive fallout from the bombs. The former is the neutron-induced activation of Japanese houses and soil on the ground surface, while the latter is the radioactivity produced by nuclear fission. The residual radioactivity within a few kilometers of the hypocenter was mainly the former. Therefore. we explored what types of neutron-induced radionuclides in the soil were the major sources of residual radiation. When materials are exposed to neutrons, some of their elements become radioactive due to neutron activation. Gritzer et al. [27] listed 28Al (half -life: 2.24 min), 56Mn (2.58 h), 24Na (15.0 h) and 46Sc (83.8 days) as the primary induced radioactive isotopes. Considering the half-lives of these radionuclides and their effective periods, 56Mn and 24Na are considered the two nuclides most likely to contribute to the radiation exposure of entrants from neutron-induced radioactivity [28].
Internal exposure
The results of this study suggest the possibility of health effects of internal exposure due to ingestion or breathing dust contaminated with these induced radioisotopes. Radionuclide 56Mn is one of the main neutron-activated emitters contained in residual radiation. The biological effects of internal exposure are assumed to be significantly different from those due to external exposure. A series of experiments using rats had been conducted to investigate the biological impacts of exposure of 56MnO2 particles. Their effects on blood chemistry, the lungs, the small intestine, and testes have been previously reported [29–33].
Future needs
It is important for Japanese people affected by the Fukushima Daiichi nuclear power plant accident [34] to clarify even low residual radiation dose could cause adverse health effects if dust contaminated with radioactive materials were inhaled. More than 10 years have passed since that disaster. There have been two conflicting opinions regarding the causal relationship between radiation exposure and health. One claims that in the case of the Fukushima disaster, the estimated dose the public was exposed to is low [35], and therefore health consequences are undetectable [36–38]. Other reports [39, 40] claim that there have been markedly higher incidence rates of thyroid cancer in the Fukushima Prefecture than in Japan overall. Regarding the report by Tsuda et al. [39], we believe it is premature to draw conclusions based on the results of the 2014 survey because of epidemiological and diagnostic issues. Health effects of internal exposure are often difficult to quantify because they vary dramatically depending on whether radiation sources accumulate only in specific tissues or enter the blood and diffuse throughout the body [41, 42]. It is hoped that more careful analyses are performed to clarify the contribution of internal exposure to the cancer risks of various organs and tissues.
Limitations
The strength of this study is that the combination of information about individual entrant’s gender, age at exposure and date of entry, was used as an alternative indicator of the exposure dose from residual radiation and ERRs were evaluated using Poisson/Cox regression methods based on the multi-stage carcinogenesis model. The limitation of our study is that it was not possible to assess the details of each individual’s dose with higher accuracy because of the lack of detailed information on the place of stay, duration of stay and activities at the time of entry into the city.
CONCLUSION
Combination of date of entry, age at entry and sex was used as an index of residual radiation dose to the early entrants to Hiroshima City and the causal relationships with solid cancer mortality risks were analyzed. The results of fitting the hazard model using equation (5) were almost consistent with crude death rates and Kaplan–Meier plots (Table 1 and Fig. 1) and they suggested that middle-aged people who entered the city on the day of the bombing were exposed to higher levels of residual radiation than other age-groups, and their ERR of solid cancer mortality was significantly higher than that of the control group. Some radioactive materials, with half-lives with the time scale of evacuation or entry, possibly contributed to their additional exposure. It is still challenging to precisely determine the internal doses to critical organs and tissues. This issue would be resolved by continuing multidisciplinary work in the fields of radiation biology, medicine, and epidemiology.
Supplementary Material
ACKNOWLEDGMENTS
This article is based on data from the ABS database of the Hiroshima University Research Institute for Radiation Biology and Medicine.
Contributor Information
Keiko Otani, The Center for Peace, Hiroshima University, Hiroshima 730-0053, Japan.
Megu Ohtaki, The Center for Peace, Hiroshima University, Hiroshima 730-0053, Japan; Professor Emeritus, Hiroshima University, Hiroshima 739-8511, Japan.
Hiroshi Yasuda, Department of Radiation Biophysics, Research Institute for Radiation Biology and Medicine, Hiroshima University, Hiroshima 734-8533, Japan.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING
This work was supported by JSPS KAKENHI Grant Numbers 26460747 (April2014–March2016), 26257501 (April 2014–March 2018) and 19H01149 (April 2019–March 2023).
SUPPLEMENT FUNDING
This work was supported by JSPS KAKENHI Grant Number JP19H01149.
REFERENCES
- 1. Young RW, Kerr GD. Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki—Dosimetry System 2002 (DS02). Hiroshima: Radiation Effects Research Foundation, 2005. [Google Scholar]
- 2. Ohtaki M. Re-construction of spatial-time distribution of black rain in Hiroshima based on statistical analysis of witness of survivors from atomic bomb. In: Aoyama M, Oochi Y (eds). Revisit the Hiroshima A-Bomb with a Database–Latest Scientific View on Local Fallout and Black Rain. Hiroshima City, 2011, 131–44 (in Japanese). [Google Scholar]
- 3. Koto H, Brown CC, Hoel DG et al. Mortality from causes other than Cancer among atomic bomb survivors, 1950-1978. Life Span Study Report 9 1981. [Google Scholar]
- 4. Cullings HM, Fujita S, Funamoto S et al. Dose estimation for atomic bomb survivor studies: its evolution and present status. Radiat Res 2006;166:219–54. [DOI] [PubMed] [Google Scholar]
- 5. Imanaka T, Endo S, Tanaka K, Shizuma K. Gamma-ray exposure from neutron-induced radionuclides in soil in Hiroshima and Nagasaki based on DS02 calculations. Radiat Environ Biophys 2008;47:331–6. [DOI] [PubMed] [Google Scholar]
- 6. Kerr G, Egbert S, Al-Nabulsi I et al. Workshop report on atomic bomb dosimetry – review of dose related factors for the evaluation of exposures to residual radiation at Hiroshima and Nagasaki. Health Phys 2015;109:582–600. [DOI] [PubMed] [Google Scholar]
- 7.Beebe GW, Kato H, Land CE. Mortality and radiation dose, October 1950-September 1966. Life Span Study Report 1970;5:61–5. [Google Scholar]
- 8. O-ho G. Statistical analysis of symptoms due to residual radiation among atomic bomb survivors. Japan Medical Journal (Nihon-Iji-Shinpo) 1957;1746:21–5 (in Japanese). [Google Scholar]
- 9. Sutou S. Rediscovery of an old article reporting that the area around the epicenter in Hiroshima was heavily contaminated with residual radiation, indicating that exposure doses of A-bomb survivors were largely underestimated. J Radiat Res 2017;58:745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sawada S. Estimation of residual nuclear radiation effects on survivors of Hiroshima atomic bombing, from incidence of acute radiation disease. Bulletin of Social Medicine 2011;29:47–62. [Google Scholar]
- 11. Tonda T, Kamada N, Ohtaki M. Statistical analysis of effect of early entrants on incidence of leukemia. Nagasaki Medical Journal 2008;83:331–4 (in Japanese). [Google Scholar]
- 12. Matsuura M, Hayakawa N, Shimokata H. Survival analyses of atomic bomb survivors in Hiroshima Prefecture, Japan, 1968-1982--cancer mortality risk among early entrants. Hiroshima J Med Sci 1995;44:29–38. [PubMed] [Google Scholar]
- 13. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodology 1972;34:187–202. [Google Scholar]
- 14. Hirota S, Yasuda H, Kawakami H, Yoshinaga S. Prospects and status of the dosimetry system for atomic bomb survivor cohort study conducted at research Institute for Radiation Biology and Medicine of Hiroshima University. J Radiat Res 2021;62:i107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muller HJ. Radiation damage to the genetic material. Sci Prog 1951;7:93–177. [PubMed] [Google Scholar]
- 16. Nording CO. A new theory on the cancer-inducing mechanism. Br J Cancer 1953;7:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. Br J Cancer 1954;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pierce DA. Age-time patterns of cancer to be anticipated from exposure to general mutagens. Biostatistics 2003;4:231–48. [DOI] [PubMed] [Google Scholar]
- 19. Ohtaki M, Tonda T, Aihara K. A two-phase Poisson process model and its application to analysis of cancer mortality among A-bomb survivors. Math Biosci 2015;268:31–7. [DOI] [PubMed] [Google Scholar]
- 20. http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/suii10/dl/s03.pdf (3 July 2021, date last accessed)
- 21. Byrd RH, Lu P, Nocedal J et al. A limited memory algorithm for bound constrained optimization. SIAM J Sci Comput 1995;16:1190–208. [Google Scholar]
- 22. Otani K, Ohtaki M, Satoh K et al. Relationship between length of A-bomb survivors health handbook possessions and mortality risk. Hiroshima J Med Sci 2012;65:259–61 (in Japanese). [Google Scholar]
- 23. http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/genbaku/index.html (3 July 2021, date last accessed)
- 24. Preston DL, Shimizu Y, Pierce DA et al. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950-1997. Radiat Res 2003;160:381–407. [DOI] [PubMed] [Google Scholar]
- 25. Vogin G, Foray N. The law of Bergonié and Tribondea nice formula for a first approximation. Int J Radiat Biol 2013;89:2–8. [DOI] [PubMed] [Google Scholar]
- 26. Ozasa K, Shimizu Y, Suyama A et al. Studies of the mortality of atomic bomb survivors, report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177:229–43. [DOI] [PubMed] [Google Scholar]
- 27. Gritzer ML, Woolson WA. Calculation of doses due to atomic bomb induced soil activation. In: US-Japan Joint Reassessment of Atomic Bomb Radiation Dosimetry in Hiroshima and Nagasaki Final Report, Vol. 2. Hiroshima: Radiation Effects Research Foundation, 1986, 342–51. [Google Scholar]
- 28. Endo S, Taguchi Y, Imanaka T et al. Activation analysis for soils of Hiroshima city and estimation of gamma-ray dose rate due to neutron induced activated soil by Hiroshima atom bomb. IPSHU Research Report Series 2012;28:25–31. [Google Scholar]
- 29. Fujimoto N, Amantayeva G, Chaizhunussova N et al. Low-dose radiation exposure with 56MnO2 powder changes gene expressions in the testes and the prostate in rats. Int J Mol Sci 2020;21:4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujimoto N, Baurzhan A, Chaizhunusova N et al. Effects of internal exposure to 56MnO2 powder on blood parameters in rats. Eur J Med 2020;52:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fujimoto N, Ruslanova B, Abishev Z et al. Biological impacts on the lungs in rats internally exposed to radioactive 56MnO2 particle. Sci Rep 2021;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kairkhanova Y, Saimova A, Uzbekov D et al. Effects of exposure to radioactive 56 mno2 powder on hyaluronan synthase 2 in the lungs of rats. Georgian Med News 2017;9:120–4. [PubMed] [Google Scholar]
- 33. Shichijo K, Fujimoto N, Uzbekov D et al. Internal exposure to neutron-activated 56Mn dioxide powder in Wistar rats—part 2: pathological effects. Radiat Environ Biophys 2017;56:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strickland E. Explainer: what went wrong in Japan’s nuclear reactors. IEEE Spectr 2011;4. [Google Scholar]
- 35. United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) . UNSCEAR 2013 Report to the Gene Assembly with Scientific Annexes, Annex B: Effects of Radiation Exposure of Children. New York: UNSCEAR, 2014. [Google Scholar]
- 36. Kamiya K, Ozasa K, Akiba S et al. Long-term effects of radiation exposure on health. Lancet 2015;386:469–78. [DOI] [PubMed] [Google Scholar]
- 37. Jordan BR. The Hiroshima/Nagasaki survivor studies: discrepancies between results and general perception. Genetics 2013;203:1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohira T, Shimura H, Hayashi F et al. Absorbed radiation doses in the thyroid as estimated by UNSCEAR and subsequent risk of childhood thyroid cancer following the great East Japan earthquake. J Radiat Res 2020;61:243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsuda T, Tokinobu A, Yamamoto E et al. Thyroid cancer detection by ultrasound among residents ages 18 years and younger in Fukushima, Japan: 2011 to 2014. Epidemiology 2016;27:316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamamoto H, Hayashi K, Scherb H. A comment on: ‘absorbed radiation doses in the thyroid as estimated by UNSCEAR and subsequent risk of childhood thyroid cancer following the great East Japan earthquake’. J Radiat Res 2021;62:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tamplin A, Cochran T. Radiation Standard for Hot Particle, a Report on the Inadequacy of Existing Radiation Protection Standards Related to Internal Exposure to Man to Insoluble Particles of Plutonium and Other Alpha-Emitting Hot Particles. Washington, D. C., USA: OSTI.GOV, 1974. [Google Scholar]
- 42. ICRP . Cancer risk from exposure to plutonium and uranium. ICRP publication 150. Ann ICRP 2021;50:1–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.