Abstract
Adenylyl cyclase 5 (ADCY5) related dyskinesia is a rare disorder characterized by early-onset paroxysmal choreoathetosis, dystonia, myoclonus, or a combination of the above, which primarily involved the limbs, face, and neck. Other common clinical features are axial hypotonia and episodic exacerbation of dyskinesia. Both sporadic and inherited cases have been reported and the predomiant mode of inheritance is autosomal dominant. Herein, we describe the first ADCY5-related dyskinesia patient in Taiwan.
Keywords: ADCY5, Dyskinesia, Whole exome sequencing, Chorea, Dystonia
Dear editor,
Adenylyl cyclase 5 (ADCY5) related dyskinesia is a rare disorder characterized by early-onset paroxysmal choreoathetosis, dystonia, myoclonus, or a combination of the above, which primarily affects the limbs, face, and neck [1]. Other common clinical features are axial hypotonia and episodic exacerbation of dyskinesia, which may precipitate by sleep, emotional stress, intercurrent illness, or without any trigger [2]. ADCY5-related dyskinesia occurs either in a sporadic or inherited pattern. The predominant mode of inheritance is autosomal dominant, although recessive inheritance has been reported [3]. To the best of our knowledge, no Taiwanese patient has been reported, we hereby describe a patient with ADCY5-related dyskinesia.
A 24-year-old female, from a non-consanguineous family, was born as a full-term baby without any perinatal injury. At 11-month-old, she developed intermittent involuntary movements including eye blinking, mouth smacking, shoulder shrugging, and four limb choreic movement. The movement could not be suppressed by herself and persisted during sleep. Her motor milestones were delayed, she started to walk at age of two and talked at 2.5 years old after early intervention. The involuntary movement progressed slowly until her adolescence and became stationary thereafter. Episodic exacerbation during stress, menstrual period, illness, or initiation after resting was noted. Although she suffered from frequent minor injuries due to falls or dyskinetic movement, she could still take care of herself without assistance and finish her high school education.
She came to our Neurological Clinic at age of 23. Neurological examination showed slow saccade, dysarthria, oculomotor and tongue impersistent, axial hypotonia, facial dyskinesia, choreoathetosis over four limbs, and neck dystonia. The remainder of the neurological examination was normal. Her parents and older brother didn’t have similar symptoms. The basic metabolic workups were normal. Electroencephalogram showed no epileptiform discharges during dyskinesia and brain magnetic resonance imaging was unremarkable.
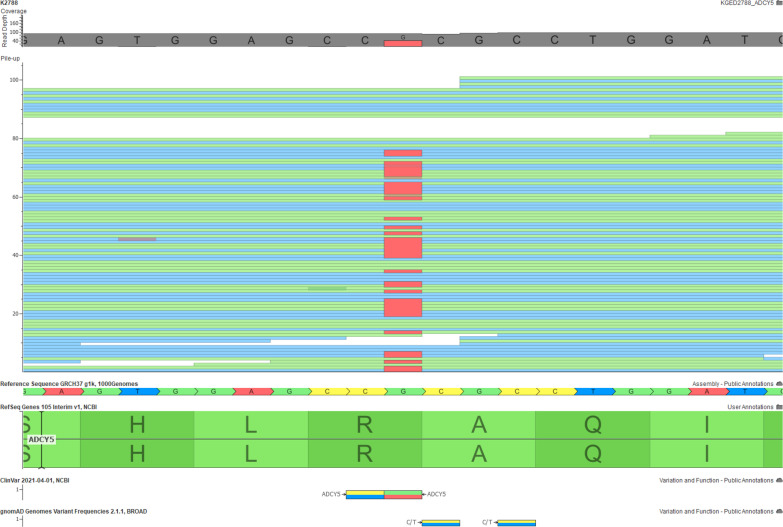
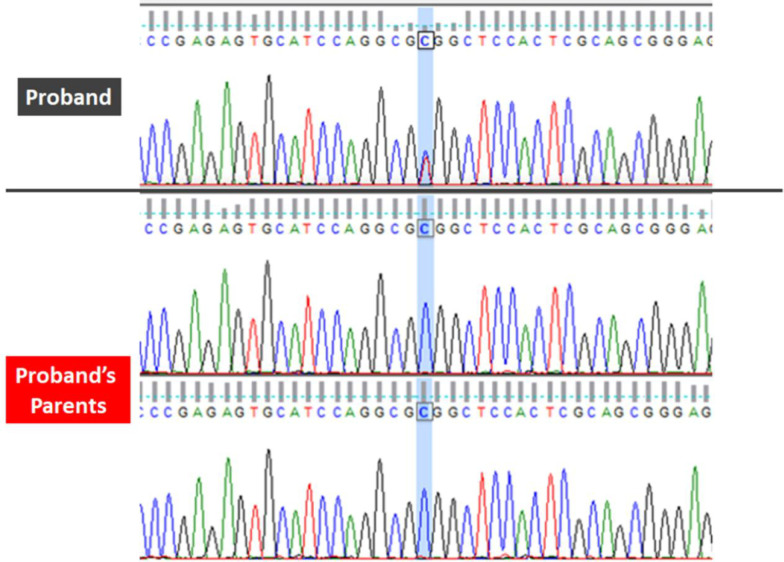
Whole exome sequencing revealed a known pathogenic heterozygous variant in the ADCY5 gene (NM_183357:Exon 2:c.1252C>T:p.Arg418Trp) using the Geneyx software (Geneyx, Inc. Israel) (Fig. 1). The variant was not presented in the gnomAD (especially the East Asian population) or the TaiwanBiobank database. None of the remaining rare variants was associated with dyskinesia or chorea. The variant was confirmed de novo by Sanger sequencing of the patient and her parents (Fig. 2). The variant was classified as pathogenic by the ACMG/AMP guideline (PS3 + PM1 + PM2 + PM6 + PP3) [4].
Fig. 1.
Whole-exome sequencing in the proband. The figure shows the alignment of the reads around the pathogenic variant (c.1252C>T:p.Arg418Trp) of the BAM file visualized using the GenomeBrowse software (Golden Helix, Inc. USA) (https://www.goldenhelix.com/products/GenomeBrowse/). The top panel illustrates the coverage of the reads, and the middle panel shows the actual mapping of the reads with blue indicating forward reads and green indicating reverse reads. The bottle panel shows the reference genome (CRCH37) and information from the gnomAD and ClinVar databases
Fig. 2.
Sanger sequencing in proband and proband’s parents. Sanger sequencing of variant c.1252C>T shows a peak of both T and C allele in the proband, whereas her parents have homozygous wildtype allele C
Several medications were prescribed, including Trihexphenidyl, Levetiracetam, Zonisamide, Propranolol, Acetazolamide, Piracetam, and Alprazolam, but no clinical improvement was observed. Intriguingly, the patient reported some benefits after consumption of coffee. Therefore, caffeine tablets were prescribed and started from 100 mg in the morning and 100 mg in the noon, which showed a partial reduction of her dyskinesia. However, when we further increased the caffeine dose to 300 mg per day, she had a paradoxical worsening of dyskinesia. She is now taking 200 mg of caffeine daily.
Adenylyl cyclases are a family of enzymes that convert adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP). ADCY5 is the major isoform expressed in the heart and the brain, which is highly expressed in the striatum and the nucleus accumbens. ADCY5 protein has two cytosolic domains C1 and C2, which subdivide into catalytic (C1a and C2a) and regulatory (C1b and C2b) subdomains. The catalytic subdomains interact to form an ATP-binding pocket. The pathogenic variant (p.Arg418Trp) is located in the C1a domain, where most pathogenic variants locate. Pathogenic variants were also found in other domains (C1b and C2a). It has been reported that variants in C1a and C2a domains cause a more severe phenotype than in the C1b domain [2]. Nearly all reported ADCY5 pathogenic variants caused gain of function change, and most cases are presented with paroxysmal hyperkinesia over limbs, face, and neck, even during sleep.
The dyskinesia is burdensome to our patient, although slowly progressive and not life-threatening, but it still affects her daily activities and results in frequent injuries. In recent two case reports, a dramatic improvement was noted after caffeine consumption [5, 6]. Partially response was also observed in our patient taking 200 mg caffeine daily, but paradoxical worsening was found at higher doses. Caffeine can antagonize A2A receptors, which may counteract the abnormal ADCY5 activity [5].
Bilateral deep brain stimulation (DBS) of the globus pallidus interna is another treatment option. A significant response of dyskinesia was noticed in eight ADCY5-related dyskinesia cases in recent studies. However, other clinical features, such as axial hypotonia, did not respond to DBS [7–10]. DBS may be an option for this patient if it is covered by the national health insurance program in the future.
In summary, we reported the first ADCY5-related dyskinesia patient in Taiwan, who presented with a partial response to caffeine. The treatment response in our patient is suboptimal, further novel therapies are needed to improve the quality of life of -patients with ADCY5-related dyskinesia.
Acknowledgements
We thank the patient and her family for participating in this study.
Abbreviations
- ADCY5
Adenylyl cyclase 5
- DBS
Deep brain stimulation
- ATP
Adenosine triphosphate
- cAMP
Cyclic adenosine monophosphate
Author contributions
SYC: study conception, collection, and analysis of clinical, genetic, and laboratory data, writing of the first draft. CJH, YTL, CHL: genetic study and data interpretation, bioinformatics analysis, and collection of clinical and laboratory data. MHT: study conception and organization, manuscript review, and critique. All authors read and approved the final manuscript.
Funding
This work was supported in part by research grant CMRPG8K0632 (MHT) from Kaohsiung Chang Gung Memorial Hospital, Taiwan. The foundation provided financial support for data collecting and had no further role in study design, analysis, and interpretation of data, the writing of the report, or in the decision to submit the paper for publication.
Availability of data and materials
All data and materials of this study are available on request from the corresponding author.
Declarations
Ethics approval and consent to participate
The study was approved by the Chang Gung Medical Foundation Institutional Review Board (IRB No.: 201801549B0). This study was carried out according to the Declaration of Helsinki. Written informed consent was obtained from the patient and her parents.
Consent for publication
Written informed consent was obtained from the participant and her parents for the publication of this report.
Competing of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hisama FM, Friedman J, Raskind WH, et al. ADCY5 Dyskinesia. 2014 Dec 18 [Updated 2020 Jul 30]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK263441/ [PubMed]
- 2.Vijiaratnam N, Bhatia KP, Lang AE, Raskind WH, Espay AJ. ADCY5-related dyskinesia: Improving clinical detection of an evolving disorder. Movement Disorders Clinical Practice. 2019;6(7):512–520. doi: 10.1002/mdc3.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohlega SA, Abou-Al-Shaar H, AlDakheel A, Alajlan H, Bohlega BS, Meyer BF, Monies D, Cupler EJ, Al-Saif AM. Autosomal recessive ADCY5-related dystonia and myoclonus: Expanding the genetic spectrum of ADCY5-related movement disorders. Parkinsonism & Related Disorders. 2019;64:145–149. doi: 10.1016/j.parkreldis.2019.02.039. [DOI] [PubMed] [Google Scholar]
- 4.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meneret A, Gras D, McGovern E, Roze E. Caffeine and the dyskinesia related to mutations in the ADCY5 gene. Annals of Internal Medicine. 2019;171(6):439. doi: 10.7326/L19-0038. [DOI] [PubMed] [Google Scholar]
- 6.Shetty K, Sarma AS, Devan M, Dalal A, Dash GK, Jannabhatla A, Patil SJ. Recurrent ADCY5 mutation in mosaic form with nocturnal paroxysmal dyskinesias and video electroencephalography documentation of dramatic response to caffeine treatment. Journal of Movement Disorders. 2020;13(3):238–240. doi: 10.14802/jmd.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, F. C., Westenberger, A., Dale, R. C., Smith, M., Pall, H. S., Perez-Dueñas, B., Grattan-Smith, P., Ouvrier, R. A., Mahant, N., Hanna, B. C., Hunter, M., Lawson, J. A., Max, C., Sachdev, R., Meyer, E., Crimmins, D., Pryor, D., Morris, J. G., Münchau, A., … Fung, V. S. (2016). Phenotypic insights into ADCY5-associated disease. Movement Disorders, 31(7), 1033–1040. 10.1002/mds.26598 [DOI] [PMC free article] [PubMed]
- 8.de Almeida Marcelino AL, Mainka T, Krause P, Poewe W, Ganos C, Kuhn AA. Deep brain stimulation reduces (nocturnal) dyskinetic exacerbations in patients with ADCY5 mutation: A case series. Journal of Neurology. 2020;267(12):3624–3631. doi: 10.1007/s00415-020-09871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dy ME, Chang FC, Jesus SD, Anselm I, Mahant N, Zeilman P, Rodan LH, Foote KD, Tan WH, Eskandar E, Sharma N, Okun MS, Fung VS, Waugh JL. Treatment of ADCY5-associated dystonia, chorea, and hyperkinetic disorders with deep brain stimulation: A multicenter case series. Journal of Child Neurology. 2016;31(8):1027–1035. doi: 10.1177/0883073816635749. [DOI] [PubMed] [Google Scholar]
- 10.Meijer IA, Miravite J, Kopell BH, Lubarr N. Deep brain stimulation in an additional patient with ADCY5-related movement disorder. Journal of Child Neurology. 2017;32(4):438–439. doi: 10.1177/0883073816681353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials of this study are available on request from the corresponding author.