Abstract
The polyketide metabolite 2,4-diacetylphloroglucinol (2,4-DAPG) is produced by many strains of fluorescent Pseudomonas spp. with biocontrol activity against soilborne fungal plant pathogens. Genes required for 2,4-DAPG synthesis by P. fluorescens Q2-87 are encoded by a 6.5-kb fragment of genomic DNA that can transfer production of 2,4-DAPG to 2,4-DAPG-nonproducing recipient Pseudomonas strains. In this study the nucleotide sequence was determined for the 6.5-kb fragment and flanking regions of genomic DNA from strain Q2-87. Six open reading frames were identified, four of which (phlACBD) comprise an operon that includes a set of three genes (phlACB) conserved between eubacteria and archaebacteria and a gene (phlD) encoding a polyketide synthase with homology to chalcone and stilbene synthases from plants. The biosynthetic operon is flanked on either side by phlE and phlF, which code respectively for putative efflux and regulatory (repressor) proteins. Expression in Escherichia coli of phlA, phlC, phlB, and phlD, individually or in combination, identified a novel polyketide biosynthetic pathway in which PhlD is responsible for the production of monoacetylphloroglucinol (MAPG). PhlA, PhlC, and PhlB are necessary to convert MAPG to 2,4-DAPG, and they also may function in the synthesis of MAPG.
Phloroglucinols are phenolic bacterial and plant metabolites with broad-spectrum antiviral, antibacterial, antifungal, antihelminthic, and phytotoxic properties. The compound 2,4-diacetylphloroglucinol (2,4-DAPG), which is produced by certain plant-associated fluorescent Pseudomonas species of worldwide origin (23, 57), is of particular significance to agriculture because of its activity in situ against a variety of root and seedling pathogens of plants (reviewed in reference 57). 2,4-DAPG production is the major determinant in the ability of P. fluorescens Q2-87 to suppress Gaeumannomyces graminis var. tritici, the fungal pathogen that causes take-all disease of wheat. The 2,4-DAPG-nonproducing Tn5 mutant Q2-87::Tn5-1 was unable to inhibit the pathogen in vitro and did not protect wheat against take-all (16, 61). Interest in 2,4-DAPG-producing pseudomonads has focused not only on their potential as introduced agents for biological control but also on their activity in natural agroecosystems. Indigenous populations of 2,4-DAPG-producing Pseudomonas spp. have a key role in the suppressiveness to take-all (take-all decline) that develops in some soils during extended monoculture of wheat in the presence of the take-all pathogen (43–45).
2,4-DAPG is thought to be derived from monoacetylphloroglucinol (MAPG), and an acetyltransferase activity capable of converting MAPG to 2,4-DAPG has been described in Pseudomonas sp. strain F113 (52). No precursors of MAPG have yet been identified, but the hydroxyl groups at alternating positions on the phloroglucinol ring are consistent with biosynthesis via a polyketide mechanism. Naturally occurring polyketides are produced by the successive condensation of small carboxylic acids in a process that resembles fatty acid synthesis. The genes encoding polyketide synthases (PKSs) show similarities to one other as well as to genes for fatty acid synthases (18, 20). Three types of PKS are known. Type I enzymes are large multifunctional proteins with domains that catalyze the individual steps of the pathway in a nonreiterative manner. In contrast, type II PKSs are complexes of four to six mono- or bifunctional proteins that can function reiteratively to assemble the polyketide molecule (20). A third kind of PKS, which we designate type III, is exemplified by members of the chalcone synthase (CHS) and stilbene synthase (STS) family from plants. These enzymes consist of a homodimeric protein that is sufficient to perform the condensation and cyclization steps needed to produce their phenolic products (30, 48). Type I and type II PKS enzyme systems have been studied extensively in Streptomyces spp. and other actinomycetes, and recent evidence indicates that both kinds of synthases also function in Pseudomonas spp. Genes characteristic of a type I PKS have been identified in DNA required for the production of the antifungal metabolite pyoluteorin by Pseudomonas fluorescens Pf-5 (35), and a type II PKS is involved in the synthesis of coronafacic acid (CFA), the polyketide component of the phytotoxin coronatine produced by Pseudomonas syringae (27, 40). A type I-like PKS coding region also has been identified adjacent to the CFA biosynthetic cluster, suggesting that CFA synthesis may involve a unique combination of mono- and multifunctional enzymes (46).
Genes sufficient to confer the ability to produce 2,4-DAPG on 2,4-DAPG-nonproducing recipient strains of Pseudomonas spp. were localized to a 6.5-kb DNA fragment from P. fluorescens Q2-87 in a previous study. Analysis of this fragment by transposon mutagenesis with Tn3HoHo1 identified a region spanning approximately 5 kb that contained at least two divergently oriented transcriptional units required for DAPG production (3). The goal of the present study was to elucidate the role of these genes in the synthesis of 2,4-DAPG and its precursor, MAPG. Nucleotide sequence analysis of the region revealed six genes organized in three transcriptional units. Four genes comprise the operon phlACBD, and expression studies indicated that the protein products of all four are required for the synthesis of both MAPG and 2,4-DAPG. The products of phlACBD resemble neither type I nor type II PKS enzyme systems. Rather, PhlD exhibits striking homology to plant chalcone synthases, indicating that phloroglucinol synthesis is mediated by a novel kind of PKS not previously described in microorganisms. The biosynthetic operon is flanked on either side by the separately transcribed genes phlE and phlF, which code respectively for putative efflux and regulatory (repressor) proteins that are not absolutely required for phloroglucinol production.
MATERIALS AND METHODS
Organisms and culture conditions.
Plasmids and bacterial strains used in this study are described in Table 1. Cultures of fluorescent Pseudomonas strains were grown at 28°C in yeast malt broth (3) for 2,4-DAPG extraction and in Luria Bertani (LB) medium (47) for other manipulations. Escherichia coli DH5α and BL21(DE3) were used as hosts for plasmid construction and protein expression studies, respectively. E. coli cultures were grown in LB medium for routine purposes, in tryptone phosphate broth (32) for protein expression, in M9 minimal medium for 35S labeling, or in M9 medium with 0.02% yeast extract for 2,4-DAPG extraction. Ampicillin was included at 100 μg/ml for routine work with plasmid-containing E. coli strains and at 400 μg/ml during protein expression. Kanamycin and tetracycline were used at 50 and 25 μg/ml, respectively.
TABLE 1.
Plasmids and strains used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| P. fluorescens | ||
| Q2-87 | Phl+ Rifr HCN+ | 42 |
| Q2-87::Tn5-1 | Phl− Rifr Kmr | 61 |
| M4-80R | Phl− Rifr HCN− | 15 |
| P. fluorescens-putida | ||
| Q69c-80 | Phl− HCN+ | 42 |
| Q69c-80::mini- | Phl+ HCN+ | 24 |
| Tn5Phl | ||
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 phoA hsdR17 (rK− mK+) supE44 λ-thi-1 gyrA96 relA1 | BRLb |
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | 54 |
| Plasmids | ||
| pIC19H | pUC8-derived lac; Apr | 28 |
| pT7-5 | ColE1; Apr | 55 |
| pMON5120 | pRK415 with 15-kb genomic fragment from Q2-87; Tcr | 61 |
| pMON5122 | pRK415 with 6.5-kb genomic fragment from Q2-87; Tcr | 61 |
| pPHL5140 | pT7-5 with 6.5-kb HindIII-EcoRI fragment from pMON5122 | This study |
| pPHL5141 | pT7-5 with 4.1-kb SalI-EcoRI fragment from PHL5122 | This study |
| pPHL5141A− | pPHL5141 with deletion in phlA | This study |
| pPHL5141B− | pPHL5141 with frameshift mutation in phlB | This study |
| pPHL5141C− | pPHL5141 with deletion in phlC | This study |
| pPHL5142A− | pPHL5141A− expressing phlCBD and missing phlA | This study |
| pPHL5142B− | pPHL5141B− expressing phlACD and missing phlB | This study |
| pPHL5142C− | pPHL5141C− expressing phlABD and missing phlC | This study |
Apr, Kmr, Rifr, and Tcr indicate resistance to ampicillin, kanamycin, rifampin, and tetracycline, respectively.
BRL, Bethesda Research Laboratory, Gaithersburg, Md.
DNA manipulations.
Plasmid DNA was isolated by the method of Birnboim and Doly (5). Restriction and ligation reactions and agarose gel electrophoresis were performed by standard methods (47) or as directed by the suppliers. DNA fragments were recovered from agarose gels by the freeze-squeeze method (56). Competent cells were prepared for transformation according to the method of Morrison (33).
Sequence analysis.
Subclones from pMON5122 and pMON5120 (Table 1) were generated by using convenient restriction sites and inserted into the cloning vector pIC19H. DNA sequences were determined at the Nucleic Acid Research Facility at Iowa State University, Ames, with an ABI automated sequencer. Oligonucleotides complementary to internal sequences were used as primers to complete the sequencing of larger fragments.
Sequence data were analyzed with the University of Wisconsin Genetics Computer Group package, version 8.0 (13). DNA sequences were compiled with GELASSEMBLE, and open reading frames (ORFs) were identified and codon usage was analyzed with MAP and FRAMES. The GenBank and EMBL databases were searched for sequence similarities by using the programs FASTA, BLAST, and MOTIFs. The PROSITE database was searched for protein domain similarities by using the ExPASy molecular biology server (1). Multiple sequences were aligned with PILEUP and PRETTYBOX (13). Kyte-Doolittle hydropathy plots were generated with PK23, a program developed by the VADMS center at Washington State University, Pullman. The significance of the similarity of a predicted protein to known proteins was determined by calculating the binary comparison score (Z score). Pairwise alignments were obtained by using the program GAP (gap weight = 3), and the resulting percent identities, percent similarities, alignment scores (A), mean random alignment scores (R), and standard deviations (SD) (n = 100) were noted. Z scores were then calculated by the equation (A − R)/SD.
RNA isolation and Northern analysis.
Total RNA was isolated with the RNeasy kit (Qiagen, Chatsworth, Calif.) from 400- or 500-μl samples of 4-ml cultures of P. fluorescens grown at 28°C with vigorous shaking. The following changes were made to the RNeasy protocol: the membrane was incubated at room temperature for 5 min after the addition of wash buffer RW1 and again after the addition of RNase-free water before centrifugation. RNA samples separated on agarose gels were transferred to BrightStar-Plus positively charged nylon membranes (Ambion, Austin, Tex.) with 7.5 mM sodium hydroxide as the transfer buffer. Probes were labeled and detected with the BrightStar nonradioactive labeling and detection system (Ambion).
Plasmid constructions.
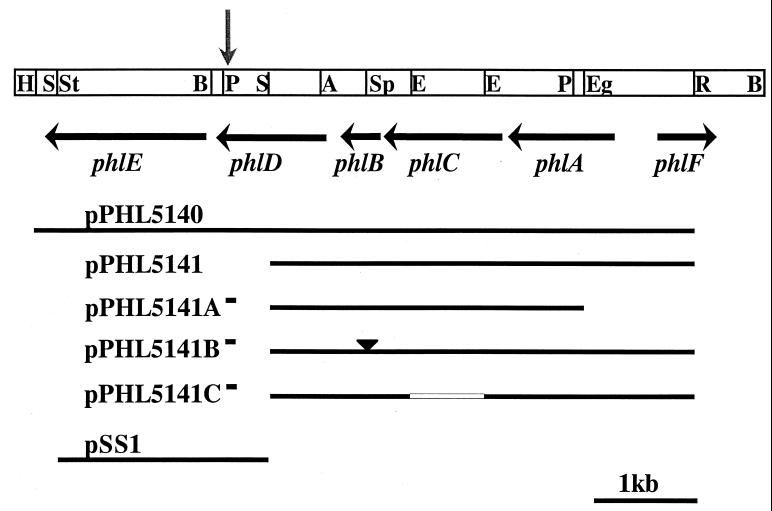
Nine plasmids were constructed for gene function analysis. Introduction of the 6.5-kb fragment from pMON5122 into pT7-5 gave pPHL5140. Plasmid pPHL5141 was created by cloning the 4.1-kb SalI-EcoRI fragment of pPHL5140 into pT7-5. Plasmid pPHL5141A− was generated by deleting the EagI-EcoRI fragment from the right end of the insert in pPHL5141. pPHL5141 was digested with SphI, the ends were filled in with the Klenow fragment of E. coli DNA polymerase, and the plasmid was religated to form pPHL5141B−. pPHL5141C− was produced by digestion of pPHL5141 with EcoRV and religation to eliminate a 0.7-kb fragment. Finally, a parallel set of three plasmids in which the 3′ end of the 7.2-kb fragment was reintroduced into the pPHL5141 derivatives was generated by a two-step cloning strategy. The SalI-StuI fragment from pPHL5140 was cloned in pIC19H to give pSS1. This fragment was then isolated as a SalI-HindIII fragment and ligated into SalI-HindIII-digested pPHL5141A−, pPHL5141B−, and pPHl5142C− to give pPHL5142A−, pPHL5142B−, and pPHl5142C−, respectively (Table 1; Fig. 1).
FIG. 1.
Genes identified in the 2,4-DAPG biosynthetic locus are indicated by black horizontal arrows. The vertical arrow indicates the site of Tn5 insertion in Q2-87::Tn5-1. Key plasmids used in assigning functions to the phl genes are shown. Construction of these plasmids is described in Materials and Methods. Restriction sites: A, AccI, B, BamHI; E, EcoRV; Eg, EagI; P, PstI; R, EcoRI; S, SalI; Sp, SphI; St, StuI.
SDS-PAGE and protein labeling.
Proteins expressed in E. coli from the nine plasmids described above were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) both with and without [35S]methionine labeling in vivo. For nonradioactive protein analysis, overnight cultures were diluted 50-fold and grown with shaking to an A600 of 0.6. Cells from 1 ml of culture were harvested and suspended in 25 to 40 μl of 1× Laemmli buffer, and a 5-μl sample was analyzed by electrophoresis on 10 or 12% acrylamide gels (47). Proteins were radiolabeled in vivo as described by Ausubel et al. (2), except that transcription from the T7 promoter was induced with 3 mM isopropyl-β-d-thiogalactoside (IPTG) for 30 min. Incubation was continued for 20 min at 42°C with rifampin (200 μg/ml), and then 10 μCi of [35S]methionine was added and incubation was continued for 5 min. Gels were stained with Coomassie brilliant blue R250 in methanol-acetic acid-water (4.5:4.5:1) for nonradioactive samples or with Coomassie brilliant blue R250 in boiling 7% acetic acid before autoradiography.
N-terminal sequencing of PhlA.
Proteins from E. coli BL21(DE3)(pPHL5141) were separated on an SDS–12% polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, Mass.) with a Mini Trans-Blot apparatus (Bio-Rad Laboratories, Hercules, Calif.). The membrane was stained with Coomassie brilliant blue R250 in methanol-acetic acid-water (4.5:4.5:1) and destained in the same solvent. After washing the membrane six times with reagent-grade water, the PhlA band was excised and the protein sequence was determined directly from the immobilized band at the Laboratory for Bioanalysis and Biotechnology at Washington State University with an Applied Biosystems 475A protein sequencer.
Extraction and detection of metabolites.
MAPG and 2,4-DAPG were extracted from 4-day-old cultures of E. coli or Pseudomonas as described previously (3). Expression of the locus in E. coli BL21(DE3) was based on the leaky lac repressor control of expression from the T7 promoter since expression in these cultures was not induced with IPTG. For analysis of the conversion of MAPG to 2,4-DAPG, MAPG was added to a concentration of 10 μg/ml to 24-h-old cultures and the cultures were incubated for 3 additional days. Metabolites were extracted as described above and analyzed by high-pressure liquid chromatography according to the method of Bonsall et al. (6).
Nucleotide sequence accession number.
The nucleotide sequence of the 7,198-bp DNA region described here is available in the EMBL, GenBank, and DDBJ data libraries under the accession no. U41818.
RESULTS
Nucleotide sequence analysis.
The nucleotide sequence of 7,198 bp of DNA including the 6.5-kb fragment from pMON5122 and about 500 bases adjacent to it from pMON5120 was determined. Six ORFs were identified with codon usage conforming to that established for Pseudomonas spp. These ORFs were designated phlA, phlC, phlB, phlD, phlE, and phlF. ORFs phlA, phlC, phlB, phlD, and phlE have a common transcriptional orientation, and phlF is oppositely oriented (Fig. 1), as predicted earlier by transcriptional analysis with Tn3HoHo1 (3). A putative ATG start codon (in boldface type) for phlA (CATTCTGGAAATG) is not preceded by a consensus ribosome binding site, but a GTG codon (in boldface type) with such a site (underlined) (GGAGGAAGTACACGTG) was identified 288 bases upstream of the ATG. Each of the other ORFs has an appropriately positioned potential ribosome binding site, except for phlE, which is preceded by a sequence rich in A and G residues (AAAGAAGGGGAAGAACATG). The characteristics of the predicted ORF products are described in Table 2.
TABLE 2.
ORFs in the 2, 4-DAPG biosynthetic cluster
| ORF | ORF length (bp) | Protein size (no. of residues) | Predicted protein mass (kDa) | Protein similar to ORF product | Accession no.a | Z score |
|---|---|---|---|---|---|---|
| phlA | 1,080 | 360 | 37.9 | ACP synthases (E. coli FabH) | P24249 | 12 |
| phlC | 1,194 | 398 | 42.5 | Mammalian SCPx (thiolase domain) | P32020 | 15 |
| phlB | 438 | 146 | 16.6 | acaC from P. furiosus | X85250 | 13 |
| phlD | 1,047 | 349 | 38.4 | CHS/STS family | S12224 | 17 |
| phlE | 1,269 | 423 | 45.2 | PMF drug efflux proteins | M80252 | 12 |
| phlF | 606 | 202 | 23.0 | Repressor proteins | X86780 | 14 |
Accession number of sequence used to calculate Z score.
The putative product of phlA shows 26% identity and 48% similarity to the 33-kDa fabH gene product β-ketoacyl-acyl carrier protein synthase III (KAS III) from E. coli (59). Similarity to KAS III enzymes from other organisms was very poor (data not shown). Further inspection revealed that PhlA lacks the active site cysteine residue (C112 of FabH). An imperfect phosphopantetheine-binding motif (IGADTINRNTAPGDL) was identified at residue 143 in which a threonine (underlined) replaces the conserved pantotheine-binding serine residue. The ORF designated phlC initiates with an ATG codon 32 bp downstream of the phlA stop codon. The predicted phlC product shows 28% identity and 50% similarity to the N-terminal thiolase domain of rat sterol carrier protein x (SCPx). PhlC carries an acetyl-coenzyme A (CoA) binding site containing the active cysteine residue as well as a glycine-rich C-terminal region conserved (36, 53) among thiolases (Fig. 2) and ketoacyl-condensing enzymes (data not shown). The ATG start codon for phlB is 11 bases downstream of phlC. No structural motifs suggestive of a function for phlB were identified. However, the predicted proteins PhlA, PhlC, and PhlB have homology with the predicted products of the aca operon from the archaebacterium Pyrococcus furiosus and with the predicted proteins MJ1545, MJ1549, and MJ1552 from Methanococcus jannaschii (7, 25). ORF phlD is separated from phlB by 155 bases and shows homology at the predicted protein sequence level to CHS/STS genes from higher plants (31% identity, 53% similarity) (Table 2). Both the active site region (for CHS, QQGC169FAG; for PhlD, QLGC138VAG) including the catalytic cysteine (underlined) (26) and a highly conserved family signature sequence (for CHS, 374VGVLFGFGPGLTVE; for PhlD, 328TGMLAAFGPGFTAE) common to CHS/STS enzymes (12) were found in PhlD. PhlD also shows similarity to three bacterial CHS homologues of unknown function (60) (GenBank accession no. L77246 and Z85982).
FIG. 2.
Important residues conserved in PhlC. (A) Alignment of cysteine 88 (∗) of PhlC with the catalytic active site cysteine of thiolases. (B) Alignment of histidine 349 (▾) and neighboring glycine residues of PhlC. Cysteine 378 (●), a key active site residue in type II thiolases, is absent from PhlC, SCPx, and AcaB. In both panels, the compared proteins are chrom_aat, Chromatium vinosum acetoacetyl-CoA thiolase (accession. no. L01112); alcal_aat, Alcaligenes eutrophus acetoacetyl-CoA thiolase (accession no. J04987); ratmit_aat, rat mitochondrial acetoacetyl-CoA thiolase (accession no. D13921); ratmit_thiol, rat mitochondrial oxoacyl-CoA thiolase (accession no. X05341); rat_pota, rat peroxisomal oxoacyl-CoA thiolase A (accession no. M32801); rat_potb, rat peroxisomal oxoacyl-CoA thiolase B (accession no. J02749); ecfada, E. coli 3-ketoacyl-CoA thiolase (accession no. P21151); caen_aat, Caenorhabditis elegans acetyl-CoA acetyltransferase (accession no. M77697); scpx, mouse sterol carrier protein 2 precursor (accession no. P32020); and acab, P. furiosus acetyl-CoA synthase. Numbers to the right of the alignment are amino acid residue numbers.
A palindromic DNA sequence (1762CCAGGGGCGGATCCGCCCCCGG) lies between phlD and phlE. PhlE is homologous (24% identity and 50% similarity) with Staphylococcus aureus NorA protein (63), a multidrug efflux transporter that belongs to the transmembrane solute facilitator superfamily of membrane permeases. PhlE retains conserved structural features of these integral membrane permeases, including a central hydrophilic loop bordered on either side by six hydrophobic α-helices (Fig. 3).
FIG. 3.
Kyte-Doolittle hydropathy plots of PhlE and S. aureus NorA protein. Both plots show central hydrophilic loops flanked by twelve hydrophobic, potential membrane spanning regions (window size = 19).
The phlF start codon was located 416 bp upstream of the start codon for phlA. The intervening region contains no ORFs, is AT-rich, and presumably contains the phlA and phlF promoters. PhlF is homologous (25% identity and 48% similarity) with a putative repressor gene in the rapamycin biosynthetic locus of Streptomyces hygroscopicus (50). Similarity with other repressor proteins, such as tetR, is concentrated within a helix-turn-helix domain (residues 34 to 64) typical of known DNA-binding regulatory proteins, as shown below. The top sequence is a consensus of helix-turn-helix domains, and X represents any amino acid (1).Consensus GYX3SIX2VX5VSXPXIYXHFXNKX2LPhlF 34 GYX3SIX2VX5ASXPXIYXWWXNKX2L 64
Northern analysis.
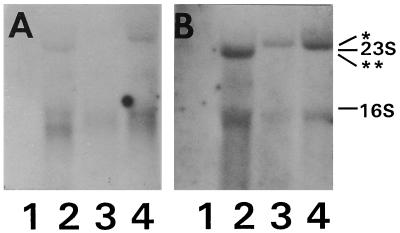
The small intergenic distances between phlA, phlC, and phlB indicated that they might form an operon. However, it was not clear whether phlD was included in this operon or was transcribed separately. To address this question, total RNA was isolated from 3- and 24-h-old cultures of Q2-87. RNA from 3-h-old cultures of Q69c-80, a 2,4-DAPG-nonproducing Pseudomonas strain, was used as a negative control. A fourth sample contained RNA from a 24-h-old culture of Q69c-80::miniTn5Phl, which includes sequences from pMON5122 on a chromosomally located mini-Tn5 transposon (24). Following electrophoresis and transfer, the RNA was hybridized either with an EcoRV fragment encompassing the central portion of phlC or with an AccI-BamHI fragment containing the entire phlD region (Fig. 4). No RNA was detected in Q69c-80 by either probe. Both probes detected RNAs of similar sizes in the 24-h Q2-87 culture; the phlC probe, which was of higher specific activity than the phlD probe, also detected this RNA in the 3-h-old culture of Q2-87 (Fig. 4). Both probes identified a slightly smaller transcript in RNA from Q69c-80::miniTn5Phl.
FIG. 4.
Northern blot analysis of phl mRNA. Total RNA was isolated at 3 h (lanes 1 and 3) and 24 h (lanes 2 and 4) from P. fluorescens Q69c-80 (lanes 1), P. fluorescens Q69c-80::miniTn5Phl (lanes 2), and P. fluorescens Q2-87 (lanes 3 and 4). The probes used were a 0.7-kb EcoRV fragment specific for phlC (A) and a 1.1-kb AccI-BamHI fragment containing the entire phlD region (B). Positions of the 23S and 16S RNAs are shown. ∗, transcript detected from wild-type Q2-87 strains; ∗∗, smaller transcript detected in Q69c-80::miniTn5Phl.
Gene function analysis.
Both MAPG and 2,4-DAPG were identified by HPLC in extracts from P. fluorescens Q2-87. Strain Q2-87::Tn5-1, which contains a transposon in phlD, produced no detectable MAPG or 2,4-DAPG. However, 2,4-DAPG was produced when the strain was provided with exogenous MAPG (Table 3), suggesting that PhlD is necessary for synthesis of MAPG and that PhlA, PhlC, and PhlB are sufficient to perform the conversion of MAPG to 2,4-DAPG. To further dissect the roles played by the different ORFs, nine plasmids were constructed in which the putative 2,4-DAPG biosynthetic genes were placed under the transcriptional control of the strong, IPTG-inducible phage T7 promoter in plasmid pT7-5. SDS-PAGE analysis helped to identify the proteins expressed by E. coli BL21(DE3) carrying these plasmids and to detect the loss of the appropriate protein on introduction of a mutation (Fig. 5).
TABLE 3.
Functional analysis of phl genes
| Strain | Metabolite detected by HPLCa
|
|||
|---|---|---|---|---|
| Exogenous MAPG | MAPGb | 2,4-DAPG | Red pigmentc | |
| Pseudomonas sp. | ||||
| Q2-87::Tn5-1 | − | − | − | − |
| Q2-87::Tn5-1 | + | NA | + | NA |
| E. coli | ||||
| pT7-5 | − | − | − | − |
| pT7-5 | + | NA | − | − |
| pPHL5140 | − | + | + | + |
| pPHL5141 | − | − | − | − |
| pPHL5141 | + | NA | + | NA |
| pPHL5141A− | − | − | − | − |
| pPHL5141A− | + | NA | − | NA |
| pPHL5141B− | − | − | − | − |
| pPHL5141B− | + | NA | − | NA |
| pPHL5141C− | − | − | − | − |
| pPHL5141C− | + | NA | − | NA |
| pPHL5142A− | − | − | − | + |
| pPHL5142B− | − | − | − | + |
| pPHL5142C− | − | − | − | + |
−, metabolite not detected; +, metabolite detected; NA, not applicable.
MAPG produced de novo by the strain.
Red pigment production was assayed on LB or KMB agar as described in reference 3.
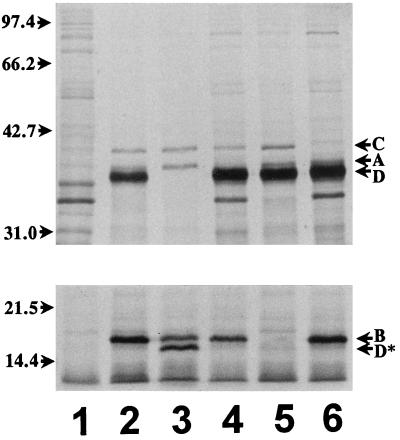
FIG. 5.
Autoradiograph of polypeptides labeled with [35S]methionine and resolved by electrophoresis in SDS-polyacrylamide gels. Samples were prepared from E. coli BL21(DE3) containing the indicated plasmids. The DNA fragments inserted in pT7-5 for gene expression are illustrated in Fig. 1. Top panel, 10% acrylamide gel; bottom panel, 12% acrylamide gel. Positions of phl gene products are indicated on the right; D∗ indicates the truncated PhlD product produced by strains expressing pPHL5141 and its derivatives. Positions of molecular size markers are given on the left in kilodaltons. Lanes: 1, pT7-5; 2, pPHL5140; 3, pPHL5141; 4, pPHL5142A−; 5, pPHL5142B−; 6, pPHL5142C−.
Plasmid pPHL5140 enabled the production of both MAPG and 2,4-DAPG. To confirm that PhlA, PhlC, and PhlB were sufficient to convert MAPG to 2,4-DAPG, pPHL5141, which contains phlA, phlC, phlB, and a truncated phlD gene encoding only the first 126 amino acid residues of PhlD, was generated. Neither MAPG nor 2,4-DAPG was detected in extracts from E. coli BL21(DE3)(pPHL5141), but MAPG added to 24-h-old cultures was converted to 2,4-DAPG. Mutations were then introduced into phlA, phlC, and phlB in pPHL5141 to determine which of them was required to carry out the conversion. pPHL5141A− lacks the ribosome binding site and the first 90 N-terminal amino acids of phlA, pPHL5141B− has a frameshift mutation in phlB, and pPHL5141C− carries a deletion in phlC. These mutations did not prevent the expression of downstream genes (Fig. 5). E. coli containing pPHL5141A−, pPHL5141B−, or pPHL5141C− failed to convert MAPG to 2,4-DAPG.
Finally, to clarify whether phlD was sufficient for the synthesis of MAPG or if phlA, phlC, or phlB also was required, phlD was reconstructed in pPHL5141A−, pPHL5141B−, and pPHL5141C− to obtain pPHL5142A−, pPHL5142B−, and pPHL5142C−. These plasmids each contain a functional phlD gene but are defective in phlA, phlB, or phlC, respectively. Cultures of E. coli BL21(DE3) expressing these plasmids produced neither MAPG nor 2,4-DAPG (Table 3), although in LB medium they exhibited a reddish color similar to that observed in uninoculated LB medium supplemented with MAPG.
Protein expression in E. coli and N-terminal sequencing of PhlA.
E. coli BL21(DE3)(pPHL5140) produced four unique proteins with relative molecular masses corresponding to those predicted for PhlA, PhlB, PhlC, and PhlD. Due to the high level of expression from the T7 promoter, all four proteins were visible on stained acrylamide gels even without radiolabeling. The same unique proteins were present in samples labeled with [35S]methionine (Fig. 5). No unique band corresponding in size to PhlE was detected by either method. PhlA and PhlD are very similar in predicted size (37.9 and 38.4 kDa, respectively) but were successfully resolved after electrophoresis in an SDS–10% polyacrylamide gel. Mutations in individual ORFs resulted in the disappearance of the corresponding protein band from the gel. PhlC was the most poorly expressed of all the proteins, while PhlB and PhlD were expressed more strongly. Strains expressing the PhlD− plasmid pPHL5141 and its derivatives produced the truncated protein PhlD*.
To identify which of the two potential start sites detected by sequence analysis of phlA was functional, the N-terminal sequence of PhlA expressed from pPHL5141 in E. coli was determined. The first eight amino acids detected, MNKVGIVS, corresponded to the predicted sequence from the GTG start site.
DISCUSSION
Six genes, designated phlA, phlC, phlB, phlD, phlE, and phlF, were identified in a 7.2-kb DNA fragment from P. fluorescens Q2-87. Each of these exhibited significant homology (Z scores > 9) with other known genes, indicating common evolutionary origins and suggesting mechanisms for the regulation, synthesis, and export of DAPG.
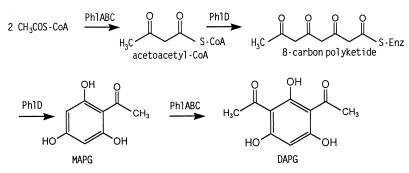
PhlD is essential for MAPG synthesis (Fig. 6). In both P. fluorescens Q2-87 and in E. coli expressing phl genes, MAPG was synthesized only in the presence of PhlD; in its absence, the cells converted exogenous MAPG to 2,4-DAPG but were unable to produce either compound themselves. The homology between PhlD and CHS/STS enzymes from plants is surprising, as until now all the microbial polyketide antibiotics have been found to be synthesized via type I or type II PKSs (10, 20, 22, 31, 35, 46). The structural similarities between PhlD and members of the CHS/STS family point to a common evolutionary origin, and the functional parallels in the roles they play in plant defense provoke speculation as to possible instances of gene exchange between plants and their bacterial colonists (4, 9).
FIG. 6.
Proposed roles of PhlA, PhlB, PhlC, and PhlD in the biosynthesis of MAPG and 2,4-DAPG. Enz, enzyme.
Unlike the multidomain (type I) and multiprotein (type II) PKSs, CHS/STS enzymes consist simply of a homodimer of 42-kDa subunits sufficient to catalyze the three condensation reactions, the cyclization reaction, and, for the STSs, the decarboxylation reaction required to generate their particular products (58; reviewed in references 30 and 48). The recruitment of such a unique enzyme for 2,4-DAPG production by strain Q2-87 suggests that other features characteristic of the CHS/STS biosynthetic mechanism might be present as well. For example, these enzymes differ from type I and type II PKSs in lacking an acyl carrier protein component; instead, they utilize CoA esters of carboxylic acids as a source of extender units. This preference may explain the absence from the phl operon of an acyl carrier protein gene comparable to those found in type II PKSs. Further, CHS/STS enzymes (as far as is known) accept only malonyl-CoA as extender units, unlike type I enzyme complexes, which can incorporate branched acyl moieties. This would mandate that 2,4-DAPG be synthesized via MAPG rather than directly by condensation of other acyl-CoA substrates. Finally, although the natural substrate of CHS/STS enzymes is 4-coumaroyl-CoA, they exhibit broad substrate specificity in vitro, producing acylphloroglucinol compounds from butyryl-CoA and hexanoyl-CoA primer molecules (19, 49). By analogy, we postulate that PhlD catalyzes the condensation of a linear primer molecule, probably acetoacetyl-CoA.
That the products of phlA, phlC, and phlB function collectively is supported both by the fact that mutations in any one of the three genes give rise to a common phenotype and by conservation of a similar gene cluster in the archaebacterium P. furiosus (25). PhlA, PhlC and PhlB appear to have a dual role in 2,4-DAPG synthesis (Fig. 6). First, all three are necessary and sufficient for the conversion of MAPG to 2,4-DAPG. This acetylation reaction corresponds to an MAPG acetyltransferase activity described previously in cell extracts of Pseudomonas sp. strain F113 (52). Second, they also appear to be required for the synthesis of MAPG, perhaps by catalyzing a condensation reaction needed to provide the primer unit for PhlD.
Sequence analysis gives only limited insight into the functions of PhlA and PhlB. PhlB lacks both recognizable motifs and similarity with other known proteins, and although PhlA is homologous with FabH from E. coli, it contains an imperfect phosphopantetheine-binding motif with a threonine residue in place of the serine through which the prosthetic group invariably is esterified. This motif is unlikely to be functional, as a similar substitution in a spinach acyl carrier protein I did not bind the phosphopantetheine group (21). PhlA also lacks the essential cysteine present in the active site region of condensing enzymes. It is significant that such cysteine-deficient ketosynthase homologues are integral to the basic architecture of bacterial type II PKSs, where they help to determine the chain length of the poly-β-ketone intermediate prior to cyclization (20). Whether PhlA functions as a chain length factor, perhaps through interactions with PhlD, remains to be determined.
Only PhlC contains structural features typical of condensing enzymes, and these features are consistent with roles in both primer unit synthesis and MAPG acetylation. For example, cysteine-88 of PhlC aligns with the catalytic cysteine in the active site of thiolases (Fig. 2A) and condensing enzymes and is the likely binding site for acetyl-CoA prior to the reaction leading to synthesis of acetoacetyl-CoA, the putative MAPG primer unit. A second conserved region includes the sequence 346GHASGCDG (Fig. 2B). It is noteworthy that this histidine residue is the only one conserved among condensing enzymes and thiolases (53). We speculate that in PhlC, it may form part of the binding site for a malonyl thioester, the extender unit in biosynthetic condensation reactions except those catalyzed by type II thiolases that use acetyl-CoA to generate acetoacetyl-CoA. Although we predict that PhlC generates acetoacetyl-CoA, as do type II thiolases, PhlC differs from the latter in lacking an equivalent to cysteine 378, a key active site residue (37) (Fig. 2B), and it therefore is unlikely that PhlC uses acetyl-CoA as an extender unit. It is curious that PhlC most closely resembles the thiolase domain of SCPx, the product of an apparent fusion of genes for thiolase and a small mammalian protein important in the intracellular transport of lipids and sterols, particularly cholesterol (36, 41, 51). Similarity between the SCPx thiolase domain and PhlC extends over the entire length of the two sequences, rather than only in the key catalytic regions, suggesting that the two proteins may have common conformational features that could facilitate an interaction between PhlC and MAPG in a manner analogous to that between SCPx and its sterol ligands.
PhlE retains structural features of integral membrane permeases including the archetypal tetracycline-H+ antiporters (34, 38, 39) and transport proteins associated with resistance and encoded within the biosynthetic clusters for polyketide antibiotics (11, 14). That PhlE functions in the export of 2,4-DAPG and/or MAPG is supported by an earlier observation (3) that certain Tn3HoHo1 insertions in phlE were correlated with reduced 2,4-DAPG production and loss of the red pigment characteristic of phloroglucinol compounds. These insertions were interspersed with others that had no effect on pigmentation, suggesting that PhlE has multiple functional domains which may correspond to the hydrophilic and transmembrane segments predicted by sequence analysis. No protein corresponding to PhlE was detected in E. coli expressing phlACBD under the control of a T7 promoter, indicating that the two are likely to be separately transcribed and that a 22-base palindrome located between phlD and phlE functions as a transcriptional terminator.
The product of the divergently transcribed phlF gene at the 5′ end of the 2,4-DAPG biosynthetic operon contains a helix-turn-helix motif strongly predictive of DNA-binding activity and similar to that of well-characterized repressor genes, including members of the TetR family. Two additional observations further support the hypothesis that PhlF is a negative regulator of 2,4-DAPG biosynthesis. First, whereas pMON5122 (which lacks the 73 codons at the 3′ end of phlF) transferred 2,4-DAPG biosynthetic capability to previously nonproducing strains of Pseudomonas, larger cloned segments containing flanking sequences that included the intact phlF gene did not. This previously led us to postulate that negative regulatory elements might reside upstream of the 2,4-DAPG locus (3). Secondly, derivatives of P. fluorescens Q69c-80 containing a single chromosomal copy of phlACBDE and the 3′-truncated phlF gene from pMON5122 produced up to several hundred-fold more 2,4-DAPG than did wild-type Q2-87 (24). Similar truncation of Tn10 and class E tetR tetracycline repressor proteins derepresses expression of the tetracycline resistance protein (17, 29). Relatively few repressor genes linked to antibiotic biosynthesis loci have been reported, and while some of these are implicated as pathway-specific negative regulators of antibiotic production (8, 62), others control the expression of adjacent resistance genes (11, 14) or have undefined functions (50). Whether PhlF is involved in the regulation of phlE expression remains to be seen.
The phl genes from P. fluorescens Q2-87 define a unique polyketide biosynthetic pathway (Fig. 6) that is conserved among plant-associated fluorescent pseudomonads (23, 45). The relative simplicity of the locus differs markedly from those of type I and type II PKSs described previously, although at least some of the biosynthetic reactions involved are likely to be mechanistically similar. It is especially curious that homologues of phlA, phlC, and phlB are found in archaebacterial genomes but not (at least until now) in eubacteria, whereas phlD homologues are present in the eubacterial genomes of Bacillus and Mycobacterium as well as in plants. This unusual assemblage of phl genes, apparently from different sources, may have arisen due to the nature of the reaction being catalyzed. Plant CHSs use aromatic products of the phenylpropanoid pathway as primers, and the requirement in bacteria for a simpler, possibly linear primer molecule may have led to the recruitment of the other three genes in the operon. To our knowledge this is the only report of such a combination of genes for the synthesis of a polyketide antibiotic. The requirement of all four proteins for the synthesis of MAPG and of PhlA, PhlC, and PhlB for the conversion of MAPG to 2,4-DAPG suggests that these proteins function as a complex to facilitate substrate transfer reactions.
ACKNOWLEDGMENTS
M. G. Bangera gratefully acknowledges the receipt of a grant from the Storkan/Hanes Research Foundation. This work was supported by grant 96-35303-3242 from the U.S. Department of Agriculture, Office of Grants and Program Systems, National Research Initiative, Competitive Grants Program.
We thank David Weller for providing Pseudomonas strains and Penny von Wettstein-Knowles, Mads Siggaard-Andersen, and Andrea Gargas for critical review of the manuscript. We thank Steve Thompson for help with sequence analysis, Robert Bonsall for assistance with HPLC analysis and Dmitri Mavrodi for technical advice.
REFERENCES
- 1.Appel R D, Bairoch A, Hochstrasser D F. A new generation of information retrieval tools for biologists: the example of the ExPASy WWW server. Trends Biochem Sci. 1994;19:258–260. doi: 10.1016/0968-0004(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Short protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1992. pp. 16.8–16.9. [Google Scholar]
- 3.Bangera M G, Thomashow L S. Characterization of a genomic locus required for synthesis of the antibiotic 2,4-diacetylphloroglucinol by the biological control agent Pseudomonas fluorescens Q2-87. Mol Plant-Microbe Interact. 1996;9:83–90. doi: 10.1094/mpmi-9-0083. [DOI] [PubMed] [Google Scholar]
- 4.Bangera M G, Weller D M, Thomashow L S. Genetic analysis of the 2,4-diacetylphloroglucinol biosynthetic locus from Pseudomonas fluorescens Q2-87, 383–386. In: Daniels M J, Downie J A, Osbourn A E, editors. Advances in molecular genetics of plant-microbe interactions. Vol. 3. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. [Google Scholar]
- 5.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonsall R F, Weller D M, Thomashow L S. Quantification of 2,4-diacetylphloroglucinol produced by fluorescent Pseudomonas spp. in vitro and in the rhizosphere of wheat. Appl Environ Microbiol. 1997;63:951–955. doi: 10.1128/aem.63.3.951-955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bult C J, White O, Olsen G J, Zhou L, Fleischmann R D, Sutton G G, Blake J A, Fitzgerald L M, Clayton R A, Gocayne J D, Kerlavage A R, Dougherty B A, Tomb J-F, Adams M D, Reich C I, Overbeek R, Kirkness E F, Weinstock K G, Merrick J M, Glodek A, Scott J L, Geoghagen N S M, Weidman J F, Fuhrmann J L, Nguyen D, Utterback T R, Kelley J M, Peterson J D, Sadow P W, Hanna M C, Cotton M D, Roberts K M, Hurst M A, Kaine B P, Borodovsky M, Klenk H-P, Fraser C M, Smith H O, Woese C R, Venter J C. Complete genome sequence of the methanogenic archaeon, Methanococcus jannaschii. Science. 1996;273:1058–1073. doi: 10.1126/science.273.5278.1058. [DOI] [PubMed] [Google Scholar]
- 8.Chater K F, Bibb M J. Regulation of bacterial antibiotic production. In: Kleinkauf H, von Döhren H, editors. Biotechnology. 6. Products of secondary metabolism. Weinheim, Germany: VCH; 1997. pp. 57–105. [Google Scholar]
- 9.Cook R J, Thomashow L S, Weller D M, Fujimoto D, Mazzola M, Bangera G, Kim D-S. Molecular mechanisms of defense by rhizobacteria against root disease. Proc Natl Acad Sci USA. 1995;92:4197–4201. doi: 10.1073/pnas.92.10.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donadio S, Staverm M J, McAlpine J B, Swanson S J, Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991;252:675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Moreno M A, Caballero J L, Hopwood D A, Malpartida F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA of Streptomyces. Cell. 1991;66:769–780. doi: 10.1016/0092-8674(91)90120-n. [DOI] [PubMed] [Google Scholar]
- 12.Fliegmann J, Schröder G, Schanz S, Britsch L, Schröder J. Molecular analysis of chalcone and dihydropinosylvin synthase from Scots pine (Pinus sylvestris) and differential regulation of these and related enzyme activities in stressed plants. Plant Mol Biol. 1992;18:489–503. doi: 10.1007/BF00040665. [DOI] [PubMed] [Google Scholar]
- 13.Genetics Computer Group. Program manual for the Wisconsin package, version 8. Madison, Wis: Genetics Computer Group; 1994. [Google Scholar]
- 14.Guilfoile P G, Hutchinson C R. Sequence and transcriptional analysis of the Streptomyces glaucescens tcmAR tetracenomycin C resistance and repressor gene loci. J Bacteriol. 1992;174:3651–3658. doi: 10.1128/jb.174.11.3651-3658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamdan H, Weller D M, Thomashow L S. Relative importance of fluorescent siderophores and other factors in biological control of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2-79 and M4-80R. Appl Environ Microbiol. 1991;57:3270–3277. doi: 10.1128/aem.57.11.3270-3277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison L A, Letendre L, Kovacevich P, Pierson E A, Weller D M. Purification of an antibiotic effective against Gaeumannomyces graminis var. tritici produced by a biocontrol agent, Pseudomonas aureofaciens. Soil Biol Biochem. 1993;25:215–221. [Google Scholar]
- 17.Heuer C, Hickman R K, Curiale M S, Hillen W, Levy S B. Constitutive expression of tetracycline resistance mediated by a Tn10-like element in Haemophilus parainfluenzae results from a mutation in the repressor gene. J Bacteriol. 1987;169:990–994. doi: 10.1128/jb.169.3.990-994.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopwood D A, Sherman D H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- 19.Hrazdina G, Kreuzaler F, Halbrock K, Grisebach H. Substrate specificity of flavonone synthetase from cell suspension cultures of parsley and structure of release products in vitro. Arch Biochem Biophys. 1976;175:392–399. doi: 10.1016/0003-9861(76)90526-9. [DOI] [PubMed] [Google Scholar]
- 20.Hutchinson C R, Fujii I. Polyketide synthase gene manipulation: a structure-function approach in engineering novel antibiotics. Annu Rev Microbiol. 1995;49:201–238. doi: 10.1146/annurev.mi.49.100195.001221. [DOI] [PubMed] [Google Scholar]
- 21.Jaworski J G, Post-Beittenmiller M A, Ohlrogge J B. Site-directed mutagenesis of the spinach acyl carrier protein-I prosthetic group attachment site. Eur J Biochem. 1989;184:603–609. doi: 10.1111/j.1432-1033.1989.tb15056.x. [DOI] [PubMed] [Google Scholar]
- 22.Katz L, Donadio S. Polyketide synthesis: prospects for hybrid antibiotics. Annu Rev Microbiol. 1993;47:875–912. doi: 10.1146/annurev.mi.47.100193.004303. [DOI] [PubMed] [Google Scholar]
- 23.Keel C, Weller D M, Natsch A, Défago G, Cook R J, Thomashow L S. Conservation of the 2,4-diacetylphloroglucinol biosynthesis locus among fluorescent Pseudomonas strains from diverse geographic locations. Appl Environ Microbiol. 1996;62:552–563. doi: 10.1128/aem.62.2.552-563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D-S, Bonsall R F, Thomashow L S, Weller D M. Construction of transgenic Pseudomonas fluorescens Q69c-80 for improved biocontrol activity to take-all. Phytopathology. 1995;85:1146. [Google Scholar]
- 25.Kletzin A, Adams M W W. Molecular and phylogenetic characterization of pyruvate and 2-ketoisovalerate ferredoxin oxidoreductases from Pyrococcus furiosus and pyruvate ferredoxin oxidoreductase from Thermotoga maritima. J Bacteriol. 1996;178:248–257. doi: 10.1128/jb.178.1.248-257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanz T, Tropf S, Marner F-J, Schröder J, Schröder G. The role of cysteines in polyketide synthases. J Biol Chem. 1991;266:9971–9976. [PubMed] [Google Scholar]
- 27.Liyanage H, Palmer D A, Ullrich U, Bender C L. Characterization and transcriptional analysis of the gene cluster for coronafacic acid, the polyketide component of the phytotoxin coronatine. Appl Environ Microbiol. 1995;61:3843–3848. doi: 10.1128/aem.61.11.3843-3848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh J L, Erfle M, Wykes E J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984;32:481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- 29.Marshall B, Morrissey S, Flynn P, Levy S B. A new tetracycline-resistance determinant, class E, isolated from Enterobacteriaceae. Gene. 1986;50:111–117. doi: 10.1016/0378-1119(86)90315-x. [DOI] [PubMed] [Google Scholar]
- 30.Martin C R. Structure, function, and regulation of chalcone synthase. Int Rev Cytol. 1993;147:233–283. doi: 10.1016/s0074-7696(08)60770-6. [DOI] [PubMed] [Google Scholar]
- 31.McDaniel R, Ebert-Khosla S, Hopwood D A, Khosla C. Engineered biosynthesis of novel polyketides. Science. 1993;262:1546–1550. doi: 10.1126/science.8248802. [DOI] [PubMed] [Google Scholar]
- 32.Moore J T, Uppal A, Maley F, Maley G F. Overcoming inclusion body formation in a high-level expression system. Protein Expr Purif. 1993;4:160–163. doi: 10.1006/prep.1993.1022. [DOI] [PubMed] [Google Scholar]
- 33.Morrison D A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- 34.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and drug efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 35.Nowak-Thompson B, Gould S J, Loper J E. Identification and sequence analysis of the genes encoding a polyketide synthase required for pyoluteorin biosynthesis in Pseudomonas fluorescens Pf-5. Gene. 1997;204:17–24. doi: 10.1016/s0378-1119(97)00501-5. [DOI] [PubMed] [Google Scholar]
- 36.Ossendorp B C, van Heusden G P H, de Beer L J, Bos K, Schouten G L, Wirtz K W A. Identification of the cDNA clone which encodes the 58-kDa protein containing the amino acid sequence of rat liver non-specific lipid-transfer protein (sterol-carrier protein 2) Eur J Biochem. 1991;201:233–239. doi: 10.1111/j.1432-1033.1991.tb16279.x. [DOI] [PubMed] [Google Scholar]
- 37.Palmer M A J, Differding E, Gamboni R, Williams S F, Peoples O P, Walsh C T, Sinskey A J, Masamune S. Biosynthetic thiolase from Zooglea ramigera: evidence for a mechanism involving cys-378 as the active site base. J Biol Chem. 1991;266:8369–8375. [PubMed] [Google Scholar]
- 38.Pao S S, Paulsen I T, Saier M H., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux pumps. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penfold C N, Bender C L, Turner J G. Characterisation of genes involved in biosynthesis of coronafacic acid, the polyketide component of the phytotoxin coronatine. Gene. 1996;183:167–172. doi: 10.1016/s0378-1119(96)00550-1. [DOI] [PubMed] [Google Scholar]
- 41.Pfeiffer S M, Furth E E, Ohba T, Chang Y J, Rennert H, Sakuragi N, Billheimer J T, Strauss J F., III Sterol carrier protein 2: a role in steroid hormone synthesis? J Steroid Biochem. 1993;47:167–172. doi: 10.1016/0960-0760(93)90071-4. [DOI] [PubMed] [Google Scholar]
- 42.Pierson E A, Weller D M. Use of mixtures of fluorescent pseudomonads to suppress take-all and improve the growth of wheat. Phytopathology. 1994;84:940–947. [Google Scholar]
- 43.Raaijmakers, J. M., R. F. Bonsall, and D. M. Weller. Effect of population density of Pseudomonas fluorescens on production of 2,4-diacetylphloroglucinol in the rhizosphere of wheat. Phytopathology, in press. [DOI] [PubMed]
- 44.Raaijmakers J M, Weller D M. Natural plant protection by 2,4-diacetylphloroglucinol-producing Pseudomonas spp. in take-all decline soils. Mol Plant-Microbe Interact. 1998;11:144–152. [Google Scholar]
- 45.Raaijmakers J M, Weller D M, Thomashow L S. Frequency of antibiotic producing Pseudomonas spp. in natural environments. Appl Environ Microbiol. 1997;63:881–887. doi: 10.1128/aem.63.3.881-887.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rangaswamy V, Mitchell R, Ullrich M, Bender C. Analysis of genes involved in biosynthesis of coronafacic acid, the polyketide component of the phytotoxin coronatine. J Bacteriol. 1998;180:3330–3338. doi: 10.1128/jb.180.13.3330-3338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring HarborNew York, N.Y.: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 48.Schröder J, Schröder G. Stilbene and chalcone synthases: related enzymes with key functions in plant-specific pathways. Z Naturforsch Sect C. 1990;45:1–8. doi: 10.1515/znc-1990-1-202. [DOI] [PubMed] [Google Scholar]
- 49.Schüz R, Heller W, Hahlbrock K. Substrate specificity of chalcone synthase from Petroselinum hortense. J Biol Chem. 1983;58:6730–6734. [PubMed] [Google Scholar]
- 50.Schwecke T, Aparicio J F, Molnar I, König A, Khaw L E, Haydock S F, Oliynyk M, Caffrey P, Cortés J, Lester J B, Böhm G, Staunton J, Leadlay P F. The biosynthetic gene cluster for the polyketide immunosuppressant rapamycin. Proc Natl Acad Sci USA. 1995;92:7839–7843. doi: 10.1073/pnas.92.17.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seedorf U, Brysch P, Engel T, Schrag K, Assmann G. Sterol carrier protein X is peroxisomal 3-oxoacyl coenzyme A thiolase with intrinsic sterol carrier and lipid transfer activity. J Biol Chem. 1994;269:21277–21283. [PubMed] [Google Scholar]
- 52.Shanahan P, Glennon J D, Crowley J J, Donnelly D F, O’Gara F. Liquid chromatographic assay of microbially derived phloroglucinol antibiotics for establishing the biosynthetic route to production, and the factors affecting their regulation. Anal Chim Acta. 1993;272:271–277. [Google Scholar]
- 53.Siggaard-Andersen M. Conserved residues in condensing enzyme domains of fatty acid synthases and related sequences. Protein Seq Data Anal. 1993;3:325–335. [Google Scholar]
- 54.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of the T7 RNA polymerase to direct the expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 55.Tabor S, Richardson C C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA. 1985;82:1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tautz D, Renz M. An optimized freeze-squeeze method for the recovery of DNA fragments from agarose gels. Anal Biochem. 1983;132:14–19. doi: 10.1016/0003-2697(83)90419-0. [DOI] [PubMed] [Google Scholar]
- 57.Thomashow L S, Weller D M. Current concepts in the use of introduced bacteria for biological disease control: mechanisms and antifungal metabolites. In: Stacey G, Keen N, editors. Plant-Microbe Interactions. Vol. 1. New York, N.Y: Chapman & Hall; 1995. pp. 187–235. [Google Scholar]
- 58.Tropf S, Kärcher B, Schröder G, Schröder J. Reaction mechanisms of homodimeric plant polyketide synthases (stilbene and chalcone synthase) J Biol Chem. 1995;270:7922–7928. doi: 10.1074/jbc.270.14.7922. [DOI] [PubMed] [Google Scholar]
- 59.Tsay J-T, Ob W, Larson T J, Jackowski S, Rock C O. Isolation and characterization of the β-ketoacyl-acyl carrier protein synthase gene (fabH) from Escherichia coli K-12. J Biol Chem. 1992;267:6807–6814. [PubMed] [Google Scholar]
- 60.Ueda K, Kim K-M, Beppu T, Horinouchi S. Overexpression of a gene cluster encoding a chalcone synthase-like protein confers redbrown pigment production in Streptomyces griseus. J Antibiot. 1995;48:638–646. doi: 10.7164/antibiotics.48.638. [DOI] [PubMed] [Google Scholar]
- 61.Vincent M N, Harrison L A, Brackin J M, Kovacevich P A, Mukerji P, Weller D M, Pierson E A. Genetic analysis of the antifungal activity of a soilborne Pseudomonas aureofaciens strain. Appl Environ Microbiol. 1991;57:2928–2934. doi: 10.1128/aem.57.10.2928-2934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang K, Han L, Vining L C. Regulation of jadomycin B production in Streptomyces venezuelae ISP5230: involvement of a repressor gene, jadR2. J Bacteriol. 1995;177:6111–6117. doi: 10.1128/jb.177.21.6111-6117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida H, Bogaki M, Nakamura S, Ubukata K, Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990;172:6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]