Abstract
Background
Immune checkpoint proteins have not been fully examined in follicular cell-derived thyroid carcinoma and medullary thyroid carcinoma (MTC). Anaplastic thyroid carcinoma (ATC) is one of the most aggressive carcinomas. Even multimodal treatment does not result in favorable clinical outcomes for patients with ATC. Anti-tumor immunity has therefore been highlighted as having therapeutic promise for ATC.
Methods
We examined a novel immune checkpoint receptor, T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif domains (TIGIT), in variable thyroid lesions: adenomatous goiter, follicular adenoma, and thyroid carcinoma (TC) using immunohistochemistry (IHC).
Results
Our IHC results showed that TIGIT expression was detected in cancer cells of MTC and high-grade TC: poorly differentiated thyroid carcinoma (PDTC) and ATC. Neoplastic cells were positive for TIGIT in four of five MTCs (80.0%), 17 of 31 ATCs (54.8%) and in 3 of 12 PDTCs (25.0%). TIGIT was not detected in any adenomatous goiters, thyroid benign tumors, or differentiated thyroid carcinoma (DTCs). Intriguingly, ATC cells showing pleomorphic/giant cell features were positive for TIGIT, while ATC cells with other cell morphologies lacked the immunoreactivity. Intra-tumoral immune cell was inclined to be enriched in TIGI-positive ATC. Although coexisting papillary thyroid carcinoma (PTC) components demonstrated high-grade microscopic features, neither the PTC nor follicular thyroid carcinoma (FTC) components expressed TIGT in any composite ATCs.
Conclusion
TIGIT was immunohistochemically found in MTC with high frequency and partially in high-grade TC. TIGIT expression in cancer cells may be beneficial for a potential utility in MTC and a subset of high-grade TC, especially ATC therapy.
Keywords: TIGIT, Anaplastic thyroid carcinoma, Poorly differentiated thyroid carcinoma, Medullary thyroid carcinoma, Immunohistochemistry
Introduction
Anaplastic thyroid carcinomas (ATCs) account for up to 2% of thyroid carcinomas (TCs) and are highly malignant epithelial tumors that result in poor patient clinical outcomes [1]. Sufficient efficacy has not been obtained despite multimodal therapeutic strategies for ATC [2]. Differentiated thyroid carcinoma (DTC) includes papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC), and comprises almost all thyroid cancers. In contrast to ATC, the prognosis of patient with DTC is greatly favorable. Poorly differentiated thyroid carcinoma (PDTC) is morphologically and behaviorally intermediate between DTC and ATC [3]. Medullary thyroid carcinoma (MTC) is a rare C-cell derived neuroendocrine tumor producing calcitonin. It is clinically diagnosed at more advanced stage and behaves more aggressively than DTC [4]. Generally, ATC and PDTC are recognized as high-risk thyroid carcinomas (TCs).
Extremely poor prognosis necessitates novel therapeutic approaches for ATC. Anti-tumor immune checkpoint proteins, such as PD-1/PDL1 and CTLA-4, are targets for cancer immunotherapy and have been examined in various malignant tumors. Twelve patients with progressive ATC were treated with kinase inhibitor plus pembrolizumab [5]. Salvage pembrolizumab therapy improved progression free survival (PFS) and overall survival (OS). Clinical trial using spartalizumab, a humanized monoclonal antibody against PD-1, started as part of a phase I/II cohort study in patients with advanced/metastatic solid tumor [6]. Nineteen percent of the patients responded to the therapy. The response rate was significantly higher in patients with PD-L1-positive ATC than negative, regardless of BRAF mutation status in ATC. More recently, Hatashima et al. confirmed clinical efficacy of pembrolizumab or nivolumab in a subset of patients with locally advanced and metastatic unresectable ATC [7].
T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif domains (TIGIT) has recently been recognized as a novel immune checkpoint receptor [8]. It binds to CD155, its ligand, and is a co-inhibitory transmembrane glycoprotein belonging to poliovirus receptor (PVR)/-nectin superfamily. TIGIT negatively regulates effector T cell activity and leads to immune tolerance for tumor cell proliferation and expansion. It has been shown to be expressed on active T cells and natural killer cells (NK cells), including tumor cells [9].
ATC generally occurs via multiple steps as consequence of the accumulation of variable genetic and epigenetic alterations. This highly aggressive carcinoma has a wide variety of characteristic microscopic features [10]. An inflammatory background is common in ATC compared to other histological subtypes of TCs. Heavy infiltration of macrophages is usually encountered in ATC, and may facilitate tumor progression [11, 12]. Based upon these genetic and morphological characteristics, an ATC-specific immune microenvironmental milieu may contribute to immune escape from cytotoxic activity and facilitate ACT cell growth.
In TCs, the representative immune checkpoint proteins, PD-1/PD-L1, have been intensively investigated in numerous previous reports [2, 13–15]. According to these previous reports, ATCs highly express PD-1/PD-L1, implying that these molecules are candidate therapeutic targets. Giannini et al. reported that expression of inhibitory immune checkpoint mediators including TIGIT was significantly high in ATCs at mRNA level [16]. Nonetheless, little is known about other immune checkpoint proteins in TCs. Therefore, we examined TIGIT expression in TCs with immunohistochemistry (IHC) using formalin-fixed paraffin-embedded tissues.
Methods
Human thyroid tissues
We examined a total of 105 surgically resected specimens and/or biopsies for thyroid nodules. The specimens consisted of seven adenomatous goiters (AGs), 13 follicular thyroid adenomas (FTAs), five medullary thyroid carcinomas (MTCs), 11 follicular thyroid carcinomas (FTCs), 28 papillary thyroid carcinomas (PTCs), 12 PDTCs, and 31 ATCs. All PDTC, ATC, and MTC samples were obtained from patients who underwent surgery or biopsy at Ito Hospital. The other thyroid tissues were surgical material from Tokyo Women’s Medical University Yachiyo Medical Center (TYMC). Tumor stages were adopted from the TMN Classification of Malignant Tumours, 8th Edition [17].
All excised materials and biopsies were routinely processed and embedded in paraffin blocks. Sections were cut 4 µm thick and then stained with hematoxylin and eosin for routine pathological diagnosis. After microscopic observation, we selected paraffin blocks that included the maximum cut surface of thyroid nodules for immunohistochemistry. Each pathological diagnosis was made based upon histopathological findings and immunohistochemical results according to the World Health Organization classification of tumours of endocrine organs [3] and recent review [10]. In particular, diagnoses of PDTC were established stringently depending upon the Turin proposal of 2007 [18]. Immunohistochemical examinations, including PAX8, were performed and all results of PDTCs were consistent with those previously reported [19]. All samples were evaluated independently by experienced pathologists (T. N. and R. K.).
Immunohistochemistry
Four-micrometer-thick sections were cut from the paraffin blocks, deparaffinized, and then pretreated with EDTA-buffer at pH 9.0 for 20 min. The sections were incubated with rabbit monoclonal antibody against TIGIT (catalog no. ab243903, Abcam, Cambridge, UK) according to the manufacturer’s instruction. Simultaneously, some ATC tissues were incubated with pancytokeratin AE1/AE3 (Dako, Carpinteria, CA) on their corresponding serial sections. We used 3,3’-diaminobenzidine tetrahydrochloride (Sigma, St Louis, MO, USA) for specific immunostaining, and finally counterstained with hematoxylin. The total staining process was carried out on a Leica BOND-MAX immunostainer (Leica Biosystems, Newcastle, UK). Tonsil tissue was used as a positive control, and an additional section from the corresponding block without primary antibody was used as a negative control for TIGIT. The same two pathologists also reviewed the positive and negative controls.
Evaluation of immunohistochemistry
We considered cells with membranous and/or cytoplasmic immunoreactivity for TIGIT to be positive, and semi-quantitatively analyzed each case by determining the percentage of positive epithelial cells. Each case was categorized into three groups: 0) less than 1%, 1 +) 1–49%, and 2 +) more than 50%.
Statistical analysis
We used χ2 tests to compare the frequencies at which differentiated TCs (PTCs and FTCs) and ATCs had TIGIT-positive cancer cells. The frequencies of differentiated TCs and high-grade TCs (PDTCs and ATCs) were also compared using the same method (Table 1). Significance was set at P < 0.05. Data analysis was performed with SPSS version 11.0 for Windows (SPSS, Tokyo, Japan).
Table 1.
Summary of TIGIT expression in epithelial component of adenomatous goiters and thyroid tumors
| histological subtype | number of case (n) | positive cases (%) |
|---|---|---|
| adenomatous goiter | 7 | 0 |
| follicular adenoma | 13 | 0 |
| medullary carcinoma | 5 | 4 (80.0%) |
| follicular carcinoma | 11 | 0*† |
| papillary carcinoma | 26 | 0*† |
| poorly differentiated carinoma | 12 | 3 (25.0%)† |
| anaplastic carcinoma | 31 | 17 (54.8%)*† |
*P < 0.05 (papillary carcinoma and follicular carcinoma versus anaplastic carcinoma)
†P < 0.05 (papillary carcinoma and follicular carcinoma versus poorly differentiated carcinoma and anaplastic carcinoma)
Results
TIGIT in non-neoplastic thyroid tissues
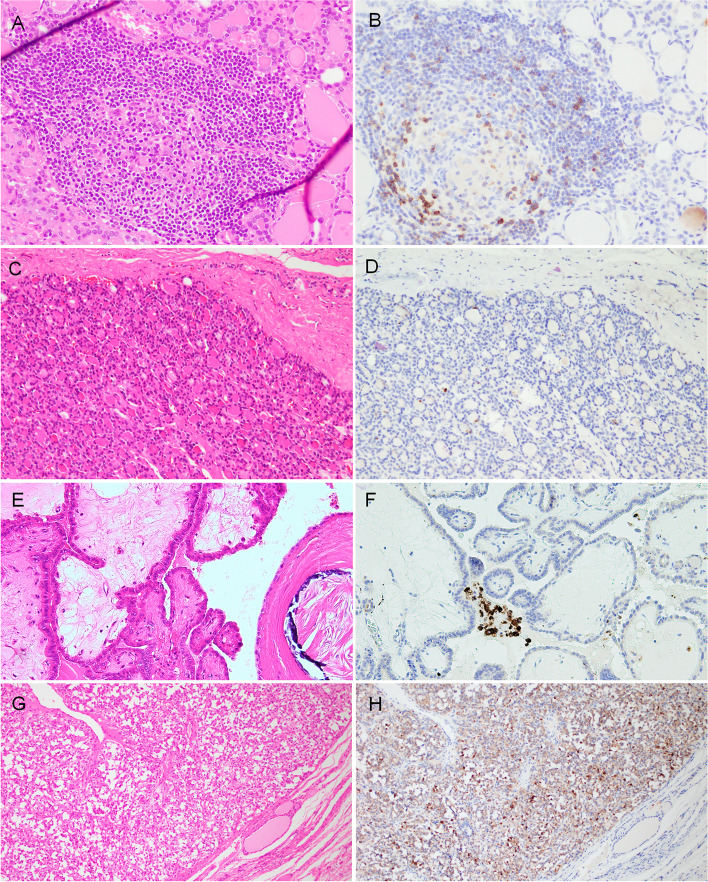
Macrophages were strongly and diffusely positive in lymphoid follicles of non-neoplastic thyroid tissue. Small-sized lymphocytes were sparsely positive. Non-neoplastic follicular cells including adenomatous goiter completely lacked immunoreactivity for TIGIT (Figs. 1A and 1B).
Fig. 1.
Representative microphotographs of TIGIT immunostaining in non-neoplastic and neoplastic thyroid tissues. Non-neoplastic thyroid tissue (H&E staining) (A). Macrophages were diffusely positive for TIGIT. TIGIT-positive small lymphocytes were scattered in the non-neoplastic thyroid tissue (B). Follicular thyroid adenoma (H&E) (C). The tumor cells lacked immunoreactivity for TIGIT (D). Papillary thyroid carcinoma (H&E) (E). These neoplastic cells were negative for TIGIT, while the macrophages were positive (F). Medullary thyroid carcinoma (H&E) (G). Tumor cells were diffusely positive for TIGIT (H)
TIGIT in the epithelial component of thyroid neoplasms
TIGIT expression in the epithelial component of variable thyroid lesions is summarized in Table 1. No epithelial expression was detected in AG, FTA (Figs. 1C and 1D) and DTCs: FTC and PTC (Figs. 1E and 1F). C cell-derived neoplastic cells were positive in four of five MTCs (Table 1, Figs. 1G and 1H). Three of 12 (25.0%) PDTCs were focally positive (Tables 1 and 2, Fig. 2). ATC cells were positive in 17 of 31 cases (54.8%) (Tables 1 and 3, Figs. 3–5). Epithelial expression of TIGIT in ATC was confirmed by pancytokeratin AE/AE3 staining in serial sections (Fig. 4). The proportion of the TIGIT-positive TC was significantly higher in ATCs and high-grade TCs (PDTC and ATC) than in DTCs (P < 0.05) (Table 1).
Table 2.
Summary of TIGIT expresion in neoplastic cells of 12 poorly differentiated thyroid carcinomas (PDTCs)
| case no | positive tumor cell morphology | TMN stage | immune cell | IHC score | ||
|---|---|---|---|---|---|---|
| type of infiltrate | density | predominant cell type | ||||
| 1 | round nuclei | T4aN0M1 | scattered | low | lymphcyte | 1 + |
| 2 | large and convoluted nuclei | T3NXM0 | focal | low | lymphcyte | 1 + |
| 3 | round neclei | T3NXM0 | scattered | low | lymphcyte | 1 + |
| 4 | round nuclei | T3N1bM0 | scattered | low | lymphcyte | 0 |
| 5 | round or oval nuclei | T3N1bM1 | scattered | low | lymphcyte | 0 |
| 6 | round or oval nuclei | T3NXM0 | scattered | low | lymphcyte | 0 |
| 7 | round or oval nuclei | T2NXN0 | scattered | low | lymphcyte | 0 |
| 8 | round nuclei | T3NXM0 | scattered | low | lymphcyte | 0 |
| 9 | round or oval nuclei | T3N1aM0 | scattered | low | lymphcyte | 0 |
| 10 | round and oval nuclei | T3NXM0 | scattered | low | lymphcyte | 0 |
| 11 | large and convoluted nuclei | T3N1aM0 | scattered | low | lymphcyte | 0 |
| 12 | round or oval nuclei | T3N0M0 | focal | high | lymphcyte | 0 |
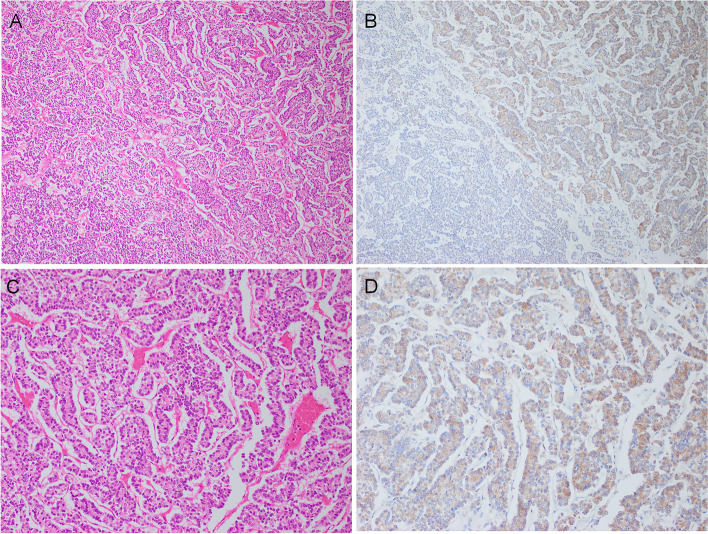
Fig. 2.
Representative immunohistochemical results of TIGIT in poorly differentiated thyroid carcinoma (PDTC). Neoplastic cells were arranged in a solid or trabecular architecture (A) (H&E, low magnification). About half of PDTC cells were positive for TIGIT in this area (upper and right) (B). TIGIT-positive area of PDTC (H&E, high magnification) (C). Cytoplasmic TIGIT reactivity was observed (D)
Table 3.
Summary of TIGIT expression in 34 anaplastic thyroid carcinomas (ATCs)
| case no | predominat tumor cell morpology | TMN stage | positive tumor cell morphology | immune cell | IHC score | ||
|---|---|---|---|---|---|---|---|
| type of infiltrate | density | predominat cell | |||||
| 1 | pleomorphic/giant | T4bN1bM1 | pleomorphic/gaint | diffuse | high | macrophage | 2 + |
| 2 | pleomorphic/giant | T4bN0MO | pleomorphic/gaint | diffuse | high | lymphocyte | 2 + |
| 3 | composite (FTC) | T4bN1bM0 | pleomorphic/gaint | diffuse | high | lymphocyte | 2 + |
| 4 | pleomorphic/giant | T4aN0M0 | pleomorphic/gaint | diffuse | high | neutrophil | 2 + |
| 5 | pleomorphic/giant | T4bN0MO | pleomorphic/gaint | diffuse | high | lymphocyte | 1 + |
| 6 | epithelioid/squamoid | T4bN1aM1 | pleomorphic/gaint | diffuse | high | lymphocyte | 1 + |
| 7 | spindle | T4bN1bM0 | pleomorphic/gaint | diffuse | low | lymphocyte | 1 + |
| 8 | composite (PTC) | T4bN1M1 | pleomorphic/gaint | diffuse | low | lymphocyte | 1 + |
| 9 | pleomorphic/giant | T4aN1bM0 | pleomorphic/gaint | diffuse | low | lymphocyte | 1 + |
| 10 | pleomorphic/giant | T4aNXMX | pleomorphic/gaint | diffuse | high | neutrophil | 1 + |
| 11 | epithelioid/squamoid | T4aN1M0 | pleomorphic/gaint | diffuse | high | lymphocyte | 1 + |
| 12 | spindle | T4aN1M0 | pleomorphic/gaint | diffuse | high | lymphocyte | 1 + |
| 13 | spindle | T4aN1bM0 | pleomorphic/gaint | diffuse | high | lymphocyte | 1 + |
| 14 | spindle | T4aNXMX | pleomorphic/gaint | diffuse | high | lymphocyte | 1 + |
| 15 | composite (FTC) | T3bNXM0 | pleomorphic/gaint | diffuse | high | macrophage | 1 + |
| 16 | pleomorphic/giant | TXNXM0 | pleomorphic/gaint | focal | low | lymphocyte | 1 + |
| 17 | spindle | N/A | pleomorphic/gaint | diffuse | low | lymphocyte | 1 + |
| 18 | pleomorphic/giant | T4bN1bM1 | diffuse | low | neutrophil | 0 | |
| 19 | epithelioid/squamoid | T4bN1M1 | diffuse | high | lymphocyte | 0 | |
| 20 | pleomorphic/giant | T4aN1aM0 | diffuse | low | macrophage | 0 | |
| 21 | spindle | T4aN1bM1 | scattered | low | lymphocyte | 0 | |
| 22 | epithelioid/squamoid | T2N1aM1 | diffuse | high | lymphocyte | 0 | |
| 23 | spindle | T1aNXM1 | diffuse | high | lymphocyte | 0 | |
| 24 | composite (PTC) | TXN1bM0 | diffuse | low | lymphocyte | 0 | |
| 25 | composite (PTC) | TXNXM1 | diffuse | high | macrophage | 0 | |
| 26 | composite (FTC) | N/A | diffuse | high | lymphocyte | 0 | |
| 27 | epithelioid/squamoid | N/A | diffuse | low | lymphocyte | 0 | |
| 28 | epithelioid/squamoid | N/A | focal | low | lymphocyte | 0 | |
| 29 | epithelioid/squamoid | N/A | diffuse | low | lymphocyte | 0 | |
| 30 | epithelioid/squamoid | N/A | diffuse | high | lymphocyte | 0 | |
| 31 | others (rhabdoid) | N/A | diffuse | low | macrophage | 0 | |
N/A: Not available
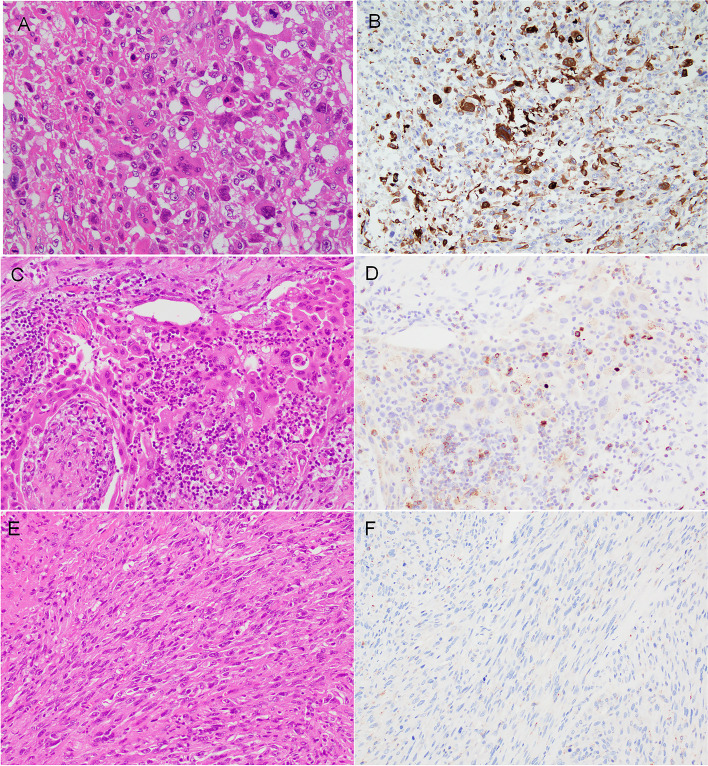
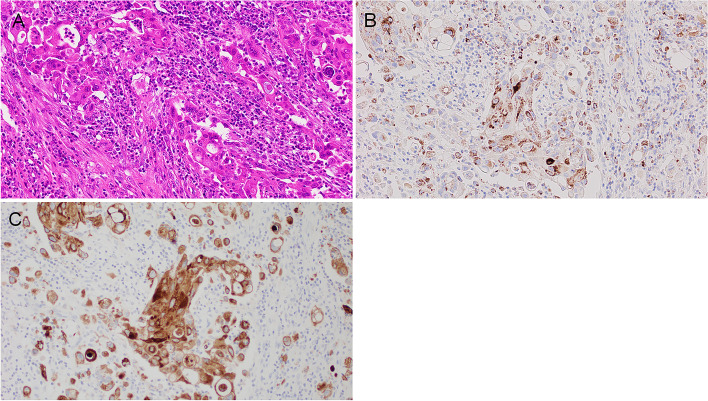
Fig. 3.
Representative results of TIGIT immunostaining in anaplastic thyroid carcinoma (ATC). Pleomorphic/giant cell-shaped tumor cells (H&E staining) (A). ATC cells were stained with strong intensity (B). Epithelioid/squamoid tumor cells (H&E) (C). The tumor cells were negative for TIGIT, while scattered lymphocytes were positive (D). Neoplastic cells displayed a spindle-shaped, sarcomatoid feature (H&E) (E). These ATC cells uniformly lacked immunoreactivity for TIGIT (F)
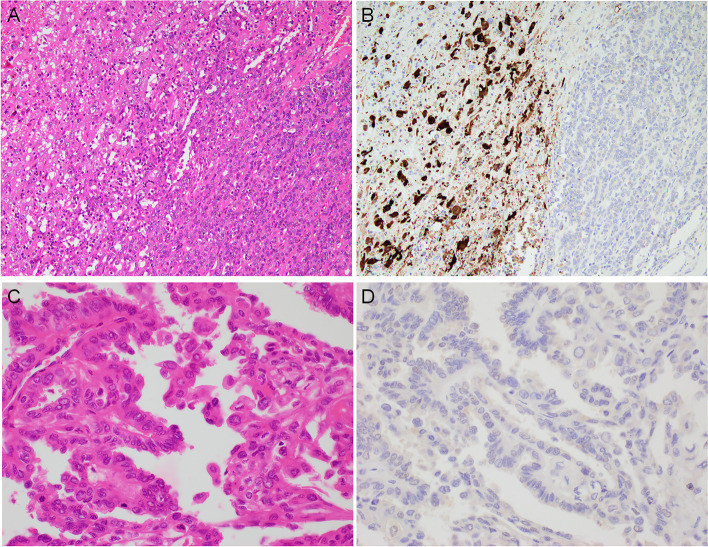
Fig. 5.
TIGIT immunohistochemical results of composite type ATCs. In H&E-stained sections, ATC components showing pleomorphic/giant cell shape (left) were concomitantly seen with adjacent FTC components (right) (A). The FTC cells were completely negative for TIGIT, while ATC cells were starkly positive (B). In PTC-composite ATC, the PTC cells showed tall cell morphology with partial hobnail structures (H&E) (C). These neoplastic cells completely lacked immunoreactivity for TIGIT (D)
Fig. 4.
Microphotographs of ATC on H&E- (A), TIGIT- (B), and AE1/AE3- (C) stained serial sections. ATC cells harbored pleomorphic large nuclei and abundant eosinophilic cytoplasm with a heavy infiltrate of immune cells (A). Tumor cells were concurrently positive for TIGIT (B) and pancytokeratin AE1/AE3 (D)
TIGIT expression in cancer cell of PDTC
Table 2 summarizes TIGIT expression in the epithelial component of PDTCs. The PTCD cells were arranged in a solid/trabecular/insular (STI) pattern. The neoplastic cells uniformly present as round or oval in most PDTCs (Fig. 2). TIGIT-positive PDTC cells occupied less than half of the whole tumor in all positive PDTCs. All positive PDTCs had IHC scores of 1 + . In regards with profile of intra-tumoral immune cell and tumor stage, there was no significant difference between TIGIT-positive group and -negative.
Microscopic features of ATC cell
Examined ATCs microscopically displayed a wide variety of tumor cell morphology, and each cell type was concomitant to a variable degree (Table 3). Based upon the morphological features of neoplastic cell, we briefly categorized five types: 1) spindle, 2) pleomorphic/giant, 3) epithelioid/squamoid, 4) composite, and 5) others. Spindle cell, pleomorphic/giant, and epithelioid/squamoid types constituted 7, 9, and 8 of 31 ATCs, respectively. A DTC component (3 PTC and 3 FTC) coexisted in 6 ATCs. These ATCs were thus classified as "composite" type. One ATC preferentially had a rhabdoid shape and was categorized into "others".
TIGIT expression in ATC
A summary of epithelial TIGIT expression in 31 ATCs is shown in Table 3. The expression clearly depended upon cell morphology (Fig. 3, Table 3). Pleomorphic/giant cell-shaped ATC cells showed immunoreactivity in 17 of 31 ATCs in various proportions (Figs. 3A and 3B, Table 3). These cells had IHC scores of 1 + and 2 + in 13 and four ATCs. Epithelioid/squamoid ATC cells were prone to show a nonspecific reactivity due to abundant eosinophilic cytoplasm and were interpreted to be negative for TIGIT (Figs. 3C and 3D). Spindle-shaped, sarcomatoid ATC cells were completely negative for TIGIT (Figs. 3E and 3F).
Intra-tumoral immune cells, such as lymphocyte, macrophage, and neutrophil, were diffuse and dense in 12 of 17 TIGIT-positive ATCs (70.6%) in contrast to six of 14 TIGIT-negative (42.9%). Regarding tumor stage, 14 of 15 TIGIT-positive ATCs (93.3%) were operated at pT4 stage, while four of six -negative (66.7%) in available cases.
TIGIT expression in composite ATCs
In six composite cases, the DTC component (three FTCs and three PTCs) was included in the same immunohistochemically stained sections. TIGIT expression was not detected in any FTC component of composite ATCs (Figs. 5A and 5B). TIGIT was also negative for three PTC components, although the components were high-grade PTC: tall cell variants with hobnail features (Figs. 5C and 5D). In summary, all composite DTC areas failed to display immunoreactivity for TIGIT.
Discussion
In this study, we examined the TIGIT expression in TCs via immunohistochemistry. Our findings showed that the expression in tumor cells was detected in most MTC, about half of ATCs, and PDTCs with lower frequency despite being negative in benign lesions/tumors and DTCs. Of note, tumor cell morphology seemed to determine TIGIT expression in ATC.
Recently, TIGIT expression has been intensively examined in malignant tumors of many organs: skin melanoma [20], Hodgkin’s lymphoma [21], hepatocellular carcinoma [22], glioblastoma [23], and gastric cancer [24]. According to these previous reports, its expression is elevated in accordance with advanced tumor stage, tumor aggressiveness, poor tumor differentiation, and high propensity for lymph node metastasis. It is also adversely correlated with patient clinical outcomes. Like these carcinomas, TIGIT mRNA expression was upregulated in ATCs [16]. Our findings confirmed the evidence by immunohistochemistry. Blockade of TIGIT with a monoclonal antibody increases antitumoral effector T cells and delays tumor growth in vitro [25]. Thus, TIGIT-targeted therapy may also suppress ATC progression and proliferation and improve the survival of ATC patients. Interestingly, our IHC revealed that four of five MTCs expressed TIGIT, while low frequency (3.0%) of MTC reported in a large series by IHC [26]. According to the report, the same monoclonal antibody against TIGIT was used for immunostaining, but combined positive score (CPS) was adopted for the evaluation of IHC results. All MTCs analyzed in this study were composed of dense neoplastic and scant immune cells and, we counted only TIGIT-positive neoplastic cells. Therefore, the results might be underestimated in the report.
Most previous reports documenting TIGIT expression have evaluated immune cells, not tumor cells [20–24]. In our study, high TIGIT expression was tended to be observed in ATCs with diffuse and dense infiltrate of immune cell and advanced TMN stage. Therefore, effector T cell may exert in TIGIT-high ATCs, although functional exhaustion of immune cells is difficult to estimate merely from morphological aspects. Several reports have found that TIGIT functionally impairs glucose metabolism in CD8 effector T cells and subsequently induces their exhaustion and inhibits their antitumor effect [24, 25]. TIGIT inhibition promotes effector T cell survival via activation of the AKT/mTOR pathway. Cytokine production is also increased by TIGIT blockade [24]. Another previous report provides evidence that TIGIT deprives NK cell function through ZAP70 and ERK1/2 [27]. In addition, ATC showed an immunosuppressed microenvironment with exhausted immune cells and decreased cytokine production [16]. An effective anti-tumor immune response may also be provoked in ATC by metabolic impairment of intra-tumoral TIGIT-positive immune cells.
Regarding TIGIT in neoplastic cells, Sun et al. reported that lung adenocarcinoma cell highly expressed TIGIT by Western blot analysis using cell lines in accordance with unfavorable clinical outcomes [9]. Several researchers have reported that tumor cell expressed other immune checkpoint receptors. Non-small-cell lung cancer cells and uveal melanoma cells expressed PD-1 [28, 29]. More recently, PD-1 expression in cancer cells have been verified in ATC [30]. In addition, cell lines from various malignant tumors generate CTLA-4, which promotes apoptosis via interaction with its ligand [31]. TIGIT is similar to CTLA-4, since it participates in a complex involving other immune checkpoint receptors (CD96/TACTILE and CD112R/PVRIG) and a competing costimulatory receptor (CD226/DNAM-1) sharing multiple ligands such as CD155 and CD122 [8]. Blockade of PD-1/PDL1 and CTLA-4 in neoplastic cells clinically has satisfactory clinical effectiveness. Therefore, it may be beneficial to develop inhibitory treatments that target TIGIT, which is expressed in MTC, PDTC, and ATC cells.
The immune system plays a role in regulating tumor initiation and progression in cancer [32]. In this study, TIGIT expression was detected in high-risk TCs (PDTC and ATC), while DTC cells were thoroughly negative. In particular, ATC cells more frequently displayed TIGIT immunoreactivity than PDTC. Interestingly, DTC components (PTC and FTC) were devoid of TIGIT in composite ATC carcinomas, although the composite PTC components displayed high-grade features, tall cell with hobnail structure. Interestingly, PDTCs infrequently expressed TIGIT. Therefore, it can raise possibility that TIGIT expression increases according to aggressiveness of follicular cell-derived thyroid cancer. TIGIT immunoreactivity may have a diagnostic value in discriminating higher risk TC including MTC from DTC.
In regard to other immune checkpoint proteins, ATC shows high PD-1 expression on tumor-infiltrating immune cells, and tumor cells highly express its ligand, PD-L1 [12]. PD-L1 expression is apparently higher in ATC than in DTC at the protein and mRNA levels [13, 14]. High PD-L1 in tumor cells and low PD-1 in immune cells are significantly related to worse prognosis [12]. Moreover, PD-1 blockade in ATC cell inhibits tumor growth via the intrinsic SHP2/Ras/MAPK signaling pathway [29]. Since the PD-1/PD-L1 pathway seems to be concurrently activated in ATC, a combination of TIGIT and PD-1/PD-L1 blockade may exert synergistic anti-tumor effects on ATC cell.
Conclusions
Immunohistochemical TIGIT expression was detected in neoplastic cells of MTC with high prevalence, about half of ATC, a quarter of PDTC. It is necessary to examine other checkpoints that constitute the complex regulatory network of TIGIT in MTC and high-grade TC, especially ATC.
Acknowledgements
We would like to thank Masaki Takahashi, Tokyo Women’s Medical University Yachiyo Medical Center (TYMC), for his excellent technique of immunohistochemistry and Takashi Amano, Ito Hospital, for his kind assistance of sample collection and preparation.
Abbreviations
- TIGIT
T-cell immunoreceptor with immunoglobulin and tyrosine-based inhibitory motif domains
- ATC
Anaplastic thyroid carcinoma
- IHC
Immunohistochemistry
- PDTC
Poorly differentiated thyroid carcinoma
- DTC
Differentiated thyroid carcinoma
- PTC
Papillary thyroid carcinoma
- MTC
Medullary thyroid carcinoma
- FTC
Follicular thyroid carcinoma
- TC
Thyroid carcinoma
- PD-1
Programmed death receptor-1
- PD-L1
Programmed death receptor-ligand 1
- CTLA-4
Cytotoxic T-lymphocyte antigen-4
Authors' contributions
TN wrote and revised the manuscript content. RK collected samples and participated in pathological diagnoses. Others reviewed and edited. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology, Japan: a Grant in-Aid for Scientific Research to Tadao Nakazawa (19K07432).
Availability of data and materials
The datasets are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients prior to surgery. The thyroid tissues were collected in accordance with the protocol approved of local ethics committees at Tokyo Women’s Medical University (Shinjuku, Tokyo, Japan; no. 5580) and Ito Hospital (Shibuya, Tokyo, Japan; no. 271). This entire study was approved by the ethics committee of Tokyo Women’s Medical University (Shinjuku, Tokyo, Japan; no. 5580).
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Keutogen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015;4(1):44–45. doi: 10.3978/j.issn.2227-684X.2014.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moriano E, Romei C, Biagini A, et al. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017;13(11):644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd RV, Osamura RY, Klöpple, Rosai J, eds. World Health Organization classification of tumours of endocrine organs. Tumours of the thyroid. Lyon: IARC Press; 2017. p 65-143.
- 4.Matias-Guiu X, De Lellis R. Medullary thyroid carcinoma: a 25-year perspective. Endocr Pathol. 2014;25(1):21–29. doi: 10.1007/s12022-013-9287-2. [DOI] [PubMed] [Google Scholar]
- 5.Iyer PC, Dadu R, Gure-Monroe M, et al. Salvage pembrolizumab added to kinase inhibitor therapy for the treatment of anaplastic thyroid carcinoma. J Immunother Cancer. 2018;6(1):68. doi: 10.1186/s40425-018-0378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capdevila J, Wirth LJ, Ernst T, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol. 2022;38(23):2620–2627. doi: 10.1200/JCO.19.02727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatashima A, Archambeau B, Armbruster H, et al. An evaluation of clinical efficacy of immune checkpoint inhibitors for patients with anaplastic thyroid carcinoma. Thyroid. 2022;32(8). 10.1089/thy.20022.0073. [Online ahead of print] [DOI] [PubMed]
- 8.Harjunpää H, Guilerey C. TIGIT emerging immune checkpoint. Clin Exp Immunol. 2020;200(2):108–111. doi: 10.1111/cei.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Luo J, Chen Y, et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma) Int Immunopharmacol. 2020;80:e106198. doi: 10.1016/j.intimp.2020.106198. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Barletta JA. Anaplastic thyroid carcinoma. Semin Diagn Pathol. 2020;37(5):248–256. doi: 10.1053/j.semdp.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Ryder M, Ghossein RA, Ricarte-Filho JCM, Knauf JA, Fagin JA. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr Relat Cancer. 2008;15(4):1069–1074. doi: 10.1677/ERC-08-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caillou B, Talbot M, Weyemi U, et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS ONE. 2011;6(7):e0022567. doi: 10.1371/journal.pone.0022567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chintakuntiawar AV, Rumilla KM, Smith CY, et al. Expression of PD-1 and PD-L1 in anaplastic thyroid carcinoma patients treated with multimodal therapy: results from a retrospective study. J Clin Endocrinol Metab. 2017;6(102):1943–50. doi: 10.1210/jc.2016-3756. [DOI] [PubMed] [Google Scholar]
- 14.Ahn S, Kim TH, Kim SW, et al. Comprehensive screening for PD-L1 expression in thyroid cancer. Endocr Relat Cancer. 2017;24(2):97–106. doi: 10.1530/ERC-16-0421. [DOI] [PubMed] [Google Scholar]
- 15.Brauner E, Gunda V, Borre PV, et al. Combining BRAF inhibitor and anti PD-L1 antibody dramatically improves tumor regression and anti-tumor immunity in an anaplastic thyroid cancer. Oncotarget. 2016;7(13):17194–17211. doi: 10.18632/oncotarget.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannini R, Moretti S, Ugolini C, et al. Immune profiling of thyroid carcinomas suggests the existence of two major phenotypes: an ATC-like and PDTC-like. J Clin Endocrinol Metab. 2019;104(8):3557–3575. doi: 10.1210/jc.2018-01167. [DOI] [PubMed] [Google Scholar]
- 17.Brierley JB, Gospodarowicz MK, Witterkind C, eds. TMN Classicication of Malignant Tumours, 8th ed. Thyroid Gland, John Wilery & Sons. p 51-53.
- 18.Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: The Turin Proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31(8):1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 19.Lai WA, Hang JF, Lui CY, et al. PAX8 expression in anaplastic thyroid carcinoma is less than those reported in early studies: a multi-institutional study of 182 cases using the monoclinal antibody MRG-50. Virchows Arch. 2020;476(3):431–437. doi: 10.1007/s00428-019-02708-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee WJ, Lee YJ, Choi ME, et al. Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytyes. J Am Acad Dermatol. 2019;81(1):e1209. doi: 10.1016/j.jaad.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Blessin NC, Simon R, et al. Expression of the immune checkpoint receptor TIGIT in Hodgkin’s lymphoma. BMC Cancer. 2018;1(18):e1209. doi: 10.1186/s12885-018-5111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duan X, Liu J, Cui J, et al. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma. Mol Med Rep. 2019;20(4):3773–3781. doi: 10.3892/mmr.2019.10641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucca LE, Lerner BA, Park C, et al. Differential expression of the T-cell inhibitor TIGIT in glioblastoma and MS. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e712. doi: 10.1212/NXI.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Zhang H, Han F, et al. CD115T/TIGIT regulates CD8+ T cell metabolism and promotes tumor progression in human gastric cancer. Cancer Res. 2017;77(22):6375–6388. doi: 10.1158/0008-5472.CAN-17-0381. [DOI] [PubMed] [Google Scholar]
- 25.Hirayama A, Kami K, Sugimoto M, et al. Quantative metabolome profiling of colon and stomach cancer microenvironment by capillary electropherasis time-of flight mass spectrometry. Cancer Res. 2009;69(11):358–373. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- 26.Shi X, Li CW, Tan LC, et al. Immune co-inhibitory receptors PD-1, CTLA-4, TIM-3, LAG-3, and TIGIT in medullary thyroid cancers: a large cohort study. J Clin Endocrinol Metab. 2021;106(1):120–132. doi: 10.1210/clinem/dgaa701. [DOI] [PubMed] [Google Scholar]
- 27.Sarhan D, Cichoki F, Zhang B, et al. Adaptive NK cells with low TIGIT expression are inherently resistant to myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5606–76. [DOI] [PMC free article] [PubMed]
- 28.lncecco AD, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non small cell lung cancer patients. Br J Cancer. 2015;112(1):85–92. [DOI] [PMC free article] [PubMed]
- 29.Jiang Z, Yan Y, Dong J, Duan L. PD-1 expression on uveal melanoma induces tumor proliferation and predicts poor patient survival. Int J Biol Markers. 2020;35(3):50–8. [DOI] [PubMed]
- 30.Loitti F, Kumar N, Prevete N, et al. PD-1 delays tumor growth by inhibiting an intrinsic SHP2/Ras/MAPK signaling in thyroid cancer cells. J Exp Clin Cancer Res. 2021;40(1): e22. [DOI] [PMC free article] [PubMed]
- 31.Contardi E, Palnisano GL, Tazzari PL, et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117(4):538–50. [DOI] [PubMed]
- 32.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.